Abstract
Introduction:
The nicotine metabolite ratio (NMR), the ratio of trans-3′-hydroxycotinine (3-HC) to cotinine, has been used as a biomarker of the rate of CYP2A6-mediated nicotine metabolism. While stable in smokers who maintain constant smoking consumption, since smoking has been shown to inhibit nicotine metabolism and this inhibition could be mediated by the nicotine in the smoke, NMR could change during nicotine reduction. The objective of this study was to determine the reproducibility (or stability) of plasma NMR in smokers of progressively reduced nicotine content (RNC) cigarettes.
Methods:
We analyzed data from subjects in a clinical trial of smoking progressively RNC cigarettes. Plasma NMR in 30 smokers whose plasma cotinine levels had decreased by at least 50% from the use of the first test cigarette (12mg nicotine content) to the final test cigarette (1mg nicotine content) was measured on 4 occasions over a period of 24 weeks.
Results:
Plasma cotinine and 3-HC decreased by an average of 85% and 84%, respectively, following the use of the first type of RNC cigarette to the last type. Plasma NMR had an average absolute change of 28.5% over the same period. Using repeated measures analysis, changes in plasma NMR over time were not significant with or without controlling for the effects of age, body mass index, gender, and race (p = .24 and p = .23, respectively). The reliability coefficient for repeated measurements of plasma NMR was .72. The average within-subject coefficient of variation for plasma NMR was 21.6% (SD = 12.0%).
Conclusion:
The plasma NMR is relatively stable over time as nicotine levels decline in smokers of progressively RNC cigarettes.
INTRODUCTION
Nicotine plays an essential role in producing tobacco dependence and regulating smoking behavior (Benowitz, 2010). Understanding the pattern, extent, and variation in nicotine metabolism and clearance is therefore important to understanding and addressing tobacco dependence. Given that nicotine is metabolized primarily by the cytochrome P450 2A6 (CYP2A6) enzyme (Hukkanen, Jacob, & Benowitz, 2005), genotyping CYP2A6 is one approach to characterize the rate of nicotine metabolism. However, since genotyping does not account for the integrated effects of endogenous (e.g., estrogen) and exogenous (e.g., diet) influences on nicotine metabolism, a more precise approach to estimate the rate of nicotine metabolism is to measure the nicotine metabolite ratio (NMR), which is the ratio of trans-3′-hydroxycotinine (3-HC) to cotinine. Cotinine, the major proximate metabolite of nicotine is further metabolized to 3-HC almost exclusively by CYP2A6 (Nakajima et al., 1996). The ratio of metabolite to parent should then reflect activity of the enzyme.
The NMR, which can be measured in saliva, urine, or plasma and is a validated measure of nicotine clearance (Dempsey et al., 2004), is expected to be stable over time among smokers who maintain constant smoking consumption. This is because the elimination rate of 3-HC is formation limited given that cotinine has a relatively long half-life (averaging 16hr) and 3-HC has a shorter half-life (5hr). Indeed, we and others have shown that the NMR measured in various biological media is stable in ad libitum smokers over study periods ranging from a few days to 44 weeks (Lea, Dickson, & Benowitz, 2006; Mooney et al., 2008; St.Helen et al., 2012). Nonetheless, it is possible that the NMR may not be a reliable measure of the rate of nicotine metabolism in smokers engaged in smoking reduction. Previous studies have shown that tobacco smoke inhibits nicotine metabolism and this inhibition could be mediated by the nicotine in smoke (Benowitz & Jacob, 1993). Therefore, there might be a change in the NMR as nicotine exposure is reduced. One study reported that the urine NMR was relatively stable in smokers during a 12-week smoking reduction period, during which the smokers were allowed to use nicotine replacement therapy (NRT) as desired (Mooney et al., 2008). Another study showed that after controlling for nicotine intake, the urine NMR did not differ significantly in smokers after 12 weeks of decreased cigarette consumption (Berg, Mason, Boettcher, Hatsukami, & Murphy, 2010). We are aware of no data on the stability of the NMR in smokers with reduced nicotine intake over time while smoking reduced nicotine content (RNC) cigarettes.
