Abstract
Unilateral spatial neglect is a common neurological syndrome following predominantly right hemisphere injuries to ventral fronto-parietal cortex. We propose that neglect reflects deficits in the coding of saliency, control of spatial attention, and representation within an egocentric frame of reference, in conjunction with non-spatial deficits of reorienting, target detection, and arousal/vigilance. In contrast to theories that link spatial neglect to structural damage of specific brain regions, we argue that neglect is better explained by the physiological dysfunction of distributed cortical networks. The ventral lesions in right parietal, temporal, and frontal cortex that cause neglect directly impair non-spatial functions and hypoactivate the right hemisphere, inducing abnormalities in task-evoked activity and functional connectivity of a dorsal frontal-parietal network that controls spatial attention. The anatomy and right hemisphere dominance of neglect follows from the anatomy and laterality of the ventral regions that interact with the dorsal attention network.
Keywords: Hemispheric dominance, stroke, neuroimaging, functional connectivity, arousal, white matter
INTRODUCTION
L J is a 58-year old school teacher, who while at school suddenly developed malaise and confusion. He was brought to the hospital for evaluation. On first appearance his affect was flat and his vigilance reduced; his responses were grammatically accurate but delayed, and when asked ‘what was the matter’ he replied he was not sure why he had been brought in. On exam his visual fields were normal but his gaze tended to deviate to the right spontaneously when looking ahead. When presented with two objects, one in each visual field, he always looked first to the right object and denied the presence of the object on the left. However, when asked, he was able to move his eyes to the left and reported seeing the object. Overall, he was very slow in reporting stimuli even in his right visual field. When searching blindfolded for objects scattered on a table with either the left or right hand, he explored mainly the right side of the table, and his search progressed to the center only when objects on the right were removed. Touches on the right hand were easily reported and localized, while touches to the left hand were inconsistently detected and reported as belonging to the examiner. Motor function was normal in terms of strength, coordination and dexterity on both sides, but L J was reluctant to use his left hand unless asked to do so. Head MRI showed a diffusion restriction in the right inferior and middle frontal gyrus and anterior insula, consistent with an acute ischemic stroke.
This clinical history illustrates some key features of unilateral spatial neglect: a reduction of arousal and speed of processing; an inability to attend and report stimuli on the side opposite the lesion (contralesional) despite apparently normal visual perception; a spatial bias for directing actions toward the hemi-space or hemi-body on the same side as the lesion (ipsilesional); and several disorders of awareness, including a degree of obliviousness toward being ill and confabulation about body ownership.
Spatial neglect is caused by lesions, typically strokes, in a number of different cortical and sub-cortical areas. Although acutely both left and right hemisphere lesions can cause neglect, only right hemisphere lesions cause severe and persistent deficits (Stone et al 1993), which is the primary basis for the widely held view that the right hemisphere is dominant for attention.
Spatial neglect is unique among the behavioral disorders resulting from focal lesions since its severity can be modulated by behavioral interventions over very short timescales (e.g. seconds). Deficits in attending to and reporting objects in contralesional space can be lessened by 1) encouraging a patient to attend to the previously ignored stimuli using verbal cues (Riddoch & Humphreys 1983), 2) presenting salient sensory stimuli, such as noises (Robertson et al 1998), 3) asking the patient to perform hand movements controlled by the injured hemisphere (Robertson & North 1992), or 4) training patients to increase their alertness (Robertson et al 1995). These observations suggest that the neural mechanisms underlying the spatial deficit can be dynamically modulated by signals from other parts of the brain reflecting endogenous or exogenous attention, movement, and arousal. Moreover, in a matter of days or weeks, most patients with spatial neglect recover from the more obvious spatial impairments, which, however, continue to negatively influence their ability to return to a productive life (Denes et al 1982, Paolucci et al 2001).
Spatial neglect has attracted tremendous interest as a model for understanding the neurological basis of awareness, cerebral lateralization, spatial cognition, and recovery of function. Yet its neural bases remain poorly understood. Here, we provide a selective review of the vast literature on neglect and present a framework for understanding the disorder.
We propose that neglect is mediated by the abnormal interaction between brain networks that control attention to the environment in the healthy brain (see (Heilman et al 1985, Mesulam 1999) for other network formulations). Although many authors have emphasized dissociations and subtypes of spatial neglect, we argue for a core set of spatial and non-spatial deficits that match the physiological properties of these networks. The core spatial deficit, a bias in spatial attention and salience mapped in an egocentric coordinate frame, is caused by the dysfunction of a dorsal frontal-parietal network that controls attention and eye movements and represent stimulus saliency. Core non-spatial deficits of arousal, reorienting, and detection reflect structural damage to more ventral regions that partly overlap with a right hemisphere dominant ventral frontal-parietal network recruited during reorienting and detection of novel behaviorally relevant events.
Second, we emphasize that the ventral lesions that result in neglect alter the physiology of structurally undamaged dorsal fronto-parietal regions, consistent with the fact that dorsal and ventral attention regions interact in the healthy brain. Physiological dysfunction in dorsal fronto-parietal regions is empirically observed not only during task performance, but also at rest, and correlates with the severity of the egocentric spatial bias. Moreover, this dysfunction decreases the top-down modulation of visual cortex, reducing its responsiveness, which can also contribute to neglect. The highly dynamic and plastic nature of the spatial deficits in neglect strongly argues that they are mediated by parts of the brain that still function, even if abnormally. Measurements of the physiology of brain regions, not just of structural damage, are essential for understanding neglect (Deuel & Collins 1983), and we emphasize neuroimaging methods that provide a window on physiological function.
Finally, perhaps the least understood clinical feature of spatial neglect is its right hemisphere lateralization. Brain imaging studies have shown that dorsal fronto-parietal regions controlling spatial attention and eye movements are largely symmetrically organized, with each hemisphere predominantly representing the contralateral side of space. In contrast, ventral regions that underlie the core non-spatial deficits observed in neglect patients are strongly right hemisphere dominant. We argue that lateralization of these latter functions, and their interaction with dorsal regions, rather than asymmetries of spatial attention per se, primarily account for the hemispheric asymmetry of neglect.
THE CORE SPATIAL DEFICIT: EGOCENTRIC BIAS
A large body of neuropsychological research has tried to characterize the deficits in neglect that are ‘spatial’ (i.e. involve predominantly one side of space). Factor analytic studies of behavioral deficits have consistently isolated at least one factor associated with impairments in attending/searching/responding to targets in contralateral space, but have yielded inconsistent conclusions with regard to other factors (Azouvi et al 2002, Halligan et al 1989, Kinsella et al 1993, Verdon et al 2010). Other studies have classified patients based on their performance on different behavioral tasks thought to isolate different subtypes, such as perceptual/attention vs. motor/intention deficits (Coslett et al 1990), but the consistency (Hamilton et al 2008), and relevance to recovery of these distinctions has been disappointing (Farne et al 2004, Rengachary et al in press). Most patients suffer from perceptual/attention deficits, and these deficits account for most of its recovery (Rengachary et al in press). We argue that at its core, spatial neglect represents a deficit of spatial attention and stimulus saliency that is mapped in an egocentric reference frame.
Gradients of Spatial Attention and Stimulus Saliency
Virtually all patients with spatial neglect manifest a lateralized bias in visual information processing that is evident both clinically and experimentally as a gradient across space (Behrmann et al 1997, Pouget & Driver 2000). Low sensitivity and responsiveness to behaviorally relevant stimuli improve as one moves from contralesional to ipsilesional locations. This bias does not reflect abnormal early visual mechanisms, as indicated by contrast sensitivity (Spinelli et al 1990), image segmentation based on low-level features in the neglected visual field (Driver & Mattingley 1998), visually evoked potentials (Di Russo et al 2008, Watson et al 1977), and functional magnetic resonance imaging (fMRI) (Rees et al 2000).
In contrast, the ‘saliency’ of objects in the neglected visual field is impaired. Saliency refers to the sensory distinctiveness and behavioral relevance of an object relative to other objects. Saliency may be expressed as the magnitude of neural activity in topographic maps (frontal, parietal, superior colliculus) that determine which objects are selected for further analysis and action. In a recent study, the saliency of objects in the ipsilesional or contralesional visual field of neglect subjects was measured by indexing the patients’ tendency to look at distinctive but task-irrelevant stimuli or at task-relevant stimuli (Bays et al 2010). The probability of an eye movement increased along a spatial gradient from contralesional to ipsilesional locations that was the same for both kinds of stimuli, suggesting that exogenous (automatic) and goal-driven components of spatial attention were equally affected (Figure 1A). The abnormally high salience of ipsilesional stimuli may prevent them from being filtered when they are task-irrelevant (Bays et al 2010, Shomstein et al 2010, Snow & Mattingley 2006) or lead to repeated re-fixations during search tasks (Husain et al 2001).
FIGURE 1. Behavioral and lesion analyses of the egocentric spatial bias in neglect patients.
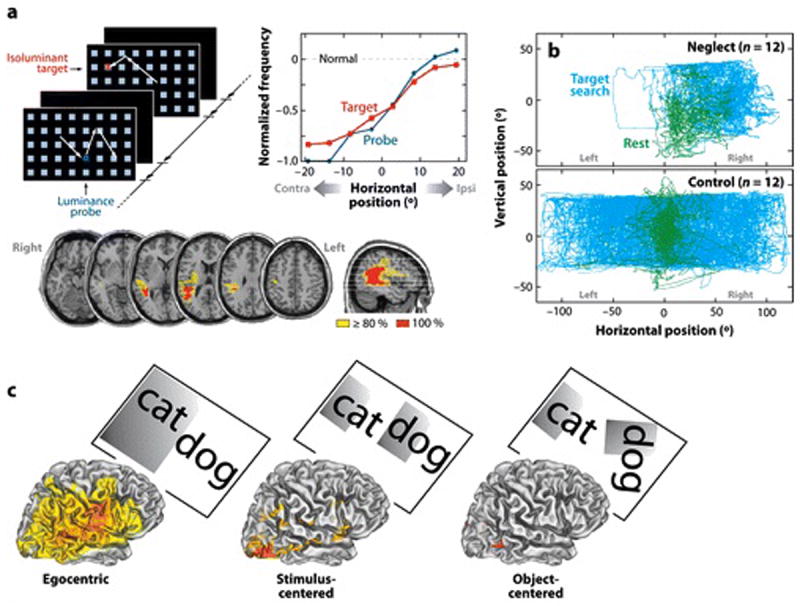
A) Effects of stimulus salience on eye movements of neglect patients. Patients were instructed to saccade to a target letter in an array. A sequence of arrays was presented, some containing a target, some containing a distinctive probe stimulus of a higher luminance or different orientation than the distracter squares, and some containing both a target and a probe. The right graph shows that targets (red line) and irrelevant but distinctive luminance probes (green line) produced a similar ipsilesional bias for saccades, indicating a similar gradient for automatic and goal-directed orienting (Bays et al 2010). B) Neglect patients show an ipsilesional gaze bias while searching for a target letter in a letter array (blue traces) and at rest (green traces). Non-neglect patients (bottom) showed no bias (Fruhmann Berger et al 2008). C) Lesions classically associated with neglect involve an ipsilesional bias within an egocentric reference frame, but not biases observed within stimulus-centered or object-centered frames. Each image shows the voxelwise lesion distribution associated with deficits within a particular reference frame (Medina et al 2008).
Importantly, a spatially lateralized bias is observed even in the absence of a stimulus. When neglect subjects searched for a non-existent object in complete darkness, search patterns as measured by eye or head position were strongly biased toward the ipsilesional field (Hornak 1992). Moreover, gaze deviations are observed tonically at rest, similar to those observed during task performance (Figure 1B)(Fruhmann Berger et al 2008).
Spatial biases in the dark during target search could reflect a reduced salience of contralesional spatial locations during task performance, but the biases observed at rest also suggest an indwelling imbalance in the mechanisms controlling gaze. These motor biases are likely associated with attentional biases because of the functional relationship between the corresponding neural systems (Corbetta et al 1998, Rizzolatti et al 1987).
An Egocentric Frame of Reference
Spatial deficits can be separated based on the reference frame in which stimuli are coded. Neglect is often egocentric (viewer-centered), with left and right hemi-spaces based on the observer’s midline (Figure 1C). Coding of the spatial midline can be further fractionated based on eye, head, or body position. While these factors have been shown to modulate the severity of neglect in individual cases, no consistent dissociation has emerged between eye-, head-, or body-centered neglect (Behrmann & Geng 2002). These null results have traditionally been explained by the large volume and heterogeneity of lesions in humans. Computational studies and physiological analyses in monkeys, however, indicate an additional factor, namely that activity in many areas reflects combinations of different egocentric reference frames (Chang & Snyder 2010, Pouget & Sejnowski 2001).
