Abstract
Introduction:
Food and Drug Administration–mandated product standards that drastically reduce nicotine content in cigarettes aim to decrease smoking and thus improve health outcomes for millions of U.S. smokers. Researchers have suggested that nicotine reduction should be implemented gradually, but a gradual nicotine reduction may shift the minimum level of nicotine required to reinforce behavior or may result in different levels of compensatory smoking behavior.
Method:
Rats were given the opportunity to acquire nicotine self-administration at 60 µg/kg/infusion nicotine with a cocktail of other tobacco constituents included as the vehicle. Rats were subsequently assigned to one of six immediate dose reductions (30, 15, 7.5, 3.75, 1.875, or 0.0 µg/kg/infusion) for 10 sessions (n = 9–15). Rats in the 30 µg/kg/infusion reduction group continued to have their nicotine dose reduced by half after at least 10 sessions at each dose until reaching 1.875 µg/kg/infusion (i.e., gradual reduction).
Results:
For both methods of reduction, reduction to 3.75 µg/kg/infusion resulted in significant decreases in behavior. Reduction to doses above 3.75 µg/kg/infusion resulted in only limited compensation. The largest compensation was temporary. There was no compensation following reduction to 3.75 µg/kg/infusion or below.
Conclusion:
This study suggests that reduction to the same nicotine dose will result in similar reductions in behavior for both gradual and immediate reductions, and both methods result in similar compensation. Future studies using humans should investigate differences in other outcomes such as withdrawal and craving.
INTRODUCTION
The Family Smoking Prevention and Tobacco Control Act (U.S. Congress, 2009) permits the regulation of tobacco products and their constituents, including the regulation of nicotine to any nonzero level. Nicotine is well established as the primary reinforcing component in cigarettes (Stolerman & Jarvis, 1995; Stolerman, Mirza, & Shoaib, 1995), and thus its regulation is an important opportunity for decreasing tobacco-related illness and deaths. If nicotine delivery maintains smoking behavior, then decreasing nicotine content to a very low level could decrease cigarette use, resulting in enormous health benefits for smokers.
Recent studies support this possibility by showing that a drastic reduction in nicotine content can result in reduced smoking rates, toxicant exposure, and dependence (Benowitz et al., 2012, 2007; Donny, Houtsmuller, & Stitzer, 2007; Hatsukami, Kotlyar, et al., 2010; Hatsukami, Perkins, et al., 2010). However, many unanswered research questions exist regarding how a nicotine reduction policy would be implemented. One issue is whether nicotine should be reduced immediately or gradually over a period of months or years. A gradual reduction was initially suggested by Benowitz and Henningfield (1994) because a dramatic reduction in nicotine content may be more aversive for smokers.
The method of reduction could impact behavior following reduction in at least two important ways: (a) the degree of compensation, and (b) the dose that will result in significant reductions in behavior. First, compensation is an increase in drug-taking behavior following a reduction in drug dose. Evidence shows that both humans and rodents are likely to compensate for small decreases in nicotine content (Hecht et al., 2004; Scherer, 1999; Shoaib, Schindler, & Goldberg, 1997). Compensation can increase the risks associated with smoking because the most harmful components of cigarettes would remain unchanged. However, most studies showing compensatory increases in smoking behavior examine small decreases in nicotine yield within a range expected to maintain dependence (Scherer, 1999). Studies examining an immediate reduction to very low doses generally fail to find significant compensation beyond the first few cigarettes (Donny et al., 2007; Donny & Jones, 2009; Hatsukami, Kotlyar, et al., 2010; Strasser, Lerman, Sanborn, Pickworth, & Feldman, 2007). Gradual and immediate reductions may produce different levels of compensation due to the differential experience with a range of nicotine doses. If compensation only occurs at doses that maintain behavior, a gradual reduction may result in more harm as it keeps individuals at doses that maintain behavior for longer. Encouragingly, Hatsukami, Kotlyar, et al. (2010) and Benowitz et al. (2012) used immediate and gradual nicotine reductions, respectively, and both found little compensation. However, methodological differences across studies make direct comparison difficult. Second, a gradual reduction may shift the dose at which significant reductions in behavior are observed. No studies have previously addressed this issue, but Cox, Goldstein, and Nelson (1984) found that self-administration behavior extinguished much more slowly if rats experienced an intermediate dose before drug was replaced with saline.
