Abstract
Helminthic infections protect mice from colitis in murine models of inflammatory bowel disease and also may protect people. Helminths like H. bakeri (Hpb) can induce Tregs. Experiments explored if Hpb infection could protect mice from colitis through activation of colonic Treg and examined mechanisms of action. We showed that Hpb infection increased the number of T cells expressing Foxp3 in the colon. More importantly, Foxp3+/IL10- and Foxp3+/IL10+ T cell subsets isolated from the colon of Hpb infected mice prevented colitis when adoptively transferred into a murine model of inflammatory bowel disease, while Tregs from uninfected mice could not provide protection. Only the transferred colonic Foxp3+/IL10- T cells from Hpb infected mice readily accumulated in the colon and MLN of recipient mice, and they reconstituted the Foxp3+/IL10- and Foxp3+/IL10+ T cell subsets. However, transferred Foxp3+/IL10+ T cells disappeared. IL10 expression by Foxp3+ T cells was necessary for colitis prevention. Thus, Hpb infection activates colonic Foxp3+ T cells making them highly regulatory. The Foxp3+ T cells that fail to express IL10 may be critical for populating the colon with the Foxp3+/IL10+ T cells, which are required to control colitis.
Keywords: helminths, Foxp3+, T cells, mucosa, colitis
INTRODUCTION
Inflammatory bowel disease (IBD) emerged as a growing health problem in highly developed countries in the latter half of the 20th Century, and it presently is advancing in developing countries. Hygiene associated with modern day living, causing alterations in intestinal flora and fauna, is postulated to be a major risk factor (1) (2). Helminthic infections are exceedingly strong inducers of regulatory circuits and cytokines. For example, Heligmosomoides polygyrus bakeri (Hpb) infection in mice promotes the production of regulatory molecules in the gut like IL10, TGFβ and PgE2 (3). Loss of helminthic infections could be one of the factors underlying the rise in IBD. Several clinical and epidemiologic studies support this concept (4)(5)(6).
Various animal models of IBD suggest that regulatory-type T cells are important for maintaining mucosal immune homeostasis and for controlling colitis (7). Hpb infection stimulates Foxp3 mRNA expression in T cells (8) and expands the number of Foxp3+ T cells in the mesenteric lymph nodes (9). Secretions from Hpb can induce T cells to express Foxp3 (9). T cells from the MLN of Hpb-infected, IL10 deficient mice transferred into helminth-naïve animals will arrest colitis attesting to the importance of T cells in the control of IBD (8). All this suggests that modulation of Treg function could be an important mechanism through which helminths work to prevent IBD.
IL10 is a regulatory cytokine important for immune homeostasis in the gut. Mice deficient in IL10 (10) or IL10R (11) develop spontaneous colitis. Humans with a mutation in the IL10R are prone to IBD further highlighting the importance of IL10 in the protection from this disease process (12). IL10 comes from several sources. However, recent evidence suggests that IL10 from Treg has particular importance in protecting the mucosa from inflammation (13).
Since Hpb can inhibit colitis and is reported to promote Treg development, we used this organism to study the effect of helminth infection on intestinal Foxp3+ T cells. Moreover, we used a Foxp3/IL10 double reporter mouse to assess the effect of Hpb infection on Treg subsets distinguished by their differential capacity to make IL10. Both subsets are naturally expressed in the colon. Experiments revealed that Hpb infection modestly increased the number of colonic T cells expressing Foxp3. Colonic Foxp3+/IL10- and Foxp3+/IL10+ T cells from Hpb-infected mice could prevent colitis when adoptively transferred into a murine model of IBD. Colonic Tregs from uninfected mice could not mediate such protection. Thus, it appears that Hpb infection activates colonic Foxp3+ T cells making them highly regulatory. The transferred colonic Foxp3+/IL10- T cells, from Hpb infected mice, readily accumulated in the colon and MLN of recipient animals reconstituting both the Foxp3+/IL10- and Foxp3+/IL10+ T cell subsets, whereas transferred Foxp3+/IL10+ T cells disappeared. However, only Foxp3+ T cells that make IL10 could prevent colitis. These additional observations suggest that Foxp3+ T cells that fail to express IL10 may be critical for populating the colon with Foxp3+/IL10+ T cells which, in turn, are the most important regulatory T cells for control of colitis.
MATERIALS AND METHODS
Mice
This study used C57BL/6 Rag2 and C57BL/6 wild-type (WT) mice obtained from Jackson Laboratory, Bar Harbor, ME. Also used were C57BL/6 OT2 CD45.1 mice (a gift of Dr. Fuhlbrigge, BWH, Boston, MA), and IL10 KO Foxp3 eGFP reporter mice (gift from Dr. Cathryn Nagler, University of Chicago, IL). Foxp3 mRFP/IL10 eGFP double reporter mice were produced by cross breeding Foxp3 mRFP and IL10 eGFP single reporter mice were obtained from (Richard Flavell, Yale University, CT). Breeding colonies were maintained in SPF facilities at Tufts University. Animals were housed and handled following national guidelines and as approved by our Animal Review Committee.
Hpb infection
For the experiments, 5- to 6-wk-old mice were colonized with 125 Hpb third stage larvae (L3) by oral gavage. And infected mice were used after two weeks. Infective, ensheathed Hpb L3 (U.S. National Helminthological Collection no. 81930) were obtained from fecal cultures of eggs by the modified Baermann method and stored at 4°C.
Dispersion of splenic T cells and mesenteric lymph node (MLN), and splenic T cell enrichment
Single cell suspensions of splenocytes and MLN cells were prepared by gentle teasing in RPMI 1640 medium (GIBCO, Grand Island, NY). The cells were washed three times in RPMI. Splenic CD4+/CD25- T cells were labeled with FITC-CD4 and PE-CD25 mAbs (eBioscience, San Diego, CA). Then, cells were sorted using FACS MoFlo MLS (multi-laser-system) (Cytomation, Fort Collins, CO. USA) with Summit V4.3 software. Viability was determined using exclusion of trypan blue dye.
Lamina propria mononuclear cells (LPMC) isolation, and LPMC and MLN cell fractionation
Gut LPMC were isolated from the colon as described (14). Foxp3 mRFP+/IL10 eGFP- T cells and Foxp3 mRFP+/IL10eGFP+ T cells from dispersed LPMC or MLN cells were isolated by FACS. The viability of the isolated cells always was greater than 95% as determined using exclusion of trypan blue dye.
Adoptive cell transfer
Rag mice of similar age (usually 5 to 6 wks old) were reconstituted i.p. with 1× 105 C57BL6 WT CD4+CD25- splenic T cells and 3×105 OT2 CD45.1 splenic T cells. In some experiments, mice also received 1×105 Foxp3+, Foxp3+/IL10-, Foxp3+/10+ or IL10KO Foxp3+ colon LPMC T cells given by i.p. injection. In other experiments, they received Foxp3+ MLN T cells.
Colitis model
Mice received CD4+CD25- splenic T cells from WT and OT2 mice. In some experiments, the animals also received unfractionated colonic Foxp3+ T cells, or colonic Foxp3+/IL10- or Foxp3+IL10+ T cells from Foxp3/IL10 double reporter mice. Some of these reporter mice were infected with Hpb for 2 wks before sacrifice. One wk after T cell transfer, Rag mice were administered piroxicam, a non-steroidal anti-inflammatory drug (NSAID), mixed into their feed for 2 wks (42mg piroxicam /250g chow wk 1, and 62mg piroxicam/250g chow wk 2). The piroxicam (Sigma) was stopped, and colitis was studied 1 wk later. Thus, it was 4 wks from the day of cell transfer until animal sacrifice. Half of the colons divided longitudinally were fixed, sectioned and stained with H&E for microscopically examined to score the severity of colitis. The other half was dissociated with collagenase to isolate LPMC, which were analyzed by flow cytometry and cultured in vitro. More details regarding the various manipulations of this model are provided in the results section.
Cell culture
Colonic LPMC from Rag mice reconstituted with C57BL/6 WT CD4+CD25- and OT2 CD4+CD25- T cells were cultured (2.5 105 cells per well) for 48h in 96-well flat-bottomed plates. Cells were cultured alone or with OVA (10 µg/ml) (Sigma). The culture medium was RPMI 1640 containing 10% FCS, 25 mM HEPES buffer, 2 mM L-glutamine, 5 × 10−5 M 2-ME, 1 mM sodium pyruvate, 100 U/ml penicillin, 5 mg/ml gentamicin, and 100 mg/ml streptomycin (complete medium) (all Life Technologies, Gaithersburg, MD)(LGM). To measure TGFβ, cells were cultured in RPMI 1640 only containing 1% FCS and 0.1% bovine serum albumin (Sigma). After culture, the supernatants were assayed for IFNγ, IL17A or IL4 using ELISA (described below).
