Abstract
Primary cilia undergo cell cycle-dependent assembly and disassembly. Emerging data suggest that ciliary resorption is a checkpoint for S phase re-entry, and that the activation of phospho(T94)Tctex-1 couples these two events. However, the environmental cues and molecular mechanisms that trigger these processes remain unknown. Here, we show that insulin-like growth-1 (IGF-1) accelerates G1-S progression by causing cilia to resorb. The mitogenic signals of IGF-1 are predominantly transduced through IGF-1 receptor (IGF-1R) on the cilia of fibroblasts and epithelial cells. At the base of the cilium, phosphorylated IGF-1R activates an AGS3-regulated Gβγ signaling pathway that subsequently recruits phospho(T94)Tctex-1 to the transition zone. Perturbing any component of this pathway in cortical progenitors induces premature neuronal differentiation at the expense of proliferation. These data suggest that during corticogenesis, a cilium-transduced, non-canonical IGF-1R-Gβγ–phospho(T94)Tctex-1 signaling pathway promotes the proliferation of neural progenitors through modulation of ciliary resorption and G1 length.
Keywords: ciliary resorption, ciliary transition zone, IGF-1, non-canonical Gβg signaling, S-phase re-entry, phospho(T94)Tctex-1, AGS3
INTRODUCTION
Primary cilia are hair-like sensory organelles that appear on G1/G0 cells, and are disassembled prior to S phase (Tucker et al., 1979). Although mutations in almost all identified genes involved in ciliary assembly have been associated with ciliopathies (Lee and Gleeson, 2011; Waters and Beales, 2011), relatively little is known about how cilia are disassembled. Recent studies have begun to identify proteins that are involved in ciliary resorption. For example, Nuclear distribution gene E homologue 1 (Nde1) negatively regulates ciliary length through its interaction with dynein light chain, LC8 (DYNLL1). Cells depleted of Nde1 develop abnormally long cilia, which leads to a delay in cell cycle re-entry (Kim et al., 2011). Our previous results showed that yet another dynein light chain protein, Tctex-1 (or DNYLT1), is phosphorylated at Thr94 and recruited to the ciliary transition zone (TZ) prior to S phase re-entry (Li et al., 2011). Suppression of phospho(T94)Tctex-1 resulted in the inhibition of both serum induced ciliary resorption and S phase progression. This reduction in cell cycle re-entry was not observed in non-ciliated cells. Conversely, overexpression of a phosphomimic mutant of Tctex-1 (i.e., T94E) accelerated the resorption of cilia and reduced G1 length (Li et al., 2011). Since phospho(T94)Tctex-1 does not bind to the dynein motor complex (Chuang et al., 2005), the role of Tctex-1 in ciliary resorption and cell cycling is dynein-independent. These data collectively suggest that ciliary resorption is a precursor of S phase entry. Furthermore, the time required for a cilium to resorb is a key determinant of G1 length.
Radial glia (RG), the proliferating progenitors of the developing neocortex, also have cilia. It has been shown that G1 length causally influences the fate choice of RG. Shortening G1 accelerates cell cycle entry and increases the proliferation rate of RG, whereas lengthening G1 drives these cells to differentiate into neurons (Calegari and Huttner, 2003; Lange et al., 2009; Pilaz et al., 2009). These findings make the developing cortex a suitable model for investigating the physiological relevance of ciliary dynamics to cell cycle progression. Our previous results showed that the ciliary TZ expression of phospho(T94)Tctex-1 in RG plays an important role in modulating G1 length and maintaining the proliferating progenitor population (Li et al., 2011). Blocking the expression of phospho(T94)Tctex-1 in RG induced premature cell cycle exit and neuronal differentiation at the expense of expanding the progenitor population. However, the environmental cues and signal transduction pathways that lead to the expression of phospho(T94)Tctex-1 in the developing brain are unknown.
Platelet derived growth factor (PDGF) has been shown to be able to induce ciliary resorption by itself (Christensen et al., 2007; Pugacheva et al., 2007; Tucker et al., 1979), but the mechanism by which PDGF receptor activation causes ciliary resorption remains unclear. Previous studies have shown that PDGF-AA mediated mitogenesis requires functional insulin-like growth factor 1 receptor (IGF-1R) (Mason and Goldman, 2002; Novosyadlyy et al., 2006). IGF-1, a ligand of IGF-1R, is a potent mitogen that accelerates G1/S progression (Hodge et al., 2004; Popken et al., 2004; Ye and D'Ercole, 2006). While IGF-1R is conventionally known as a tyrosine kinase receptor (Ullrich et al., 1985), it also has non-canonical G protein coupled receptor (GPCR) activity (Dalle et al., 2001; Hallak et al., 2000; Luttrell et al., 1995). Expression of a scavenger of Gβγ subunits, βARK-ct (Koch et al., 1994), inhibits IGF-1 stimulated mitogenesis (Dalle et al., 2001; Luttrell et al., 1995).
Tctex-1 is synonymous with Activator of G protein Signaling 2 (AGS2), which was first isolated as a GPCR-independent activator of G protein signaling through a functional screen in yeast (Takesono et al., 1999). Activator of G protein Signaling 3 (AGS3), another protein involved in non-canonical Gβγ signaling, was isolated in the same screen (Takesono et al., 1999). Tctex-1 binds directly to the Gβ subunit of heterotrimeric G proteins; Gβγ competes with dynein intermediate chain for Tctex-1 binding in order to regulate the availability of dynein-free Tctex-1 (Sachdev et al., 2007; Takesono et al., 1999). AGS3, on the other hand, binds to Gαi. The GoLoco/G protein regulatory (GPR) motifs of AGS3 stabilize the GDP-bound form of Gαi, preventing its re-association with Gβγ (Ghosh et al., 2003; Oner et al., 2010; Peterson et al., 2000). Similar to Tctex-1 silencing (Li et al., 2011), the inhibition of Gβγ activation (via βARK-ct overexpression or AGS3 silencing) pushes cortical progenitors out of division and into differentiation mode (Sanada and Tsai, 2005).
In the present study, we identify and characterize a pathway in which IGF-1 transmits signals through the primary cilium in order to mediate ciliary resorption and cell cycle entry. This pathway involves the activations of IGF-1R, non-canonical Gβγ signaling, and phospho(T94)Tctex-1 at the base of the cilium.