Nonhuman animal research is an important tool for addressing research questions that are difficult to address in human populations (Donny et al., 2012). Animal self-administration models allow for tight experimental control over the pharmacological and behavioral history of research subjects. For example, compliance is a concern when asking human subjects to switch cigarette brands and could differ depending on the method of reduction; however, this is not an issue in animal self-administration research. In addition, many of the critical pharmacological and environmental factors that contribute to human smoking can be studied in the self-administration paradigm. This present study aimed to include several of these factors so that these results may be most generalizable to a nicotine reduction policy in human smokers. One factor that may influence the dose that produces significant reductions in behavior or the degree of compensation is the non-nicotine constituents in tobacco smoke. Research has shown that several constituents other than nicotine may contribute to the reinforcing potential of nicotine or may function as reinforcers on their own (Bardo, Green, Crooks, & Dwoskin, 1999; Belluzzi, Wang, & Leslie, 2005; Clemens, Caillé, Stinus, & Cador, 2009; Guillem et al., 2005; Villégier, Lotfipour, McQuown, Belluzzi, & Leslie, 2007). These constituents include the minor alkaloids (myosmine, nornicotine, cotinine, anabasine, anatabine), the β-carbolines (harman and norharman), and acetaldehyde. A cocktail of these constituents can be used as the vehicle for studying nicotine self-administration. Another factor that may influence the degree of compensation or the dose that produces significant reductions in behavior is the continued presence of cues that have been paired with nicotine. The self-administration paradigm can mimic a smoker’s experience with cues by pairing arbitrary stimuli with drug infusions.
This present study aimed to determine whether the method of nicotine reduction (gradual versus immediate) affected the minimum dose for maintaining self-administration behavior or the level of compensation following reduction. Rats were trained to self-administer nicotine, along with a cocktail of other cigarette constituents, at a relatively high dose of nicotine (60 μg/kg/infusion). After stable self-administration was established, animals were randomly assigned either to continue receiving the same nicotine dose or to receive an immediate reduction to one of six nicotine doses (30, 15, 7.5, 3.75, 1.875, 0.0 µg/kg/infusion). Rats in the 30 µg/kg/infusion group continued to have their nicotine dose reduced gradually (30 → 15 → 7.5 → 3.75 → 1.875 µg/kg/infusion). The behavior of the rats in the gradual reduction group was compared at each nicotine dose with the behavior of the rats that were reduced to that dose immediately. The doses of the other constituents and the cue conditions were held constant across the entire experiment as may be the case if a policy were enacted in which the nicotine content in cigarettes was reduced. Although it is not clear how the other constituents or cues may have influenced the results of this present study, including them allows us to better model the experience of a human smoker experiencing a reduction in nicotine content.
METHOD
Subjects
A total of 108 male Sprague-Dawley rats (Harlan-Farms) weighing between 175 and 212g (M = 194, SD = 6.85) on the day after arrival were used as subjects. Rats were housed individually in wire mesh, hanging cages in a temperature-controlled room (68–70 °F). Rats were kept on a reverse light–dark 12:12hr schedule. Rats had unlimited access to water throughout the experiment in their home cages. Rats received ad libitum chow for the first 7 days while habituating to individual home cages and being handled and weighed daily. Rats were implanted with jugular catheters beginning on the 8th day after their arrival and were changed to a 20g/day feed schedule on this day for the remainder of the study. Rats were allowed to recover for at least 5 days following surgery.
Apparatus
Eighteen operant chambers (30.5cm × 24.1cm × 21.0cm; ENV-008 CT; Med-Associates) were used in this present experiment. Each chamber was closed inside a sound-attenuating cubicle with a ventilation fan. Chambers contained two nose-poke holes, each 2.5cm in diameter on the right side of the chambers located 5cm from the chamber base to the base of the hole. A white stimulus light, 3.5cm in diameter, was located 6.25cm above the top of each nose poke. Each chamber also contained a house light on the same wall. The house light was illuminated red and located 1cm below the ceiling of the chamber. During sessions, rats were connected to a swivel system that delivered intravenous infusions while allowing for unrestricted movement within the chamber.
Drugs
Nicotine hydrogen tartrate salt (Sigma) was dissolved in 0.9% saline. The doses of nicotine used for self-administration were 60, 30, 15, 7.5, 3.75, and 1.875 µg/kg/infusion (free base). As discussed above, to better approximate the effects of nicotine within the context of tobacco smoke, we utilized a vehicle that contained tobacco constituents hypothesized to potentially play a role in the abuse liability of cigarettes. This cocktail of other cigarette constituents contained acetaldehyde, harman, norharman, anabasine, nornicotine, myosmine, cotinine (Sigma), and anatabine (Toronto Research Chemicals), which were all dissolved in 0.9% saline. The concentrations of the other constituents were as follows: 16 μg/kg/infusion (acetaldehyde), 0.1 μg/kg/infusion (harman), 0.3 μg/kg/infusion (norharman), 0.9 μg/kg/infusion (anabasine and nornicotine), and 0.09 μg/kg/infusion (myosmine, cotinine, and anatabine), and they were chosen to be proportional to the content found in cigarette smoke given 30 µg/kg/infusion nicotine (Herraiz, 2004). All drug solutions were adjusted to a 7.0 (±0.2) pH with dilute NaOH and HCl. All solutions were sterilized by being passed through a 0.22 μm filter. During sessions, infusions were delivered in less than 1 s at a volume of 0.1ml/kg/infusion.
