Abstract
The extrinsic sensory innervation of the gastrointestinal tract is the conduit through which the gut and the central nervous system communicate. The hindbrain receives information directly from the bowel via the vagus nerve, while information from spinal afferents arrives in the central nervous system through the dorsal root ganglia. This review focuses on the molecular development of these vagal and spinal innervations, with an emphasis on mechanisms that involve axon guidance. During development, axons from both the nodose ganglia and dorsal root ganglia grow into the gut, innervate their appropriate enteric targets and avoid inappropriate cells in the gut wall. These developmental outcomes suggest that both attractive and repellent molecules are important in establishing the normal pattern of the extrinsic sensory innervation. In the fetal mouse gut, the guidance of vagal sensory axons is mediated by axon guidance molecules, such as netrin and the netrin receptor, deleted in colorectal cancer (DCC), as well as extracellular matrix molecules, such as laminin-111. Dorsal root ganglion neurons are known to express, and their axons to respond to, axon guidance molecules. The question of whether or not these molecules are involved in guiding spinal afferents to the bowel, however, has not yet been examined. It is anticipated that a better understanding of how vagal and spinal afferents innervate the fetal gut and reach specific enteric locations will provide deeper insights into the underlying mechanisms of normal and abnormal sensation from the gastrointestinal tract.
Keywords: Vagus nerves, Spinal nerves, Nodose ganglia, Dorsal root ganglia, Gastrointestinal tract, Axon guidance
1. Introduction
The gut has both an extrinsic and intrinsic innervations. The intrinsic innervation is provided by neurons of the enteric nervous system (ENS), which unlike other divisions of the peripheral nervous system, can regulate the function of its target organ without input from the central nervous system (CNS) (Gershon, 2005). Although the ENS can control gut behavior independently, its activity is normally coordinated with the activity of the critical extrinsic innervation, which provides communication between the bowel and the CNS. The extrinsic innervation consists of vagal and spinal sensory nerves (Blackshaw et al., 2007), vagal and sacral parasympathetic motor axons, and axons from sympathetic neurons in prevertebral ganglia (Langley, 1921). Normal homeostasis is dependent on this extrinsic innervation, which is involved in satiety, nausea, sphincter function and detection of visceral pain. Gastrointestinal (GI) motility and secretion can also be enhanced or inhibited by extrinsic nerves. Most of the extrinsic axons that supply the gut are sensory; up to 75% of vagal axons carry sensory information from the bowel to the brain (Blackshaw et al., 2007; Powley and Phillips, 2002). Vagal sensory fibers are thought to mediate satiety, nausea and maintenance of homeostasis. Perception of visceral pain generally relies upon spinal afferents from the dorsal root ganglia (DRG), but strong vagal afferent stimulation can also elicit pain (Blackshaw et al., 2007).
2. The extrinsic sensory innervation of the gut
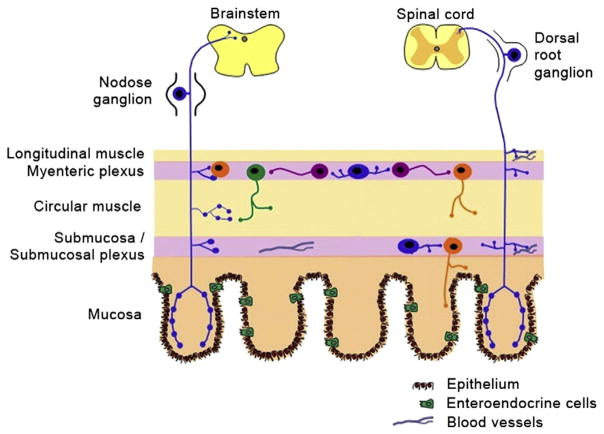
Sensory information from the bowel is conveyed to the CNS by two separate extrinsic pathways. The vagal sensory innervation sends information to the brain via the nodose ganglia. Input to the spinal cord comes from the sensory innervation provided by the DRG (Fig. 1). Axons from neurons in the thoracic and lumbar DRG travel via the sympathetic chain and grow into the gut along the splanchnic nerves, traversing the celiac ganglion to reach the esophagus, stomach and small intestine and traveling through the mesenteric ganglia to reach the colon. Axons from neurons in sacral DRG follow the parasympathetic pelvic splanchnic nerves to supply the colon and rectum (Furness et al., 1999). While these two pathways overlap for the majority of the length of the gut, the vagal afferents are thought to signal mainly from the upper GI regions and the pelvic afferents from the colorectal region (Blackshaw et al., 2007).