Neglect can also be allocentric, where the midline is defined from the central axis of a stimulus, irrespective of its position in the environment (stimulus-centered, Figure 1C) or of both its position and orientation (object-centered, Figure 1C). The great majority of patients with spatial neglect, however, suffer from egocentric deficits. Marsh and Hillis studied 100 consecutive cases of right hemisphere acute stroke, and found that while 17% and 34% showed visual and tactile egocentric neglect, respectively, the corresponding figures for allocentric neglect were only 4% and 2% (Marsh & Hillis 2008). Allocentric and egocentric neglect rarely co-occur clinically and are dissociated anatomically (Medina et al 2008, Verdon et al 2010). While egocentric neglect is associated with regions classically damaged in spatial neglect, i.e. inferior parietal lobule (IPL), superior temporal gyrus (STG), and inferior frontal gyrus (IFG), stimulus- and object-centered neglect is associated with damage of inferior temporal regions (Figure 1C).
Therefore, egocentric spatial deficits in neglect correspond much more closely to the clinical syndrome both behaviorally and anatomically than allocentric deficits, and represent core features of the syndrome.
Does Spatial Neglect Involve Only Attention/Salience?
An important question is whether an attention/saliency account of the spatial component of neglect leaves out other impaired perceptual-cognitive functions, particularly visuospatial short-term memory (VSTM) and spatial cognition.
An influential account proposes that neglect is a deficit in forming, storing, or manipulating the left side of mental images or information in VSTM, termed representational neglect (i.e. representation within VSTM) (Bisiach & Luzzatti 1978, Della Sala et al 2004). Most or all of the empirical findings that support an attentional/saliency interpretation of the spatial deficit in neglect patients can also be explained within a representational framework. This duality reflects the fact that mechanisms for spatial attention, VSTM, and imagery are closely related, as shown by psychological and physiological studies (Awh & Jonides 2001, Kosslyn et al 2001), and entry into VSTM is often considered a normal consequence of conscious perception. Not surprisingly, representational neglect is nearly always observed in association with perceptual neglect (Bartolomeo et al 1994). The few cases in which a dissociation has been reported (e.g. (Guariglia et al 1993, Ortigue et al 2001)) used pencil-and-paper tasks with lower sensitivity than computerized tasks (Rengachary et al 2009), and did not control for potential biases/differences in eye movements (Fruhmann Berger et al 2008, Hornak 1992) and cognitive load or time-on-task, which affect visuo-spatial biases (Dodds et al 2008). Regardless, both the low frequency of dissociations between perceptual and representational neglect and the likelihood that each is mediated by overlapping neural systems suggest that this distinction, while very important theoretically, may not be critical for first-order identification of the psychological and neural mechanisms that are damaged in the large majority of patients with spatial neglect. Accordingly, in this review, we do not distinguish ‘representational’ and ‘spatial attention’ formulations of the core egocentric deficit underlying spatial neglect.
Some neglect patients exhibit deficits in VSTM that may not show a contralesional-to-ipsilesional gradient and could be separate from a contralesional attention/salience/VSTM deficit. VSTM deficits are observed for stimuli presented along a central, vertical axis (Malhotra et al 2005), while a more specialized deficit has also been reported in trans-saccadic spatial memory (Mannan et al 2005), possibly reflecting saccadic remapping mechanisms (Vuilleumier et al 2007). When coupled with a bias to attend to ipsilesional locations, full-field VSTM and trans-saccadic deficits can exacerbate contralesional neglect by leading to multiple refixations of already-searched, ipsilesional objects (Husain et al 2001, Mannan et al 2005). However, a recent study reported that both VSTM deficits and visual search performance were worse in the contralesional visual field. This result is consistent with the functional similarity and neural overlap of mechanisms for attention/perception and working memory (Kristjansson & Vuilleumier 2010).
Spatial cognition is involved in many tasks used to assess spatial neglect, in addition to spatial attention. Line bisection, for example, requires fine judgments of spatial extent or position. Patients with neglect typically show rightward biases in line bisection (Bisiach et al 1983) or underestimate the size of objects placed on the left side of space (Milner et al 1998). These perceptual deficits are sometimes thought to reflect a distortion of the horizontal dimension of space (Bisiach et al 2001). However extensive investigations of eye movements during line bisection clearly indicate that neglect patients fail to explore the left side of lines, most often judging as midpoint the leftmost position fixated (Ishiai et al 2006). Moreover, their subjective midline judgment is strongly biased by the position of the rightward line endpoint (McIntosh et al 2005). These findings suggest a more parsimonious explanation of line bisection errors based on the relative saliency of the right vs. left side of lines (Figure 1A), and tonic oculomotor biases (Figure 1B). Another aspect of spatial cognition, processing of global stimulus structure, is also impaired in at least some patients (Delis et al 1986). A resulting local bias could increase the tendency to search near the current focus of attention, exacerbating a bias to attend to ipsilesional locations (Robertson & Rafal 2000).
Overall, the egocentric spatial deficit in neglect reflects impairments in a set of related mechanisms for spatial attention, salience, and VSTM, and perhaps spatial cognition. A recent study has shown that this deficit, when assessed using even a very simple task, is highly associated with clinical judgments of neglect. In a paradigm involving simple reaction time to a cued target, performance differences for left and right field targets discriminated acute and chronic neglect patients from healthy controls with better accuracy than a variety of standard neuropsychological tests of neglect (Rengachary et al 2009). Notably, this task did not involve spatial imagery, spatial cognition (line bisection, clock-drawing, copying tasks), or shape identification in a cluttered field (cancellation tasks), and involved minimal VSTM demands.
Summary
Spatial neglect is characterized by a spatial gradient of impaired attention/saliency/representation within an egocentric reference frame. The saliency deficit reflects both task and sensory factors and is linked with indwelling motor imbalances that produce resting ipsilesional deviations in eye, head, and body movements. Abnormal inter-hemispheric interactions likely play a role in producing the spatial gradient. The gradient fluctuates depending on arousal and task instructions, suggesting that the underlying neural mechanisms are modulated by signals from other parts of the brain and are dysfunctional rather than obliterated by structural damage.
ANATOMY AND PHYSIOLOGY OF THE EGOCENTRIC SPATIAL BIAS
We next discuss anatomical studies of neglect. The most commonly damaged brain regions do not contain physiological signals that can mediate the egocentric spatial bias. In contrast, physiological studies of spatial attention in healthy adults have highlighted the importance of a dorsal frontal-parietal attention network that typically is not structurally damaged in neglect patients. The mismatch between anatomical lesions and physiology may be explained by recent physiological studies of the dorsal network in neglect patients.
Structural Damage Does Not Explain the Egocentric Spatial Bias
Spatial neglect was first associated with damage to parietal cortex (Critchley 1953), especially IPL (Mort et al 2003, Vallar & Perani 1987). However, subsequent studies have also emphasized STG (Karnath et al 2001) and IFG (Husain & Kennard 1996), and convincing evidence exists that damage to other regions, including anterior insula and middle frontal gyrus (MFG), sometimes produces neglect. Interestingly, the distribution of cortical damage is similar irrespective of the behavioral criteria (e.g. neglect severity, clinical diagnosis, comparison of neglect vs. no-neglect individuals) used to group the lesions (Figure 2A). Importantly, neglect patients, especially in severe cases, have white matter fiber damage, which can disconnect frontal, temporal, and parietal cortex (Bartolomeo et al 2007, Gaffan & Hornak 1997, He et al 2007, Thiebaut de Schotten et al 2005). White matter damage most commonly involves a dorsal region lateral to the ventricle where arcuate and superior longitudinal fasciculi (II and III) run parallel in an anterior-to-posterior direction (Figure 2C) (Doricchi & Tomaiuolo 2003). Finally, neglect can be also caused by damage to subcortical nuclei (pulvinar, caudate, putamen)(Karnath et al 2002, Vallar & Perani 1987) that cause cortical hypo-activation of regions important for the genesis of neglect (Karnath et al 2005, Perani et al 1987).
FIGURE 2. Relationship between anatomical distribution of lesions associated with neglect, attentional networks, and damage to fiber tracts.
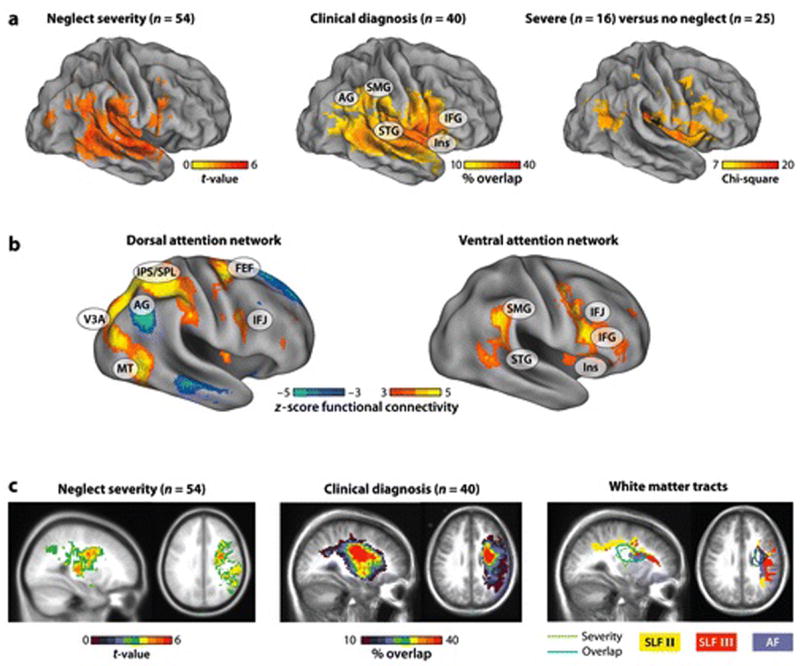
A) Anatomical regions associated with neglect, as shown by lesion-symptom mapping (left panel)(Karnath et al in press), overlap of lesions in patients diagnosed with neglect (middle panel)(A.R. Carter, G.L. Shulman, and M. Corbetta, unpublished data), and comparisons of groups of patients with severe neglect vs. no neglect (right panel)(Verdon et al 2010). The three cortical distributions are quite similar and emphasize ventral regions in IPL (Angular and Supramarginal Gyri), TPJ, STG, and VFC. B) The dorsal (left panel) and ventral (right panel) attention networks as determined by resting state functional connectivity in 25 healthy controls. The ‘yellow-orange’ voxels indicate regions with strong and significant temporal correlation at rest. The ‘blue’ voxels in the left panel indicate regions negatively correlated with the dorsal network, corresponding to the default network. The anatomical distributions shown in Figure 2A match the distribution of the ventral but not dorsal attention networks. Seed regions for the connectivity analysis were determined by meta-analyses of task activation paradigms (shown in Figures 3A and 6A). Dorsal seed regions were determined from the activations evoked by a central cue to shift attention and from comparisons of activations evoked by peripheral cues to shift vs. maintain attention. Ventral seed regions were determined from comparisons of activations to targets presented at unattended and attended locations. C) Slice representations from the anatomical distributions shown in the left (Karnath et al in press) and middle panels (A.R. Carter, G.L. Shulman, and M. Corbetta unpublished data) of Figure 2A indicate that anatomical damage includes both gray and white matter. White matter tracts corresponding to the arcuate fasciculus (AF) and superior longitudinal fasciculus (SLF) II and III, as determined by diffusion tensor imaging (DTI) in 30 healthy controls, are shown in the right panel (M.Glasser and K.Patel, unpublished data). The regions of maximal damage in neglect patients, shown in the left and middle panels, are outlined in green and blue. Lesion damage overlaps most strongly with the AF, but also with SLF II and III. These tracts connect regions within and across networks.
While the detailed profile of behavioral deficits undoubtedly depends on the site of the lesion (Medina et al 2008, Verdon et al 2010), and some lesion sites are more likely than others to produce neglect, the striking fact remains that neglect of the left field can be caused by many different right hemisphere lesions. Attempts to identify a critical region based on structural damage alone inevitably must explain away a large number of reported lesions that produce neglect and yet do not involve the supposedly critical region.
Moreover, regions that are commonly damaged in spatial neglect do not contain the physiological signals expected based on deficits in spatial attention, eye movements, and coding of salience. These regions are not usually recruited in neuroimaging experiments that isolate those processes and none have yet been shown in humans to contain maps of space. Right ventral frontal cortex, for example, has been associated with target detection (Stevens et al 2005), task control and error detection (Dosenbach et al 2006), and response inhibition (Aron et al 2004).
To resolve this paradox, we have proposed that the heterogeneity of lesions in spatial neglect patients masks a greater uniformity at the level of physiology, with common physiological abnormalities in remote neural systems specialized for spatial processing (Corbetta et al 2005, He et al 2007). Next, we review evidence in healthy subjects for a dorsal frontal-parietal attention network that houses the physiological signals impaired in spatial neglect.