Procedures
Catheter Implantation
Rats were anesthetized with isoflurane and implanted with jugular catheters. For the first 4 days after surgery, the cannulae were flushed once daily with 0.1ml of sterile saline containing heparin (30U/ml), timentin (66.67mg/ml), and streptokinase (9333U/ml) to maintain catheter patency and prevent infection. After this initial postsurgery time period, the daily flushing solution contained only heparin and timentin. Three patency tests were conducted during the experiment: two using up to 60mg/rat of chloral hydrate (on the first day of Reductions 2 and 5), and one using 5mg/kg of methohexital (at the end of the experiment). At the end of the experiment, all rats that had failed patency tests were sacrificed following the infusion of a black dye, and their catheters were examined. Rats that had working catheters with no observable leaks via visual inspection were included in the analyses (N = 8), whereas all others that had visually confirmed catheter failures were dropped from the analyses (N = 46) from their first failed patency test.
Habituation
Each rat was placed in their assigned operant chamber for a 20-min period during which time a red house light illuminated the chamber, and the nose-poke operanda were removed from the chamber.
Acquisition Phase
On the first day of the self-administration phase, one poke in the right (active) nose poke resulted in one 60 μg/kg infusion of nicotine along with a cocktail of other constituents for all rats (a fixed ratio (FR) 1 schedule of reinforcement). Each infusion was paired with a 15-s presentation of the stimulus light. Each infusion also resulted in a 1-min time out during which nose poking had no programmed consequences. Nose pokes in the left (inactive) nose poke never resulted in a consequence. After one session on an FR1 schedule of reinforcement, the ratio was escalated to an FR2 for seven sessions followed by an FR5 for nine sessions. During this portion of the experiment, sessions lasted 1hr and were conducted 5 days/week. Sessions were conducted between 7:30 a.m. and 3 p.m., and each session occurred at approximately the same time each day.
Reduction 1
On the 10th day of FR5, all rats were assigned to one of seven groups, matched for the average number of infusions over the last three sessions. One group, referred to as the “constant” group, continued to receive the same solution as before (60 μg/kg/infusion nicotine + cocktail). The other six groups had their nicotine dose reduced to one of six nicotine doses (30, 15, 7.5, 3.75, 1.875, and 0.0 μg/kg/infusion) but continued to receive the same cocktail of constituents and presentation of the cue light. Rats were given 10 sessions of experience at their assigned doses. Starting in this phase of the experiment, sessions were conducted 7 days/week. Sample sizes in the Acquisition and Reduction 1 phases were between 9 and 15.
Reductions 2–5
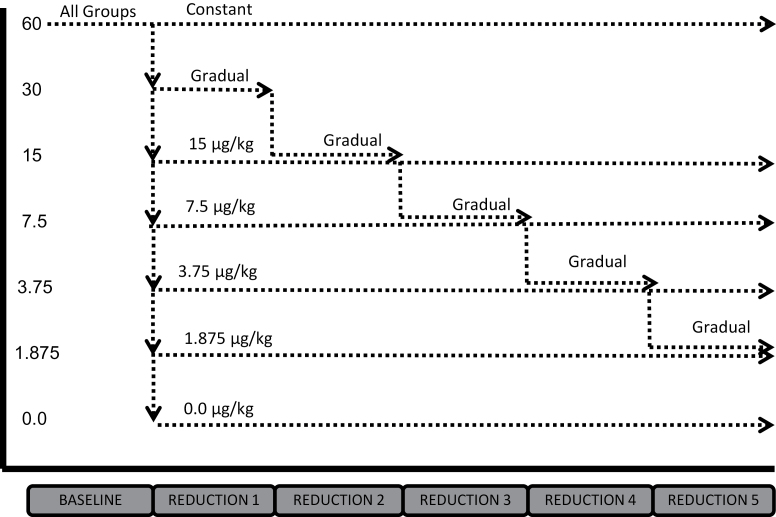
After 10 days of experience at their assigned nicotine dose, the 30 μg/kg/infusion group (now referred to as the “gradual” group) had their nicotine dose reduced again by half. Nicotine dose continued to be reduced by half after at least 10 sessions of experience at each dose, resulting in this group experiencing the following doses (in order): 30 μg/kg/infusion (10 sessions), 15 μg/kg/infusion (10 sessions), 7.5 μg/kg/infusion (10 sessions), 3.75 μg/kg/infusion (12 sessions), and 1.875 μg/kg/infusion (10 sessions). Sample size was 13 for Reductions 2–4 and 12 for Reduction 5. All other groups remained at their assigned doses for Reductions 2–5. Sample sizes were between 9 and 12 for Reductions 2–4 and between 5 and 9 for Reduction 5. Figure 1 shows the order of conditions for each group in the experiment.
Figure 1.