Fig. 1.
Diagram illustrating the extrinsic sensory innervation of the gastrointestinal tract. Adapted from Furness (Furness et al., 1999) and Wright (Wright et al., 2008). This material is reproduced with permission of the American Physiological Society and of Wiley-Blackwell.
The nodose ganglia and DRG have different developmental origins. The neurons of the nodose ganglia are derived from the placodal ectoderm while DRG neurons differentiate from cells that migrated from the thoracic level of the neural crest (Le Douarin and Kalcheim, 1999). Molecular profiling studies have shown that many genes, including G protein-coupled receptors and ion channels, are differentially expressed in adult mouse nodose ganglia and thoracic DRG (Peeters et al., 2006). Axons from both the nodose ganglia and the DRG grow into the gut during development to find their correct targets in the bowel wall, but given the described similarities and differences between the two types of ganglia, it is reasonable to expect that there will be similarities and differences in terms of the molecules and mechanisms by which each is guided.
3. Molecular development of enteric vagal afferents
Defining the timing of descent of vagal sensory axons has been important for understanding the molecular mechanisms that underlie the development of the vagal sensory innervation of the gut. Research in the late 1980s and 1990s revealed that vagal axons first enter the mouse stomach at E11 (Baetge and Gershon, 1989), the rat stomach at E12 (Rinaman and Levitt, 1993), and were present in the mouse esophagus at E12 (Sang and Young, 1998). These observations were extended by studies in which the liphophilic dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) was applied to the nodose ganglia of embryonic day (E) 12, E14 and E16 fetal mice (Ratcliffe et al., 2006). This work showed that the stomach receives vagal sensory fibers by E12 and that the celiac ganglion and duodenum are innervated by E14. DiI-labeled vagal axons reach the distal small intestine by E16 and, at the same stage, form a plexus in the esophageal musculature (Ratcliffe et al., 2006). Vagal terminals in the developing mouse stomach were also recently characterized using DiI (Murphy and Fox, 2007). These detailed experiments revealed that nerve bundles containing DiI-labeled axons had formed the vagal gastric branches, extended from the esophagus toward the greater curvature, and progressed beyond the greater curvature by E14.5/E15.5. Vagal axon bundles showed an adult-like distribution throughout the stomach wall by E16.5.
Once they have reached the gut, vagal sensory axons grow to provide innervation to three different enteric locations (Fig. 2) (Blackshaw et al., 2007; Powley and Phillips, 2002). Long terminals, described as intramuscular arrays (IMAs), lie between the cells of the external muscle. Mechanosensitive large intraganglionic laminar endings (IGLEs) are associated with the connective tissue sheaths of ganglia (Zagorodnyuk et al., 2001) and sensory endings occur within the lamina propria of the mucosa. The correlation of the location of these endings with their function highlights the importance of each type of ending being able to reach its appropriate target. IMAs appear to be stretch receptors, tension in the gut wall activates IGLEs (Phillips and Powley, 2000), and the sensory projections in the lamina propria correspond to functional mucosal receptors (Blackshaw et al., 2007). DiI-labeling has demonstrated that IGLE-like terminals first appear in the mouse esophagus at E15 and by E18 are more differentiated (Sang and Young, 1998). In the stomach, putative precursors to IGLEs and IMAs are first detected at E16.5/E17.5, becoming more frequent and more mature by P0 to P2 (Murphy and Fox, 2007).
Fig. 2.