A Dorsal Fronto-Parietal Network for Spatial Attention, Stimulus Salience, and Eye Movements
Regions in dorsal frontal and parietal cortex, including bilateral medial intraparietal sulcus (mIPS), SPL, precuneus, supplementary eye field (SEF) and frontal eye field (FEF), respond to symbolic cues to shift attention voluntarily to a location (e.g. (Corbetta et al 2000, Hopfinger et al 2000, Kastner et al 1999))(Figure 3A). In some studies additional responses are reported in lateral prefrontal cortex, including the inferior frontal sulcus/junction (IFS/IFJ) (Sylvester et al 2007) and MFG (Hopfinger et al 2000). The ‘standard’ regions are also recruited when attention is shifted to salient objects based on task relevance and sensory distinctiveness (Shulman et al 2009) (Figure 3B). Dorsal fronto-parietal regions (mIPS, precuneus/SPL, FEF, SEF, DLPFC) are recruited during visually- and memory-guided saccades, with almost complete overlap of attention and eye movement related activations (Corbetta et al 1998), and in some regions sensory signals are remapped during eye movements (Merriam et al 2003). Both body-centered and stimulus-centered coding has been reported in IPS/SPL (Galati et al 2010).
FIGURE 3. Physiology of spatial attention in healthy adults.
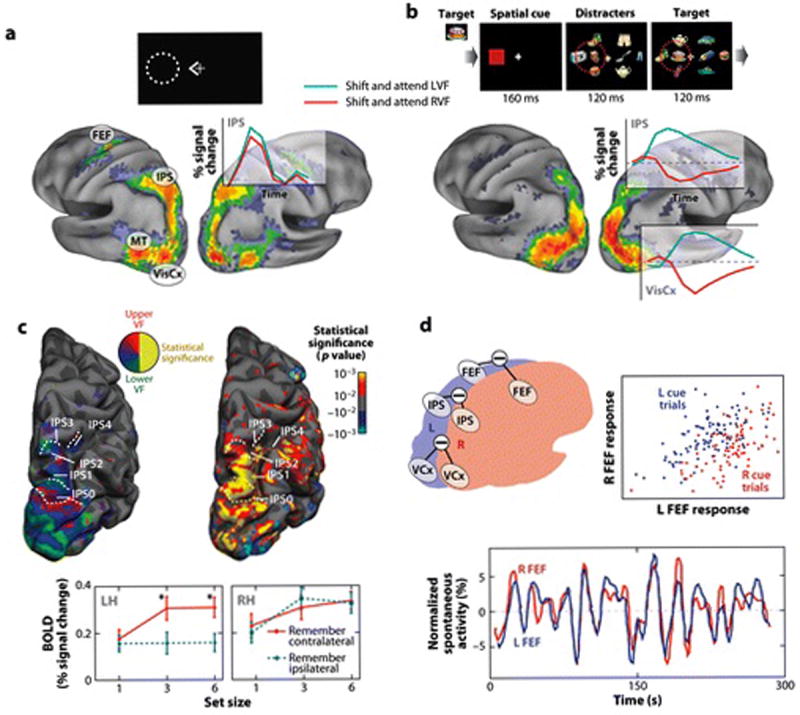
A) Dorsal fronto-parietal regions are activated following a central cue to shift attention. The statistical map shows the z-map from a meta-analysis of four experiments (n=58) in which BOLD activity was measured following a central cue to shift attention to a peripheral location (Astafiev et al 2003, Corbetta et al 2000, Kincade et al 2005). The time course of the response to the cue shows bilateral activity from right IPS with a contralateral preponderance indicating spatial selectivity. B) Occipital and dorsal fronto-parietal regions show spatially selective attentional modulations following a stimulus-driven shift of attention (from a meta-analysis of two experiments (n=47), (Shulman et al 2009) and (A. Tosoni, G.L. Shulman, D.L.W. Pope, McAvoy, M.P., and M. Corbetta, unpublished data). Subjects were cued to attend left or right to detect targets in a rapid-serial-visual-presentation (RSVP) stream presented among distracter streams. The z-map indicates voxels showing contralateral activity > ipsilateral BOLD activity following a shift of attention to the peripheral cue (red square). Note the strong spatially selective response in right IPS and visual cortex for shifting and attending to contralateral rather than ipsilateral stimulus streams. Also, maps for purely endogenous (Panel A) vs. stimulus-driven (Panel B) shifts of attention are very similar. C) Contralateral topographic maps in dorsal parietal cortex. The left image shows five contralateral polar angle maps along R IPS. The right image shows the activations in these maps during a VSTM task in which subjects remembered the orientation and location of target lines presented among distracters. The bottom graph shows a comparison of the magnitude of contralateral and ipsilateral activations in left and right IPS maps as a function of VSTM load. While left and right IPS contains contralateral polar angle maps (left IPS not shown), right IPS was equally activated by VSTM load in the contralateral and ipsilateral hemifieds but left IPS was only modulated by load in the contralateral hemifield (Sheremata et al 2010). This pattern of activity matches that postulated by the ‘standard’ model for neglect (Mesulam 1981). D) Inter-hemispheric coding of spatial attention. BOLD activity was measured following an auditory cue to attend to a peripheral location. The top right graph shows the magnitude of activity in L and R FEF on a trial-to-trial basis following leftward (blue dots) and rightward (red dots) cues. Activity in L and R FEF is highly correlated across trials, but a contralateral signal is superimposed on the positively correlated ‘noise’ (i.e. blue dots plot above red dots). This correlated ‘noise’ is partly explained by the presence of strong correlations at rest (bottom graph) between homologous regions (e.g. left-right FEF) or parts of maps (e.g. left-right fovea in V1). The locus of attention is only weakly predicted (AUC value=~.60, chance=.50) by ‘reading out’ activity only from the portion of the map in visual cortex or area (e.g. FEF) coding for the attended location. The prediction increases significantly (AUC=~.80) when subtracting activity from the attended-minus-unattended homologous portion of map or area in the two hemispheres (Sylvester et al 2007).
We previously proposed that these fronto-parietal regions constituted a dorsal cortical network for the control of spatial and featural attention and stimulus-response mapping (Corbetta & Shulman 2002). Subsequent work demonstrated that at rest, many of these regions show highly correlated activity (Figure 2B), consistent with the notion that they represent a separate functional-anatomical network analogous to sensory and motor systems (Fox et al 2006, He et al 2007). Importantly, these dorsal fronto-parietal regions are not generally damaged in neglect patients, as shown in the comparison of Figures 2A and 2B.
Recent physiological studies of these dorsal fronto-parietal regions, discussed next, have suggested two mechanisms for coding the locus of behaviorally relevant stimuli that may provide insights into the pathogenesis of spatial neglect.
Topographic Maps of Contralateral Space
Computational theories show that the co-occurrence of topographic maps, eye/attention, and saliency signals is useful for stimulus selection (Koch & Ullman 1985). Correspondingly, many dorsal fronto-parietal regions modulated by spatial attention, eye movements, and salience also contain polar-angle maps of the contralateral hemifield (Figure 3C, left image) (Hagler & Sereno 2006, Sereno et al 2001, Swisher et al 2007): medial IPS, precuneus, medial parieto-occipital cortex, SPL, FEF, and IFS/IFJ. Polar angle maps of the ipsilateral hemifield, however, have not been reported, indicating no evidence for separate topographic representations of both visual fields within a hemisphere, a longstanding explanation for the right hemisphere dominance of neglect (see below).
Conversely, topographic maps have not been reported in ventral regions typically damaged in neglect patients, consistent with the lack of evidence for their involvement in spatial attention. However, this null result should be treated cautiously. Maps in these regions may only be imaged with appropriate tasks or may be masked by a larger-scale organization involving other variables, such as eye position (Siegel et al 2003). Moreover, the absence of a topographic map does not imply null spatial coding, as in rat hippocampus and entorhinal cortex (OKeefe 2006). Spatial coding in ventral regions may occur at a scale and organization that is amenable to multivariate analyses of activation patterns, but not to standard retinotopic mapping procedures.
Inter-hemispheric Control of the Locus of Attention
Inter-hemispheric competition may play a key role in the efficient control of spatial attention by the dorsal network (Kinsbourne 1987). Sylvester and colleagues (Sylvester et al 2007) found that attention-related BOLD activity in dorsal fronto-parietal and occipital regions contralateral to an attended location was only modestly predictive of whether a left or right field location had been cued on that trial. However, predictability was greatly increased by subtracting the activity of homologous left and right hemisphere regions, largely because activations in the two hemispheres showed strong positive trial-to-trial correlations (Figure 3D, right graph). Differencing the signals between the two hemispheres eliminated this common ‘noise’ (Figure 3D, left). High inter-hemispheric correlation of activity also occurs at rest (Figure 3D, bottom), indicating tonic inter-hemispheric interactions.
Computational studies show that when signals in two brain regions are negatively correlated, as is the case with spatially selective signals in the two hemispheres, positive correlations in the noise increase the amount of information that is encoded by the corresponding neurons (Averbeck et al 2006). The locus of attention may be efficiently coded by the two hemispheres through a difference signal implemented by interactions either via the corpus callosum (Innocenti 2009), or subcortical routes. Enhanced prediction of the locus of attention based on an inter-hemispheric difference signal also has been observed with EEG (Thut et al 2006) and single unit recordings in monkey posterior parietal cortex (Bisley & Goldberg 2003).
A prediction of this model is that abnormalities in the computation of the locus of attention should correlate with abnormal inter-hemispheric interactions or response imbalances between the left and right hemisphere dorsal attention network. The next section discusses the physiological activity of these regions in neglect patients.
Physiological Correlates of the Egocentric Spatial Bias in Neglect
Patients with spatial neglect following lesions to right ventral cortex and underlying white matter have shown two types of physiological abnormalities in the structurally intact, dorsal attention network. First, at three weeks post-stroke, a widespread cortical hypo-activation during a spatial attention task was observed more strongly in the right than left hemispheres (Figure 4A), a finding consistent with other studies in the literature (Pizzamiglio 1998, Thimm et al 2008). The cortical hypo-activation was associated with a large inter-hemispheric imbalance of activity in dorsal parietal (Figures 4B). The inter-hemispheric imbalance normalized at the chronic stage (9 months post-stroke) in parallel with an overall improvement of cortical activity and spatial neglect (Figure 4A,B) (Corbetta et al 2005). Interestingly, activity in ipsilesional occipital visual cortex is abnormal, showing reductions in magnitude and spatial selectivity (Corbetta et al 2005) that are particularly marked under high attentional loads (Vuilleumier et al 2008). These impairments in sensory-evoked activity, possibly reflecting abnormal top-down control from fronto-parietal cortex (Bressler et al 2008), may further lessen the saliency of contralesional stimuli.
FIGURE 4. Physiology of egocentric spatial bias in neglect patients.
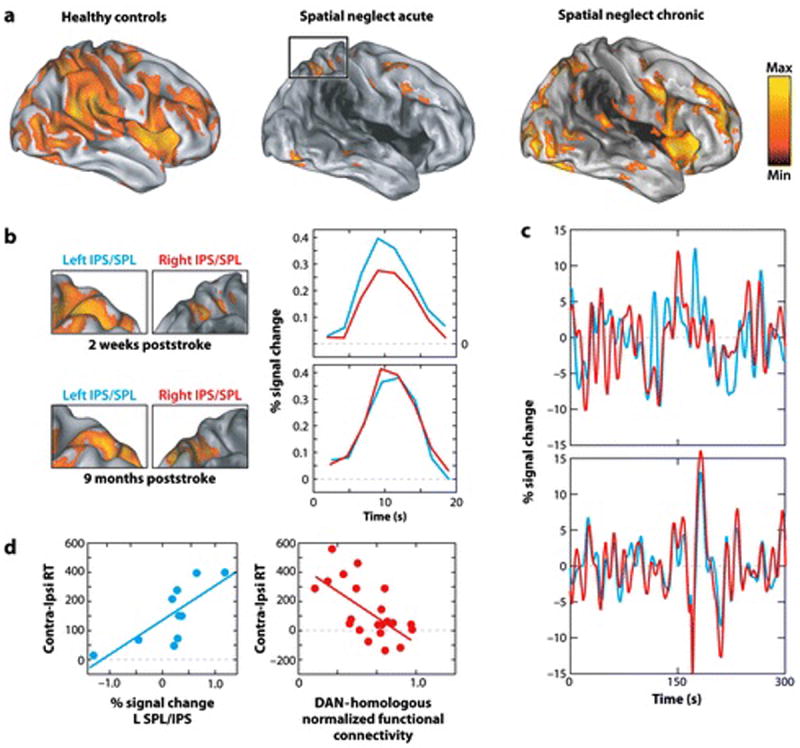
A) Statistical map of BOLD activations during a Posner spatial attention task (same as in Figure 3A), in which subjects are cued to a peripheral location and detect a subsequent target. Right hemisphere acute neglect patients show hypo-activation of both hemispheres (right>left) that partly recovers at the chronic stage. The dark shading in the anatomical image indicates the distribution of structural damage. (Corbetta et al 2005) B) As a result of right hemisphere hypo-activation, acute patients show a large imbalance of BOLD activity in IPS/SPL, with much greater left than right hemisphere activity. This imbalance normalizes at the chronic stage. The left columns show statistical maps of activity in parietal cortex, the right column shows the averaged time course of activity time-locked to the presentation of the cue over a trial of the Posner task in left (blue line) and right (red line) parietal cortex. (Corbetta et al 2005). C) Acute neglect patients (top graph) show low correlations in BOLD spontaneous activity between homologous regions of left (red lines) and right (blue lines) parietal cortex, but the correlation recovers at the chronic stage (bottom graph)(He et al 2007). D) Abnormal physiological signals in the dorsal attention network of acute neglect patients are functionally significant. Left graph: Left parietal activity was stronger in subjects with more severe neglect, as indexed by the difference in response times to contralesional vs. ipsilesional visual targets. Right graph: reduced inter-hemispheric correlation within fronto-parietal regions of the dorsal attention network correlates with the severity of neglect of the left visual field, as indexed by longer RTs to contralesional vs. ipsilesional visual targets (Carter et al 2010).