Sandwich ELISAs
ELISAs were performed using paired antibodies mostly from (R & D Systems, Minneapolis, MN) according to manufacturer’s instructions. The IL17 ELISA was done using primary capture mAb (MAB721) and biotinylated anti-IL17A mAb (BAF421) (R&D Systems). The IL-4 ELISA used the primary capture mAb (MAB404) and biotinylated anti-IL4 mAb (BAF404) (R&D Systems). To measure IFNγ, plates were coated with a mAb to IFNγ (HB170, ATCC) and incubated with supernatant. IFNγ was detected with polyclonal rabbit anti-IFNγ (gift from Dr. Mary Wilson, University of Iowa) followed by biotinylated goat anti-rabbit IgG (AXcell, Westbury, NY). TGFβ ELISA reagents were capture mAb (MAB240) and biotinylated anti-TGFβ Ab (BAF240) also from R&D. Cells for the TGFβ ELISA were grown in low serum RPMI medium, which was acidified, neutralized and then assayed.
Flow cytometry analysis
Isolated LPMC were washed twice and adjusted to 107 cells/ml in LGM and stained with saturating amounts of conjugated mAb for 30 min at 4°C. Following staining, cells were washed twice and re-suspended in LGM for analysis by a BD LSR II Flow Cytometer (BD Bioscience, Mountain View, CA) and analyzed by Summit V4.3 software. Before adding labeled mAb, each tube received 1 µg of anti-Fc mAb (eBioscience, San Diego, CA) to block nonspecific binding of conjugated Abs to FcRs. The mAbs used for staining or cell sorting were anti-CD4-FITC, −APC or −PECy5; anti-CD25-PE; anti-CD45.1-APC, -CD8-APC and -gamma/delta-APC (all from eBioscience). Usually, 106 cells were stained, and 105 cell were analyzed.
Immunohistochemical staining for Foxp3 and IL10 in colon tissue sections
Foxp3RFP/IL10eGFP reporter mice were left uninfected or infected with Hpb for 2 wks. Colons then were fixed in 4% paraformaldehyde (Electron Microscopy Science, Hatfield, PA, cat # 15710) in PBS for 2 h on ice, washed 6 times in PBS, and stored in 30% sucrose in PBS overnight at 4°C. The tissue then was placed in OCT fluid (Tissue Tek, Torrence, CA, #4583), frozen in liquid nitrogen and stored at −80°C.
Staining was performed on 4 um thick frozen tissue sections. Primary antibodies were sheep anti-eGFP (Thermo Fisher, Rockford, IL, # OSS00005W) and rabbit anti-RFP (Life Technologies, Carlsbad, CA #R10367) polyclonal Abs. Secondary antibodies were donkey anti-sheep Alexa fluor 488 (#713–545–147)(green) and donkey anti-rabbit Alexa fluor 594 (#711–585–152)(red) affinity purified abs both from Jackson Immuno Research, West Grove, PA. Frozen sections were permeabilized with 0.1% Triton X in PBS for 10 min at room temperature, rinsed with PBS and then treated with PBS containing 5% normal donkey serum (Jackson Immuno Research) for 1 h. Slides were washed 3 times in PBS with Triton X 0.1%. Slides were incubated with primary antibodies at 1:500 on the same tissue sections for 4 h at room temperature and again washed 3 times with PBS with Triton X. Then, slides were incubated with secondary antibodies at 1:200 for 1 h in the dark at room temperature and again washed as described above. Control slides were treated similarly except no primary antibody was used or they were treated with nonspecific sheep and rabbit Abs. After the incubations and washings, slides were coverslipped using Fluoromount G (Southern Biotech, Birmingham, AL) and viewed using a fluorescent microscope with appropriate filters for the two Alexa flours in the Tufts Center for Neuroscience Research P30 NS047243.
Quantitative Real-Time RT-PCR
Total RNA was isolated from individual samples using Quick-RNA Mini Prep (ZymoResearch, CA) as per manufacturer’s instructions. RNA was reverse-transcribed using RT2 Easy First Strand Kit (Qiagen, CA). Real-time quantitative RT-PCR was performed by Taqman analysis using an ABI 7300 instrument (Applied Biosystems, NY). GAPDH levels were used to normalize the data. Taqman real-time probes for Foxp3, IL10, IL4, TGF-β1, IFN-γ and IL17 were obtained from Applied Biosystems, NY. Using the average mean cycle threshold (Ct) value for GAPDH and the gene of interest for each sample, the equation 1.8e (Ct GAPDH – Ct gene of interest) X 104 was used to obtain normalized values.
Histological evaluation
A pathologist blinded to the experimental condition graded the severity of the colonic inflammation using a well describe 4-point scale (15).
Statistical analysis
Data are means ±SE of multiple determinations. Difference between two groups was compared using Student’s t-test. Multiple group comparisons used analysis of variation and Dunnett’s t-test. P values <0.05 were considered significant.
RESULTS
Hpb infection induced an increase in the proportion of colonic LPMC CD4+ T cells expressing Foxp3
T cells that express Foxp3 are plentiful in the gut and help to limit mucosal immune responses. Since Hpb can protect mice from colitis (14,16), it was determined if Hpb infection increased the relative number of Foxp3+ T cells in the colonic mucosa of healthy C57BL/6 Foxp3 reporter transgenic mice. Foxp3mRFP/IL10eGFP double reporter mice were used to allow visualization and then isolation of Treg subsets distinguished by their differential capacity to express IL10.
In the colon, TI and MLN of healthy transgenic mice, Foxp3mRFP was seen only in T cells, and nearly all the Foxp3+ T cells were CD4+ (>97%). In the colonic LP, about 25% of the CD4+ T cells were Foxp3+, and about 63% of them also expressed IL10 (Figure 1). In the TI, Foxp3+ T cells comprised only 10% of the total CD4+ T cell population and compared to the colon, a smaller proportion of these cells expressed IL10 (about 40%). IL10eGFP also was seen in some CD4+ T cells that were negative for Foxp3 expression, appearing in similar proportions in the colon and TI, relative to the total CD4+ T cell subset. The MLN contained very few Foxp3+/IL10+ or Foxp3-/IL10+ CD4+ T cells (<1% of total CD4+ T cells). A small number of CD8+ T cells and γδ T cells in the colon, TI and MLN also expressed Foxp3 (<1% of each subset) (data not shown). There were no Foxp3+/IL10+ T cells in these two T cell subsets.
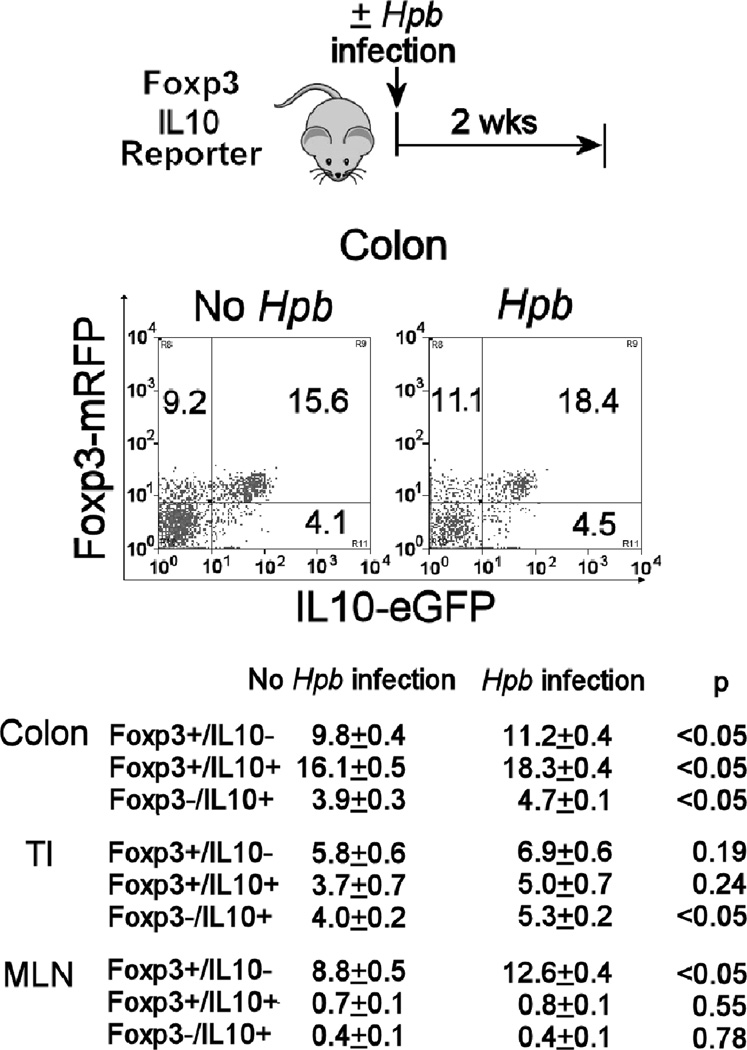
Figure 1. Hpb infection increased the number of Foxp3+ Tregs in the colon of Foxp3/IL10 reporter mice.