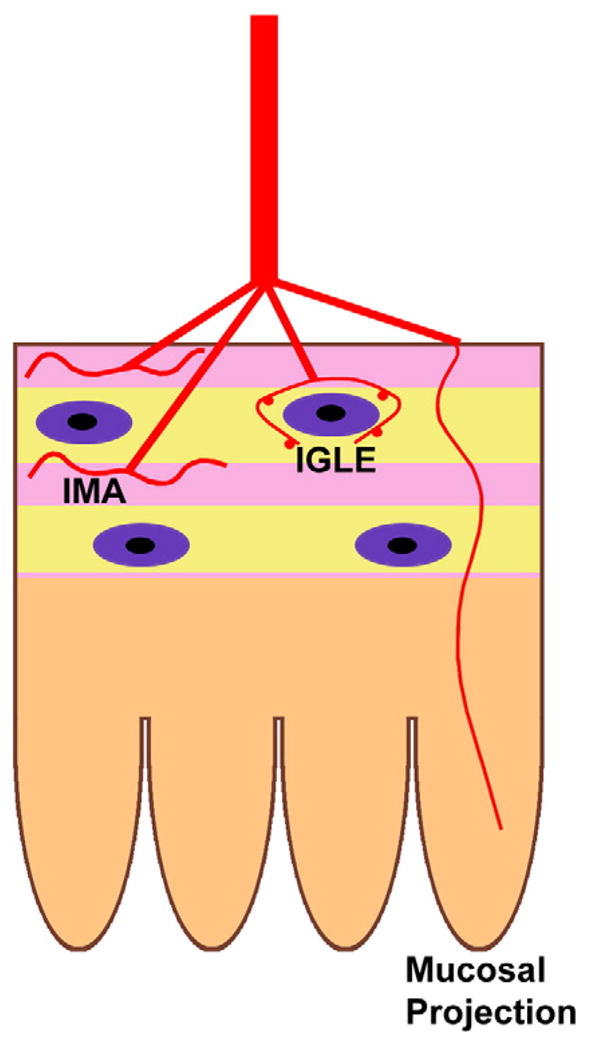
Diagram illustrating the three different locations where vagal sensory endings terminate in the bowel wall. Intramuscular arrays (IMAs) are located within either the circular or longitudinal muscle layers, intraganglionic endings (IGLEs) are situated at the surface of a myenteric ganglion and mucosal projections extend into the lamina propria.
After first finding the bowel during development, vagal sensory axons must then be guided to their correct locations in the gut wall. Netrins have been identified as important axon guidance molecules in the CNS, where they act through both attractive (Astic et al., 2002; Serafini et al., 1996) and repellent (Colamarino and Tessier-Lavigne, 1995) mechanisms. Like many signaling molecules, netrins occur in the gut as well as in the brain (Gershon and Ratcliffe, 2004; Young et al., 2004). In the gut, netrins act through their receptor, deleted in colorectal cancer (DCC), to ensure that neural crest-derived neuronal and glial precursors reach the submucosal and pancreatic plexuses (Jiang et al., 2003). More recent work has established that vagal sensory axons are attracted to the fetal bowel through a mechanism that involves netrin acting on its receptor, DCC (Ratcliffe et al., 2006). These studies showed that DCC was expressed in nodose ganglia and that its expression was developmentally regulated; DCC immuno-reactivity was also demonstrated in descending vagal axons (Fig. 3). The outer mesenchyme and mucosa of the developing foregut expressed netrin-1 and vagal axons labeled by applying DiI to the nodose ganglion extended first into a netrin-1 producing zone and then toward a netrin-1 source. Co-cultures of explants of nodose ganglia with either foregut or netrin-1-secreting EBNA cells in vitro revealed that growing nodose neurites were attracted to the foregut and to netrin-1-secreting cells, and that this targeted growth was blocked by the addition of antibodies to DCC to the culture media (Ratcliffe et al., 2006).
Fig. 3.