Secondly, neglect patients have shown anomalies in the pattern of spontaneous activity fluctuations within the dorsal attention network (Figure 4C). Coherence between left and right parietal regions was disrupted three weeks post-stroke and improved over time in parallel with the improvement of neglect (Figure 4C)(He et al 2007).
Dorsal fronto-parietal abnormalities in both task-evoked activity and resting coherence are functionally significant. Left parietal activity is stronger in subjects with more severe neglect, as indexed by the difference in response times to contralesional vs. ipsilesional visual targets (Figure 4D)(Corbetta et al 2005). Stronger left than right hemisphere activation of parietal and occipital regions may reflect a biased representation of stimulus salience and the locus of spatial attention (Bisley & Goldberg 2003, Sylvester et al 2007). This interpretation is consistent with trans-cranial magnetic studies, in which inactivation of left posterior parietal cortex reduced left field neglect (Brighina et al 2003, Koch et al 2008). Secondly, at rest, significant correlations are found throughout the dorsal attention network between reductions in inter-hemispheric coherence and the magnitude of the spatial bias (Figure 4D)(Carter et al 2010, He et al 2007). The anomalies in spontaneous activity may underlie the lateral rotation of the eyes, head, and body in neglect patients at rest (Fruhmann Berger et al 2008), which likely reflect biased coding of the locus of attention and salience, and contribute to the observed abnormalities in task-evoked responses.
Therefore, in structurally intact regions of the dorsal attention network, ventral lesions induce changes in BOLD resting functional connectivity and task-evoked activity that reflect abnormal inter-hemispheric interactions and response balances (Kinsbourne 1987), which plausibly explain the egocentric spatial bias in neglect. Recent studies of resting blood perfusion, which assess physiological function, have associated spatial deficits in neglect patients with hypo-perfusion of right IPL (Hillis et al 2005), STG (Zopf et al 2009), and IFG/anterior insula (Medina et al 2008), but not of dorsal fronto-parietal regions. However, these studies did not assess the functional relationship or coherence between regions, corresponding to the resting BOLD measure of the dorsal network that shows abnormalities, and did not measure task-evoked signal changes, corresponding to the other BOLD measure of dorsal network function that shows abnormalities. Moreover, the task-evoked changes measured in BOLD studies are quite small, well under 1% (Corbetta et al 2005), and may not be detected with less sensitive perfusion methods.
Summary
The extant literature strongly supports the presence of physiological signals (spatial attention, eye movement, saliency, maps of contralateral space, inter-hemispheric interactions, egocentric frame of reference) in the dorsal frontal-parietal network that are likely highly relevant to the pathogenesis of spatial neglect. Recent neuroimaging results potentially resolve the paradox that ventral regions traditionally associated with neglect do not contain physiological signals that could account for an egocentric spatial bias, while dorsal fronto-parietal regions that contain these signals are not typically damaged in strokes that cause neglect. However, only a few BOLD imaging studies have been conducted, involving relatively small patient samples. Further studies will be necessary to determine if similar changes in dorsal network physiology are observed across the different lesions that cause spatial neglect.
The above neuroimaging results, moreover, do not explain why lesions that cause neglect occur ventrally and in the right hemisphere. The right hemisphere dominance of spatial neglect remains after more than 50 years of research the most puzzling aspect of this syndrome. These issues are considered next.
RIGHT HEMISPHERE DOMINANCE OF SPATIAL NEGLECT
Right Hemisphere Lateralization of Spatial Deficits
The right hemisphere dominance of neglect might reflect a corresponding asymmetry for spatial attention, the primary mechanism underlying the egocentric spatial deficit, and for related functions of VSTM and spatial cognition. Perhaps the most widely accepted, ‘standard’ theory of neglect postulates that the right hemisphere controls shifts of attention to both sides of space while the left hemisphere only controls attention to the right side (Mesulam 1981). Damage to the right hemisphere impairs attention to the left hemifield while damage to the left hemisphere can be compensated. A second, ‘opponent-process’ theory proposes that each hemisphere promotes orienting in a contralateral direction, but the strength of this bias is stronger in the left than right hemispheres (Kinsbourne 1987). Left hemisphere lesions cause only mild right spatial neglect because the unopposed orienting bias generated by the right hemisphere is relatively weak.
Empirical support for each theory from neuroimaging studies is surprisingly modest. Mapping studies have reported contralateral retinotopic maps in both hemispheres but no evidence of ipsilateral maps in the right hemisphere. Most neuroimaging studies of cueing paradigms have reported largely bilateral dorsal fronto-parietal activations for directing spatial attention to locations in either visual field (e.g. (Hopfinger et al 2000, Kastner et al 1999, Shulman et al 2010, Sylvester et al 2007)). Several studies, however, have reported larger activations for contralateral than ipsilateral stimuli (‘contralateral bias’) in some left than right dorsal regions (e.g. (Szczepanski et al 2010, Vandenberghe et al 2005)). These asymmetries in contralateral bias are consistent with either the standard or opponent process theory, depending on whether the bias in the right hemisphere was completely absent (standard theory) or was simply present to a lesser degree (opponent process). Given the large number of discrepant findings, however, it will be important to identify the factors controlling when the specific hemispheric asymmetry postulated by either theory is observed.
A recent study suggested that one factor might involve high loading of VSTM (Sheremata et al 2010). Under high-load VSTM conditions that involved spatial filtering of distracters, left and right hemisphere activations in regions of IPS, which contained strictly contralateral polar-angle maps, nevertheless showed the postulated visual field profile (Figure 3C). Despite this intriguing result, however, the presence of the ‘standard’ visual field organization under high VSTM loads does not satisfactorily explain the laterality of neglect. Contralesional neglect is correlated with conditions that do not involve high VSTM loads, such as simple detection of a single visual target (Rengachary et al 2009). Similarly, gaze biases that correlate with contralesional neglect are present even at rest.
Finally, the laterality of contralesional neglect could partly reflect a similar laterality for aspects of spatial cognition. Variants of line bisection tasks qualitatively produce right dominant activity in IPS/SPL after they are referenced to control tasks that largely subtract out activations from shifting and maintaining attention (Fink et al 2001). Similarly, Sack and colleagues observed right hemisphere dominance during variants of a ‘clock’ task, in which subjects judge the angle formed by hour and minute hands (Sack 2009). To explain the laterality of contralesional neglect, however, mechanisms of spatial cognition must also show the visual field organization postulated by the standard attention theory, for which there is only limited evidence (Kukolja et al 2006).
Most importantly, right lateralization of spatial attention, VSTM, and spatial cognition has primarily been observed in dorsal parietal regions, leaving unanswered the critical question of why ventral (e.g. IFG or IPL) rather than dorsal (e.g. IPS/SPL) right hemisphere lesions cause neglect.
Right Hemisphere Lateralization and Anatomy of Core Non-Spatial Deficits
An alternative explanation that addresses this question, and is fully compatible with either the presence or absence of hemispheric asymmetries in dorsal visuospatial mechanisms, links the laterality of neglect to several ‘non-spatial’ behavioral deficits that are commonly observed in neglect patients (Heilman et al 1987, Husain & Rorden 2003, Robertson 2001). ‘Non-spatial’ refers to deficits that are nominally present across the visual field (e.g. the putative full-field VSTM and global processing deficits discussed earlier). In practice, a non-spatial deficit is rarely if ever shown to be of equal severity in the two hemifields, and measurements across the visual field are often not made, mainly because of the difficulty of separating non-spatial deficits from the egocentric spatial bias (but see (Duncan et al 1999)). More commonly, conclusions are based on finding a deficit relative to control subjects for stimuli located centrally or in the ipsilesional field, although this procedure does not control for spatial gradients that extend across the visual field (e.g. Figure 1A).
Below we briefly review three core non-spatial deficits consistently observed in neglect patients. These deficits are of particular interest since, unlike the egocentric spatial mechanisms discussed above, their physiology maps closely onto the anatomy of neglect, with clear involvement of ventral right hemisphere regions. We discuss evidence for the interaction of these ventral regions, damaged in neglect, with the dorsal attention network, a critical link for understanding the pathophysiology and right hemisphere dominance of the neglect syndrome.
Reorienting of Attention
Michael Posner and colleagues reported that neglect patients are impaired in reorienting to unexpected events (Posner et al 1984). Patients showed especially large deficits in detecting contralesional targets when they were expecting an ipsilesional target, suggesting a deficit in disengaging attention from the ipsilesional field. A comparison of TPJ/STG vs SPL patients originally localized disengagement/reorienting deficits to right TPJ/STG (Friedrich et al 1998). A recent study confirmed that a reorienting deficit was stronger for contralesional than ipsilesional targets following TPJ lesions, but the magnitude of the ipsilesional deficit was substantially increased following VFC lesions (Rengachary et al in press)(Figure 5A), indicating a bilateral deficit in reorienting.
FIGURE 5. Behavioral analyses of ‘non-spatial’ deficits in neglect patients and healthy adults.
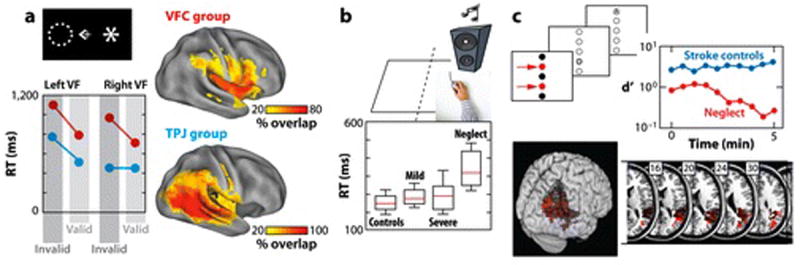
A) Reorienting deficits in neglect patients with VFC and TPJ lesions (Rengachary et al in press). Patients detected a visual target (the asterisk) that occurred in a validly cued location (indicated by the dotted circle) or at an invalidly cued location (shown in the figure). Both TPJ and VFC patients showed large contralesional deficits in reorienting, as indexed by longer RTs to unattended (invalid) than attended (valid) targets. The VFC group additionally showed reorienting deficits in the ipsilesional field and larger overall detection deficits. Similar results were observed for accuracy (not shown), but the TPJ group showed evidence of a small reorienting deficit in the ipsilesional field. B) Detection deficits in neglect patients. Neglect patients show abnormally slow simple RTs to an ipsilesional auditory stimulus (Samuelsson et al 1998). Controls were healthy age- and gender-matched subjects. The mild and severe groups consisted of non-neglect patients with minor and major right hemisphere strokes. C) Arousal deficits in neglect patients. Parietal neglect patients show a vigilance decrement (red curve) in a task that involved detection of letter targets in two locations (indicated by arrows) within a central column. No deficit is observed in right hemisphere patients without neglect (green curve). The anatomical images show the association of damaged voxels in right TPJ with the vigilance decrement, with darker areas indicating a weaker association (Malhotra et al 2009).