The objective of our study was to assess the reproducibility (stability) of plasma NMR in smokers of progressively RNC cigarettes during a 24-week period. Given the increasing use of the NMR in treatment and research (Strasser et al., 2011; West, Hajek, & McRobbie, 2011), data on its reproducibility in nicotine reducers will inform its applicability as a biomarker of nicotine metabolism.
METHODS
Study, Subjects, and Experimental Protocol
This study was a clinical trial of RNC cigarettes in which smokers were randomly assigned to a control or research arm after a 2-week baseline period in which they smoked their usual brand of cigarettes. The control group smoked their usual brand of cigarettes throughout the study, and the research group smoked five types of progressively lower RNC cigarettes (12, 8, 4, 2, and 1mg nicotine). The first four levels of RNC cigarettes were smoked for 4 weeks each, and the lowest RNC cigarette was smoked for 6 months. The present analysis focuses on the first 24 weeks of the RNC group. Details of the parent study have been described in a previous publication (Benowitz et al., 2012).
Subjects were recruited by newspaper advertisements and were healthy smokers of 10 or more cigarettes per day (CPD) for the past year. We limited our analysis to smokers assigned to the RNC group whose plasma cotinine levels had decreased by at least 50% by the time they were assigned the last level of RNC (1mg nicotine) compared with when they smoked the first level of RNC (12mg nicotine). This was done to ensure that the smokers included in the analysis demonstrated substantially reduced nicotine intake. Plasma samples collected at Weeks 1, 8, 16, and 24 were assayed for concentrations of cotinine and 3-HC. The study was approved by the Institutional Review Board at the University of California, San Francisco, and written informed consent was obtained from each participant.
Analytical Chemistry
Analyses of plasma cotinine and 3-HC concentrations were carried by liquid chromatography-tandem mass spectrometry as described previously (Jacob et al., 2011). In quality control (QC) tests of 176 QC plasma samples over a time span of about 6 years, the coefficients of variation (CVs) for assays of plasma cotinine, 3-HC, and NMR were 8.7%, 10.7%, and 8.2%, respectively. Additional precision, accuracy, and limit of quantitation data for the assays used are presented elsewhere (Jacob et al., 2011).
Statistical Analysis
We assessed the stability (reproducibility) or changes in plasma NMR over time three ways: using the reliability coefficient, repeated measures analysis, and CV. Plasma NMR data were approximately log-normally distributed and therefore log-transformed for statistical tests. We computed a reliability coefficient, the ratio of between-sample variance to the sum of between- and within-sample variances, for plasma log-NMR from mixed-model analysis. We used mixed effects repeated measures analysis with subjects as a random effect and no covariates included. Age, body mass index (BMI), gender, and race, which were obtained at baseline, were included as covariates in another model. Pairwise comparisons between plasma NMR at all measurement occasions were performed, and we used Tukey’s method to adjust the error rate. Post-hoc power analysis of 30 subjects, 4 measurement occasions, and correlations of .6 between measurements showed that a repeated measures analysis had a power of 82.2% to detect significant changes in plasma NMR across the measurement occasions. Finally, we computed the average within-subject CV for plasma NMR (untransformed data). All analyses were carried out using SAS v. 9.3 (SAS Institute, Inc.), and statistical tests were considered significant at α = .05.
RESULTS
Of 53 smokers enrolled in the RNC arm of the clinical trial, plasma cotinine levels decreased by at least 50% during tapering in 30 smokers. Of these, 17 were females and the races were as follows: White (n = 22), Black (n = 3), Asian (n = 1), American Indian-Alaska Native (n = 1), and mixed race (n = 3). At baseline, the average age was 40.0±11.6 years (mean ± SD), and the average BMI was 27.5±7.3. The average baseline cigarette consumption was 21.0±7.4 CPD. On average, the participants had a moderate Fagerström Test for Nicotine Dependence score of 5.6±1.8.