Some Foxp3/IL10 double reporter mice were infected with Hpb for 2 wks before isolation of colonic LPMC for flow analysis (Hpb), while age-matched control mice remained uninfected (no Hpb). Isolated colonic LPMC were analyzed for Foxp3 and IL10 expression using FACS. The FACS plot shows the results from a single representative experiment gated on the CD4+ T cell subset. Data in Table I are mean percentages +/− SE of CD4+ colonic LP T cells expressing Foxp3 and/or IL10 from 3 separate experiments. Each group in each individual experiment used pooled cells from at least 3 individual mice.
Reporter mice were infected with Hpb for 2 wks. As compared to age-matched uninfected control animals, there was a modest, but significant increase in the proportion of colonic LP CD4+ T cells that were Foxp3+/IL10- or Foxp3+/IL10+ (Figure 1). Also, the proportion of Foxp3- CD4+ T cells expressing IL10 increased slightly in the colon and TI as well. In the MLN, the Foxp3+/IL10- CD4+ T cell subset expanded relative to the total CD4+ T cell population, while there were no changes in Foxp3+/IL10+ or Foxp3-/IL10+ CD4+ T cells. The proportion of CD8+ and γδ T cells expressing Foxp3 did not change after Hpb infection (data not shown). In the colon and TI, Hpb infection did not induce inflammation or alter the composition of the LPMC isolated from these tissues. Thus, Hpb infection induced a small but significant increase in total CD4+ Foxp3+ T cell number in these tissues.
Foxp3mRFP+ and IL10eGFP+ cells are mostly in the lamina propria
Fluorescent immunohistochemistry was used to localize the Foxp3+ and IL10+ T cells to specific regions of the colon. Sections of colon from uninfected mice were treated with anti-eGFP and −mRFP Abs to identify the cells expressing IL10 (eGFP+) and Foxp3 (mRFP+). The lamina propria contained many Foxp3+ T cells. IL10+ cells were also present, and the IL10+ staining localized mostly to the Foxp3+ T cells. There were few such cells seen outside of the lamina propria (data not shown). The distribution of Foxp3+ T cells in colon of Hpb-infected mice appeared similar to that of uninfected animals (data not shown).
RT-PCR analysis of cytokine expression in colonic Foxp3+IL10- and Foxp3+IL10+
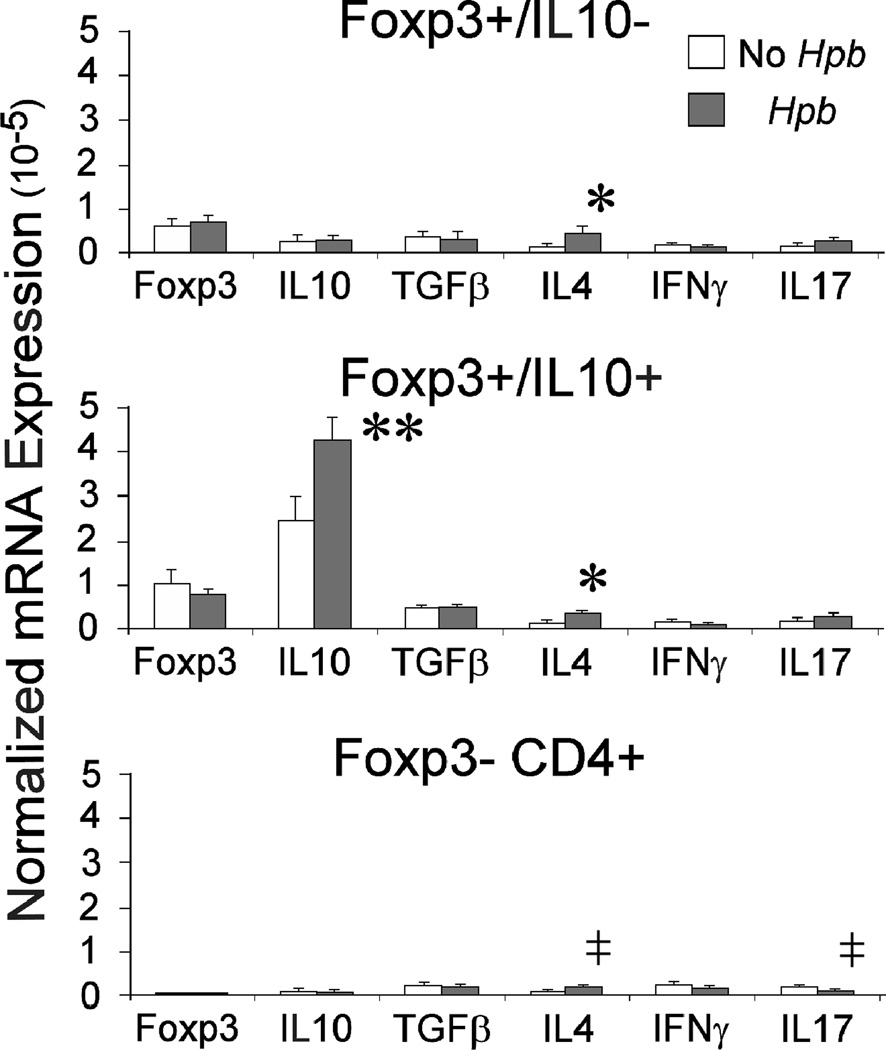
RNA was extracted from Foxp3+/IL10-, Foxp3+/IL10+ and Foxp3- T cells isolated from dispersed colonic LP cells using FACS. Reverse transcribed RNA was analyzed for Foxp3, IL10, TGFβ1, IFNγ and IL17 transcripts using RT-PCR. As expected, Foxp3+ T cells, identified by mRFP+ fluorescence, contained Foxp3 transcripts, which were nearly undetectable in the Foxp3- T cells. The Foxp3+IL10+ (eGFP+) T cells had many more IL10 transcripts compared to the other two cell subsets. Hpb infection increased IL10 expression in the Foxp3+IL10+ T cell subset. Foxp3+IL10- and Foxp3+IL10+ T cells expressed TGFβ transcripts. Hpb infection did not alter TGFβ expression in any of the cell types. The low level of IL4 detected in all three T cell subsets increased following Hpb infection. IFNγ and IL17 were expressed at low levels in the three T cell subsets. Hpb infection caused a decrease in the relative expression of IL17 only in the Foxp3- CD4+ T cells. (Figure 2)
Figure 2. Cytokine expression in colonic Foxp3+/IL10-, Foxp3+/IL10+ and Foxp3- CD4+ T cells before and after Hpb infection.
Some Foxp3/IL10 double reporter mice were infected with Hpb for 2 wks before isolation of colonic LPMC (Hpb), while age-matched control mice remained uninfected (no Hpb). Foxp3+/IL10-, Foxp3/IL10+ and Foxp3- CD4+ T cells were isolated from the colon using FACS. RNA was extracted from each subset and converted to cDNA. RT-PCR was used to determine the relative expression of Foxp3, IL10, TGFβ1, IL4, IFNγ and IL17 transcripts in the three cell subsets before and after infection. Each experiment used 3–4 mice, and data are means +/−SE of 3 independent experiments.* IL4, No Hpb vs. Hpb, p<0.02. ** IL10, No Hpb vs. Hpb, p<0.01. ‡IL4 or IL17, No Hpb vs. Hpb, p<0.05.
