Nearly one million Americans suffer a myocardial infarction each year, many of whom progress to heart failure, the single most common hospital discharge diagnosis in those over age 65 (ref. 1). The adult human heart has a limited regenerative response to injury such that the loss or dysfunction of cardiomyocytes results in reduced pump function, often culminating in heart failure, life-threatening arrhythmias and sudden death. Numerous clinical trials over the past decade have introduced a variety of autologous stem and progenitor cell types into failing human hearts as a strategy for regenerating new myocardium, but exogenous stem cells seem to give rise to few if any new muscle cells, bringing into question the biological basis for the limited functional improvement. Thus, there is still a dire need for innovative strategies for heart regeneration and repair.
A series of recent studies in rodents has reported the ability of exogenous transcription factors and miRNAs to reprogram cardiac fibroblasts into cardiomyocytes2–6, resulting in dramatic improvement of cardiac contractility after myocardial infarction2,3,6. Much work remains to optimize such reprogramming methods and to define the mechanistic basis for functional improvement in this setting, but this initial evidence suggests a potentially transformative new approach for heart repair.
Whereas skeletal and smooth muscle cells can be generated from fibroblasts by ectopic expression of single transcription factors7,8, the cardiac muscle phenotype has proven more elusive, as no single factor has been shown to be capable of generating cardiomyocytes from fibroblasts. An important step toward possible therapeutic generation of cardiomyocytes was provided by Ieda et al.9, who showed that three transcription factors—Gata4, Mef2c and Tbx5 (together referred to as GMT)—could activate cardiac gene expression in cultured mouse fibroblasts with a low efficiency of between 5 and 15%. Activation of cardiac genes by these factors seems to require precise levels of expression of the factors. Inclusion of a fourth factor, Hand2, in the GMT cocktail substantially increases reprogramming efficiency2. Several cardiac miRNAs have also been reported to activate cardiac gene expression in fibroblasts with low efficiency4. Because cardiac transcription factors and miRNAs function within complex regulatory networks involving feed-forward and autoregulatory interactions, it is likely that multiple combinations of these cardiac regulators may initiate the cardiac phenotype.
Reprogramming by cardiac transcription factors and miRNAs seems to involve direct conversion of fibroblasts toward a cardiomyocyte-like fate without transition through a stem cell intermediate. This approach therefore differs from reprogramming methods that involve the generation of induced pluripotent stem cells and subsequent commitment to the cardiac lineage. Direct cardiac reprogramming of fibroblasts also circumvents potential teratogenicity and immunogenicity of induced pluripotent stem cells.
Induced cardiac-like myocytes (iCLMs) seem to be relatively immature, and only a very low fraction show action potentials and strong contractility, well-developed sarcomeres, and binucleation, characteristics of adult cardiomyocytes. Thus, maturation to adult cardiac phenotypes may require prolonged periods in culture or additional factors not yet identified. Initial efforts to reprogram human fibroblasts to a cardiac fate have recently found a set of at least five factors different from the factor combination in mouse fibroblasts that can activate cardiac gene expression in adult human cardiac and dermal fibroblasts and in neonatal human foreskin fibroblasts10. Cardiac reprogramming of human fibroblasts is slower and less efficient than in mouse fibroblasts, perhaps reflecting stable epigenetic events that need to be overcome.
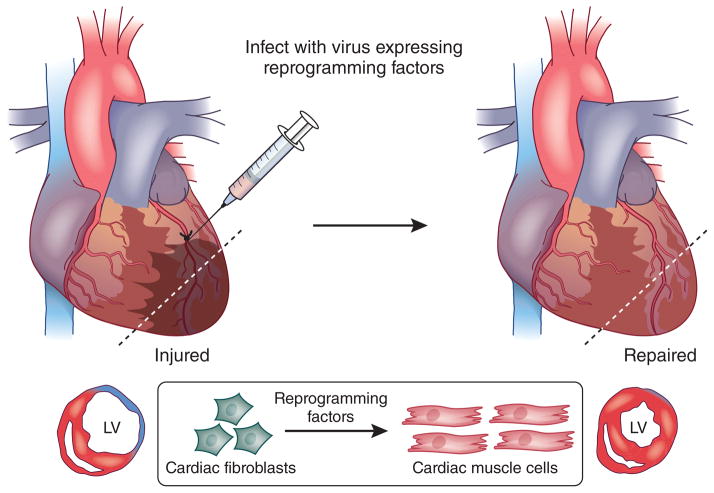
Nearly half of the cells in the heart are fibroblasts, and their activation during heart disease leads to fibrosis, which impedes contractility and contributes to conduction abnormalities. Thus, targeting activated cardiac fibroblasts after injury to induce heart repair is particularly attractive (Fig. 1). Retroviruses, which infect only proliferating cells, were used to introduce GMT and GHMT into fibroblasts in the infarct zone of mice after myocardial infarction2,3,5. Lineage-tracing studies with fibroblast markers indicated that newly generated iCLMs were derived from fibroblasts.
Figure 1.
Heart repair by in vivo reprogramming of nonmyocytes into iCLMs. After myocardial infarction, a viral cocktail of cardiac transcription factors or miRNAs is directly injected into the border zone adjacent to the infarcted myocardium. The forced expression of reprogramming factors in the heart after myocardial infarction generates iCLMs in situ and leads to the improvement of contractile function and reduction of scar formation. LV, left ventricle.