RESULTS
IGF-1 induces ciliary resorption via the activation of IGF-1R
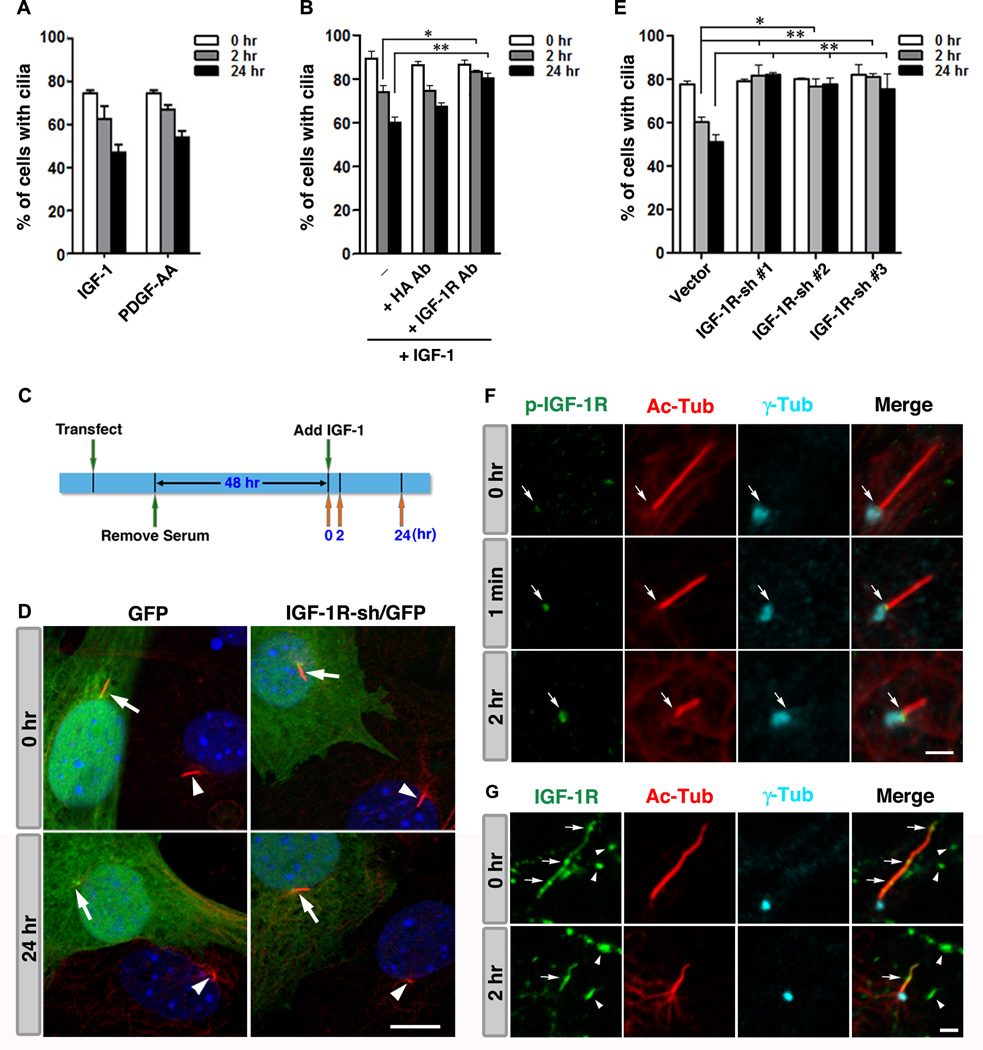
Previous studies have confirmed that certain non-transformed cell types (i.e., human retinal pigment epithelial cells (RPE-1), 3T3, and mouse embryonic fibroblasts (MEF)) can be starved to induce quiescence and the formation of cilia. Subsequent re-addition of serum triggers biphasic ciliary resorption, which peaks at 2 hr and 24 hr following stimulation (Li et al., 2011; Tucker et al., 1979). The first wave of cilium shortening occurs at mid/late G1 phase preceding S phase entry, whereas the second wave occurs at the G2/M transition (Tucker et al., 1979). We first tested whether or not IGF-1 stimulation is able to induce the resorption of cilia in RPE-1 cells. IGF-1 (10 nM) induced ciliary resorption to roughly the same degree as PDGF-AA (50 ng/mL) (Fig. 1A), while lower concentrations of IGF-1 (1–5 nM) were found to be insufficient (data not shown).
Fig. 1. IGF-1 triggers ciliary resorption.
(A) Biphasic ciliary resorption profiles of RPE-1 cells treated with IGF-1 or PDGF-AA. (B) Serum starved cells were treated with or without 1 µg/ml of anti-IGF-1R αIR3 Ab or anti-hemagglutinin (HA) Ab 15 min prior to and during IGF-1 treatment. (C) A timeline depicting the sequence of events in a typical ciliary assembly/disassembly assay involving gene manipulation. (D) Representative images of 3T3 cells transfected with IGF-1R-sh/GFP or GFP alone at 0 hr and 24 hr following IGF-1 treatment. Arrows and arrowheads point to the cilia in GFP+ transfected, and GFP− non-transfected cells, respectively. (E) Percentages of GFP+ transfected cells displaying cilia. (F) Immunolabeling of phospho-IGF-1R (green), Ac-Tub (red), and γ- Tub (cyan) in RPE-1 cells prior to IGF-1 stimulation (0 hr), 1 min, and 2 hr after IGF-1 addition. Arrows point to the ciliary TZ. (G) Immunolabeling of (pan)IGF-1R (green), Ac-Tub (red), and γ-Tub (cyan) in RPE-1 cells immediately after serum deprivation (0 hr) and 2hr after IGF-1 addition. Arrows and arrowheads point to ciliary and plasma membrane localizations of IGF-1R, respectively. Data are presented as mean ± SEM (**p<0.01; *p<0.05; one-way ANOVA; n=3 experiments). Scale bars: 10 µm (D); 2 µm (F, G). See also Fig. S1.
We used three independent methods to verify that IGF-1 mediates ciliary resorption specifically through IGF-1R. First, we demonstrated that IGF-1 induced ciliary resorption was effectively blocked by an IGF-1R neutralizing antibody (Ab), but not by a control (anti-hemagglutinin) Ab (Fig. 1B). Next, we performed ciliary assembly/disassembly assays in cells transfected with constructs encoding shRNA against IGF-1R and green fluorescent protein (IGF-1R-sh/GFP), or a construct encoding GFP only (vector control). Fractions of total GFP+ cells exhibiting cilia were scored at 0 hr, 2 hr, and 24 hr following IGF-1 stimulation (Fig. 1C). IGF-1R silencing did not affect the formation of cilia, but significantly inhibited IGF-1 induced resorption of cilia (Fig. 1D, 1E). This inhibition was consistently observed in cells transfected with three independent, validated IGF-1R-sh constructs (i.e., confirmed by qRT-PCR to reduce endogenous IGF-1R expression by ~50%), ruling out the possibility of off-target effects (Fig. 1E).
Shortly following IGF-1 stimulation, activated IGF-1R (phosphorylated at Tyr1131/1135/1136 (Kato et al., 1994)) signals became increasingly enriched at the ciliary transition zone (TZ), located between the γ-tubulin (γ-Tub)-labeled basal body and acetylated-α-tubulin (Ac-Tub)-labeled ciliary axoneme (Fig. 1F). Phospho-IGF-1R became prominent at the base of the cilium 2 hr after IGF-1 treatment, a time point that correlates with the first wave of ciliary resorption.
Using an anti-(pan)IGF-1R Ab, we found that IGF-1R localized to both the cell surface (arrowheads, Fig. 1G) and primary cilium (arrows, Fig. 1G) of almost all RPE-1 cells at various time points before and after IGF-1 stimulation. IGF-1R signals on the cell surface, which often appeared as clusters, were not particularly enriched near the base of the cilium (arrowheads, Fig. 1G). The treatment of cells with dynasore blocked dynamin-mediated clathrin-dependent endocytosis (Macia et al., 2006), but did not affect the IGF-1 stimulated appearance of phospho-IGF-1R at the base of cilium (Fig. S1). Hence, phospho-IGF-1R at the base of the cilium is not likely to be derived from the translocation of surface IGF-1R via ligand-activated endocytosis. On the other hand, IGF-1R on the ciliary membrane exhibited an uneven spatial distribution pattern, suggesting that these receptors undergo dynamic bidirectional transport (Fig. 1G, arrows).