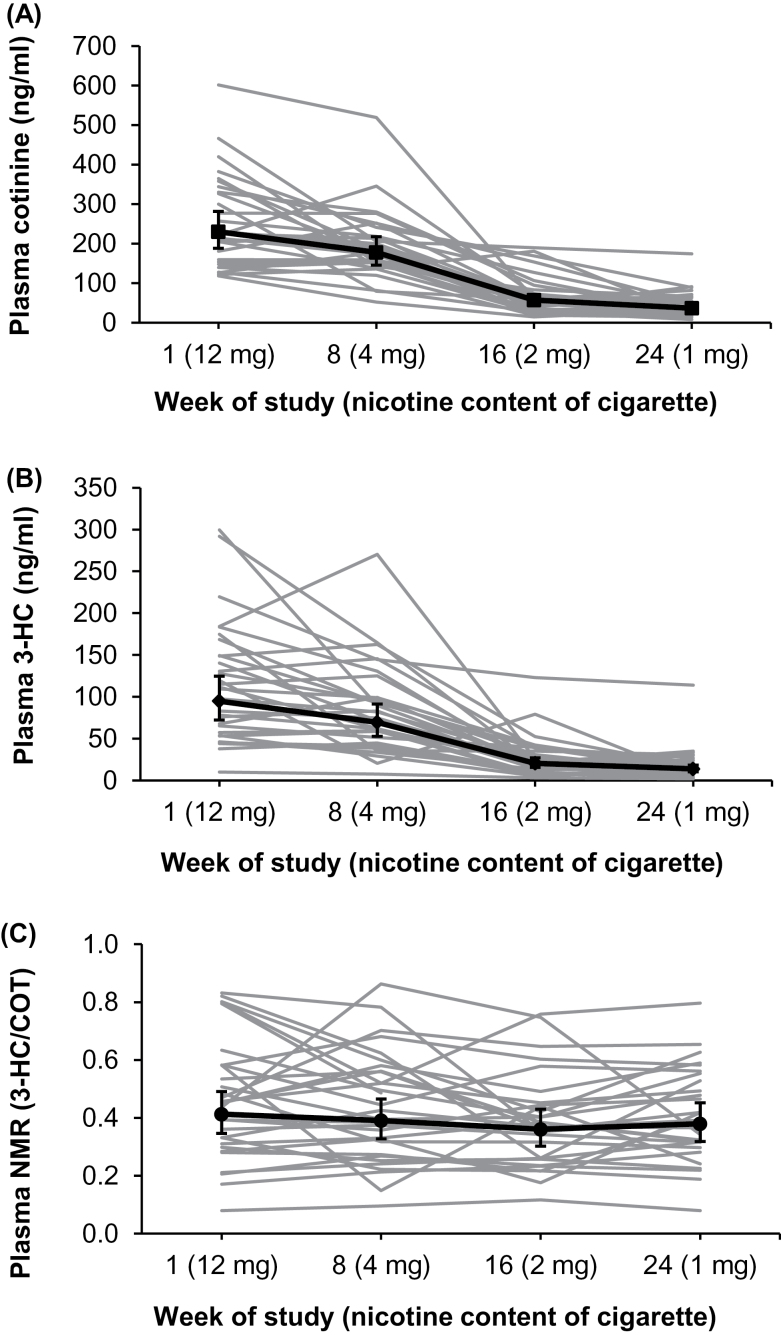
The geometric means and 95% CIs of plasma cotinine, 3-HC, and NMR (including individual subject cotinine, 3-HC, and NMRs over time) are presented in Figure 1. Twenty-nine participants had biomarker data for all four measurement occasions and one had data for three of four times. Plasma cotinine and 3-HC decreased by an average of 85% and 84%, respectively, following the use of the first type of RNC cigarette to the last type. Plasma NMR had an average absolute change of 28.5% over the same period. The distribution of the absolute change in plasma NMR following the use of the first type of RNC cigarette to the last type was as follows: 0%–10% change, 6 subjects; 11%–20%, 3 subjects; 21%–30%, 8 subjects; 31%–40%, 6 subjects; 41%–50%, 4 subjects; and >50%, 3 subjects.