DCC immunoreactivity (green) in E13 fetal mouse gut. (A) DCC immunoreactivity (arrows) is seen in paraesophageal anterior and posterior vagal trunks. (B) DCC-immunoreactive trunks are seen at the esophago-gastric junction, extending from the esophagus into the stomach. In the stomach, DCC-positive processes defasciculate and fine processes with the morphology of growth cones (inset) travel into the outer gastric mesenchyme. Scale bar: 25 μm (A); 50 μm (B); 20 μm (inset). Reproduced from Ratcliffe (Ratcliffe et al., 2006). Copyright [2006, John Wiley & Sons, Inc.]. This material is reproduced with permission of John Wiley & Sons, Inc.
Not only were vagal sensory axons attracted to the gut and to specific locations within it, but the axons also failed to enter most of the bowel wall. Vagal sensory fibers thus appear to arrive at their correct enteric targets using both attractive and repellent cues. Chemorepulsion can be mediated by Slit proteins, which act on Robo receptors (Stein and Tessier-Lavigne, 2001). The mesoderm of the chick bowel contains Slits1–3 (De Bellard et al., 2003), the fetal mouse gut expresses mRNA encoding Slits 1–3 and transcripts encoding Robo1 occur in the developing nodose ganglia (Ratcliffe et al., 2005). These observations suggest that chemorepulsion based on Slit/Robo could play a role in targeting vagal sensory innervation to specific cell types within the bowel wall.
Besides axon guidance molecules, components within the extra-cellular matrix can also influence growing axons (Höpker et al., 1999). Laminins in the extracellular matrix not only act as adhesion molecules but also play roles in cell signaling (Colognato and Yurchenco, 2000). In particular, netrin-1-mediated attraction can be converted to repulsion by laminin-111 (Höpker et al., 1999). The fetal mouse gut expresses laminin-111 (Simon-Assmann et al., 1998), as do regions of the fetal gut where vagal sensory axons terminate (Ratcliffe et al., 2008). These findings suggest that laminin-111 could act as a stop signal for vagal axons entering the gut wall. In fetal mouse gut, in which DiI had been used to label vagal sensory axons, the laminin-rich sheaths of developing enteric ganglia, where IGLEs form, were found to be the targets for vagal sensory innervation (Fig. 4) (Ratcliffe et al., 2008). The addition of soluble laminin to co-cultures of E14 fetal mouse nodose ganglia with netrin-1-secreting cells reversed the preferential growth of nodose axons toward the source of netrin-1. Similarly, the attraction of nodose axons towards a co-cultured explant of foregut was changed to repulsion by adding soluble laminin. These co-culture studies, while demonstrating that the netrin-mediated attraction of nodose axons is inhibited by laminin, represent an artificial situation in which laminin is a circulating soluble protein (Ratcliffe et al., 2008). Laminin in situ is membrane-bound and concentrated in the periganglionic and smooth muscle basal laminae; vagal axons thus are not simply repelled by a gradient of laminin but come into contact with laminin-rich targets, which can terminate the extension of vagal neurites.
Fig. 4.

Vagal sensory terminals in the E16 gut visualized via bilateral application of DiI (red) to the nodose ganglia. Sections are double labeled with antibodies to laminin (green). Nuclei are identified by staining DNA with bisbenzimide (blue; B, C). (A). Laminin-immunoreactivity is most prominent in the bowel and pancreas in a coronal section of abdomen. Yellow arrows indicate the locations of micrographs in B and C. (B). Punctate, DiI-labeled vagal sensory terminals of axons are seen in the myenteric plexus of the stomach. These terminals lie close to a ganglion, possibly forming an IGLE. The DiI-labeled sensory terminals occur very close to, but do not cross the areas that contain highly concentrations of laminin immunoreactivity (white arrows). (C). DiI-labeled sensory terminals extend into the outer mesenchyme of the wall of the small bowel; the sensory terminals travel between but do not invade areas of concentrated laminin (white arrows). Scale bar: 200 μm (A); 25 μm (B); 10 μm (C). Reproduced from Ratcliffe (Ratcliffe et al., 2008). Copyright [2008, John Wiley & Sons, Inc.]. This material is reproduced with permission of John Wiley & Sons, Inc.