IGF-1 induces ciliary resorption and S phase entry by activating phospho(T94)Tctex-1 at the ciliary TZ
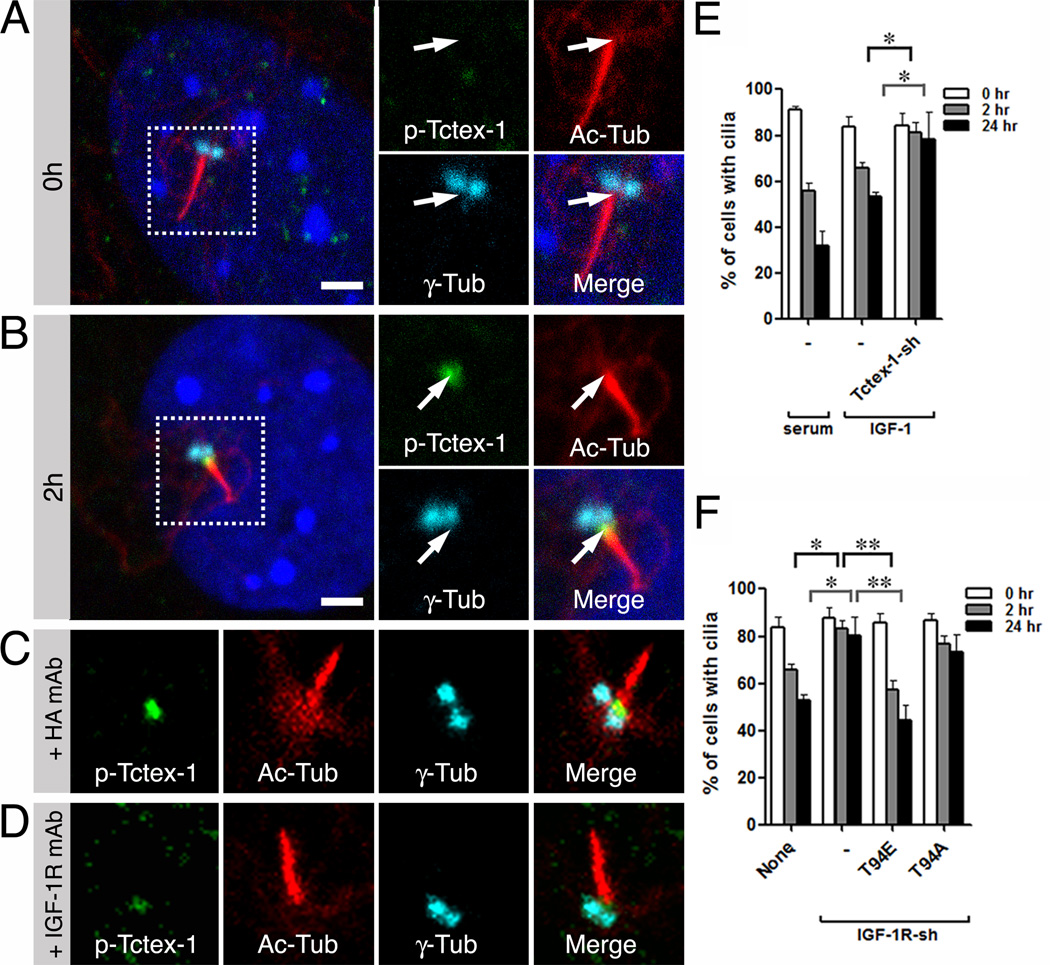
The spatial and temporal expression patterns of phospho-IGF-1R described above are strikingly similar to those of phospho(T94)Tctex-1 (Li et al., 2011). This led us to examine whether or not the ciliary and mitogenic effects of IGF-1 require the downstream activation of phospho(T94)Tctex-1. First, we showed that IGF-1 alone is sufficient to induce the expression of phospho(T94)Tctex-1 at the base of the cilium (Fig. 2A, 2B). Second, IGF-1R neutralizing Ab, but not control Ab, was able to suppress the activation of phospho(T94)Tctex-1 at the TZ (Fig. 2C, 2D). Third, we showed that cells transfected with Tctex-1-sh/GFP were unable to resorb cilia in response to IGF-1 stimulation (Fig. 2E),
Fig. 2. Phospho(T94)Tctex-1 is a downstream effector of IGF-1 induced ciliary resorption.
(A, B) Representative images of cells immunolabeled for phospho(T94)Tctex-1, Ac-Tub, and γ-Tub in quiescent RPE-1 cells (A; 0 hr) or IGF-1 treated cells (B; 2 hr). (C, D) Representative images of cilia in IGF-1 stimulated RPE-1 cells incubated with control HA Ab (C) or anti-IGF-1R αIR3 Ab (D), and immunolabeled with indicated Abs. (E) Percentages of control- and Tctex-1-sh/GFP- transfected 3T3 cells displaying cilia after serum or IGF-1 treatments. (F) Biphasic ciliary resorption profiles of IGF-1 treated cells that were non-transfected, or transfected with IGF-1R-sh/GFP alone (−) or together with Tctex-1 mutant T94E or T94A. Data are presented as mean ± SEM (**p<0.01; *p<0.05; one-way ANOVA; n=3 experiments). Scale bars: 2 µm (A, B).
At the functional level, we found that the ectopic expression of T94E alone reduced the minimum concentration of IGF-1 that was necessary to induce ciliary resorption from 10 nM to 1 nM (data not shown). Co-expression with T94E, but not T94A (the non-phosphorylatable mutant form of Tctex-1), was able to rescue the defects in ciliary resorption caused by reductions in IGF-1R expression (Fig. 2F).
The mitogenic signals provided by IGF-1 are transmitted through primary cilia and phospho(T94)Tctex-1
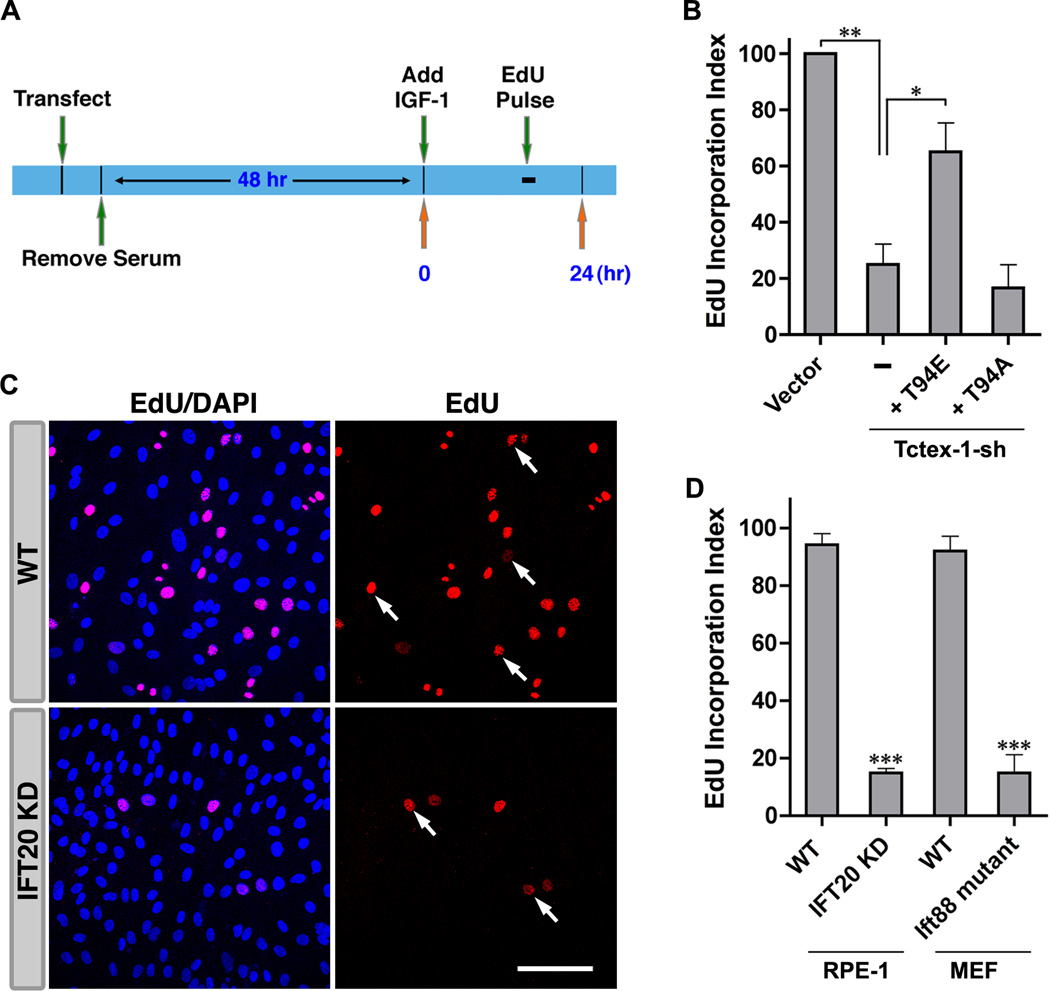
Using a 5-ethynyl-2'-deoxyuridine (EdU) incorporation assay (Fig. 3A), we found that Tctex-1 suppression significantly blocked IGF-1 induced S phase entry. This phenotype was effectively rescued by co-expression with T94E, but not T94A (Fig. 3B), suggesting that IGF-1 triggers S phase entry through a pathway that involves the downstream activation of phospho(T94)Tctex-1.
Fig. 3. The mitogenic signals of IGF-1 are transduced through cilia, and are phospho(T94)Tctex-1-dependent.