There are a few aspects of these studies that warrant consideration. First, the reprogramming efficiency in vivo seems to be higher compared to in vitro, suggesting that the milieu of the intact heart may favor reprogramming in ways that cannot be reproduced in culture. Second, introduction of reprogramming factors results in dramatic functional improvement after myocardial infarction2,3, indicating that, at least in mice, the impact of cardiac reprogramming exceeds the relatively modest and transient effects observed with autologous stem cell transplantation. Finally, the extent of functional improvement after in vivo reprogramming is greater than expected, given the relatively modest number of mature cardiomyocytes generated. This may suggest that reprogramming factors enhance cardiac function through mechanisms beyond simply reprogramming of fibroblasts toward a cardiomyocyte cell fate, perhaps also promoting neoangiogenesis, preventing cardiomyocyte death and/or inhibiting fibroblast proliferation.
Although these initial studies point to a potentially promising new approach for heart repair, numerous technical and biological hurdles remain to be overcome. The efficiency of the reprogramming process remains relatively low, and reprogrammed cells show a spectrum of intermediate phenotypes, reflecting incomplete conversion to a mature cardiac phenotype. The latter issue is of concern, given the propensity of arrhythmias to arise from zones of cardiomyocyte heterogeneity11. The long-term stability and integration of reprogrammed cardiomyocytes with native cardiomyocytes also remains to be shown. Further optimization of reprogramming of human fibroblasts and demonstration of the therapeutic efficacy and safety of this approach in large animals is needed.
Cells from the cardiac conduction system and vasculature are also lost after cardiac injury, and full restoration of cardiac function after injury will therefore require recreation of multiple cell types. Smooth muscle, endothelial and angioblast-like progenitor cells have been efficiently generated by reprogramming8,12,13 and inclusion of a vascular endothelial growth factor–expressing virus with GMT enhances functional recovery of mice after myocardial infarction, possibly through neovascularization of the injured myocardium6. In addition, forced expression of Tbx3, activated Notch or Tbx18 in working cardiomyocytes is sufficient to generate conduction system cells in vitro14–16 and in vivo16.
Reprogramming experiments in rodents requires open chest surgery to directly inject viruses into the infarct zone; in humans, direct delivery of reprogramming factors during coronary artery bypass graft surgery could be a starting point. Given the potential for teratogenic viral insertions in the genome, as well as other complications associated with viral delivery, it will be important to develop nonintegrative methods for safe clinical application. Replacing cardiogenic transcription factors with small molecules or synthetic oligonucleotides with cardiogenic activity has long-term therapeutic possibilities; their combination with catheter-based delivery during a percutaneous coronary artery intervention after myocardial infarction could also reach widespread and effective use for intervention after heart attack.
Whereas studies thus far have been limited to the reprogramming of fibroblasts to cardiomyocytes within the infarct zone of hearts after myocardial infarction, it will be of interest to determine whether this approach can also be applied to other forms of acquired and inherited forms of heart disease associated with loss or dysfunction of cardiomyocytes. As most heart diseases are associated with an increase in cardiac fibrosis, this approach may extend beyond post–myocardial infarction therapy. Given our desperate need for entirely new heart repair strategies, further studies are warranted to resolve the current challenges facing in vivo reprogramming approaches. Cellular reprogramming, perhaps in combination with biological scaffolds or other bioengineering strategies, has the potential to provide an alternative or complementary heart repair strategy to cell transplantation–based approaches, which have been in clinical trials for nearly a decade.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Competing financial interests
Declaration: R.T.L. is a founder of and consultant to Provasculon. C.L.M. is a founder of and consultant to Pluriomics.
References
- 1.Roger VL, et al. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian L, et al. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song K, et al. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayawardena TM, et al. Circ Res. 2012;110:1465–1473. doi: 10.1161/CIRCRESAHA.112.269035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inagawa K, et al. Circ Res. 2012;111:1147–1156. doi: 10.1161/CIRCRESAHA.112.271148. [DOI] [PubMed] [Google Scholar]
- 6.Mathison M, et al. J Am Heart Assoc. 2012;1:e005652. doi: 10.1161/JAHA.112.005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis RL, Weintraub H, Lassar AB. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Wang D-Z, Teg Pipes GC, Olson EN. Proc Natl Acad Sci USA. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ieda M, et al. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam Y-J, et al. Proc Natl Acad Sci USA. (in the press) [Google Scholar]
- 11.Liao SY, et al. Heart Rhythm. 2010;7:1852–1859. doi: 10.1016/j.hrthm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Margariti A, et al. Proc Natl Acad Sci USA. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurian L, et al. Nat Methods. 2013;10:77–83. doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakker ML, et al. Cardiovasc Res. 2012;94:439–449. doi: 10.1093/cvr/cvs120. [DOI] [PubMed] [Google Scholar]
- 15.Rentschler S, et al. Circulation. 2012;126:1058–1066. doi: 10.1161/CIRCULATIONAHA.112.103390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapoor N, Liang W, Marban E, Cho HC. Nat Biotechnol. 2013;31:54–62. doi: 10.1038/nbt.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]