Order of self-administration dose across the six phases of the experiment for each of the seven groups. All doses are µg/kg/infusion, and all groups received a vehicle containing a cocktail of other tobacco constituents (see text for details).
Data Analysis
Immediate reduction was analyzed over the 10 sessions of Reduction 1 in three ways. Infusions earned on the first day of reduction were compared with the average earned infusions over the last three prereduction baseline sessions as an index of initial compensation for each group using a paired-sample t-test. To examine the change in behavior following the first day of reduction, paired-sample t-tests compared the earned infusions on the first day of reduction with the average earned infusions over the last three sessions of Reduction 1. Finally, stable behavior at each dose was examined by comparing average earned infusions over the last three sessions of Reduction 1 with the average earned infusions over the last three sessions of baseline using paired-sample t-tests. The same three analyses were then conducted for the gradual reduction group alone for each reduction using the last three sessions of their baseline, the first session of each reduction, and the last three sessions of each reduction phase. Because the immediate reduction groups experienced extended exposure to a single dose following reduction, earned infusions over the last three sessions of the entire experiment were compared with the last three sessions of Reduction 1 using a paired-sample t-test to determine if behavior was stable.
If earned infusions are believed as analogous to cigarettes per day in human smoking, a proportion of baseline score may be believed as a measure of risk for increased or decreased harm. Thus, to compare the two reduction methods, proportion of baseline scores was calculated for each rat for each method of reduction at each of the four doses using the same timepoints described above (with one exception, see below). By using the last three sessions of Reduction 1 to calculate proportion of baseline scores for stable behavior in the immediate reduction group, even though the immediate reduction group went on to experience many more sessions at this dose, the number of sessions experienced at a given dose was comparable with the gradual reduction group. Because the gradual reduction group experienced 12 sessions at this dose, sessions 10, 11, and 12 following Reduction 1 were used to calculate stable-behavior proportion of baseline scores for both methods of reduction. Independent-sample t-tests compared the scores of the two methods at each dose.
If a gradual reduction delays the reduction in self-administration behavior, then overall exposure would be increased compared with immediate reduction. One way to quantify exposure is to assess total infusions from the onset of change for both conditions. Total infusions for the constant group represent no change in policy. Independent-sample t-tests compared the sum of all infusions earned in the entire experiment from the first day of reduction between each reduction group and the constant group. The scores for the gradual reduction group were then compared with the 1.875 µg/kg/infusion group because that is the final dose that the gradual reduction group reached.
RESULTS
Acquisition Phase