(A) Timeline depicting the sequence of events in a typical S phase entry EdU experiment. (B) Quantification of EdU incorporation in cells transfected with indicated plasmids. Incorporation of EdU in GFP control-transfected cells was taken to be 100%. (C) Representative images of EdU-labeled WT and IFT20 KD RPE-1 cells after 16 hr of IGF-1 treatment. Red: EdU; Blue: DAPI; Arrows: examples of EdU positive nuclei. (D) Quantification of EdU incorporation in indicated cell types. For each cell type, incorporation of EdU in parental (WT) cells was taken to be 100%. Data are presented as mean ± SEM (***p<0.001; **p<0.01; *p<0.05; one-way ANOVA; n=3 experiments). Scale bars: 100 µm (C). See also Fig. S4.
To assess whether or not IGF-1 transmits its mitogenic signals through primary cilia, we examined the rates of IGF-1 induced S phase progression in two IFT mutant cell lines — RPE-1 stable cell line with Ift20 suppressed (Follit et al., 2006) and Ift88 mutant MEF cells derived from Ift88Δ2–3βGal mice (Jia et al., 2009; Murcia et al., 2000), both of which are unable to form cilia (Jia et al., 2009; Li et al., 2011; Murcia et al., 2000). Significantly fewer IFT mutant cells were able to enter S phase in response to IGF-1 relative to their parental, wild type counterparts (Fig. 3C, 3D). These results suggest that IGF-1 mediated mitogenic signaling requires the presence of a functional cilium.
A non-canonical Gβγ signaling pathway activates phospho(T94)Tctex-1 prior to the resorption of cilia
Previous studies have shown that IGF-1 binding leads to an increase in association between IGF-1R and GαiGDP, which sustains the intracellular pool of free Gβγ (Dalle et al., 2001; Hallak et al., 2000; Luttrell et al., 1995). It is possible that this pool of Gβγ is important for generating the dynein-free Tctex-1 that is necessary for Thr94 phosphorylation (Sachdev et al., 2007). Since IGF-1R has no reported guanine nucleotide exchange (GEF) factor activity toward Gαi, we surmise that downstream to the activation of IGF-1R, AGS3 might play a role in stabilizing GαiGDP to maintain free Gβγ (Takesono et al., 1999). In support of this model, GFP-IGF-1R, but not GFP alone, co-immunoprecipitated with AGS3 in transfected 293T cells that co-expressed Gαi (Fig. 4A). Furthermore, Gβ (Fig. 4B) and AGS3 (Fig. 4C) were also concentrated at the base of the cilium (closely apposing the γ-Tub-labeled basal body) at both 0 hr and 2 hr time points in RPE-1 cells.
Fig. 4. Gβγ signaling mediates ciliary resorption via the recruitment of phospho(T94)Tctex-1 to the ciliary TZ.
(A) Immunoblots of total cell lysates (TCL) or GFP Ab immunoprecipitates of 293T cells transfected with indicated plasmids, and then stimulated with IGF-1. (B, C) Immunolabeling of γ-Tub (cyan), Ac-Tub (red), and Gβ (green in B) or AGS3 (green in C) in RPE-1 cells post serum treatment. (D) Biphasic ciliary resorption profiles of 3T3 cells transfected with indicated plasmids. Fractions of GFP+ transfected cells displaying cilia at indicated time points following serum addition are shown. (E) The relative (Rel.) intensity of phospho(T94)Tctex-1 immunolabeling at the ciliary TZ of transfected cells is shown. The immunofluorescence intensity of phospho(T94)Tctex-1 in control non-transfected cells was taken to be 100%. (F, G) Cells were transfected with βARK-ct/GFP (E) or AGS3-sh/GFP (F), then immunolabeled with phospho(T94)Tctex-1 (red) and γ and 2 are enlarged views of boxed regions 1 and 2, respectively. Data are represented as mean ± SEM (***p<0.001; **p<0.01; *p<0.05; one-way ANOVA; n=3 experiments). Scale bars: 5 µm (low power panel of C); 2 µm (high power panel of C, G).
At the functional level, we performed ciliary assembly/disassembly assays in cells depleted of free Gβγ subunits via the transfection of either βARK-ct/GFP or AGS3-shRNA/GFP. Compared to GFP-transfected control cells, fewer cells transfected with either construct were able to form cilia following 48 hr of serum starvation (Fig. 4D), indicating that Gβγ signaling is important for ciliogenesis. However, the cilia formed in these cells failed to resorb upon treatment with serum (Fig. 4D). Co-transfection with T94E, but not T94A, was able to specifically rescue the defects in biphasic ciliary resorption (but not ciliogenesis) caused by Gβγ removal (Fig. 4D).
Finally, we observed that the intensity of phospho(T94)Tctex-1 labeling at the ciliary TZ was significantly weaker in cells transfected with βARK-ct/GFP or AGS3-sh/GFP than in neighboring non-transfected GFP− cells following serum treatment (Fig. 4E–G). Taken together, these data suggest that depleting free Gβγ inhibits the downstream TZ activation of phospho(T94)Tctex-1 and hence, ciliary resorption.
IGF-1R mediated non-canonical Gβγ signaling maintains the proliferating progenitor population during corticogenesis
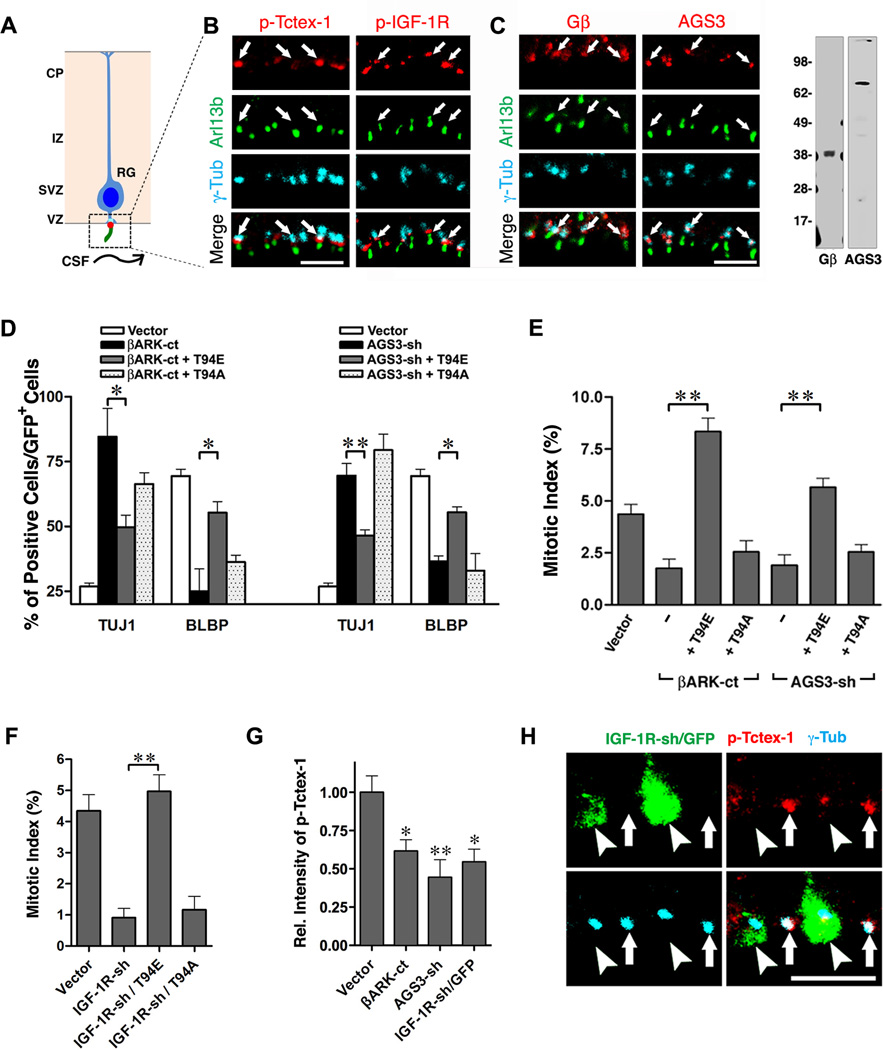
Previously, we proposed that RG receive cues from the ventricle via primary cilia, allowing for subsequent recruitment of phospho(T94)Tctex-1, which ensures timely ciliary resorption and proliferation (Li et al., 2011) (Fig. 5A). A recent study showed that cerebrospinal fluid (CSF) circulating through the ventricles contains IGF-1R ligands (i.e., IGF-1, IGF-2) (Lehtinen et al., 2011). Furthermore, immunoEM reveals prominent IGF-2 signal on the cilia of RG in the developing neocortex (Lehtinen et al., 2011).