Detection of Behaviorally Relevant Stimuli
Right hemisphere and neglect patients show deficits in target detection in even the simplest paradigms. Simple auditory reaction time (RT), for example, is much slower following right than left hemisphere damage (Howes & Boller 1975). Related differences have been reported for auditory stimuli presented at ipsilesional locations in right hemisphere patients with neglect vs. without neglect (Samuelsson et al 1998), indicating that the RT slowing may reflect damage to right hemisphere brain regions specifically associated with neglect (Figure 5B). Impairments are sometimes reported even for accurate detection of suprathreshold stimuli presented centrally (Malhotra et al 2009) or ipsilesionally (although only acutely (Rengachary et al in press)), indicating that RT slowing likely does not reflect motor difficulties with the ipsilesional or ‘good’ hand. RT slowing could reflect deficits in arousal and processing capacity (Duncan et al 1999, Husain et al 1997), although the latter effects occur following both left and right hemisphere damage (Peers et al 2005).
Arousal and Vigilance
Reduced arousal and vigilance is an important component of the neglect syndrome following right hemisphere injury. Clinically, patients with neglect and right hemisphere injuries suffer from lower arousal than patients with similar lesions in the left hemisphere. Arousal refers to the combination of autonomic, electrophysiological, and behavioral activity that is associated with an alert state, while vigilance refers to the ability to sustain this state over time.
Kenneth Heilman and colleagues have argued that neglect patients have decreased arousal due to hypo-activation of the right hemisphere (Heilman et al 1987) (see Figure 4A for a striking example) (Corbetta et al 2005). For instance, patients with right as opposed to left hemisphere damage do not show the typical slowing of heart rate following a cue that signals a subsequent target (Yokoyama et al 1987) and show reduced GSRs to electrical stimulation (Heilman et al 1978). Lesions studies have associated right frontal damage with decreased arousal (Wilkins et al 1987) and a decrement over time in sustaining attention and detecting targets (vigilance decrement) (Rueckert & Grafman 1996).
Importantly, there is evidence for interaction between arousal/vigilance and spatial deficits. A non-speeded, auditory ‘counting’ test of arousal discriminated neglect from non-neglect patients in a right hemisphere group with heterogeneous lesions, indicating a strong linkage between arousal and spatial deficits in neglect (Robertson et al 1997). A recent study reported a specific association between damage to right TPJ cortex and vigilance decrements, but only when attention had to be sustained to a spatial location, not for targets presented at random locations (Figure 5C) (Malhotra et al 2009). The interaction between mechanisms underlying non-spatial and spatial deficits is likely critical for the pathogenesis and right hemisphere lateralization of spatial neglect (see below).
Right Hemisphere Lateralization and Physiology of Core Non-Spatial Deficits
The above results indicate that neglect patients show ‘non-spatial’ deficits in reorienting, target detection, and arousal that are likely right hemisphere dominant. We next show that, correspondingly, physiological signals mediating these functions in the healthy brain are right lateralized and occur in ventral regions typically damaged in neglect (IPL, STG, and IFG). Interestingly, the lateralization of these processes is supported by similar findings in other species (see Side Bar).
SIDE BAR.
Right Hemisphere Dominance in Vertebrates
While right lateralization in humans is often considered a byproduct of left hemisphere dominance for language, comparative studies suggest the basic specialization of each hemisphere may have been present early in vertebrate evolution. MacNeilage and colleagues, summarizing this work, write “…. the right hemisphere, the primary seat of emotional arousal, was at first specialized for detecting and responding to unexpected stimuli in the environment” (MacNeilage et al 2009).
The latter sentence describes the human ventral attention network (Corbetta et al 2008, Corbetta & Shulman 2002). When visual input is confined to the left eye/right hemisphere of chicks, their behavior is greatly affected by salient or novel stimuli (Rogers & Anson 1979). Feeding behavior, for example, is disrupted by the presence of brightly colored pebbles scattered among grains (Rogers et al 2007). Similarly, during feeding a simulated hawk is detected faster when it appears in the left than right monocular fields (Rogers 2000).
Behavioral asymmetries in chicks arise partly from asymmetric light exposure prior to hatching. Following exposure, connections from the ipsilateral visual field innervate more the right than left hyperstriatum (Rogers & Sink 1988), reminiscent of the ‘standard’ neglect theory. This physiology, however, varies widely across avian species. In pigeons, the asymmetry is reversed and is mediated by tectofugal rather than thalamofugal pathways (Valencia-Alfonso et al 2009).
In mammals, right hemisphere dominance for several ‘non-spatial’ functions may partly reflect asymmetric brainstem projections. The locus coeruleus/noradrenergic system in rats shows an asymmetric organization (Robinson 1985) that Posner and Petersen (Posner & Petersen 1990) have linked to right lateralization of arousal. Recent evidence also points toward locus coeruleus/noradrenergic involvement in reorienting and target detection, two other lateralized, ‘non-spatial’ functions associated with neglect (Aston-Jones & Cohen 2005, Bouret & Sara 2005, Corbetta et al 2008).
The similar right hemisphere specializations in animals and humans may reflect convergent evolution rather than homology, but raise the possibility that the lateralization underlying human neglect is of longstanding origin.
Reorienting of Attention
Neuroimaging studies of healthy adults have shown that reorienting to stimuli in either visual field that are presented outside the focus of attention (‘stimulus-driven’ reorienting) recruits a right lateralized ‘ventral attention’ network in TPJ (including separate foci in SMG and STG) (Shulman et al 2010) and VFC (Insula, IFG, MFG), in conjunction with the dorsal network (Figure 6A) ((Corbetta & Shulman 2002). While TPJ is uniformly activated by stimulus-driven reorienting, however, VFC is mainly activated when reorienting is unexpected and requires cognitive control (Shulman et al 2009) or is coupled to a response. Because one or both conditions usually apply in real-world situations, the two regions are typically co-activated and a similar network is observed in the resting state (Figure 2B) (Fox et al 2006, He et al 2007).
FIGURE 6. Physiological analyses of ‘non-spatial’ deficits in neglect patients and healthy adults.
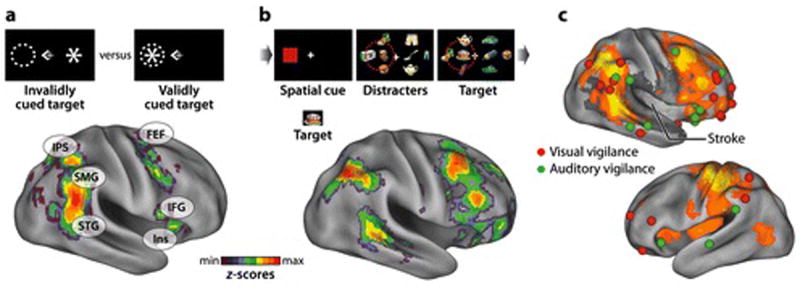
A) Physiology of reorienting in healthy adults. BOLD activity was compared for visual targets that occurred in invalidly cued (left panel) vs. validly cued (right panel) locations. The statistical map shows the z-map from a meta-analysis (n=58) of four experiments (Astafiev et al 2003, Corbetta et al 2000, Kincade et al 2005). Strong activations are observed in right TPJ, extending from posterior temporal cortex (STG) to ventral IPL (SMG), and in right IFG/insula. These ventral regions are co-activated with dorsal attention regions in IPS and FEF. B) Physiology of detection in healthy adults. A spatial cue directed subjects to attend to a rapid-serial-visual-presentation (RSVP) stream in the left or right visual fields that might contain a target. The z-map shows all voxels with greater right than left hemisphere BOLD activity to detected targets. The map includes ventral voxels that showed significant activations from reorienting in panel D, but also additional regions in prefrontal cortex. The dorsal attention network, however, largely does not show an asymmetry. C) Physiology of arousal in healthy adults. A meta-analysis of foci from 5 experiments reporting activations that index arousal/vigilance, visual (red spheres) or auditory (green spheres)(Coull et al 1998, Foucher et al 2004, Paus et al 1997, Sturm et al 1999, Sturm et al 2004). The right hemisphere foci are superimposed on the average of z-maps for reorienting and detection-related hemispheric asymmetries from panels A and B. The dark shading shows the distribution of neglect lesions from a recent neglect study (Rengachary et al 2009). Many more foci are observed in the right than left hemispheres. Foci are clustered around the TPJ and insula/frontal operculum regions that are activated by reorienting and target detection, but many prefrontal foci are anterior to both distributions. Few foci occur in dorsal fronto-parietal regions, indicating that arousal-related activations overlap more the ventral than dorsal attention network.
Importantly, the cortical anatomy of the ventral attention network includes the primary regions damaged in neglect (Figure 2B, Figure 6A) and matches the localization of the reorienting/disengagement deficit (Figure 5A), indicating a clear convergence between studies in neglect patients and healthy adults (Fox et al 2006, Friedrich et al 1998, He et al 2007, Rengachary et al in press, Shomstein et al 2010, Shulman et al 2010).
Detection of Behaviorally Relevant and Novel Stimuli
The ventral (and dorsal) attention network is activated by detection of behaviorally relevant stimuli that are unattended or unexpected with respect to a wide range of attributes, not just their location, as demonstrated by ‘oddball’ tasks, in which subjects report infrequent targets that differ in some feature (e.g. color, tone frequency) from a standard stimulus (Corbetta & Shulman 2002). Oddball detection, however, activates not only the ventral attention network but also additional regions in frontal, parietal, and temporal cortex that show right hemisphere dominance in direct inter-hemispheric voxelwise comparisons (Stevens et al 2005). Similar although not identical right hemisphere regions are activated by novel stimuli that are task irrelevant (Stevens et al 2005).
Right hemisphere dominance during target detection is observed in regions that are frequently associated with neglect (IPL, STG, IFG) and for visual targets in both left and right hemifields (Shulman et al 2010), consistent with the ‘non-spatial’ deficit in neglect (Figure 6B). Unlike the simple detection tasks studied in neglect patients, however, the above paradigms presented both targets and non-targets, and the frequency of targets was relatively low.
Arousal and Vigilance
Neuroimaging studies of arousal and vigilance using simpler auditory and visual detection paradigms, more similar to those adopted in neglect subjects, have qualitatively reported right hemisphere dominance (direct inter-hemispheric comparisons were not conducted), usually in lateral prefrontal, insula/frontal operculum, and TPJ regions (Coull et al 1998, Foucher et al 2004, Pardo et al 1991, Paus et al 1997, Sturm et al 1999, Sturm et al 2004). Figure 6C shows that arousal-related activations are recorded more frequently in ventral cortex of the right than left hemisphere, and are not frequently reported in dorsal fronto-parietal cortex, indicating a much stronger overlap with ventral than dorsal attention networks. Importantly, arousal-related activations in the TPJ and insula/frontal operculum overlap with regions that are damaged in spatial neglect, and recruited during reorienting and target detection, although prefrontal activations are localized more anteriorly.
Summary
The widespread view that attention in healthy adults is right-hemisphere dominant is more consistent with the evidence for right lateralization in ventral fronto-parietal cortex of reorienting, detection, and arousal than the more modest evidence for right lateralization in dorsal fronto-parietal cortex of the mechanisms controlling spatial attention. The main conclusion is that the right hemisphere ventral regions (IPL, STG, IFG/insula) associated with neglect underlie the above non-spatial functions impaired in patients.
VENTRAL AND DORSAL ATTENTION NETWORKS AND SPATIAL NEGLECT
What remains to be considered is how damage to these ventral regions produces the documented abnormalities in the dorsal attention network that likely underlie the egocentric spatial bias. We next consider behavioral evidence for interactions between non-spatial and spatial functions that map to ventral and dorsal regions, and physiological studies that have attempted to identify the specific pathways that might mediate these interactions. Finally, we present a novel physiological model of spatial neglect based on the structural-physiological interaction of ventral and dorsal attention networks.
Interactions between Ventral and Dorsal Mechanisms
Recent behavioral evidence in healthy adults indicates that arousal, a right lateralized, non-spatial function impaired in neglect patients, interacts with spatial attention, the primary spatial function impaired in neglect patients. Healthy adults show a slight tendency to attend to the left side of an object (Nicholls et al 1999), but this bias is reduced or shifted to the right under conditions of low arousal (Bellgrove et al 2004, Manly et al 2005, Matthias et al 2009). The specific form of the interaction between arousal and spatial attention predicts that increases in arousal should bias attention to the left visual field, ameliorating left field neglect.
Ian Robertson and colleagues observed this result in two important studies. Increases in either phasic (Robertson et al 1998) or sustained arousal (Robertson et al 1995) decreased neglect of the contralesional field, consistent with a direct effect of the activation of ventral right hemisphere mechanisms on the dorsal attention network. Biases in spatial attention have also been observed immediately following target detection, another right lateralized, non-spatial function damaged in neglect patients (Perez et al 2009). Perez and colleagues suggest that conditions that reduce processing capacity, such as the attentional blink and low arousal, bias spatial attention to the right.