While the current review focuses on axon guidance molecules, it is important to note that members of the neurotrophin family have also been implicated in vagal development (Liu and Jaenisch, 2000). More recent work has focused on brain-derived neurotrophic factor (BDNF), which has previously been shown to have effects on vagal sensory, but not motor, development (Jones et al., 1994). In mice lacking BDNF, the vagal sensory development of the stomach was found to be abnormal: the gastric population of IGLEs was decreased by 50% at birth, the population of IMAs, while normal in density, were abnormal in morphology with stunted telodendria, and the vagal innervation of the antrum was disorganized (Fox and Murphy, 2008; Murphy and Fox, 2010). These findings, in particular the loss of IGLEs and the altered antral innervation, suggest that BDNF may act not only as a survival factor for vagal sensory axons, but may also play a role in guiding vagal fibers to their correct locations in the stomach wall (Murphy and Fox, 2010).
4. Molecular development of enteric spinal afferents
Similar to vagal afferents, DRG axons target specific locations in the myenteric plexus, submucosa and mucosa. Spinal afferents are also associated with blood vessels in the mesentery and in the submucosa (Zagorodnyuk et al., 2010). Whether nodose and DRG axons differ in their expression of DCC and in their requirement for netrins remain open questions. It is also not known if these two types of axons follow common signals to reach the gut and then different signals to reach their respective innervation targets.
Chemorepellent molecules are known to affect the initial trajectories of DRG axons, preventing them from entering “non-target” tissues (Masuda and Shiga, 2005). Whether these molecules are involved in targeting vagal innervation within the GI tract have not yet been investigated. Netrin-1 is involved in preventing DRG axons from prematurely extending collaterals into the dorsal spinal cord (Watanabe et al., 2006). In this situation, netrin-1 is an inhibitory cue and growing DRG axons express UNC5A and UNC5C netrin receptors (but not UNC5B). While DCC mediates attraction in response to netrins (Keino-Masu et al., 1996; Stein and Tessier-Lavigne, 2001), the effect of netrins is converted to repulsion when DCC is co-expressed with UNC5 proteins (Hong et al., 1999); furthermore, if UNC5A is internalized, allowing plasmalemmal DCC to operate independently of UNC5, then netrin-mediated repulsion is lost and the response is converted to attraction (Bartoe et al., 2006). These findings suggest that the UNC5 family of receptors may also be involved in guiding extrinsic nerves to and/or within the gut. If DRG axons supplying the gut expressed only DCC, then they would probably be attracted to enteric cells that secrete netrins, as are nodose axons. In contrast, these same netrin-secreting cells would repel DRG axons if the axons also expressed UNC5 receptors in addition to DCC. It is not yet known whether DCC, receptors of the UNC5 family or both occur in thoracic and/or lumbosacral DRG neurons that innervate the bowel. Sensory neurons express Robo1 and Robo2 and DRG express Slit1 and Slit2 (Brose et al., 1999). Absence of Slit/Robo signaling in vivo results in an abnormal distribution of DRG axons in the spinal cord (Ma and Tessier-Lavigne, 2007). Since fetal chick and mouse bowel express mRNA transcripts that encode Slit proteins (De Bellard et al., 2003; Ratcliffe et al., 2005), it is conceivable that Slit/Robo could be involved in the establishment of the spinal afferent innervation of the GI tract.
5. Relationship between extrinsic sensory nerves and the enteric nervous system
The normal function of the gut requires both components of innervation, the intrinsic and extrinsic, to be intact and working in coordination. For example, both vagal and spinal afferents have endings in the myenteric plexus (Peeters et al., 2006). How the connections between the extrinsic and intrinsic nervous systems form, and how the development of each affects the other, is unknown. The enteric ganglia are derived from cells that have migrated from the neural crest during fetal life (Le Douarin and Kalcheim, 1999). Axons growing out from the CNS frequently follow the pathways that were previously traveled by crest-derived cells and by often responding to similar environmental cues (Rickmann et al., 1985). Studies that have demonstrated that axon guidance molecules play important roles in routing crest-derived cells to their correct destinations in the bowel (De Bellard et al., 2003; Jiang et al., 2003), therefore, not only support the idea that extrinsic nerves follow similar cues as the migrating crest-derived cells, but raise the possibility that the crest-derived cells themselves might produce some of the guiding cues. Vagal sensory axons reach the stomach and small bowel later in gestation than crest-derived cells in mice (Baetge and Gershon, 1989; Ratcliffe et al., 2006) and, of interest, the developmental pattern of the vagal innervation in zebrafish also seems to parallel that of the enteric innervation (Olsson et al., 2008). It is possible that because enteric neurons synthesize netrins, netrin-secreting neurons could help guide vagal axons to their appropriate innervation targets within the gut wall (Ratcliffe et al., 2007).