All rats that passed the first patency test were included in this portion of the experiment (N = 90). A criterion for acquiring self-administration was set at an average of five infusions over the last 3 days before reduction and at least twice as many average active as inactive nose pokes over that same time period. As the purpose of the experiment was to examine behavior following nicotine reduction after self-administration of a higher nicotine dose, the eight rats failing to meet this criterion were excluded from this portion, as well as all other portions of the experiment. A paired-sample t-test confirmed that the average number of active responses over the last three baseline sessions (M = 84.44, SD = 34.11) was significantly higher than the average number of inactive responses (M = 4.51, SD = 4.28), p < .01. The average number of infusions over this period was 12.85 (SD = 4.00). A one-way analysis of variance confirmed that there were no significant differences between groups at the end of acquisition (i.e., following randomization when all groups had the same history), p > .05.
Immediate Reduction
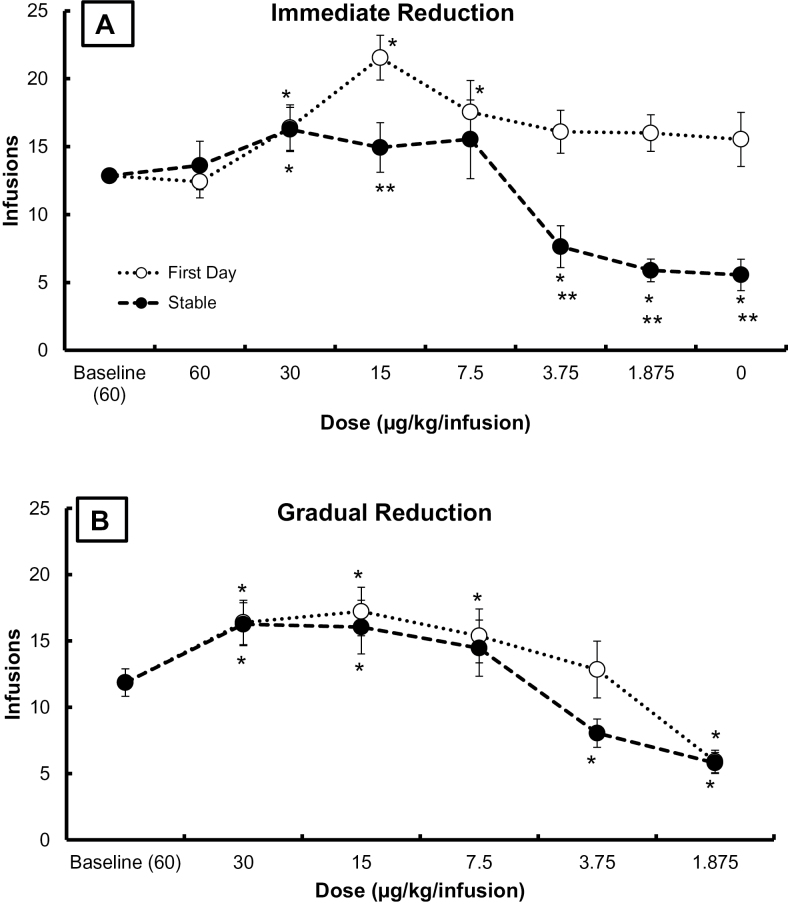
Figure 2A displays the average (±SEM) number of infusions earned for all rats at baseline (three sessions prior to reduction), on the first session following immediate reduction (open symbols), and on the last three sessions of Reduction 1 for each group (closed symbols). Immediate reduction in nicotine dose resulted in an increase in the number of infusions on the first day for the 30, 15, and 7.5 µg/kg/infusion groups. No other groups had significant changes from baseline. By the last three sessions of Reduction 1, behavior had stabilized. Stable behavior was significantly greater than baseline at 30 µg/kg/infusion but significantly less than baseline at 3.75, 1.875, and 0.0 µg/kg/infusion. No other groups significantly changed from baseline over the stable sessions. Direct comparison of the first day to the last 3 days revealed that the number of infusions earned at the end of Reduction 1 were significantly less than on the first day for the 15, 3.75, 1.875, and 0.0 µg/kg/infusion groups. Behavior in the immediate groups was fairly stable in Reductions 2–5. There was a significant decrease in earned infusions at the end of the experiment compared with the last 3 days of Reduction 1 for the 1.875 µg/kg/infusion group only, p < .01. Earned infusions were comparable for all other groups over the same timeframe, p >.05.
Figure 2.
(A) Infusions earned on the first day of initial reduction (open symbols) and the average infusions earned over the last three sessions of Reduction 1 (closed symbols) for each group. Baseline infusions earned are shown for all rats at training dose (60 µg/kg/infusion). All groups have at least nine rats. (B) Infusions earned on the first day of each reduction (open symbols) and the average earned infusions over the last three sessions at each dose (closed symbols) for the gradual reduction group. Group size for the gradual reduction group is 15 rats at 60 and 30 µg/kg/infusion, 13 rats at 15, 7.5, and 3.75 µg/kg/infusion, and 12 rats at 1.875 µg/kg/infusion. Error bars are standard error of the mean in both graphs. Significant change from baseline is denoted by *. Significant change from first day to stable behavior is denoted by **. All ps < 0.05. Data from the 30 µg/kg/infusion dose are the same in both (A) and (B).
Gradual Reduction
Figure 2B displays the average (±SEM) number of infusions earned for rats in the gradual reduction group at baseline (60 μg/kg/infusion; immediately prior to first reduction), as well as number of infusions earned over the first (open symbols) and last three sessions of each reduction (closed symbols). Infusions on the first day of reduction to 30 (reported above), 15, and 7.5 μg/kg/infusion were significantly greater than baseline infusions. On the first day of reduction to 1.875 μg/kg/infusion, infusions were significantly less than at baseline. Infusions over the last three sessions at each reduction were significantly greater than baseline for 30 (reported above) and 15 μg/kg/infusion and significantly less than baseline for 3.75 and 1.875 μg/kg/infusion. The change in infusions from the first to the last three sessions of each reduction was not significant at any dose (trend at 3.75 µg/kg/infusion, p = .058).