Figure 1.
Plasma concentrations of cotinine over a 24-week period in smokers of progressively reduced nicotine content cigarettes showing individual subjects and geometric means and 95% CI for all subjects (A); plasma trans-3′-hydroxycotinine concentrations for individual subjects and geometric means and 95% CI for all subjects (B); and individual plasma nicotine metabolite ratio and geometric mean and 95% CI for all subjects (C).
The reliability coefficient for repeated measurements of plasma NMR was .72. Changes in plasma NMR over the 24 weeks were nonsignificant with or without covariates included in the model (p = .24 and p = .23, respectively). Further, the average within-subject CV for plasma NMR was 21.6% (SD = 12.0%). The distribution of within-subject CVs for plasma NMR was as follows: 0%–10% CV, 4 subjects; 11%–20%, 12 subjects; 21%–30%, 9 subjects; 31%–40%, 1 subject; 41%–50%, 3 subjects; and >50%, 1 subject.
To test whether changes in NMR were related to changes in nicotine or cigarette consumption, we computed the correlations between within-subject CV of plasma NMR, percent change in plasma NMR from smoking the first type of RNC to the last type, and percent change in plasma cotinine from the first RNC to the last, as well as test whether CPD changed over time. The within-subject CV for plasma NMR and the percent change in plasma NMR were not significantly related to the percent change in plasma cotinine (Spearman correlation [r s]: –.05, p = .78 and .02, p = .90, respectively). Average self-reported number of cigarettes smoked over past 24hr before the four measurement occasions were as follows: 22.7±7.7, 23.8±10.3, 22.3±9.5, and 21.7±10.3, respectively, and did not differ significantly from each other in pairwise comparisons (p > .51).
DISCUSSION
In a 24-week study of 30 smokers of progressively RNC cigarettes, our results indicate that measurement of plasma NMR at one occasion seems to be a reliable estimate of the true NMR value for populations of nicotine reducers. The variability within subjects accounted for about 28% of the total variability observed in plasma NMR measurements, results that were comparable with the variability observed in plasma NMR in ad libitum smokers (St.Helen et al., 2012). The average within-subject CV for plasma NMR (22%) also indicated relative stability, and we saw no significant changes in plasma NMR over time using repeated measures analysis.
The stability of the plasma NMR in nicotine reducers due to smoking of RNC cigarettes has not been reported previously. As mentioned before, one study showed relative stability of the urine NMR in smokers over a 12-week smoking reduction period, which followed 8 weeks of ad libitum smoking (Mooney et al., 2008). However, the subjects in that study concurrently used NRT for nicotine not obtained through smoking. As a result, the subjects in that study cannot be accurately characterized as nicotine reducers. A second study showed that urine NMR was stable after controlling for nicotine intake in smokers who reduced their nicotine intake through smoking reduction (Berg et al., 2010).
While we observed relatively stable NMR on average, substantial variation was observed in a few subjects (Figure 1). For example, plasma NMR changed by more than 50% during nicotine tapering in three subjects (10% of study population), and the intraindividual CV was greater than 50% in one subject (3.3% of study population). There are several possible sources of intraindividual variability in NMR of nicotine reducers. First, since smoking inhibits nicotine metabolism, reduction in cigarette consumption over time might lead to enhanced rates of nicotine metabolism, reflected as higher plasma NMRs. However, the observation of no average change in NMR among all subjects and no significant correlations between percent changes in plasma cotinine and percent changes and CV of plasma NMR argue against this explanation. Second, since the stability of NMR depends on steady state levels of cotinine and therefore consistent smoking behavior and cigarette nicotine content, changes in cigarette consumption, smoking pattern, or nicotine content of cigarettes in the days before sample collection might lead to 3-HC not reaching steady state and increased variability in plasma NMR (Lea et al., 2006). However, cigarette consumption, measured as CPD over past 24hr before sample collection, did not change over the study period. Temporal changes in 3-HC clearance from other competing pathways such as glucuronidation or renal clearance could also contribute to interindividual variability in NMR (Hukkanen et al., 2005). Variations in laboratory measurements of these biomarkers may also contribute to the overall variation in plasma NMR, although the assays used in this study are highly precise (CVs ≤ 10.7%) and likely contributed minimal variation to plasma NMR.