6. Clinical implications
Functional gastrointestinal disorders (FGID) are common in both children and adults, causing discomfort and disability (Drossman, 2006; Rasquin et al., 2006). Many FGID symptoms, including bloating, diarrhea, constipation, nausea, vomiting and pain, are consistent with dysfunction in sensation and/or motility in the GI tract (Kellow et al., 2006). Specifically, visceral hypersensitivity and vagal dysfunction are documented features of irritable bowel syndrome (IBS) (Price et al., 2006; Spaziani et al., 2008; Wilder-Smith and Robert-Yap, 2007). Although IBS has generally been considered an adult problem, it is now clear that IBS has developmental roots (Chitkara et al., 2008; Khan et al., 2007) and it thus follows that if IBS can be present early in life, then in some patients, the underlying defect could have occurred in the fetal or early post-natal period. The biopsychosocial model of FGID supports the concept that early life events, such as genetic and environmental factors, play an important role in increasing susceptibility to gut dysfunction, including the development of visceral hypersensitivity (Fig. 5). Further exploration into the molecular mechanisms by which vagal and spinal afferents innervate the fetal gut and reach specific enteric locations may represent novel avenues in which to understand and characterize FGIDs as well as other potential congenital disorders of GI motility.
Fig. 5.

Diagram illustrating the biopsychosocial theory of the pathogenesis of FGID. Physiological, psychosocial and early life factors are interrelated and can influence the clinical expression and outcome of disordered GI motility and sensation. Adapted from Drossman (Drossman, 2006).
Acknowledgments
Supported by the Canadian Association of Gastroenterology (CAG)/Canadian Institutes of Health Research (CIHR)/AstraZeneca Operating Grant and by the McMaster Children’s Hospital.
References
- Astic L, Pellier-Monnin V, Saucier D, Charrier C, Mehlen P. Expression of netrin-1 and netrin-1 receptor, DCC, in the rat olfactory nerve pathway during development and axonal regeneration. Neuroscience. 2002;109:643–656. doi: 10.1016/s0306-4522(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Dev Biol. 1989;132:189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- Bartoe JL, McKenna WL, Quan TK, Stafford BK, Moore JA, Xia J, Takamiya K, Huganir RL, Hinck L. Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J Neurosci. 2006;26:3192–3205. doi: 10.1523/JNEUROSCI.3469-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptor and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Chitkara DK, Talley NJ, Schleck C, Zinsmeister AR, Shah ND, Locke GR., III Recollection of childhood abdominal pain in adults with functional gastrointestinal disorders. Scand J Gastroenterol. 2008:1–7. doi: 10.1080/00365520802555975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The axonal chemoattractant netrin-1 is also a chemorepellent for trochlear motor axons. Cell. 1995;81:621–629. doi: 10.1016/0092-8674(95)90083-7. [DOI] [PubMed] [Google Scholar]
- Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- De Bellard ME, Rao Y, Bronner-Fraser M. Dual function of Slit2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells. J Cell Biol. 2003;162:269–279. doi: 10.1083/jcb.200301041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Fox EA, Murphy MC. Factors regulating vagal sensory development: potential role in obesities of developmental origin. Physiol Behav. 2008;94:90–104. doi: 10.1016/j.physbeh.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Clerc N. Nutrient tasting and signaling mechanisms in the gut. II The intestine as a sensory organ: neural, endocrine, and immune responses. Am J Physiol. 