Comparing Immediate With Gradual Reduction
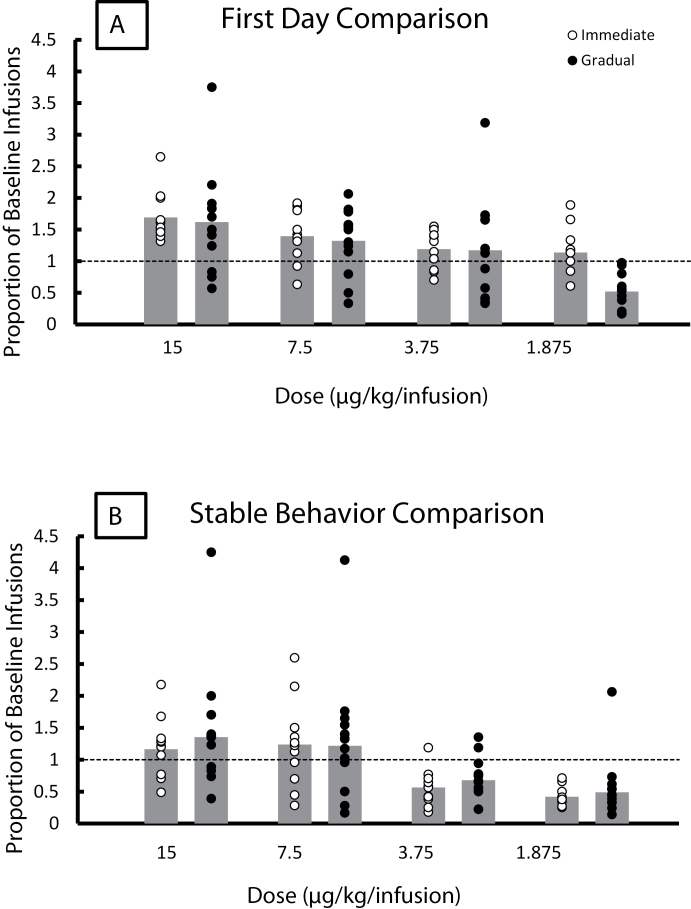
Figure 3A shows the proportion of baseline infusions for the immediate (open symbols, left bars) and gradual (filled symbols, right bars) groups at each of the four doses tested (15, 7.5, 3.75, and 1.875 µg/kg/infusion) on the first day of each reduction. Bars represent group averages, and circles represent individual rats in each group. Proportion of baseline infusions was significantly less for the gradual reduction method at the 1.875 µg/kg/infusion dose but was not significantly different at any other dose (ps > 0.05). Figure 3B shows the proportion of baseline infusions for the stable behavior for the immediate (open symbols, left bars) and gradual (closed symbols, right bars) groups at each of the four doses tested (15, 7.5, 3.75, and 1.875 µg/kg/infusion) for both methods of reduction. The proportion of baseline infusions was not significantly different between the two methods at any of the doses tested (ps > .05).
Figure 3.
Proportion of baseline scores for immediate reduction (open symbols, left hand bars) and gradual reduction (filled symbol, right hand bars). Bars represent group averages; symbols represent individual rats. Group sizes are at least nine across all groups. (A) First day of each reduction. Infusions earned on the first day are divided by the average infusions earned over the last 3 days of baseline. Significantly fewer infusions were earned for the gradual reduction method at the 1.875 µg/kg/infusion dose (t 19 = 4.696, p < .05). (B) Stable behavior. Infusions earned over the last 3 days of each reduction are divided by infusions earned on the last 3 days before initial reduction for the gradual reduction group. Scores for the immediate reduction groups are calculated in the same way using the three sessions of comparable experience at each dose.
Table 1 shows the total number of infusions earned over all sessions from the first day of reduction (52 sessions) for each group. The 3.75, 1.875, and vehicle groups all earned significantly fewer infusions than the constant group. The 1.875 group earned significantly fewer infusions than the gradual reduction group.
Table 1.
Earned Infusions Over the Entire Experiment (52 Sessions) for Each Group
| Group | Mean infusions | SE infusions | Range infusions |
|---|---|---|---|
| Constant | 725.75 | 60.89 | 489–997 |
| Gradual | 653.92 | 72.46 | 182–1206 |
| 15 | 805.85 | 195.17 | 330–1353 |
| 7.5 | 640.83 | 207.82 | 114–1456 |
| 3.75* | 322.44 | 52.53 | 155–329 |
| 1.875* | 212.00 | 31.20 | 125–323 |
| Vehicle* | 266.78 | 81.99 | 111–910 |
Note. *The 3.75 (t 15 = 5.043, p < .0001), 1.875 (t 13 = 7.180, p < .001), and vehicle (t 15 = 4.399, p < .001) reduction groups all earned significantly less infusions than the constant group. The 1.875 group earned significantly less infusions than the gradual reduction group, (t 17 = 4.472, p < .001).
Individual Differences
Immediate reduction to 15, 7.5, 3.75, and 1.875 µg/kg/infusion resulted in a decrease in stable earned infusions for 27, 36, 91, and 100% of rats in each group, respectively. Gradual reduction to the same doses resulted in a decrease in infusions for 31, 31, 85, and 92% of rats, respectively. Of the 12 rats in the gradual reduction group assessed at all doses, the peak number of earned infusions during stable behavior was 30 μg/kg/infusion for four rats, 15 μg/kg/infusion for five rats, and 7.5 μg/kg/infusion for two rats (with the additional rat having the same average infusions at the 15 and 7.5 μg/kg/infusion doses). No rat displayed a maximal rate of infusions below 7.5 μg/kg/infusion.