There is currently only limited evidence of the anatomy and physiology underlying the interactions between non-spatial and spatial mechanisms observed in behavioral studies of healthy adults, and the effects of ventral lesions on dorsal physiology observed in neglect patients. Both may involve similar pathways. Studies in healthy adults have suggested possible linkages between ventral and dorsal regions that run through lateral frontal cortex. A region near IFJ, for example, which shows resting-state connectivity with both dorsal and ventral networks (Figure 2B) (He et al 2007) and shares task-evoked properties with each network (Asplund et al 2010), may act as a ‘pivot’ point. Ventral frontal lesions that include IFJ produce greater deficits in spatial attention than temporoparietal lesions (Figure 5A)(Rengachary et al in press).
Putative ventral-dorsal linkages have also been evaluated by measuring the relationship between ventral to pivot-region functional connectivity and connectivity within the dorsal network. Impaired functional connectivity between STG/TPJ and MFG, for example, is correlated with impaired inter-hemispheric connectivity between left and right posterior IPS/SPL, which in turn relates to the magnitude of the spatial deficit (He et al 2007). The same study also assessed structural damage to a white matter tract that putatively connected ventral/pivot regions to the dorsal network, and observed the resulting effects on dorsal network connectivity and spatial behavioral biases. Neglect patients that suffered damage to the superior longitudinal fasciculus showed reduced inter-hemispheric functional connectivity in posterior parietal cortex and more severe spatial neglect. This may explain why patients with more severe neglect have more involvement of the white matter tracts connecting parietal, temporal, and frontal regions (Gaffan & Hornak 1997, Karnath et al in press, Verdon et al 2010) (Figure 2C). Consistent with this latter result, stimulating a white matter tract that connects right frontal and parietal cortex produced rightward deviations in bisection performance (Thiebaut de Schotten et al 2005). Finally, the neural mechanisms behind ventral-dorsal interactions are unknown but may depend on synchronization of neural activity in ventral and dorsal regions that is time-locked to behaviorally relevant events (Daitch et al 2010).
These results are provisional and exploratory, but suggest that in neglect patients, both the severity of spatial biases and the inter-hemispheric functional connectivity of dorsal regions are related to the functional connectivity or integrity of ventral/pivot regions and to the integrity of white matter tracts that likely connect ventral and dorsal regions.
A Physiological Framework for Understanding Spatial Neglect
Our review suggests the following account of spatial neglect, as observed in LJ’s case report. LJ’s reduced vigilance and slowness in responding to targets, even in his right visual field, reflected impairments in arousal, reorienting, and the detection of novel and behaviorally important stimuli. These non-spatial processes are directly damaged by stroke and other focal brain injuries in neglect patients (in LJ a ventral frontal stroke involving the anterior insula), and, correspondingly, all involve in healthy adults right hemisphere ventral regions that are commonly associated with neglect, including superior temporal cortex, TPJ, IPL, and VFC/insula. These non-spatial mechanisms directly interact with spatial attention mechanisms, providing a link between damage to ventral regions and the abnormal physiology of dorsal regions. Damage to right hemisphere ventral regions, which impairs arousal, reorienting and detection, hypo-activates the right hemisphere, reducing interactions between the ventral and dorsal attention network and between regions of the ipsilesional (right) dorsal network. The result is unbalanced inter-hemispheric physiological activity in the dorsal network, both at rest and during a task, in a direction that favors the left hemisphere. As the locus of attention is coded by mechanisms that take into account activity from both sides of the brain, this imbalance drives spatial attention and eye movements to the right visual field (Figures7A and 7B). This spatial bias explains LJ tendency to look and explore first the right visual field, and his inability to detect stimuli in the left visual field.
FIGURE 7. Pathophysiology of spatial neglect.
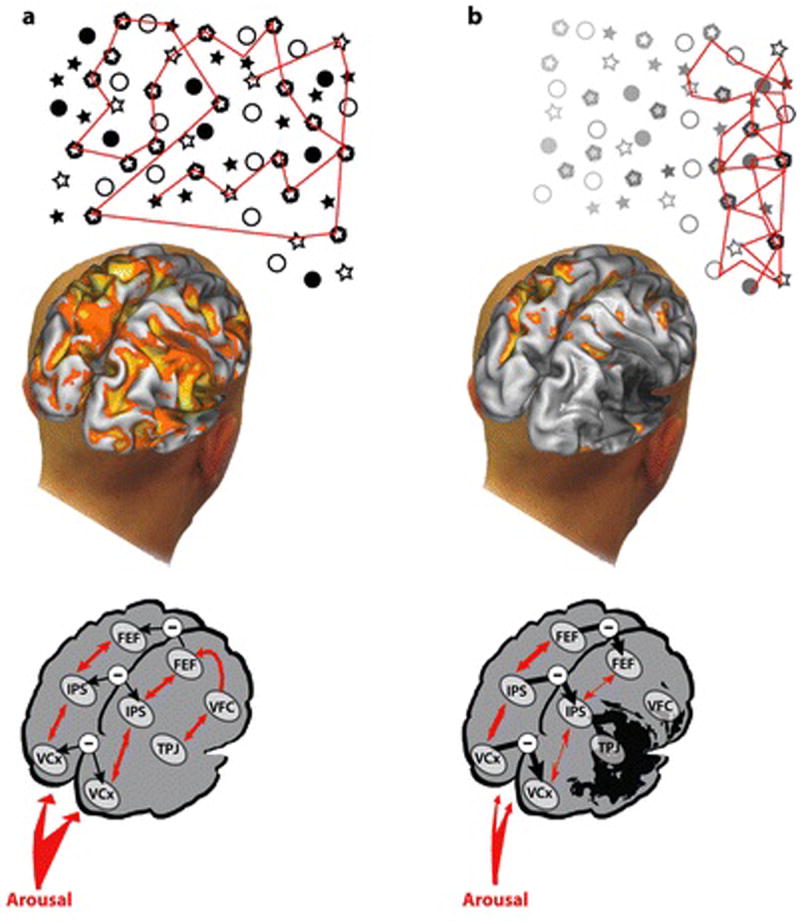
A) In the healthy brain, activity during visual search is symmetric, and inter-hemispheric interactions between left and right dorsal attention and visual occipital areas are balanced. Each side of the dorsal attention network direct shifts of attention and eye movements contralaterally, and the locus of spatial attention is coded by a differencing mechanism that takes into account activity from both hemispheres, as described in Figure 3D and in (Sylvester et al 2007). Balanced interhemispheric activity results in a normal eye movement search pattern, shifts of attention, and coding of stimulus saliency. The ventral network is lateralized to the right hemisphere due to a slight asymmetric (right>left) arousal input from the brainstem LC/NE system, and interacts with the dorsal network (right>left). Accordingly, decreases in arousal shifts spatial attention rightward because of greater left than right activity in the dorsal attention network, while under normal conditions spatial attention shows a slight leftward bias due to slightly greater right than left dorsal activity. B) In a patient with a ventral stroke, direct damage of ventral regions causes a reduction of arousal, target detection, and reorienting that leads to a bilateral visual field impairment. Abnormal ventral-to-dorsal interactions cause a dorsal imbalance with a relative over-activation of left dorsal spatial maps, leading to tonic and task-dependent rightward spatial biases in attention, eye movements, and stimulus salience.
The right hemisphere dominance of neglect follows from the specific biases produced by right lateralized non-spatial mechanisms on the direction of spatial attention, reflecting the inter-hemispheric balance of activity in the dorsal network. Right hemisphere lesions may also impair mechanisms that do not directly affect the inter-hemispheric balance of activity within the dorsal attention network, but increase the behavioral effects produced by the extant stroke-induced imbalance. Full-field deficits in VSTM, trans-saccadic memory, and global perception may act in this fashion (Husain et al 2001, Robertson & Rafal 2000). Finally, while physiological evidence that the right but not left hemisphere directs spatial attention to both visual fields has not been reported in the majority of studies, the predicted responses are sometimes observed (Sheremata et al 2010), suggesting that hemispheric asymmetries in dorsal attentional mechanisms may contribute to the laterality of neglect even if they do not account for its ventral anatomy.
An important future goal is to identify the basis of right hemisphere lateralization of non-spatial mechanisms underlying reorienting, detection, and arousal. Several authors (Corbetta et al 2008, Posner & Petersen 1990) have argued that this lateralization reflects asymmetries in cortical modulation from the locus coeruleus/nor-epinephrine (LC/NE) system (Robinson 1985). Lesions to right ventral cortex may damage mechanisms normally receiving strong LC/NE inputs, such as IPL (Morrison & Foote 1986), and cause widespread cortical hypo-activation.
Problems and Omissions
While we propose that the dorsal fronto-parietal network underlies control of overt and covert spatial orienting, unilateral lesions of IPS and SPL in humans are traditionally associated with optic ataxia, i.e. difficulties in pointing rather than a general egocentric spatial bias. However, carefully placed lesions in monkey dorsal areas (e.g. LIP) cause contralesional deficits in visual search and memory-guided saccades (Wardak et al 2004). Similarly, recent lesion studies in humans involving careful psychophysical testing found that lesions centered in SPL or in the white matter connecting IPS/SPL to FEF (Superior longitudinal Fasciculus) cause deficits in goal-driven shifts of attention (Shomstein et al 2010) and abnormal capture by irrelevant distracters (Ptak & Schnider 2010, Shomstein et al 2010), consistent with the proposed role of the dorsal attention network in directing spatial attention and coding of stimulus saliency.
The more important point, however, is that damage to dorsal regions alone is insufficient to produce the full neglect syndrome. We argue that the full syndrome depends on a combination of right hemisphere hypo-activation, associated with damage to mechanisms for reorienting, detection, and arousal in ventral fronto-parietal regions, and a resulting imbalance in the dorsal network, generating an egocentric spatial bias. The latter emphasis on the dysfunction of dorsal fronto-parietal cortex is necessarily provisional, since it partly reflects the lack of evidence that in the healthy human brain, ventral regions control spatial attention and eye movements or contain topographic maps.
An important topic that we have omitted due to space limitation concerns the role of subcortical nuclei in spatial neglect. Similarly, the current review did not consider how visuospatial deficits interact with body representations, which may underlie some of the striking symptoms shown by neglect patients, such as their denial of symptoms and confabulations about body ownership (e.g. LJ).
SUMMARY POINTS.
The primary spatial impairment in neglect patients is a failure to attend to the contralesional side of space within a reference frame centered on the observer.
This spatial deficit is observed both at rest and during task performance.
The spatial deficit reflects tonic and task-evoked interhemispheric imbalances of activity within the dorsal attention network.
The dorsal attention network may be physiologically impaired across the wide variety of right hemisphere ventral fronto-parietal lesions that can produce neglect.
The right hemisphere dominance of neglect primarily reflects the laterality of ‘non-spatial’ mechanisms for reorienting, detection, and arousal in right ventral fronto-parietal cortex, rather than the laterality of mechanisms for spatial attention within dorsal fronto-parietal cortex.
The activation of non-spatial mechanisms directly biases spatial attention, corresponding to the interaction of ventral and dorsal fronto-parietal regions.
Damage to right ventral fronto-parietal cortex in neglect patients impairs non-spatial functions, hypoactivates the right hemisphere, and unbalances the activity of the dorsal attention network.
Ventral-dorsal interactions link the ventral lesions that cause neglect to the egocentric spatial bias that is the hallmark of the neglect syndrome.
FUTURE ISSUES.
What is the anatomy and physiology of the interaction between dorsal and ventral networks? Are there critical frontal regions and fiber tracts that link the two networks? How is activity in the two networks synchronized?
Is the right hemisphere dominance of mechanisms for arousal, reorienting, and detection related to asymmetries in the locus coeruleus/noradrenaline system?
Are inter-hemispheric imbalances in the attention-related activity of dorsal fronto-parietal regions present across the wide variety of right hemisphere lesions that can cause neglect?
Do the ventral fronto-parietal regions associated with neglect contain spatial maps that are involved in controlling attention, independently or in conjunction with the dorsal network?
Under what conditions does the dorsal attention network consistently show hemispheric asymmetries in visual field organization?
What non-spatial manipulations bias spatial attention to the right hemifield in healthy adults? Are saliency and eye movement deficits related to arousal deficits?
Acknowledgments
We acknowledge the help of many colleagues who generously made their data available for this review. Drs. Bays and Driver, University College London, for Figure 1A; Drs. Karnath and Fruhmann-Berger, University of Tubingen, for Figure 1B; Drs. Medina, Pavlak, and Hillis, Johns Hopkins and University of Pennsylvania, for Figure 1C; Drs. Karnath, University of Tubingen, Verdon and Vuilleumier, University of Geneva, for Figure 2A; Dr. Karnath for Figure 2C; Drs. Sheremata and Somers, Boston University, for Figure 3C; Dr. Samuelsson, University of Goteborg, for Figure 5B; Drs Malhotra and Husain, University College London, for Figure 5C. Drs. Jon Driver, David Somers, and Patrick Vuilleumier, as well as Dr. Michael Posner of University of Oregon, and Dr. John Duncan of the MRC, Cambridge, also made useful suggestions concerning the manuscript. Finally, we thank Dr. Serguei Astafiev for help in preparing the figures.