Fig. 5. Non-canonical IGF-1R-Gβγ signaling pathway regulates the recruitment of phospho(T94)Tctex-1 and cell fate choice of RG.
(A) Schematic diagram of RG in the developing neocortex. Each RG has a single primary cilium, which projects from its apical endfeet and extends into the ventricles, contacting CSF. Immunolabeling of boxed area is shown in (B, C). (B) Enlarged views of ventricle surfaces that are triple labeled with phospho(T94)Tctex-1 (red) or phospho-IGF-1R (red) together with Arl13b (green) and γ-Tub (cyan). (C) Left: enlarged views of the ventricle surfaces immunolabeled with Gβ (red), or AGS3 (red) together with Arl13b (green) and γ-Tub (cyan). Right: Gβ and AGS3 Abs each recognized a single band of expected size in mouse embryonic brain lysates on immunoblots. (D) Percentage of total transfected GFP+ cells that were neurons (Tuj1+) or RG (BLBP+). (E) Mitotic indices of GFP+ cells transfected with indicated plasmids. More than 400 cells in 4 independent animals were scored. (F) Mitotic index. Fractions of GFP+ /PH3+ out of total GFP+ cells are shown as means ±SEM. More than 600 GFP+ cells in at least 3 experiments were scored. (G) Quantification of the relative (Rel.) intensity of phospho(T94)Tctex-1 of cells transfected with indicated plasmids. The intensity in vector control-transfected cells was taken to be 100%. (H) Representative images of ventricle surfaces containing IGF-1R-sh/GFP transfected cells. Arrowheads and arrows point to GFP+ transfected cells and GFP− non-transfected cells. Data are presented as mean ± SEM (**p<0.01; *p<0.05; one-way ANOVA). Scale bars: 5 µm (B, C, H). See also Fig. S2 and S3.
We set out to determine whether or not our proposed IGF-1R-Gβγ-phospho(T94)Tctex-1 signaling pathway exhibits the same functional hierarchy in the developing brain as that seen in vitro. First, we found that phospho-IGF-1R was co-enriched with phospho(T94)Tctex-1 near the ciliary TZ region of RG (between Arl13b-labeled cilia and γ-Tub labeled basal bodies) (Fig. 5B, Fig. S2A). No significant phospho-IGF-1R signal was found in any other region of the cortex (Fig. S2B), suggesting that IGF-1R activation occurs predominantly through cilia in vivo as well. Furthermore, using antibodies validated for specificity, we showed that the immunoreactivity of both Gβ and AGS3 were concentrated at the basal body in RG (Fig. 5C).
The majority of RG division occurs at the ventricular zone (VZ) (Fig. S2C). Post-mitotic neuronal progenies migrate away from the VZ, halt temporarily at the intermediate zone (IZ), and get incorporated into laminated cortical plates according to their birthdates (Fig. S2C). We performed knockdown and rescue experiments in E13.5 mouse cortices via in utero electroporation (IUE). About 40 hrs post-transfection, the majority of RG transfected with GFP alone produced daughter RG, characterized by the expression of brain lipid-binding protein (BLBP) (Fig. 5D) and the possession of nuclei distributed between the VZ and IZ (Fig. S2D). Similar to Tctex-1 silencing (Fig. S2D; Li et al., 2011), suppression of Gβγ (via the expression of either βARK-ct or AGS3) caused RG to differentiate into Tuj1+ neurons (Fig. 5D) that migrated to the IZ (Fig. S2E, S2F). Consistently, significantly fewer cells transfected with βARK-ct-IRES-GFP or AGS3-shRNA-IRES-GFP were mitotic (i.e., phosphohistone-3 (PH3) positive) compared to cells transfected with control vectors (Fig. 5E, Fig. S2E, S2F).
RG transfected with IGF-1R-shRNA/GFP behaved almost identically to RG in which Tctex-1 or Gβγ signaling was suppressed. RG with IGF-1R suppressed exhibited significantly lower mitotic indices (Fig. 5F) due to their premature exit from the cell cycle (Fig. S3A, S3C). These cells adopted neuronal fates and migrated to the IZ (Fig. S3B).
Remarkably, co-expression of T94E, but not T94A, effectively rescued the majority of phenotypic deviations (e.g., neuron/progenitor ratio, mitotic index) caused by βARK-ct expression (Fig. 5D, 5E, Fig. S2E), AGS3 silencing (Fig. 5D, 5E, Fig. S2F), or IGF-1R silencing (Fig. 5F, Fig. S3A–C). Finally, we found that the cells with Gβγ or IGF-1R suppressed (that still had their processes contacting the ventricle surface) exhibited significantly weaker phospho(T94)Tctex-1 signals relative to neighboring non-transfected cells (Fig. 5G, 5H, Fig. S2G).
DISCUSSION
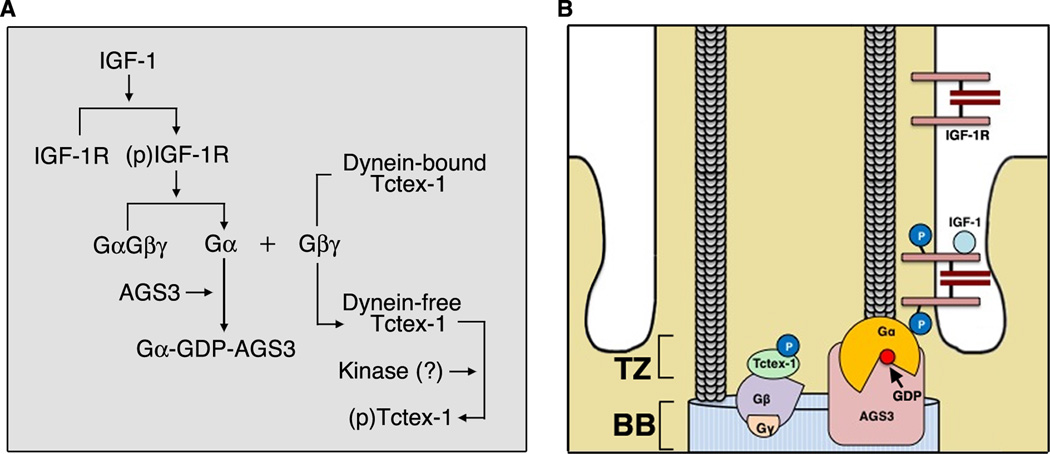
Our data support a model in which IGF-1 promotes cell cycle entry by mediating ciliary resorption through a non-canonical Gβγ signaling axis. Fig. 6 depicts our proposed stepwise process based on data from our previous and current studies: (1) Upon ligand binding, ciliary IGF-1R translocates to the base of the cilium; (2) Here, AGS3 binds and stabilizes Gαi-GDP bound to activated IGF-1R in order to sustain the release of Gβγ; (3) Gβγ competes with dynein intermediate chain for Tctex-1 binding, generating a pool of dynein-independent Tctex-1; (4) Tctex-1 is phosphorylated at Thr94 and recruited to the ciliary TZ, where it induces the first wave of ciliary resorption and primes cells to re-enter S phase.
Fig. 6. Working Model.
(A) Sequential diagram of our proposed signaling pathway. Activated IGF-1R binds to the Gα subunit of the heterotrimeric G protein complex. AGS3 binds and stabilizes GDP-bound Gα. Free Gβγ triggers the release of Tctex-1 from the dynein complex, enabling subsequent phosphorylation of Tctex-1. (B) Spatial diagram of our working model. BB: basal body.