Colonic Foxp3+ T cells from Hpb infected mice prevented colitis and reduced the release of IFNγ and IL17 from the colonic LPMC
To study colitis, experiments employed a well established Rag CD4+ CD25- T cell transfer model of IBD (17). Many such models develop colitis inconsistently. To enhance expression of disease, 1 wk after cell transfer, mice were fed a NSAID (piroxicam) for 2 wks. This resulted in all animals developing severe colitis that was evident 1 wk thereafter stopping the NSAID (Four wks after cell transfer). The NSAID disrupts the production of protective arachadonic acid metabolites in the mucosa (15) making the animals more prone to IBD. This is relevant to human IBD, since administration of many types of NSAIDs worsen the disease (18) (19). We also adoptively transferred CD4+ CD25- OT2 T cells into the Rag mice concomitantly with the other cells so that we could study an antigen specific T cell response in the colonic LPMC. OT2 T cells are transgenic T cells that response to OVA. Isolated LPMC from these T cell reconstituted Rag animals respond to OVA with IFNγ and IL17 release. These cytokines were of particular interest, since it is well appreciated that these cytokines help drive the disease in human IBD and in many animal models of this condition (20)(21).
Using this model, it was asked if the Hpb infection affected the functionality of the colonic Foxp3+ T cells isolated from WT mice with regard to their capacity to prevent IBD. Foxp3+ T cells from the colon of Hpb infected or uninfected WT reporter mice were adoptively transferred into Rag mice along with the appropriate splenic CD4+CD25-T cells. Another group of Rag mice received the appropriate CD4+CD25- splenic T cells, but no colonic Foxp3+ T cells. The Rag mice were give piroxicam and sacrificed 4 wks after cell transfer to assess the severity of the colitis. Figure 3 shows that only colonic Foxp3+ T cells from Hpb infected mice protected the mice from IBD.
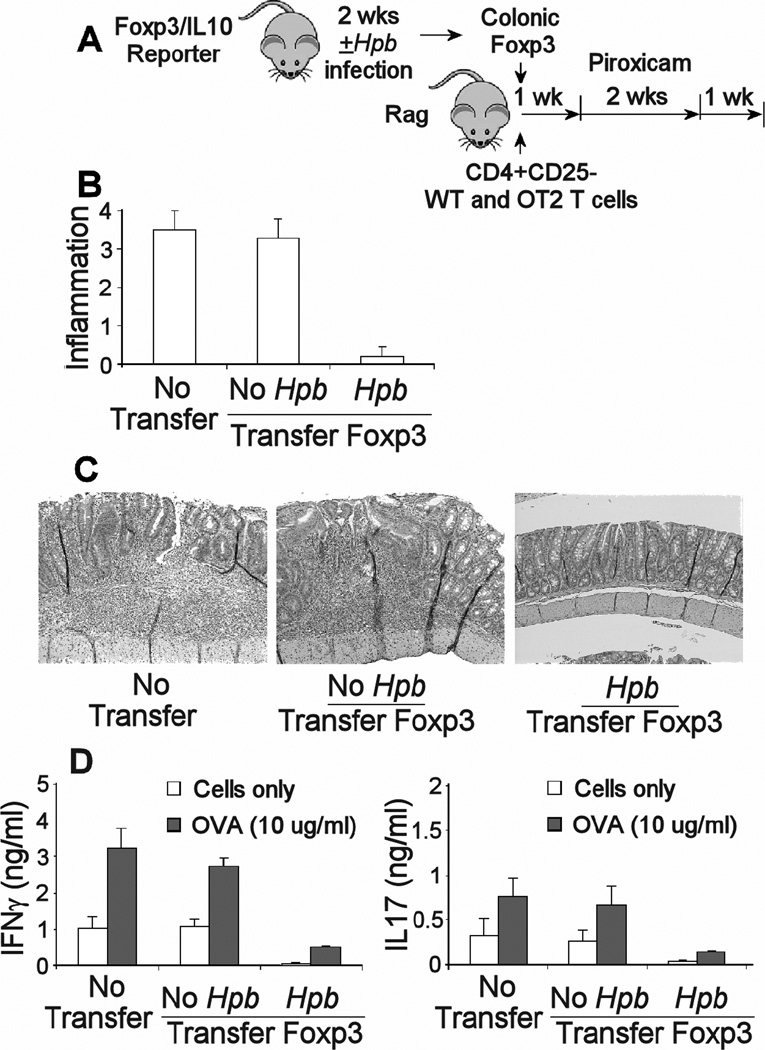
Figure 3. Colonic Foxp3+ T cells from Hpb infected mice blocked the development of colitis in a CD4+CD25- T cell transfer model of IBD.
A) Some Foxp3/IL10 reporter mice were infected with Hpb for 2 wks, while age matched control mice we no infected. Then, Foxp3+ T cells were isolated from dispersed colonic LPMC using FACS. These cells from either Hpb infected (Hpb) or uninfected animals (no Hpb) were adoptively transferred via i.p. injection into Rag mice along with CD4+ CD25- WT and OT2 splenic T cells. After 1 wk, the mice received piroxicam in their food for 2 wks to stimulate colitis. The animals were sacrificed 1 wk after stopping the piroxicam to evaluate colitis severity. B) Colitis was scored for severity on a 4-point scale in stained histological sections. C) The pictures in panel C represent the severity of inflammation in colons of mice receiving no Foxp3+ T cells (no transfer), or adoptively transferred colonic Foxp3+ T cells from either uninfected (no Hpb) or Hpb infected (Hpb) mice. The sections, stained with H&E, were photographed at 40x. D) Dispersed colonic LPMC were cultured 48h in vitro without or with OVA (10 ug/ml) to stimulate cytokine release. Culture supernatants were assayed for IFNγ and IL17 using ELISA. Data are means +/− SE from 3 separate experiments. Each experiment used 5 mice/group. B) No transfer or No Hpb transfer Foxp3 vs. Hpb transfer Foxp3, p <0.01. No transfer vs. No Hp/transfer Foxp3, p NS. D), no transfer or no Hpb/transfer Foxp3 vs. Hp/transfer Foxp3, unstimulated or OVA-stimulated, p<0.01. No transfer vs. No Hpb/transfer Foxp3, p NS. Unstimulated vs. OVA stimulated, p<0.01.
Colonic LPMC we isolated from the colitis-induced Rag mice 4 wks after cell transfer and cultured with or without OVA. Colonic LPMC cultured without antigen produced substantial amounts of IFNγ and IL17, but much more in the presence of OVA. Only adoptive transfer of colonic Foxp3+ T cells from Hpb infected reporter animals into Rag recipients decreased constitutive and OVA-stimulated cytokine release from the colon LPMC (Figure 3). Cultures also were assayed for IL4 and TGFβ, which were only detected at <100 pg/ml. Neither Treg transfer nor OVA or anti-CD3/CD28 stimulation changed the rate of IL4 or TGFβ secretion (data not shown).
The loss of LPMC OVA responsiveness after adoptive transfer of colonic Treg from Hpb-infected mice could have signified that Treg transfer interfered with normal OT2 T cell accumulation in the LP rather than inhibited their function. T cells from C57BL/6 mice express the molecule CD45.2. We reconstituted Rag mice with CD4+ CD25- OT2 T cells from transgenic C57BL/6 mice expressing CD45.1 so that OT2 cells within the isolated LPMC could be distinguished from the other T cells through differential CD45 display.
Compared to Rag mice receiving no supplemental Treg, the relative number of colonic LP cells in the lymphoid gate expressing CD45.1 did not diminish in Rag mice reconstituted with colonic Foxp3+ T cells. In colitic Rag mice that did not receive colonic Foxp3+ T cell transfer, the % of dispersed colonic LPMC in the lymphoid gate that expressed CD45.1 was 4.4+0.7%. The relative number remained the same after either transfer of colonic Foxp3+ T cells from mice without or with Hpb infection (not infected vs. infected, 4.4+0.3% vs. 4.5%+0.8%. (Data are means + SE from each of 9 independent experiments.) This suggests that the low cytokine response to OVA stimulation in vitro is a result of loss of OT2 T cell responsiveness, not due to lack of OT2 T cell number. Mice with colitis develop a mononuclear infiltrate in the lamina propria. These data also suggest that the mice with colitis have more T cells and OT2 T cells in the colonic lining than mice with blunted colitis as a result of Treg transfer.
Fluorescent colonic Foxp3+ T cells from Hpb infected reporter mice readily accumulated in the colon and MLN of Rag recipients
It also was determined if colonic reporter Foxp3+ T cells from Hpb infected mice transferred into Rag recipients led to accumulation of fluorescent Foxp3+ T cells in colons of the cell recipients. Following such transfers, the colons of Rag mice contained large numbers of CD4+Foxp3+/IL10- and Foxp3+/IL10+ T cells (Figure 4). All tissues were examined at the usual time of sacrifice for this colitis model (4 wks after Foxp3 T cell transfer). Examination of the MLN yielded similar results, although, compared to the colon, the relative number of CD4+ T cells expressing Foxp3 was lower in this tissue (Figure 4).