This work was supported by the National Institute of Child Health and Human Development (NICHD) RO1 HD061117-05A2 and the National Institute of Mental Health (NIMH) RO1 MH 71920-06.
GLOSSARY
- contralesional
the side of space opposite a brain lesion.
- unilateral spatial neglect
a failure to attend and respond to contralesional stimuli.
- saliency
a measure of the sensory and behavioral relevance of a stimulus.
- egocentric spatial bias
a failure to attend to contralesional stimuli within a reference frame centered on the observer.
- dorsal attention network
regions centered around IPS, SPL, and FEF, involved in selectively attending to stimuli and linking them to responses.
- ventral attention network
regions centered around TPJ and ventral frontal cortex, involved in reorienting to salient stimuli outside the focus of attention.
- functional connectivity
the correlation over time of the spontaneous activity between different brain regions.
ACRONYMS
- fMRI
functional magnetic resonance imaging
- TMS
transcranial magnetic stimulation
- BOLD
blood oxygenation level dependent
- IPS
intraparietal sulcus
- IPL
inferior parietal lobule
- SPL
superior parietal lobule
- STG
superior temporal gyrus
- FEF
frontal eye field
- IFG
inferior frontal gyrus
- TPJ
temporo-parietal junction
Contributor Information
Maurizio Corbetta, Email: mau@npg.wustl.edu, Departments of Neurology, Radiology, and Anatomy and Neurobiology, Washington University School of Medicine, 314-362-4530, 4525 Scott Avenue, St. Louis, MO 63110.
Gordon L. Shulman, Email: gordon@npg.wustl.edu, Department of Neurology, Washington University School of Medicine, 314-362-8880, 4525 Scott Avenue. St. Louis, MO 63110.
References
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13:507–12. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–99. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7:358–66. doi: 10.1038/nrn1888. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–26. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. 2002;73:160–6. doi: 10.1136/jnnp.73.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomeo P, D’Erme P, Gainotti G. The relationship between visuospatial and representational neglect. Neurology. 1994;44:1710–4. doi: 10.1212/wnl.44.9.1710. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17:2479–90. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bays PM, Singh-Curry V, Gorgoraptis N, Driver J, Husain M. Integration of goal- and stimulus-related visual signals revealed by damage to human parietal cortex. J Neurosci. 2010;30:5968–78. doi: 10.1523/JNEUROSCI.0997-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrmann M, Geng JJ. What is ‘left’ when all is said and done? Spatial coding and hemispatial coding. In: Karnath HO, Milner D, Vallar G, editors. The Cognitive and Neural Bases of Spatial Neglect. New York: Oxford University Press; 2002. pp. 85–100. [Google Scholar]
- Behrmann M, Watt S, Black S, Barton J. Impaired visual search in patients with unilateral neglect: an oculographic analysis. Neuropsychologia. 1997;35 doi: 10.1016/s0028-3932(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Dockree PM, Aimola L, Robertson IH. Attenuation of spatial attentional asymmetries with poor sustained attention. Neuroreport. 2004;15:1065–9. doi: 10.1097/00001756-200404290-00027. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Bulgarelli C, Sterzi R, Vallar G. Line bisection and cognitive plasticity of unilateral neglect of space. Brain Cogn. 1983;2:32–8. doi: 10.1016/0278-2626(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129–33. doi: 10.1016/s0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Neppi-Modona M, Ricci R. Space anisometry in unilateral neglect. In: Karnath HO, Milner D, Vallar G, editors. The Cognitive and Neural Bases of Spatial Neglect. New York: 2001. pp. 145–52. [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–6. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–82. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–61. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, et al. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci Lett. 2003;336:131–3. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SW, Snyder LH. Idiosyncratic and systematic aspects of spatial representations in the macaque parietal cortex. Proc Natl Acad Sci U S A. 2010;107:7951–6. doi: 10.1073/pnas.0913209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–73. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–97. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–10. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Bowers D, Fitzpatrick E, Haws B, Heilman KM. Directional hypokinesia and hemispatial inattention in neglect. Brain. 1990;113:475–86. doi: 10.1093/brain/113.2.475. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frackowiak RS, Frith CD. Monitoring for target objects: activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–34. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Critchley M. The Parietal Lobes. London: Edward Arnold; 1953. [Google Scholar]
- Daitch AL, Snyder AZ, Astafiev SV, Bundy D, Freudenburg Z, et al. Temporal dynamics of stimulus-driven attention shifts as studied through the combined use of ECoG and fMRI; Presented at Society for Neuroscience; San Diego, California. 2010. [Google Scholar]
- Delis DC, Robertson LC, Efron R. Hemispheric specialization of memory for visual hierarchical stimuli. Neuropsychologia. 1986;24:205–14. doi: 10.1016/0028-3932(86)90053-9. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Logie RH, Beschin N, Denis M. Preserved visuo-spatial transformations in representational neglect. Neuropsychologia. 2004;42:1358–64. doi: 10.1016/j.neuropsychologia.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Denes G, Semenza C, Stoppa E, Lis A. Unilateral spatial neglect and recovery from hemiplegia: a follow-up study. Brain. 1982;105:543–52. doi: 10.1093/brain/105.3.543. [DOI] [PubMed] [Google Scholar]
- Deuel RM, Collins RC. Recovery from unilateral neglect. Experimental Neurology. 1983;81:733–48. doi: 10.1016/0014-4886(83)90340-0. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Aprile T, Spitoni G, Spinelli D. Impaired visual processing of contralesional stimuli in neglect patients: a visual-evoked potential study. Brain. 2008;131:842–54. doi: 10.1093/brain/awm281. [DOI] [PubMed] [Google Scholar]
- Dodds CM, van Belle J, Peers PV, Dove A, Cusack R, et al. The effects of time-on-task and concurrent cognitive load on normal visuospatial bias. Neuropsychology. 2008;22:545–52. doi: 10.1037/0894-4105.22.4.545. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport. 2003;14:2239–43. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Mattingley JB. Parietal neglect and visual awareness. Nature Neuroscience. 1998;1:17–22. doi: 10.1038/217. [DOI] [PubMed] [Google Scholar]
- Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. Systematic analysis of deficits in visual attention. J Exp Psychol Gen. 1999;128:450–78. doi: 10.1037//0096-3445.128.4.450. [DOI] [PubMed] [Google Scholar]
- Farne A, Buxbaum LJ, Ferraro M, Frassinetti F, Whyte J, et al. Patterns of spontaneous recovery of neglect and associated disorders in acute right brain-damaged patients. J Neurol Neurosurg Psychiatry. 2004;75:1401–10. doi: 10.1136/jnnp.2002.003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: an fMRI study of normal volunteers. Neuroimage. 2001;14:S59–S67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Otzenberger H, Gounot D. Where arousal meets attention: a simultaneous fMRI and EEG recording study. Neuroimage. 2004;22:688–97. doi: 10.1016/j.neuroimage.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich FJ, Egly R, Rafal RD, Beck D. Spatial attention deficits in humans: A comparison of superior parietal and temporal-parietal junction lesions. Neuropsychology. 1998;12:193–207. doi: 10.1037//0894-4105.12.2.193. [DOI] [PubMed] [Google Scholar]
- Fruhmann Berger M, Johannsen L, Karnath HO. Time course of eye and head deviation in spatial neglect. Neuropsychology. 2008;22:697–702. doi: 10.1037/a0013351. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120(Pt 9):1647–57. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- Galati G, Pelle G, Berthoz A, Committeri G. Multiple reference frames used by the human brain for spatial perception and memory. Exp Brain Res. 2010;206:109–20. doi: 10.1007/s00221-010-2168-8. [DOI] [PubMed] [Google Scholar]
- Guariglia C, Padovani A, Pantano P, Pizzamiglio L. Unilateral neglect restricted to visual imagery. Nature. 1993;364:235–7. doi: 10.1038/364235a0. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr, Sereno MI. Spatial maps in frontal and prefrontal cortex. Neuroimage. 2006;29:567–77. doi: 10.1016/j.neuroimage.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Halligan PW, Marshall JC, Wade DT. Visuospatial neglect: underlying factors and test sensitivity. Lancet. 1989;2:908–11. doi: 10.1016/s0140-6736(89)91561-4. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Coslett HB, Buxbaum LJ, Whyte J, Ferraro MK. Inconsistency of performance on neglect subtype tests following acute right hemisphere stroke. J Int Neuropsychol Soc. 2008;14:23–32. doi: 10.1017/S1355617708080077. [DOI] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Schwartz HD, Watson RT. Hypoarousal in patients with the neglect syndrome and emotional indefference. Neurology. 1978;28:229–32. doi: 10.1212/wnl.28.3.229. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford; 1985. pp. 243–93. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Goldberg ME. Attention: behaviour and neural mechanisms. In: Plum F, Mountcastle VB, Geiger ST, editors. The Handbook of Physiology Section 1: The Nervous System. Volume V. Higher Functions of the Brain Part 2. Bethesda, MD: American Physiological Society; 1987. pp. 461–81. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 2005;25:3161–7. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–91. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Hornak J. Ocular exploration in the dark by patients with visual neglect. Neuropsychologia. 1992;30:547–52. doi: 10.1016/0028-3932(92)90057-s. [DOI] [PubMed] [Google Scholar]
- Howes D, Boller F. Simple reaction time: Evidence for focal impairment from lesions of the right hemisphere. Brain. 1975;98:317–32. doi: 10.1093/brain/98.2.317. [DOI] [PubMed] [Google Scholar]
- Husain M, Kennard C. Visual neglect associated with frontal lobe infarction. J Neurol. 1996;243:652–7. doi: 10.1007/BF00878662. [DOI] [PubMed] [Google Scholar]
- Husain M, Mannan S, Hodgson T, Wojciulik E, Driver J, Kennard C. Impaired spatial working memory across saccades contributes to abnormal search in parietal neglect. Brain. 2001;124:941–52. doi: 10.1093/brain/124.5.941. [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385:154–6. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Innocenti GM. Dynamic interactions between the cerebral hemispheres. Exp Brain Res. 2009;192:417–23. doi: 10.1007/s00221-008-1484-8. [DOI] [PubMed] [Google Scholar]
- Ishiai S, Koyama Y, Seki K, Hayashi K, Izumi Y. Approaches to subjective midpoint of horizontal lines in unilateral spatial neglect. Cortex. 2006;42:685–91. doi: 10.1016/s0010-9452(08)70405-2. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Ferber S, Himmelbach M. Spatial awareness is a function of the temporal not the posterior parietal lobe. Nature. 2001;411:950–3. doi: 10.1038/35082075. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus and pulvinar. Brain. 2002;125((Pt) 2):350–60. doi: 10.1093/brain/awf032. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Zopf R, Johannsen L, Fruhmann Berger M, Nèagele T, Klose U. Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain. 2005;128((Pt) 10):2462–9. doi: 10.1093/brain/awh629. [DOI] [PubMed] [Google Scholar]
- Karnath OH, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute vs. chronic spatial neglect. Brain. doi: 10.1093/brain/awq355. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–61. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. The Journal of Neuroscience. 2005;25(18):4593–604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsbourne M. Mechanisms of unilateral neglect. In: Jeannerod M, editor. Neurophysiological and Neuropsychological Aspects of Spatial Neglect. Amsterdam: Elsevier Science; 1987. pp. 69–86. [Google Scholar]
- Kinsella G, Olver J, Ng K, Packer S, Stark R. Analysis of the syndrome of unilateral neglect. Cortex. 1993;29:135–40. doi: 10.1016/s0010-9452(13)80217-1. [DOI] [PubMed] [Google Scholar]
- Koch C, Ullman S. Shifts in visual attention: towards the underlying circuitry. Human Neurobiology. 1985;4:219–27. [PubMed] [Google Scholar]
- Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, et al. Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain. 2008;131:3147–55. doi: 10.1093/brain/awn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL. Neural foundations of imagery. Nat Rev Neurosci. 2001;2:635–42. doi: 10.1038/35090055. [DOI] [PubMed] [Google Scholar]
- Kristjansson A, Vuilleumier P. Disruption of spatial memory in visual search in the left visual field in patients with hemispatial neglect. Vision Res. 2010;50:1426–35. doi: 10.1016/j.visres.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Marshall JC, Fink GR. Neural mechanisms underlying spatial judgements on seen and imagined visual stimuli in the left and right hemifields in men. Neuropsychologia. 2006;44:2846–60. doi: 10.1016/j.neuropsychologia.2006.06.029. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, Rogers LJ, Vallortigara G. Origins of the left & right brain. Sci Am. 2009;301:60–7. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132:645–60. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra P, Jager HR, Parton A, Greenwood R, Playford ED, et al. Spatial working memory capacity in unilateral neglect. Brain. 2005;128:424–35. doi: 10.1093/brain/awh372. [DOI] [PubMed] [Google Scholar]