DISCUSSION
This present study found significant decreases in nicotine self-administration, similar to vehicle substitution, when the nicotine dose was reduced to 3.75 µg/kg/infusion or below. In contrast, doses at or above 7.5 µg/kg/infusion produced similar or greater rates of infusions relative to maintenance at 60 µg/kg/infusion. Importantly, these dose–response relations were similar, regardless of whether nicotine dose was reduced immediately or gradually. Reduction from 60 µg/kg/infusion to doses above 3.75 µg/kg/infusion resulted in very little compensation, and the largest compensation was temporary. However, reduction to doses of 3.75 µg/kg/infusion or less did not result in compensation. Additionally, reduction to doses of 3.75 µg/kg/infusion or less produced decreases in behavior almost exclusively, whereas the response to reduction was more variable when reduction to doses of 7.5 µg/kg/infusion or above. The method of reduction did not have a significant impact on the change in self-administration behavior on the first day of reduction or after behavior had become stable. The only difference between the two methods was on the first day of reduction to the lowest dose tested (1.875 µg/kg/infusion) (see Figure 3), and this difference in the proportion of baseline infusions was due to the gradual reduction group already having very low behavior at the end of the previous reduction.
Policy Implications
The results of this present study are encouraging, regarding a policy implementing nicotine reduction in cigarettes or other tobacco products. Marked reduction in nicotine dose decreased self-administration for almost all individuals without compensation. This present study also suggests that because gradual and immediate reductions result in similar self-administration behavior at low doses, a gradual reduction may result in more overall exposure because it keeps individuals at intermediate doses for longer. There were not substantial differences in the degree of compensation or the dose required to maintain behavior. However, gradual reduction would require that smokers are kept at intermediate doses for an unspecified time, which could carry significant risks associated with greater cumulative exposure to cigarettes. The difference in total earned infusions over the whole experiment between the rats in the gradual group and the rats in the comparable immediate group illustrates the increase in exposure that could be caused by choosing a gradual reduction.
There was a substantial amount of variability in the response of the subjects to nicotine reduction, which may be the result of variability in subjects’ individual dose–response curves. Indeed, the rats in the gradual reduction group differed in the dose that resulted in their peak responding. There is likely to be even more variability in a human population with more genetic and historical variability. Factors predicting response to nicotine reduction should be further explored. Despite the variability in response to reduction, if nicotine was reduced to a low enough dose, harm may be decreased across almost the entire population.
Limitations and Future Directions
Several methodological factors may have contributed to the results of this present study and should be considered when conducting future extensions of the research. First, this present study utilized certain non-nicotine tobacco constituents as part of the vehicle (Belluzzi et al., 2005; Clemens et al., 2009; Guillem et al., 2005; Villégier et al., 2007). This present study represents the first time that this particular formulation of constituents has been examined in an animal model. The findings presented here, including the nicotine dose that produced a significant decrease in behavior, may be specific to the self-administration of nicotine in combination with these particular constituents. Although it is unclear how the other constituents may have affected the results of this present study, they would be present in cigarettes if a nicotine reduction policy was enacted, and thus, their inclusion was important for modeling a policy scenario. Second, this present study also utilized an environmental cue (stimulus light onset) paired with each infusion. Environmental cues are likely to partially support behavior as conditioned reinforcers as a consequence of being paired with nicotine. Third, pharmacological exposure to nicotine and/or reinforcement history associated with the nose-poke operant may have influenced the response to nicotine reduction. More specifically, the number of days, 1-hr sessions, or the nicotine dose used for self-administration prior to reduction may have affected the nicotine dose that produced decreases in self-administration behavior (3.75 µg/kg/infusion or below). A critically related and currently unanswered question is whether the dose–response curve for acquisition is similar or different than the dose–response curve for reduction. This question has important clinical implications when considering how current smokers might be affected by nicotine reduction differently from individuals who initiate use for the first time at reduced nicotine levels. Finally, the rats used in this present study were all male adults. Many individuals begin smoking as adolescents, and some researchers have found sex differences in nicotine self-administration (Perkins, Donny, & Caggiula, 1999), making the study of females and adolescents particularly important.
CONCLUSION
Gradual and immediate nicotine reduction in cigarettes should eventually be compared in a population of current smokers. The present data suggest that there are unlikely to be differences in the nicotine dose that produces significant decreases in smoking behavior or the level of compensation between the methods. However, a gradual reduction will keep smokers at intermediate doses for a longer period of time, necessarily increasing their exposure to the harmful effects of smoking. In this present study, we were unable to compare more subjective outcomes such as withdrawal and craving. Future studies should evaluate other potential beneficial or harmful effects of gradual versus immediate reduction to provide a better empirical basis for action by the Food and Drug Administration.
FUNDING
This work was supported by the National Institute on Drug Abuse (U54 DA031659) awarded to E.C.D.
DECLARATION OF INTERESTS
None declared.
ACKNOWLEDGMENT
The authors would like to thank Maysa Gharib, Angela Lutheran, Nana Marfo, Melinda Moran, and Richard Jacobson for their assistance in data collection.