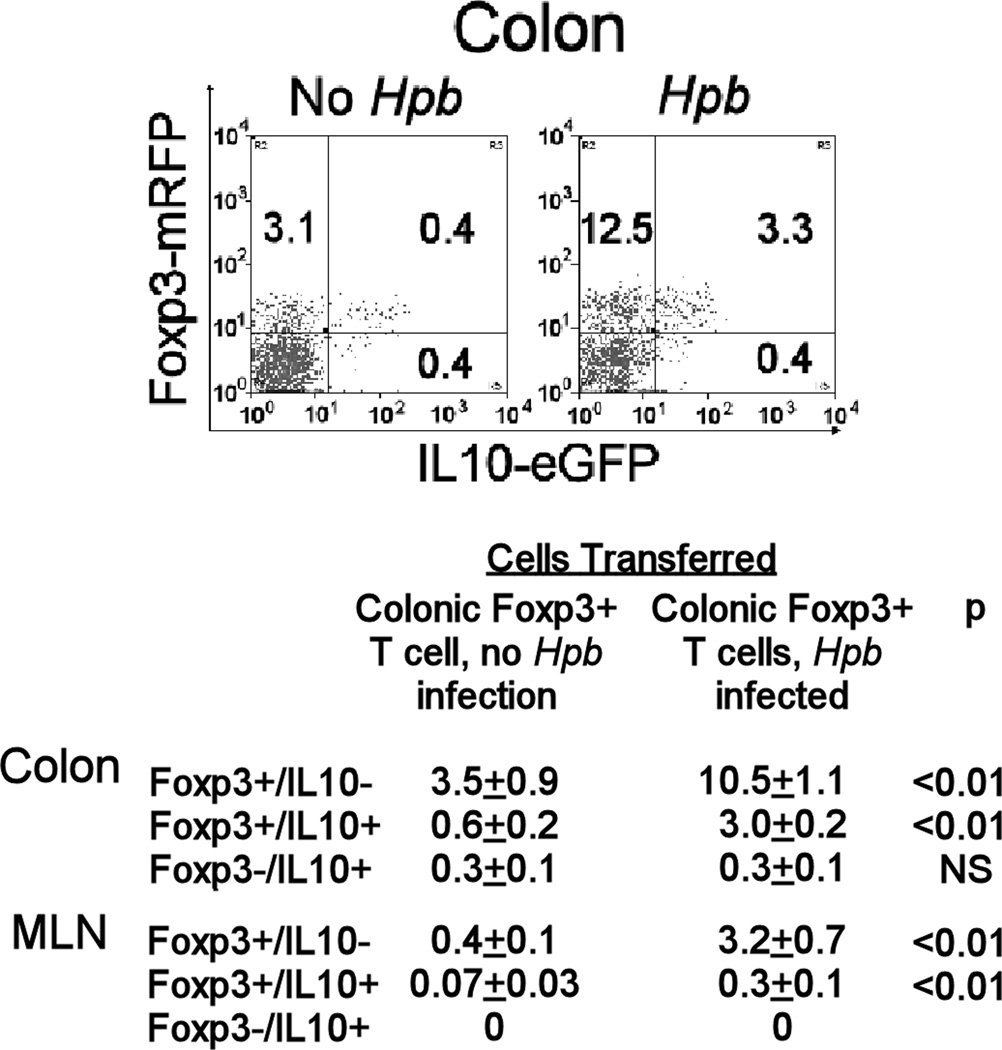
Figure 4. Colonic LPMC Foxp3+ T cells from Hpb-infected mice readily accumulated in Rag intestine after adoptive transfer.
Rag mice received colonic LP Foxp3+ T cells from Foxp3/IL10 reporter mice that were infected for 2 wks with Hpb, or they received similar cells from age-matched, uninfected animals. Using FACS, colonic LPMC cells were analyzed for Foxp3 and IL10 expression 4 wks after cell transfer. The FACS plot shows the results from a single representative experiment gated on the colonic CD4+ T cell subset. Data in Table II are mean percentages +/− SE of CD4+ colonic LP or MLN T cells expressing Foxp3 and/or IL10 from 3 separate experiments. Each group in each individual experiment pooled cells from at least 3 individual mice.
Transfer of colonic Foxp3+ T cells from uninfected reporter mice into Rag recipients resulted in proportionately fewer T cells in the colon that were Foxp3+/IL10- compared to mice receiving cells from infected animals (about 70% less) (Figure 4). Moreover, there were nearly no Foxp3+/IL10+ T cells present. Results were similar for MLN. Also, noted was a small, but definite CD4+ T cell subset that was Foxp3-/IL10+.
Rag that received colonic Treg from Hpb-infected animals developed much less colitis than mice receiving Treg from uninfected mice. The colons of the colitic mice yielded about 30% more LPMC upon dissolution (Treg, no Hpb infection vs. Treg, Hpb infection; 8.2+1.2 vs. 5.7+0.5 ×106 cells/colon, +SD, N=6). CD4+ T cells were present in similar proportions in LPMC isolates from mice receiving Treg from either infected or uninfected mice (No Treg vs. Treg, Hpb, no Hpb; 9.7+2 vs. 8.7+2.6%, +SD, N=6). This suggests that there was an absolute as well as a relative increase in the number of Treg in the colons of mice transferred Tregs from Hpb-infected reporter mice.
Foxp3+ T cells from MLN also inhibit colitis after Hpb infection
Hpb infection also affected the functionality of the MLN Foxp3+ T cells. Adoptive transfer of Foxp3+ T cells from MLN of Hpb-infected reporter mice into Rag recipients block colitis. (Colitis score: No cell transfer, 3.7+0.2, vs. Foxp3+ T cells transfer from Hpb infected mice, 0.36+0.1, p <0.01; +SE, 3 separate experiments) Colitis was only minimally affected with transfer of MLN Foxp3+ T cells from uninfected animals. (Colitis score: Foxp3+ T cell transfer from uninfected mice, 2.9+0.3, vs. no cell transfer, p< 0.05).
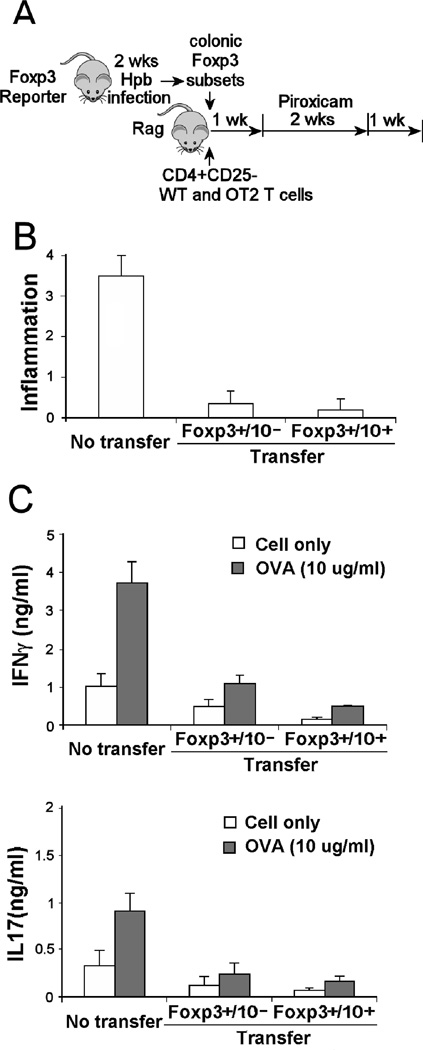
Colonic Foxp3+IL10- and Foxp3+IL10+ T cell subsets protected from colitis with comparable efficiency
The colonic Foxp3+ T cells adoptively transferred into Rag mice to prevent colitis were composed of two subsets distinguished by their capacity to express IL10. Experiments ascertained if the Foxp3+ IL10- and Foxp3+ IL10+ T cell subsets, obtained from the colon of Hpb infected mice, would provide similar levels of colitis protection. Experiments showed that both subsets afforded comparable protection (Figure 5), and reduced constitutive and OVA-stimulated cytokine release from the colonic LPMC isolated from the colitic mice (Figure 5).
Figure 5. Both colonic Foxp3+ IL10- and Foxp3+ IL10+ T cells from Hpb infected mice blocked colitis.