Cilia dependent vs. independent control of cell proliferation
Emerging data suggest that in cycling cells, ciliary disassembly acts as a “checkpoint” to monitor G1/S progression (Kim et al., 2011; Li et al., 2011). Here, we show that in non-transformed epithelial and fibroblast cells, IGF-1 transmits its proliferative signals primarily through the cilium by modulating its disassembly, which in turn, times the G1/S transition of the cell. Compromising the formation of cilia in IFT mutant cells eliminates their ability to proliferate in response to IGF-1. This suggests that IGF-1R associated with the ciliary membrane represents the major sensor of IGF-1 in wild type cells.
Our findings support the theory that cilia are important organelles which transmit mitogenic signals required for cell division (reviewed by (Christensen et al., 2007)). This leads us to question why IGF-1 induces cell cycle entry by preferentially activating IGF-1Rs on cilia over those on the cell surface. We speculate that the close proximity of key effectors of ciliary resorption (this paper; Li et al., 2011) to centrosomal proteins required for S phase entry which localize to the base of the cilium (Doxsey et al., 2005) is necessary to ensure the temporal coupling of these two cellular events.
While non-ciliated IFT mutant cells lose their ability to respond to IGF-1 (Fig. 3C, 3D), it is interesting to note that these mutant cells retain their capacity to enter the cell cycle in response to serum (Fig. S4), which contains numerous other growth factors. In fact, these cells entered S phase at a ~2-fold higher rate relative to their wild type, ciliated counterparts (Fig. S4). These results suggest that while IFT mutant cells have lost their ability to sense IGF-1, they respond with higher sensitivity to alternative, serum-derived extracellular cues through signaling pathways that are normally less active in wild type, ciliated cells. This finding might help to address why cilia have opposing effects on cell growth, depending on the context (Han et al., 2009; Wong et al., 2009).
Finally, many types of cancerous/transformed cells lack cilia (Han et al., 2009; Seeley et al., 2009; Wong et al., 2009; Yuan et al., 2010), but express elevated levels of IGF-1R and its effectors, including insulin receptor substrate and Ras/PI3K/Akt signaling components (Chang et al., 2002; Schubbert et al., 2007; Shimizu et al., 2004). These cells must respond to IGF-1 via cilia-independent signaling pathways transduced by IGF-1Rs on the cell surfaces. In support of this hypothesis, IGF-1 is known to have distinct effects on the growth of normal and transformed cells (Baserga et al., 2003).
Regulation of ciliary resorption is important for maintaining neural progenitor population
The cerebral cortex is formed via the coordination between lateral expansion of neural progenitors and their differentiation. During early cortical development, the majority of RG divide to produce identical daughters that expand the mitotic progenitor population, whereas late progenitors tend to adopt neuronal fates. A better understanding of the mechanisms that underlie the switch between self-renewal and neuronal differentiation in RG is essential for elucidating the factors which control brain size (Bond and Woods, 2006). Recent data suggest that CSF-borne IGF-1R ligands are an important source of proliferative cues sensed by receptors on the cilia of RG. Here, a hierarchical signaling cascade involving AGS3 and Gβγ recruits phospho(T94)Tctex-1 to the ciliary TZ in order to control the timing of ciliary resorption and hence, S phase re-entry. The expression of IGF-1R ligands in CSF is temporally dynamic, peaking during times of neurogenesis (Lehtinen et al., 2011). This likely represents a mechanism for regulating progenitor population according to stage of development.
While it remains a technical challenge to quantitatively evaluate the disassembly of RG cilia in situ, we have shown in the past that suppressing molecules known to mediate ciliary resorption (e.g., AurA kinase, HDAC6 (Li et al., 2011)) unanimously blocks the S phase re-entry of RG. Furthermore, mouse and human genetics studies have shown that mutations in genes encoding proteins involved in ciliary resorption (e.g., Nde1, Inositol-1,4,5-trisphosphate 5-phosphatase) are associated with microcephaly (Bakircioglu et al., 2011; Bielas et al., 2009; Jacoby et al., 2009). Microcephaly and mental retardation have also been reported in patients with mutations in Igf1 or Igf1R (Walenkamp et al., 2005; Walenkamp and Wit, 2006). Lastly, the human Tctex-1 (TCTEL1) gene has been mapped to a chromosomal region that is deleted in a subset of patients with mental retardation (Watanabe et al., 1996). Taken together, these findings suggest that the regulation of ciliary dynamics in RG plays an important role in determining the population of proliferating progenitors and hence, the overall size of the brain.
Perspectives
IGF-1R signaling is highly conserved across species, and is responsible for a diverse set of physiological roles including lifespan, growth and metabolism. Interestingly, it has been shown that lifespan regulation of C. elegans depends on both the IGF-1R homologue DAF-2 and sensory cilia (Apfeld and Kenyon, 1999). Additionally, cilium-dependent IGF-1R signaling is required to induce the differentiation of preadipocytes (Zhu et al., 2009). Collectively, these and our present studies lead us to speculate that cilia-associated IGF-1R signaling mediates a large and diverse repertoire of cellular functions, some of which have yet to be identified.
EXPERIMENTAL PROCEDURES
Reagents
Primary Abs used were: IGF-1R neutralizing mouse Ab αIR3 (Kull et al., 1983) EMD Millipore, Temecula, CA); hemagglutinin Ab (Abcam, Cambridge, MA), IGF-1Rβ subunit rabbit Ab (for immunostaining; Santa Cruz, SC713), phospho-IGF-1R Ab (Tyr1131/1135/1136; Abcam), Ac-Tub mouse IgG2b (Sigma), AGS3 Ab (gift of Joe Blumer; (Blumer et al., 2002), Gβ rabbit Abs (clone T20 (Santa Cruz, Santa Cruz, CA) and gift of Tom Sakmar), Arl13b rabbit Ab (Gift from Kathyrn Anderson; (Caspary et al., 2007)), BLBP rabbit Ab (Gift from Nathaniel Heintz; Feng et al., 1994), BrdU rat Ab (Harlan, Loughborough, England), dynein intermediate chain mouse Ab (EMD Millipore, Billerica, MA), GFP rabbit Ab (Invitrogen, Grand Island, NY), GFP chicken Ab (Abcam), GFP mouse Ab (EMD Millipore), γ-Tub mouse Ab (clone GTU-88, IgG1, Sigma), Ki67 rabbit Ab (Novocastra, Newcastle, UK), Nestin mouse Ab (clone 401, Developmental Studies Hybridoma Bank, Iowa City, IA), P-H3 rabbit Ab (EMD Millipore), anti-phospho(T94)Tctex-1 rabbit Ab (Li et al., 2011), and Tuj1 mouse Ab (Covance, Princeton, NJ). Alexa-conjugated streptavidin or secondary Abs (anti-mouse, anti-rabbit, anti-rat, anti-IgG2b) were purchased from Invitrogen; biotinylated anti-IgG1 and Cy5-conjugated streptavidin were purchased from Jackson Lab (used as 1:200; West Grove, PA). In this study, negative controls involved omission of the primary Ab or use of Ab that had been pre-absorbed with antigen. Co-labeling of Ac-Tub (mouse IgG2b) and γ-Tub (mouse IgG1) in this study involved using isotype-specific mouse IgGs.
All plasmids used for overexpression experiments in cultures were under the CMV promoter. The expression constructs used in IUE were cloned into pCAG vector (gift of Dr. C. Cepko). All knockdown constructs were in pU6 vector (gift of Dr. Y. Shi; (Xia et al., 2003). Plasmid encoding human or mouse Tctex-1-sh, control-sh, WT, T94E, or T94A (in pCAG-IRES-GFP) have been described (Li et al., 2011). βARK-ct (gift from R. J. Lefkowitz; Koch et al., 1994b), AGS3 (gift from J. Blumer; (Blumer et al., 2003)), GFP-(human)IGF-1R (gift from Drs. Le Roith and Santigo Quiroga (Buckley et al., 2002)), AGS3-sh (gift from Dr. L.-H. Tsai; (Sanada and Tsai, 2005)), and IGF-1R-sh-IRES-GFP were also used. IGF-1R shRNA #3 was used for the majority of our experiments (The targeting sequencing of IGF-1R is 5’-GGGAATGGGTCGTGGACAGAT).