REFERENCES
- Bardo M. T., Green T. A., Crooks P. A., Dwoskin L. P. (1999). Nornicotine is self-administered intravenously by rats. Psychopharmacology, 146, 290–296 [DOI] [PubMed] [Google Scholar]
- Belluzzi J. D., Wang R., Leslie F. M. (2005). Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology, 30, 705–712 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Dains K. M., Hall S. M., Stewart S., Wilson M., Dempsey D., Jacob P., III (2012). Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiology, Biomarkers & Prevention, 21, 761–769.10.1158/1055–9965.EPI-11–0644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N. L., Hall S. M., Stewart S., Wilson M., Dempsey D., Jacob P., III (2007). Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiology, Biomarkers & Prevention, 16, 2479–2485.10.1158/1055–9965.EPI-07-0393 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Henningfield J. E. (1994). Establishing a nicotine threshold for addiction. The implications for tobacco regulation. The New England Journal of Medicine, 331, 123–125.10.1056/NEJM199407143310212 [DOI] [PubMed] [Google Scholar]
- Clemens K. J., Caillé S., Stinus L., Cador M. (2009). The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. International Journal of Neuropsychopharmacology, 12, 1355–1366.10.1017/S1461145709000273 [DOI] [PubMed] [Google Scholar]
- Cox B. M., Goldstein A., Nelson W. T. (1984). Nicotine self-administration in rats. British Journal of Pharmacology, 83, 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny E. C., Houtsmuller E., Stitzer M. L. (2007). Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction (Abingdon, England), 102, 324–334.10.1111/j.1360-0443.2006.01670.x [DOI] [PubMed] [Google Scholar]
- Donny E. C., Jones M. (2009). Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug and Alcohol Dependence, 104, 23–33.10.1016/j.drugalcdep.2009.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny E. C., Taylor T. G., LeSage M. G., Levin M., Buffalari D. M., Joel D., Sved A. F. (2012). Impact of tobacco regulation on animal research: New perspectives and opportunities. Nicotine & Tobacco Research, 14, 1319–1338.10.1093/ntr/nts162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K., Vouillac C., Azar M. R., Parsons L. H., Koob G. F., Cador M., Stinus L. (2005). Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. Journal of Neuroscience, 25, 8593–8600.10.1523/JNEUROSCI.2139-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D. K., Kotlyar M., Hertsgaard L. A., Zhang Y., Carmella S. G., Jensen J. A, … Hecht S. S. (2010). Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction (Abingdon, England), 105, 343–355.10.1111/j.1360-0443.2009.02780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D. K., Perkins K. A., Lesage M. G., Ashley D. L., Henningfield J. E., Benowitz N. L, … Zeller M. (2010). Nicotine reduction revisited: Science and future directions. Tobacco Control, 19, e1–10.10.1136/tc.2009.035584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S. S., Murphy S. E., Carmella S. G., Zimmerman C. L., Losey L., Kramarczuk I, … Hatsukami D. K. (2004). Effects of reduced cigarette smoking on the uptake of a tobacco-specific lung carcinogen. Journal of the National Cancer Institute, 96, 107–115 [DOI] [PubMed] [Google Scholar]
- Herraiz T. (2004). Relative exposure to beta-carbolines norharman and harman from foods and tobacco smoke. Food Additives and Contaminants, 21, 1041–1050.10.1080/02652030400019844 [DOI] [PubMed] [Google Scholar]
- Perkins K. A., Donny E., Caggiula A. R. (1999). Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine & Tobacco Research, 1, 301–315 [DOI] [PubMed] [Google Scholar]
- Scherer G. (1999). Smoking behaviour and compensation: A review of the literature. Psychopharmacology, 145, 1–20 [DOI] [PubMed] [Google Scholar]
- Shoaib M., Schindler C. W., Goldberg S. R. (1997). Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology, 129, 35–43 [DOI] [PubMed] [Google Scholar]
- Stolerman I. P., Jarvis M. J. (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl), 117, 2–10; discussion 14–20 [DOI] [PubMed] [Google Scholar]
- Stolerman I. P., Mirza N. R., Shoaib M. (1995). Nicotine psychopharmacology: Addiction, cognition and neuroadaptation. Medicinal Research Reviews, 15, 47–72 [DOI] [PubMed] [Google Scholar]
- Strasser A. A., Lerman C., Sanborn P. M., Pickworth W. B., Feldman E. A. (2007). New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug and Alcohol Dependence, 86, 294–300.10.1016/j.drugalcdep.2006.06.017 [DOI] [PubMed] [Google Scholar]
- U.S. Congress (2009). Family Smoking Prevention and Tobacco Control Act. U.S. Government Printing Office; Retrieved from http://www.gpo.gov/ [Google Scholar]
- Villégier A. S., Lotfipour S., McQuown S. C., Belluzzi J. D., Leslie F. M. (2007). Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology, 52, 1415–1425.10.1016/j.neuropharm.2007.02.001 [DOI] [PubMed] [Google Scholar]