A)Foxp3/IL10 reporter mice were infected with Hpb of 2 wks. Then, Foxp3+/IL10- and Foxp3/IL10+ T cells were isolated from dispersed colonic LPMC using FACS. These cells were adoptively transferred via i.p. injection into Rag mice along with CD4+ CD25- WT and OT2 splenic T cells. After 1 wk, the mice received piroxicam in their food for 2 wks to stimulate colitis. The animals were sacrificed 1 wk after stopping the piroxicam (diagram displays experimental design). B) Colitis was scored for severity on a 4-point scale in stained histological sections. C) Dispersed colonic LPMC were cultured 48h in vitro without or with OVA (10 ug/ml) to stimulate cytokine release. Culture supernatants were assayed for IFNγ and IL17 using ELISA. Data are means +/− SE from 3 separate experiments. Each experiment used 5 mice/group. For (B), No transfer vs. Foxp3+IL10- or Foxp3+IL10+, p<0.01. Foxp3+IL10- vs. Foxp3+IL10+, p NS. For (C), No transfer vs. Foxp3+IL10- or Foxp3+IL10+, unstimulated or OVA-stimulated, p<0.01. Foxp3+IL10- vs. Foxp3+IL10+, IFNγ, p <0.05. Unstimulated vs. OVA stimulated, p<0.05.
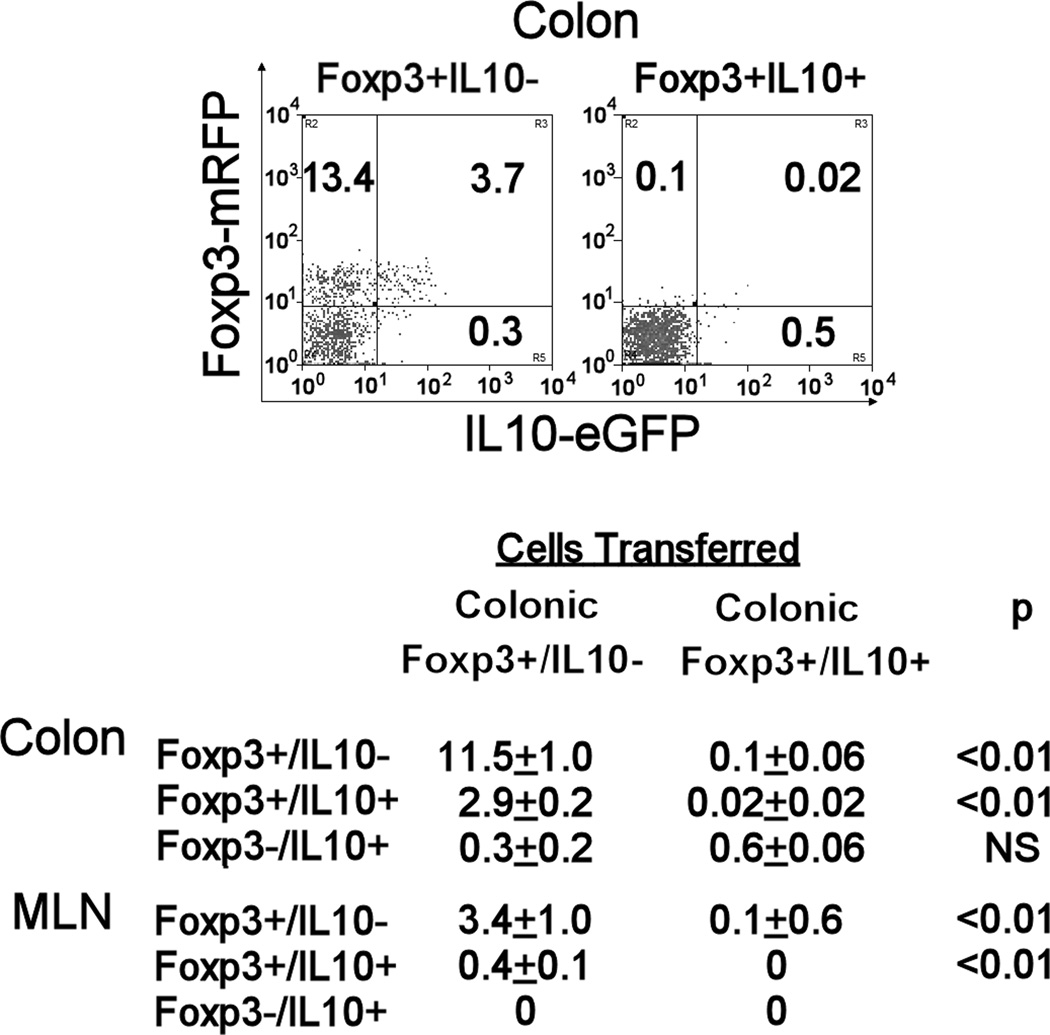
Transfer of colonic Foxp3+IL10- T cells from Hpb infected reporter mice into Rag recipients resulted in accumulation of both Foxp3+/IL10- and Foxp3+/IL10+ T cells in the colon and MLN of the recipient animals
The above observation that Foxp3+/IL10- T cells can protect mice from colitis was unexpected, since previous studies showed that Foxp3+ T cells that make IL10 are important for controlling immune responses in the intestinal mucosa (13). Thus, further studies determined if transfer of Foxp3+ T cells that cannot produce IL10 will result in the accumulation of both Foxp3+ T cell subsets in the colon of our Rag recipients.
Rag mice that received colonic Foxp3+IL10- T cells from Hpb infected reporter mice readily acquired large numbers of fluorescent CD4+ Foxp3+IL10- and Foxp3+IL10+ T cells in the colon (Figure 6) and MLN (Figure 4) at the standard time of sacrifice (4 wks). In these tissues, the relative number of CD4+ T cells expressing Foxp3 with or without IL10 was similar to that observed in Rag mice reconstituted with unfractionated colonic Foxp3+ T cells (Figure 4, Hpb).
Figure 6. In the Rag colitis model, the proportion of colonic and MLN CD4+ T cells that were Foxp3+/IL10- or Foxp3+/IL10+ substantially increased after the transfer of colonic Foxp3+/IL10- T cells from Hpb-infected reporter mice.
Rag mice received Foxp3+/IL10- T cells or Foxp3+/IL10+ T cells derived from colons of Foxp3/IL10 reporter mice infected for 2 wks with Hpb. These cells were adoptively transferred via i.p. injection into Rag mice along with CD4+ CD25- WT and OT2 splenic T cells. After 1 wk, the mice received piroxicam in food for 2 wks to stimulate colitis. LPMC isolated from Rag colon 4 wks after cell transfer were analyzed for Foxp3 and IL10 expression using FACS. The FACS plot shows the results from a representative experiment gated on colonic CD4+ T cells. In Table III, data from 3 experiments are expressed as mean percentages +/− SE of colonic LP or MLN CD4+ T cells expressing Foxp3 and/or IL10. Cells for each analysis were pooled from 3–4 individual mice.
We also ascertained if transfer of Foxp3+IL10+ T cells would yield similar results. A surprising outcome was that Rag recipients of colonic Foxp3+IL10+ T cells, examined 4 wks after transfer, displayed essentially no fluorescent Foxp3+T cells in the colon and MLN (Figure 6). They were absent from the TI also. Examination of colons at earlier time points after cell transfer (wk 1 and 2) revealed such cells, but in small numbers (about 1% of CD4+ T cells). Thus, transferring just colonic Foxp3+/IL10+ T cells failed to stably reconstitute the Foxp3 compartment.