CONCLUSION
Repeated measurements of plasma NMR were relatively stable during a 24-week period in a sample of smokers of progressively RNC cigarettes. While significant intraindividual variation was observed in a few subjects, measurement of plasma NMR at one occasion seems to be a reliable estimate of the true NMR value for populations of nicotine reducers.
FUNDING
The study was supported by U.S. Public Health Service grants CA78603 and R25 CA113710 from the National Cancer Institute, DA02277 and DA12393 from the National Institute on Drug Abuse, National Institutes of Health, and S10 RR026437 from the Flight Attendant Medical Research Institute.
DECLARATION OF INTERESTS
NLB is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. No potential conflicts of interest were disclosed by the other authors.
ACKNOWLEDGMENTS
The authors thank Dr. Faith Allen for data management, Christopher Havel for lab analyses, Drs. Delia Dempsey and Katherine Dains for assistance in the clinical study, and Marc Olmsted for editorial assistance.
REFERENCES
- Benowitz N. L. (2010). Nicotine addiction. The New England Journal of Medicine, 362, 2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Dains K. M., Hall S. M., Stewart S., Wilson M., Dempsey D., Jacob P. (2012). Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiology Biomarkers & Prevention, 21, 761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Jacob P., III (1993). Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clinical Pharmacology and Therapeutics, 53, 316–323 [DOI] [PubMed] [Google Scholar]
- Berg J. Z., Mason J., Boettcher A. J., Hatsukami D. K., Murphy S. E. (2010). Nicotine metabolism in African Americans and European Americans: Variation in glucuronidation by ethnicity and UGT2B10 haplotype. The Journal of Pharmacology and Experimental Therapeutics, 332, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D., Tutka P., Jacob P., Allen F., Schoedel K., Tyndale R. F., Benowitz N. L. (2004). Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics, 76, 64–72 [DOI] [PubMed] [Google Scholar]
- Hukkanen J., Jacob P., III, Benowitz N. L. (2005). Metabolism and disposition kinetics of nicotine. Pharma cological Reviews, 57, 79–115 [DOI] [PubMed] [Google Scholar]
- Jacob P., Yu L., Duan M., Ramos L., Yturralde O., Benowitz N. L. (2011). Determination of the nicotine metabolites cotinine and trans-3’-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: Biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. Journal of Chromatography B, 879, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea R. A., Dickson S., Benowitz N. L. (2006). Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: Prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. Journal of Analytical Toxicology, 30, 386–389 [DOI] [PubMed] [Google Scholar]
- Mooney M. E., Li Z. Z., Murphy S. E., Pentel P. R., Le C., Hatsukami D. K. (2008). Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiology, Biomarkers & Prevention, 17, 1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M., Yamamoto T., Nunoya K., Yokoi T., Nagashima K., Inoue K., … Kuroiwa Y. (1996). Characterization of CYP2A6 involved in 3’-hydroxylation of cotinine in human liver microsomes. Journal of Pharmacology and Experimental Therapeutics, 277, 1010–1015 [PubMed] [Google Scholar]
- St.Helen G., Novalen M., Heitjan D. F., Dempsey D., Jacob P., III, Aziziyeh A., … Benowitz N. L. (2012). Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiology, Biomarkers and Prevention, 21, 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. A., Benowitz N. L., Pinto A. G., Tang K. Z., Hecht S. S., Carmella S. G., … Lerman C. E. (2011). Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiology, Biomarkers & Prevention, 20, 234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West O., Hajek P., McRobbie H. (2011). Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology, 218, 313–322 [DOI] [PubMed] [Google Scholar]