- Manly T, Dobler VB, Dodds CM, George MA. Rightward shift in spatial awareness with declining alertness. Neuropsychologia. 2005;43:1721–8. doi: 10.1016/j.neuropsychologia.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Mannan SK, Mort DJ, Hodgson TL, Driver J, Kennard C, Husain M. Revisiting previously searched locations in visual neglect: role of right parietal and frontal lesions in misjudging old locations as new. J Cogn Neurosci. 2005;17:340–54. doi: 10.1162/0898929053124983. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Hillis AE. Dissociation between egocentric and allocentric visuospatial and tactile neglect in acute stroke. Cortex. 2008;44:1215–20. doi: 10.1016/j.cortex.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias E, Bublak P, Costa A, Muller HJ, Schneider WX, Finke K. Attentional and sensory effects of lowered levels of intrinsic alertness. Neuropsychologia. 2009;47:3255–64. doi: 10.1016/j.neuropsychologia.2009.08.004. [DOI] [PubMed] [Google Scholar]
- McIntosh RD, Schindler I, Birchall D, Milner AD. Weights and measures: a new look at bisection behaviour in neglect. Brain Res Cogn Brain Res. 2005;25:833–50. doi: 10.1016/j.cogbrainres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, et al. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J Cogn Neurosci. 2008;21:2073–84. doi: 10.1162/jocn.2008.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Spatial updating in human parietal cortex. Neuron. 2003;39:361–73. doi: 10.1016/s0896-6273(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–25. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–46. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Harvey M, Pritchard CL. Visual size processing in spatial neglect. Exp Brain Res. 1998;123:192–200. doi: 10.1007/s002210050561. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic and tectal visual structures in old and new world monkeys. Journal of Comparative Neurology. 1986;243:117–28. doi: 10.1002/cne.902430110. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, et al. The anatomy of visual neglect. Brain. 2003;126:1986–97. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Nicholls ME, Bradshaw JL, Mattingley JB. Free-viewing perceptual asymmetries for the judgement of brightness, numerosity and size. Neuropsychologia. 1999;37:307–14. doi: 10.1016/s0028-3932(98)00074-8. [DOI] [PubMed] [Google Scholar]
- OKeefe J. Hippocampal neurophysiology in the behaving animal. In: Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The Hippocampus Book. Oxford University Press; 2006. pp. 475–548. [Google Scholar]
- Ortigue S, Viaud-Delmon I, Annoni JM, Landis T, Michel C, et al. Pure representational neglect after right thalamic lesion. Ann Neurol. 2001;50:401–4. doi: 10.1002/ana.1139. [DOI] [PubMed] [Google Scholar]
- Paolucci S, Antonucci G, Grasso MG, Pizzamiglio L. The role of unilateral spatial neglect in rehabilitation of right brain-damaged ischemic stroke patients: a matched comparison. Arch Phys Med Rehabil. 2001;82:743–9. doi: 10.1053/apmr.2001.23191. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Paus T, Zatorre RJ, Hofle N, Zografos C, Gotman J, et al. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience. 1997:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJ, Rorden C, Cusack R, Bonfiglioli C, et al. Attentional Functions of Parietal and Frontal Cortex. Cereb Cortex. 2005 doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Perani D, Vallar G, Cappa S, Messa C, Fazio F. Aphasia and neglect after subcortical stroke: a clinical/cerebral perfusion correlation study) Brain. 1987;110:1211–29. doi: 10.1093/brain/110.5.1211. [DOI] [PubMed] [Google Scholar]
- Perez A, Peers PV, Valdes-Sosa M, Galan L, Garcia L, Martinez-Montes E. Hemispheric modulations of alpha-band power reflect the rightward shift in attention induced by enhanced attentional load. Neuropsychologia. 2009;47:41–9. doi: 10.1016/j.neuropsychologia.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Pizzamiglio L. Recovery of neglect after right hemispheric damage: H215O positron emission tomographic activation study. Archives of Neurology. 1998;55:561–68. doi: 10.1001/archneur.55.4.561. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. Journal of Neuroscience. 1984;4:1863–74. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Driver J. Relating unilateral neglect to the neural coding of space. Curr Opin Neurobiol. 2000;10:242–9. doi: 10.1016/s0959-4388(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Pouget A, Sejnowski TJ. Simulating a lesion in a basis function model of spatial representations: comparison with hemineglect. Psychol Rev. 2001;108:653–73. doi: 10.1037/0033-295x.108.3.653. [DOI] [PubMed] [Google Scholar]
- Ptak R, Schnider A. The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci. 2010;30:12557–65. doi: 10.1523/JNEUROSCI.2722-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000;123(Pt 8):624–33. doi: 10.1093/brain/123.8.1624. [DOI] [PubMed] [Google Scholar]
- Rengachary J, d’Avossa G, Sapir A, Shulman GL, Corbetta M. Is the posner reaction time test more accurate than clinical tests in detecting left neglect in acute and chronic stroke? Arch Phys Med Rehabil. 2009;90:2081–8. doi: 10.1016/j.apmr.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M. A behavioral analysis of spatial neglect and its recovery after stroke. Front Hum Neurosci. doi: 10.3389/fnhum.2011.00029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch MJ, Humphreys GW. The effect of cueing on unilateral neglect. Neuropsychologia. 1983;21:589–99. doi: 10.1016/0028-3932(83)90056-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Robertson I, North N. Spatio-motor cueing in unilateral left neglect: The role of hemispace, hand and motor activation. Neuropsychologia. 1992;30:553–63. doi: 10.1016/0028-3932(92)90058-t. [DOI] [PubMed] [Google Scholar]
- Robertson I, Tegner R, Tham K, Lo A, Nimmo-Smith I. Sustained attention training for unilateral neglect: Theoretical and rehabilitation implications. Journal of Clinical and Experimental Neuropsychology. 1995;17:416–30. doi: 10.1080/01688639508405133. [DOI] [PubMed] [Google Scholar]
- Robertson IH. Do we need the “lateral” in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. Neuroimage. 2001;14:S85–90. doi: 10.1006/nimg.2001.0838. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Manly T, Beschin N, Daini R, Haeske-Dewick H, et al. Auditory sustained attention is a marker of unilateral spatial neglect. Neuropsychologia. 1997;35:1527–32. doi: 10.1016/s0028-3932(97)00084-5. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–72. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Robertson LC, Rafal RD. Disorders of Visual Attention. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. Cambridge: MIT Press; 2000. pp. 633–50. [Google Scholar]
- Robinson RG. Cerebral Lateralization in Nonhuman Species. New York: Academic Press; 1985. Lateralized behavior and neurochemical consequences of unilateral brain injury in rats; pp. 135–56. [Google Scholar]
- Rogers LJ. Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 2000;73:236–53. doi: 10.1006/brln.2000.2305. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew RJ, Johnston AN. Light experience and the development of behavioural lateralization in chicks III. Learning to distinguish pebbles from grains. Behav Brain Res. 2007;177:61–9. doi: 10.1016/j.bbr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Anson JM. Lateralisation of function in the chicken fore-brain. Pharmacol Biochem Behav. 1979;10:679–86. doi: 10.1016/0091-3057(79)90320-4. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Sink HS. Transient asymmetry in the projections of the rostral thalamus to the visual hyperstriatum of the chicken, and reversal of its direction by light exposure. Exp Brain Res. 1988;70:378–84. doi: 10.1007/BF00248362. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Grafman J. Sustained attention deficits in patients with right frontal lesions. Neuropsychologia. 1996;34:953–63. doi: 10.1016/0028-3932(96)00016-4. [DOI] [PubMed] [Google Scholar]
- Sack AT. Parietal cortex and spatial cognition. Behav Brain Res. 2009;202:153–61. doi: 10.1016/j.bbr.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Samuelsson H, Hjelmquist EK, Jensen C, Ekholm S, Blomstrand C. Nonlateralized attentional deficits: an important component behind persisting visuospatial neglect? J Clin Exp Neuropsychol. 1998;20:73–88. doi: 10.1076/jcen.20.1.73.1481. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–4. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Sheremata SL, Bettencourt KC, Somers DC. Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load. J Neurosci. 2010;30:12581–8. doi: 10.1523/JNEUROSCI.2689-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Lee J, Behrmann M. Top-down and bottom-up attentional guidance: investigating the role of the dorsal and ventral parietal cortices. Exp Brain Res. 2010 doi: 10.1007/s00221-010-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, Franke D, Pope DL, Snyder AZ, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29:4392–407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci. 2010;30:3640–51. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RM, Raffi M, Phinney RE, Turner JA, Jando G. Functional architecture of eye position gain fields in visual association cortex of behaving monkey. J Neurophysiol. 2003;90:1279–94. doi: 10.1152/jn.01179.2002. [DOI] [PubMed] [Google Scholar]
- Snow JC, Mattingley JB. Goal-driven selective attention in patients with right hemisphere lesions: how intact is the ipsilesional field? Brain. 2006;129:168–81. doi: 10.1093/brain/awh690. [DOI] [PubMed] [Google Scholar]
- Spinelli D, Guariglia C, Massironi M, Pizzamiglio L, Zoccolotti P. Contrast sensitivity and low spatial frequency discrimination in hemi-neglect patients. Neuropsychologia. 1990;28:727–32. doi: 10.1016/0028-3932(90)90127-a. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Calhoun VD, Kiehl KA. Hemispheric differences in hemodynamics elicited by auditory oddball stimuli. Neuroimage. 2005;26:782–92. doi: 10.1016/j.neuroimage.2005.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SP, Halligan PW, Greenwood RJ. The incidence of neglect phenomena and related disorders in patients with an acute right or left hemisphere stroke. Age and Ageing. 1993;22:46–52. doi: 10.1093/ageing/22.1.46. [DOI] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, et al. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Sturm W, Longoni F, Fimm B, Dietrich T, Weis S, et al. Network for auditory intrinsic alertness: a PET study. Neuropsychologia. 2004;42:563–8. doi: 10.1016/j.neuropsychologia.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. J Neurosci. 2007;27:5326–37. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, Corbetta M. Asymmetry of anticipatory activity in visual cortex predicts the locus of attention and perception. Journal of Neuroscience. 2007 doi: 10.1523/JNEUROSCI.3759-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S. Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci. 2010;30:148–60. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, et al. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005;309:2226–8. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Thimm M, Fink GR, Sturm W. Neural correlates of recovery from acute hemispatial neglect. Restor Neurol Neurosci. 2008;26:481–92. [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26:9494–502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia-Alfonso CE, Verhaal J, Gunturkun O. Ascending and descending mechanisms of visual lateralization in pigeons. Philos Trans R Soc Lond B Biol Sci. 2009;364:955–63. doi: 10.1098/rstb.2008.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of spatial neglect in humans. In: Jeannerod M, editor. Neurophysiological and Neuropsychological Aspects of Spatial Neglect. North-Holland: Elsevier Science Publishers; 1987. pp. 235–58. [Google Scholar]
- Vandenberghe R, Geeraerts S, Molenberghs P, Lafosse C, Vandenbulcke M, et al. Attentional responses to unattended stimuli in human parietal cortex. Brain. 2005;128:2843–57. doi: 10.1093/brain/awh522. [DOI] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain. 2010;133:880–94. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Schwartz S, Verdon V, Maravita A, Hutton C, et al. Abnormal attentional modulation of retinotopic cortex in parietal patients with spatial neglect. Curr Biol. 2008;18:1525–9. doi: 10.1016/j.cub.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Sergent C, Schwartz S, Valenza N, Girardi M, et al. Impaired perceptual memory of locations across gaze-shifts in patients with unilateral spatial neglect. J Cogn Neurosci. 2007;19:1388–406. doi: 10.1162/jocn.2007.19.8.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–8. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]
- Watson RT, Miller BD, Heilman KM. Evoked potential in neglect. Arch Neurol. 1977;34:224–7. doi: 10.1001/archneur.1977.00500160038006. [DOI] [PubMed] [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–65. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Jennings R, Ackles P, Hood P, Boller F. Lack of heart rate changes during an attention-demanding task after right hemisphere lesions. Neurology. 1987;37:624–30. doi: 10.1212/wnl.37.4.624. [DOI] [PubMed] [Google Scholar]
- Zopf R, Berger MF, Klose U, Karnath HO. Perfusion imaging of the right perisylvian neural network in acute spatial neglect. Front Hum Neurosci. 2009;3:15. doi: 10.3389/neuro.09.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