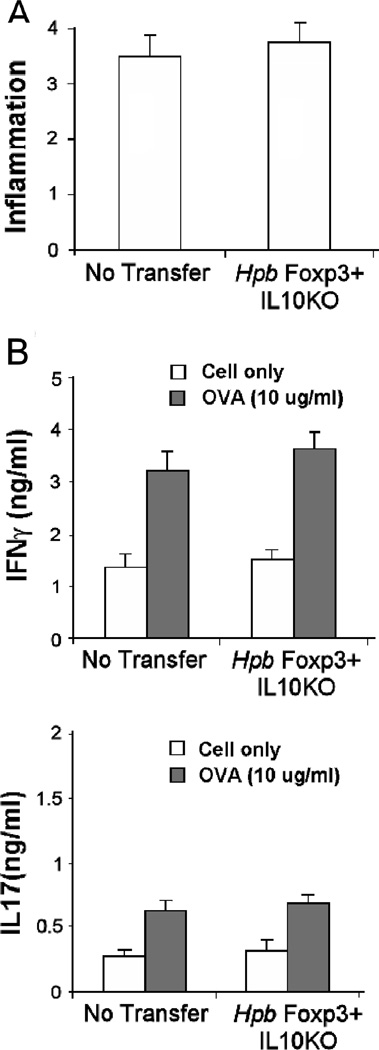
Adoptive transfer of colonic Foxp3+ T cells from Hpb infected IL10−/− mice into Rag recipients resulted in Tregs accumulating within their tissues, but failed to protect them from colitis
To further explore the importance of IL10 in prevention of colitis, IL10−/− Foxp3eGFP reporter mouse were colonized for 2 wks with Hpb. Foxp3+ T cells isolated from the colon of these animals adoptively transferred into Rag recipients did not prevent colitis (Figure 7). Colonic LPMC, isolated from the Rag recipients of IL10−/− Foxp3+ T cells, cultured in vitro produced amounts of IFNγ and IL17 without or with OVA stimulation similar to that of control mice.
Figure 7. Foxp3+ T cells from the colon of IL10 KO mice infected with Hpb could not prevent colitis.
Foxp3+IL10 KO reporter mice were infected with Hpb of 2 wks. Then, Foxp3+ T cells were isolated from dispersed colonic LPMC using FACS. These cells were adoptively transferred via i.p. injection into Rag mice along with CD4+ CD25- WT and OT2 splenic T cells. Control mice (No Transfer) received no Foxp3+ T cells. After 1 wk, the mice received piroxicam in their food for 2 wks to stimulate colitis. The animals were sacrificed 1 wk after stopping the piroxicam. A) Colitis was scored for severity on a 4-point scale in stained histological sections. B) Dispersed colonic LPMC were cultured 48h in vitro without or with OVA (10 ug/ml) to stimulate cytokine release. Culture supernatants were assayed for IFNγ and IL17 using ELISA. Data are means +/− SE from 3 separate experiments. Each experiment used 5 mice/group. No transfer vs. Foxp3+IL10KO, A) inflammatory score or B) unstimulated or OVA-stimulated, p NS.
In the Rag mice that received IL10−/− Foxp3+ T cells, colon and MLN were examined for the presence of Foxp3eGFP+ CD4+ T cells at the 4-wk time of sacrifice. Dispersed colonic LPMC and MLN cells were examined by flow cytometry. Fluorescent IL10−/− Foxp3+ CD4+ T cells were numerous in both tissues. (Mean percentage of CD4+ T cells expressing Foxp3eGFP: Colon, 6.8±1.8 and MLN, 3.4±1.7. Data are means of 3 experiments ±SE.)
DISCUSSION
This study made several important observations. As revealed by the double reporter mice, the LP of WT mouse colon contains large numbers of CD4+ T cells that express Foxp3, and the majority of the Foxp3+ T cells also express IL10. Relatively few Foxp3- T cells in the colon expressed IL10 suggesting that colonic Tregs are the major source of this cytokine in this tissue. This was confirmed by RT-PCR analysis. While Hpb infection modestly expanded the number of CD4+ Foxp3+ T cells in the colon and MLN, the major effect of this infection was to activate or modulate colonic and MLN Treg making them more capable at preventing colitis.
Foxp3+ T cells that make IL10 appear to be critically important for protection from colitis. Our study showed that colonic Foxp3+ T cells from Hpb-infected IL10−/− mice fail to restrain colitis in our adoptive transfer model of IBD. Other studies using a similar animal model of IBD also support this contention (22,23). Also, mice bearing a conditional deletion of the IL10 allele limited to Foxp3 expressing T cells develop spontaneous colitis (13). In our experiments, the transfer of colonic or MLN Foxp3+ T cells from Hpb infected reporter mice into colitis-induced, Rag recipients resulted in a much greater accumulation of Foxp3+/IL10+ T cells in the colon and MLN of the recipient animals. Thus, in our colitis model, failure to adequately reconstitute Foxp3+/IL10+ T cells within the tissues of recipient animals could be one of the reasons colonic and MLN Foxp3+ T cells from uninfected WT mice failed to control colitis. In a recent report, homing and expansion of Foxp3+ Tregs within the LP was required for oral tolerance (24). It is tempting to speculate that, in our IBD model, the failure to replete the IL10 expressing Tregs in the colon was of most importance.
It was interesting to note that transfer of Foxp3+/IL10- T cells from Hpb-infected WT mice reconstituted the Foxp3+/IL10- and the Foxp3+/IL10+ CD4+ T cell subsets in the colon and MLN of recipient colitis-induced Rag animals. There even appeared a small subset of Foxp3- T cells that expressed IL10. However, donor WT colonic Foxp3+/IL10+ T cells appeared briefly in recipient colon and MLN and then disappeared. Thus, it is possible that the colonic Foxp3+/IL10- T cell is a distinct Treg subset that retains the capacity to expand and persist in the recipient mice, whereas, Foxp3+/IL10+ T cells lack this capacity. This could signify that in the colon, Foxp3+/IL10- T cells have limited regulatory activity, but are important for replenishing the less sustainable Foxp3+/IL10+ Treg subset critical for colitis protection.
Adoptive transfer of the colonic Foxp3+/IL10+ T cells from Hpb-infected WT animals was sufficient to prevent colitis over the 4-wk interval of observation, although, they could not reconstitute the Treg population long-term. However, colonic Foxp3+/IL10+ T cells from uninfected WT mice could not mediate this protection. In recipient mice, colonic Foxp3+/IL10- T cells from uninfected mice, as opposed to similar cells from Hpb-infected mice, were less able to reconstitute the Foxp3+/ IL10+ Treg subset or control colitis. This could infer that Hpb infection affects the function of both Foxp3+/IL10+ and Foxp3+/IL10- T cell subsets.
What Hpb does to colonic Treg to promote their function remains speculative. Only a limited number of molecular pathways involved in Tregs activation have been characterized or proposed. GATA-3 is a transcription factor important for initiating and maintaining the expression of IL4, IL5 and IL13. It also appears that GATA-3 helps to control IL10 expression (25). Recent data suggested that GATA-3 plays an important role in promoting Treg function (26)(27). However, GATA-3 also may serve an inhibitory role in Treg differentiation (28) suggesting that the role of GATA-3 in Treg differentiation and function, may be quite complex. The DNA-binding inhibitor Id3 also has a role in Treg generation through enriching the binding of the transcription factor E2A to the Foxp3 promoter region perhaps through relief of GATA-3 inhibition (28). Many of these factors appeared to be integrated into the TGF-β signaling pathway, which is important for Treg differentiation (29). Foxo proteins can serve as coactivators downstream of the TGF-β signaling pathway and affect the differentiation of Foxp3+ Treg (30). Loss of Foxo protein expression in lymphocytes of mice with Gimap5 gene mutation may be the mechanism promoting spontaneous colitis in these animals (31). ICOS is a member of the CD28 superfamily, and stimulation via ICOS can enhance IL10 secretion. ICOS signaling has been reported to be an important factor in promoting Treg function and survival (32). Further studies will be required to determine if these or other regulatory pathways mediate Hpb-induced Treg activation.
It is not known how Hpb interfaces with colonic Treg to enhance their anti-colitogenic function. Hpb can release soluble factors that elicit the expansion of regulatory-type T cells (9)(33)(34). Dendritic cell (DC) subsets are important for driving T cell differentiation in the gut and MLN, and can promote Treg development (35). Undefined Hpb products have been shown to modulate DC function rendering them capable of driving the CD4+ T cells into the Treg phenotype (33). Natural infection is associated with expansion within the MLN of a CD11clow CD 103+DC subset associated with induction of Treg responses in vitro and in vivo (36). Since Foxp3+ T cells and other regulatory T cell subsets can ameliorate colitis in murine models of IBD, and tolerogenic DC can expand regulatory T cells in vitro and protect mice from colitis (37), it is reasonable to assume that tolerogenic DC/T cell interactions are an important part of the process.
Hpb inhabits mostly the proximal small bowel, while it acts distally on the colon to limit disease. The process of protection likely requires some form of communication between the parasite and the host. Hpb releases factors that could have immune modulatory functions (38)(39). For instance, Hpb secretions contain homologues to MIF and C-type lectin receptors. Such molecules could interact with intestinal epithelial cells and DC that penetrate the epithelial barrier locally near the infection or far distally within the gut. Also, Hpb infection affects the composition of the intestinal flora with unknown consequences (40). The factors produced by Hpb that alter DC function and how these factors reach their cellular targets are not yet explored.