Cell culture, transfection, cilium assembly/disassembly assay, EdU incorporation index, and co-immunoprecipitation
IFT20 KD-RPE-1 (Follit et al., 2006) was a gift from G. Pazour (University of Massachusetts Medical School). Wild-type MEF and Ift88 mutant MEF (Ift88Δ2–3βGal) cells were gifts from A. Liu (Pennsylvania State University, (Jia et al., 2009)). All cells were transfected using nucleofection (Amaxa, Gaithersburg, MD); cells receiving more than one plasmid were routinely immunolabeled to confirm high (>90%) double or triple transfection efficiency. Standard methods were used for immunoprecipitation and immunoblotting. Immunoblots were quantified using the Odyssey Infrared system (LI-COR, Lincoln, NB). A cilium assembly/disassembly assay was performed exactly as previously described (Pugacheva et al., 2007). Briefly, 18-hr post-transfected or non-transfected cells were starved in serum-free medium for 48 hr to induce cilium formation (and gene expression). Assay medium containing either 10% serum, 10 nM IGF-1 (Sigma), 50 ng/ml PDGF-AA (EMD Millipore) was then added to the cells to induce ciliary resorption. Cells were harvested at various time points for immunolabeling. In neutralization experiments, Abs (1 µg/ml) were added to cells at the time of growth factor addition, and replenished once 12 hr post serum addition.
To quantify EdU incorporation, experiments were carried out in synchronized cells. 18 hr post-transfected cells were growth arrested by a 48 hr serum starvation period. Cells were then cultured in regular medium for 12–16 hr, and pulse labeled with 1 hr EdU (10 µM) followed by a 7–11 hr chase. These cells were subsequently stained using a CLICK-it reaction following manufacturer’s instructions (Invitrogen). Co-immunoprecipiation was carried out in 293T cells that were transfected with indicated plasmids, serum starved, and then treated with IGF-1 for 12 hr. Harvested cell lysates were subjected to immunoprecipitation using anti-GFP antibody coupled on Protein G Dynabeads® (Invitrogen). Immunoblots of immuoprecipitates were quantified using the Odyssey Infrared system.
IUE, immunohistochemistry of mouse brain slices, and quantitative analyses
IUE procedures were performed on E13.5 CD1 mouse brains as described (Li et al., 2011). At specific time points, electroporated brains were harvested, fixed with 4% paraformaldehyde overnight at 4°C, embedded in low melting agarose, and sectioned by vibratome. Sectioned brain slices were subsequently subjected to immunostaining using standard protocols. For the co-labeling of phospho(T94)Tctex-1 and phospho-IGF-1R, sections were first incubated with Ab against phospho(T94)Tctex-1, followed by excess biotinylated goat anti-rabbit Ab. The sections were then paraformaldehyde fixed, incubated with anti-phospho-IGF-1R Ab and γ-Tub Abs, and detected using Alexa488-conjugated anti-rabbit, Cy5-conjugated anti-mouse Abs, and Alexa568 conjugated streptavidin. Analysis of the immunostained sections was carried out on a Leica TCS SP2 spectral confocal system. All the quantification studies were carried out in transfected cells localized within the dorsolateral neocortex to avoid possible variation within particular brain regions. At least 3 independent brains were analyzed for each DNA construct. In order to improve observer objectivity, image capture and analysis were done at separate times in a double-blind fashion. To score images, the GFP channel was first judged independently, followed by judgments of the other markers. For Ki67 and BrdU-nuclear labeling, small punctae or signals that were not compliant with the DAPI nuclear labeling were ignored. All animal manipulations were performed in accordance with the guidelines for animal experiments established by the Institutional Animal Care and Use Committees of Weill Medical College of Cornell University.
Mitotic index, cell-cycle exit and cell-cycling analyses in brain slices
The mitotic index of treated brain slices was determined by the ratios of GFP and PH3-double positive cells to total GFP+ transfected cells in the VZ/subventricular zone. Cell cycle analysis was performed as described (Li et al., 2011). Briefly, pregnant mice were injected with BrdU (50 mg/kg body weight) 24 hr after IUE, and fetal brains were harvested 24 hr after BrdU treatment. Brain slices were then immunolabeled for GFP, BrdU and Ki67. The cell-cycle-exit index of GFP-transfected cells was determined as the ratio of GFP-labeled cells that exited the cell cycle (GFP+, BrdU+ and Ki67−) to total GFP-labeled cells with BrdU incorporation (GFP+ and BrdU+).
Statistical analyses
Statistical analyses were performed with GraphPad software (GraphPad Prism v4.0, GraphPad Software Inc., San Diego, CA, USA). Data are presented as the mean ± SEM from at least three representative independent experiments. T-test was used for the comparison of two groups. One-way analysis of variance (ANOVA) was applied for the comparisons in which only one independent variable was being analyzed. The Dunnet test (as a post hoc test) was used to compare data samples versus control. Statistical significance was defined as p < 0.05, 0.01 or 0.001.
Supplementary Material
The relationship between ciliary resorption and S-phase entry is unclear. Yeh et al. show that activation of IGF-1 receptor initiates a non-canonical Gβγ signaling cascade at the primary cilium, culminating in the recruitment of phospho(T94)Tctex-1 and the resorption of the cilium. This ciliary pathway regulates progenitor proliferation in the developing neocortex.
The mitogenic activity of IGF-1 requires a primary cilium
IGF-1 induces ciliary resorption by activating a non-canonical Gβγ pathway
IGF-1 mediated ciliary resorption and S phase entry are phospho-Tctex-1 dependent
This IGF-1R-Gβγ-phospho(T94)Tctex-1 pathway maintains neural progenitor population
ACKNOWLEDGEMENTS
We are indebted to the following grant support: Tri-Institutional Starr Foundation, NYSTEM, NIH (EY11307, EY016805), RPB (to CHS) and Tohoku University (to MS). We thank Drs. Kathy Anderson, Joe Blumer, Greg Pazour, Aiming Liu, Thomas Sakmar, Robert Hevner, Yang Shi, Nathaniel Heintz, Connie Cepko, Li-Huei Tsai, and Robert Lefkowitz for reagents. We thank Drs. M. Elizabeth Ross, Francis Lee, Tao Sun, Leonidas Tsiokas and Bryan Tsao for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Apfeld J, Kenyon C. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Bakircioglu M, Carvalho OP, Khurshid M, Cox JJ, Tuysuz B, Barak T, Yilmaz S, Caglayan O, Dincer A, Nicholas AK, et al. The Essential Role of Centrosomal NDE1 in Human Cerebral Cortex Neurogenesis. Am J Hum Genet. 2011;88:523–535. doi: 10.1016/j.ajhg.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. International journal of cancer Journal international du cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer JB, Bernard ML, Peterson YK, Nezu J, Chung P, Dunican DJ, Knoblich JA, Lanier SM. Interaction of activator of G-protein signaling 3 (AGS3) with LKB1, a serine/threonine kinase involved in cell polarity and cell cycle progression: phosphorylation of the G-protein regulatory (GPR) motif as a regulatory mechanism for the interaction of GPR motifs with Gi alpha. J Biol Chem. 2003;278:23217–23220. doi: 10.1074/jbc.C200686200. [DOI] [PubMed] [Google Scholar]
- Blumer JB, Chandler LJ, Lanier SM. Expression analysis and subcellular distribution of the two G-protein regulators AGS3 and LGN indicate distinct functionality. Localization of LGN to the midbody during cytokinesis. J Biol Chem. 2002;277:15897–15903. doi: 10.1074/jbc.M112185200. [DOI] [PubMed] [Google Scholar]
- Bond J, Woods CG. Cytoskeletal genes regulating brain size. Curr Opin Cell Biol. 2006;18:95–101. doi: 10.1016/j.ceb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Buckley DA, Loughran G, Murphy G, Fennelly C, O'Connor R. Identification of an IGF-1R kinase regulatory phosphatase using the fission yeast Schizosaccharomyces pombe and a GFP tagged IGF-1R in mammalian cells. Molecular pathology: MP. 2002;55:46–54. doi: 10.1136/mp.55.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F, Huttner WB. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J Cell Sci. 2003;116:4947–4955. doi: 10.1242/jcs.00825. [DOI] [PubMed] [Google Scholar]
- Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chang Q, Li Y, White MF, Fletcher JA, Xiao S. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: therapeutic implications. Cancer research. 2002;62:6035–6038. [PubMed] [Google Scholar]
- Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Caceres A, Sung CH. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell. 2005;9:75–86. doi: 10.1016/j.devcel.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle S, Ricketts W, Imamura T, Vollenweider P, Olefsky JM. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J Biol Chem. 2001;276:15688–15695. doi: 10.1074/jbc.M010884200. [DOI] [PubMed] [Google Scholar]
- Doxsey S, Zimmerman W, Mikule K. Centrosome control of the cell cycle. Trends Cell Biol. 2005;15:303–311. doi: 10.1016/j.tcb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Peterson YK, Lanier SM, Smrcka AV. Receptor- and nucleotide exchange-independent mechanisms for promoting G protein subunit dissociation. J Biol Chem. 2003;278:34747–34750. doi: 10.1074/jbc.C300271200. [DOI] [PubMed] [Google Scholar]
- Hallak H, Seiler AE, Green JS, Ross BN, Rubin R. Association of heterotrimeric G(i) with the insulin-like growth factor-I receptor. Release of G(betagamma) subunits upon receptor activation. J Biol Chem. 2000;275:2255–2258. doi: 10.1074/jbc.275.4.2255. [DOI] [PubMed] [Google Scholar]
- Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nature medicine. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge RD, D'Ercole AJ, O'Kusky JR. Insulin-like growth factor-I accelerates the cell cycle by decreasing G1 phase length and increases cell cycle reentry in the embryonic cerebral cortex. J Neurosci. 2004;24:10201–10210. doi: 10.1523/JNEUROSCI.3246-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Jia J, Kolterud A, Zeng H, Hoover A, Teglund S, Toftgard R, Liu A. Suppressor of Fused inhibits mammalian Hedgehog signaling in the absence of cilia. Dev Biol. 2009;330:452–460. doi: 10.1016/j.ydbio.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Faria TN, Stannard B, Roberts CT, Jr, LeRoith D. Essential role of tyrosine residues 1131, 1135, and 1136 of the insulin-like growth factor-I (IGF-I) receptor in IGF-I action. Mol Endo. 1994;8:40–50. doi: 10.1210/mend.8.1.7512194. [DOI] [PubMed] [Google Scholar]
- Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, Katsanis N, Obara T, Tsiokas L. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci U S A. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull FC, Jr, Jacobs S, Su YF, Svoboda ME, Van Wyk JJ, Cuatrecasas P. Monoclonal antibodies to receptors for insulin and somatomedin-C. J biol chem. 1983;258:6561–6566. [PubMed] [Google Scholar]
- Lange C, Huttner WB, Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Lee JE, Gleeson JG. A systems-biology approach to understanding the ciliopathy disorders. Genome Med. 2011;3:59. doi: 10.1186/gm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, Tomizawa K, Kaitsuka T, Sung CH. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat cell biol. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ. G beta gamma subunits mediate mitogen-activated protein kinase activation by the tyrosine kinase insulin-like growth factor 1 receptor. J Biol Chem. 1995;270:16495–16498. doi: 10.1074/jbc.270.28.16495. [DOI] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Developmental cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Mason JL, Goldman JE. A2B5+ and O4+ Cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left-right axis determination. Development. 2000;127:2347–2355. doi: 10.1242/dev.127.11.2347. [DOI] [PubMed] [Google Scholar]
- Novosyadlyy R, Dudas J, Pannem R, Ramadori G, Scharf JG. Crosstalk between PDGF and IGF-I receptors in rat liver myofibroblasts: implication for liver fibrogenesis. Lab Invest. 2006;86:710–723. doi: 10.1038/labinvest.3700426. [DOI] [PubMed] [Google Scholar]
- Oner SS, An N, Vural A, Breton B, Bouvier M, Blumer JB, Lanier SM. Regulation of the AGS3.G{alpha}i signaling complex by a seven-transmembrane span receptor. The Journal of biological chemistry. 2010;285:33949–33958. doi: 10.1074/jbc.M110.138073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson YK, Bernard ML, Ma H, Hazard S, 3rd, Graber SG, Lanier SM. Stabilization of the GDP-bound conformation of Gialpha by a peptide derived from the G-protein regulatory motif of AGS3. The Journal of biological chemistry. 2000;275:33193–33196. doi: 10.1074/jbc.C000509200. [DOI] [PubMed] [Google Scholar]
- Pilaz LJ, Patti D, Marcy G, Ollier E, Pfister S, Douglas RJ, Betizeau M, Gautier E, Cortay V, Doerflinger N, et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci U S A. 2009;106:21924–21929. doi: 10.1073/pnas.0909894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken GJ, Hodge RD, Ye P, Zhang J, Ng W, O'Kusky JR, D'Ercole AJ. In vivo effects of insulin-like growth factor-I (IGF-I) on prenatal and early postnatal development of the central nervous system. Eur J Neurosci. 2004;19:2056–2068. doi: 10.1111/j.0953-816X.2004.03320.x. [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Menon S, Kastner DB, Chuang JZ, Yeh TY, Conde C, Caceres A, Sung CH, Sakmar TP. G protein beta gamma subunit interaction with the dynein light-chain component Tctex-1 regulates neurite outgrowth. EMBO J. 2007;26:2621–2632. doi: 10.1038/sj.emboj.7601716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Tsai LH. G Protein betagamma Subunits and AGS3 Control Spindle Orientation and Asymmetric Cell Fate of Cerebral Cortical Progenitors. Cell. 2005;122:119–131. doi: 10.1016/j.cell.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu C, Hasegawa T, Tani Y, Takahashi F, Takeuchi M, Watanabe T, Ando M, Katsumata N, Fujiwara Y. Expression of insulin-like growth factor 1 receptor in primary breast cancer: immunohistochemical analysis. Human pathology. 2004;35:1537–1542. doi: 10.1016/j.humpath.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Takesono A, Cismowski MJ, Ribas C, Bernard M, Chung P, Hazard S, 3rd, Duzic E, Lanier SM. Receptor-independent activators of heterotrimeric G-protein signaling pathways. J Biol Chem. 1999;274:33202–33205. doi: 10.1074/jbc.274.47.33202. [DOI] [PubMed] [Google Scholar]
- Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Bell JR, Chen EY, Herrera R, Petruzzelli LM, Dull TJ, Gray A, Coussens L, Liao YC, Tsubokawa M, et al. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. Nature. 1985;313:756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Karperien M, Pereira AM, Hilhorst-Hofstee Y, van Doorn J, Chen JW, Mohan S, Denley A, Forbes B, van Duyvenvoorde HA, et al. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J Clin Endocrinol Metab. 2005;90:2855–2864. doi: 10.1210/jc.2004-1254. [DOI] [PubMed] [Google Scholar]
- Walenkamp MJ, Wit JM. Genetic disorders in the growth hormone - insulin-like growth factor-I axis. Horm Res. 2006;66:221–230. doi: 10.1159/000095161. [DOI] [PubMed] [Google Scholar]
- Watanabe TK, Fujiwara T, Shimizu F, Okuno S, Suzuki M, Takahashi E, Nakamura Y, Hirai Y. Cloning, expression, and mapping of TCTEL1, a putative human homologue of murine Tcte1, to 6q. Cytogenet Cell Genet. 1996;73:153–156. doi: 10.1159/000134329. [DOI] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatr Nephrol. 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XG, Zhou H, Ding H, Affar el B, Shi Y, Xu Z. An enhanced U6 promoter for synthesis of short hairpin RNA. Nucleic Acids Res. 2003;31:e100. doi: 10.1093/nar/gng098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, D'Ercole AJ. Insulin-like growth factor actions during development of neural stem cells and progenitors in the central nervous system. J Neurosci Res. 2006;83:1–6. doi: 10.1002/jnr.20688. [DOI] [PubMed] [Google Scholar]
- Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon YJ, Steg AD, Serra R, Frost AR. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Shi S, Wang H, Liao K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J Cell Sci. 2009;122:2760–2768. doi: 10.1242/jcs.046276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.