Acknowledgments
Supported by NIH grants DK38327, DK058755, DK091987, and Broad Foundation, Schneider Family, Friedman Family, Gilman Family
Abbreviations
- APC
Antigen presenting cells
- DC
Dendritic cells
- Hpb
Heligmosomoides polygyrus bakeri
- IBD
Inflammatory bowel disease
- LP
Lamina propria
- LPMC
Lamina propria mononuclear cells
- MLN
Mesenteric lymph node
- NSAID
Non-steroidal anti-inflammatory drug
- Treg
Regulatory T cells
- RPMI
RPMI 1640 medium
- TI
Terminal ileum
Contributor Information
Long Hang, Email: lhang@tuftsmedicalcenter.org.
Arthur M. Blum, Email: ablum@tuftsmedicalcenter.org.
Tommy Setiawan, Email: tsetiawan@tuftsmedicalcenter.org.
Joseph P. Urban, Jr., Email: joe.urban@ARS.USDA.GOV.
Korynn M. Stoyanoff, Email: kstoyanoff@tuftsmedicalcenter.org.
Joel V. Weinstock, Email: jweinstock2@tuftsmedicalcenter.org.
Reference List
- 1.Elliott DE, Urban JF, Jr, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn's disease? FASEB J. 2000;14:1848–1855. doi: 10.1096/fj.99-0885hyp. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflam. Bowel Dis. 2009;15(1):128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- 3.Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Inf Immun. 2007;75(9):4655–4663. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buning J, Homann N, von Smolinski D, Borcherding F, Noack F, Stolte M, Kohl M, Lehnert H, Ludwig D. Helminths as governors of inflam. bowel dis. Gut. 2008;57:1182–1183. doi: 10.1136/gut.2008.152355. [DOI] [PubMed] [Google Scholar]
- 5.Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Science Translational Medicine. 2010;2:60–88. doi: 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- 6.Kabeerdoss J, Pugazhendhi S, Subramanian V, Binder HJ, Ramakrishna BS. Exposure to hookworms in patients with Crohn's disease: a case-control study. Alimentary Pharmacology & Therapeutics. 2011;34:923–930. doi: 10.1111/j.1365-2036.2011.04824.x. [DOI] [PubMed] [Google Scholar]
- 7.Boden EK, Snapper SB. Regulatory T cells in inflammatory bowel disease. Current Opinion in Gastroenterology. 2008;24(6):733–741. doi: 10.1097/mog.0b013e328311f26e. [DOI] [PubMed] [Google Scholar]
- 8.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 9.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, Belkaid Y, Rudensky AY, Maizels RM. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF- pathway. J. Exp. Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. J. Exp. Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, Hatscher N, Pfeifer D, Sykora KW, Sauer M, Kreipe H, Lacher M, Nustede R, Woellner C, Baumann U, Salzer U, Koletzko S, Shah N, Segal AW, Sauerbrey A, Buderus S, Snapper SB, Grimbacher B, Klein C. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 15.Berg DJ, Zhang J, Weinstock JV, Ismail HF, Earle KA, Alila H, Pamukcu R, Moore S, Lynch RG. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterol. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 16.Elliott DE, Metwali A, Leung J, Setiawan T, Blum AM, Ince MN, Bazzone LE, Stadecker MJ, Urban JF, Jr, Weinstock JV. Colonization with Heligmosomoides polygyrus suppresses mucosal IL-17 production. J. Immunol. 2008;181:2414–2419. doi: 10.4049/jimmunol.181.4.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjellev S, Lundsgaard D, Poulsen SS, Markholst H. Reconstitution of Scid mice with CD4+CD25- T cells leads to rapid colitis: an improved model for pharmacologic testing. International Immunopharmacology. 2006;6:1341–1354. doi: 10.1016/j.intimp.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, Bjornsson E, Bjarnason I. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clinical Gastroenterology & Hepatology. 2006;4:196–202. doi: 10.1016/s1542-3565(05)00980-8. [DOI] [PubMed] [Google Scholar]
- 19.Chan SS, Luben R, Bergmann MM, Boeing H, Olsen A, Tjonneland A, Overvad K, Kaaks R, Kennedy H, Khaw KT, Riboli E, Hart AR. Aspirin in the aetiology of Crohn's disease and ulcerative colitis: a European prospective cohort study. Alimentary Pharmacology & Therapeutics. 2011;34:649–655. doi: 10.1111/j.1365-2036.2011.04784.x. [DOI] [PubMed] [Google Scholar]
- 20.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflam. Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 21.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 22.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J. Immunol. 2006;177(9):5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunological Reviews. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 24.Hadis U, Wahl B, Schulz O, Hardtke-Wolenski M, Schippers A, Wagner N, Muller W, Sparwasser T, Forster R, Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 25.Shoemaker J, Saraiva M, O'Garra A. GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J. Immunol. 2006;176:3470–3479. doi: 10.4049/jimmunol.176.6.3470. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfert EA, Grainger JR, Bouladoux N, Konkel JE, Oldenhove G, Ribeiro CH, Hall JA, Yagi R, Naik S, Bhairavabhotla R, Paul WE, Bosselut R, Wei G, Zhao K, Oukka M, Zhu J, Belkaid Y. Gata3 controls foxp3[Superscript plus sign] Regulatory t cell fate during inflammation in mice. J. Clin. Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, Zhang P, Zamarron BF, Yu D, Wu Y, Zhuang Y, Gutkind JS, Chen W. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nature Immunology. 2011;12:86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nature Immunology. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 30.Harada Y, Harada Y, Elly C, Ying G, Paik JH, DePinho RA, Liu YC. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J. Exp. Med. 2010;207:1381–1391. doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aksoylar HI, Lampe K, Barnes MJ, Plas DR, Hoebe K. Loss of immunological tolerance in Gimap5-deficient mice is associated with loss of Foxo in CD4+ T cells. J. Immunol. 2012;188:146–154. doi: 10.4049/jimmunol.1101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornete M, Sgouroudis E, Piccirillo CA. ICOS-dependent homeostasis and function of Foxp3+ regulatory T cells in islets of nonobese diabetic mice. J. Immunol. 2012;188:1064–1074. doi: 10.4049/jimmunol.1101303. [DOI] [PubMed] [Google Scholar]
- 33.Segura M, Su Z, Piccirillo C, Stevenson MM. Impairment of dendritic cell function by excretory-secretory products: a potential mechanism for nematode-induced immunosuppression. Eur. J. Immunol. 2007;37(7):1887–1904. doi: 10.1002/eji.200636553. [DOI] [PubMed] [Google Scholar]
- 34.Rausch S, Huehn J, Kirchhoff D, Rzepecka J, Schnoeller C, Pillai S, Loddenkemper C, Scheffold A, Hamann A, Lucius R, Hartmann S. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Inf. Immunity. 2008;76:1908–1919. doi: 10.1128/IAI.01233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith KA, Hochweller K, Hämmerling GJ, Boon L, MacDonald AS, Maizels RM. Chronic helminth infection promotes immune regulation in vivo through dominance of CD11clo CD103 dendritic cells. J. Immunol. 2011;186:40–49. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanishi H, Murakami H, Ikeda Y, Abe M, Kumagi T, Hiasa Y, Matsuura B, Onji M. Regulatory dendritic cells pulsed with carbonic anhydrase I protect mice from colitis induced by CD4+CD25- T cells. J. Immunol. 2012;188:2164–2172. doi: 10.4049/jimmunol.1100559. [DOI] [PubMed] [Google Scholar]
- 38.Moreno Y, Gros PP, Tam M, Segura M, Valanparambil R, Geary TG, Stevenson MM. Proteomic analysis of excretory-secretory products of Heligmosomoides polygyrus assessed with next-generation sequencing transcriptomic information. PLoS Neglected Tropical Diseases [electronic resource] 2011;5:e1370. doi: 10.1371/journal.pntd.0001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hewitson JP, Harcus Y, Murray J, van AM, Filbey KJ, Grainger JR, Bridgett S, Blaxter ML, Ashton PD, Ashford DA, Curwen RS, Wilson RA, Dowle AA, Maizels RM. Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of venom allergen-like (VAL) proteins. Journal of Proteomics. 2011;74:1573–1594. doi: 10.1016/j.jprot.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walk ST, Blum AM, Ewing SA, Weinstock JV, Young VB. Alteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrus. Inflamm. Bowel Dis. 2010;16:1841–1849. doi: 10.1002/ibd.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]