1999;277:G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol. 2005;39:S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Ratcliffe EM. Developmental biology of the enteric nervous system: pathogenesis of Hirschsprung’s disease and other congenital dysmotilities. Semin Pediatr Surg. 2004;13:224–235. doi: 10.1053/j.sempedsurg.2004.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne MES. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Höpker V, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu M, Gershon MD. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SSY, Culotti JG, Tessier-Lavigne M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Kellow JE, Azpiroz F, Delvaux M, Gebhart GF, Mertz HR, Quigley EM, Smout AJ. Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology. 2006;130:1412–1420. doi: 10.1053/j.gastro.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Khan S, Campo J, Bridge JA, Chiappetta LC, Wald A, di Lorenzo C. Long-term outcome of functional childhood constipation. Dig Dis Sci. 2007;52:64–69. doi: 10.1007/s10620-006-9308-9. [DOI] [PubMed] [Google Scholar]
- Langley JN. The Autonomic Nervous System, Part 1. W. Heffer; Cambridge: 1921. [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. 2. Cambridge University Press; Cambridge, U.K: 1999. [Google Scholar]
- Liu X, Jaenisch R. Severe peripheral sensory neuron loss and modest motor neuron reduction in mice with combined deficiency of brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5. Dev Dyn. 2000;218:94–101. doi: 10.1002/(SICI)1097-0177(200005)218:1<94::AID-DVDY8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Shiga T. Chemorepulsion and cell adhesion molecules in patterning initial trajectories of sensory axons. Neurosci Res. 2005;51:337–347. doi: 10.1016/j.neures.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Murphy MC, Fox EA. Anterograde tracing method using DiI to label vagal innervation of the embryonic and early postnatal mouse gastrointestinal tract. J Neurosci Meth. 2007;163:213–225. doi: 10.1016/j.jneumeth.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MC, Fox EA. Mice deficient in brain-derived neurotrophic factor have altered development of gastric vagal sensory innervation. J Comp Neurol. 2010;518:2934–2951. doi: 10.1002/cne.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neurol. 2008;508:756–770. doi: 10.1002/cne.21705. [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Aerssens J, de Hoogt R, Stanisz A, Gohlmann HW, Hillsley K, Meulemans A, Grundy D, Stead RH, Coulie B. Molecular profiling of murine sensory neurons in the nodose and dorsal root ganglia labeled from the peritoneal cavity. Physiol Genomics. 2006;24:252–263. doi: 10.1152/physiolgenomics.00169.2005. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Powley TL, Phillips RJ. Musings on the wanderer: what’s new in our understanding of vago-vagal refexes? I Morphology and topography of vagal afferents innervating the GI tract. Am J Physiol. 2002;283:G1217–G1225. doi: 10.1152/ajpgi.00249.2002. [DOI] [PubMed] [Google Scholar]
- Price DD, Zhou Q, Moshiree B, Robinson ME, Verne GN. Peripheral and central contributions to hyperalgesia in irritable bowel syndrome. J Pain. 2006;7:529–535. doi: 10.1016/j.jpain.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe EM, Chen JJ, Gershon MD. Chemorepulsion in the guidance of vagal sensory axons that innervate the fetal gut: expression of Slit-1 in the gut and its receptor, Robo-1 in nodose ganglia. Gastroenterology. 2005;128:A-6. [Google Scholar]
- Ratcliffe EM, Setru SU, Chen JJ, Li ZS, D’Autreaux F, Gershon MD. Netrin/DCC-mediated attraction of vagal sensory axons to the fetal mouse gut. J Comp Neurol. 2006;498:567–580. doi: 10.1002/cne.21027. [DOI] [PubMed] [Google Scholar]
- Ratcliffe EM, Anderson M, Chalazonitis A, Gershon MD. Netrin biosynthesis by enteric neurons: roles in normal guidance of vagal sensory axons and their mislocation in aganglionic gut. Gastroenterology. 2007;132:A-81. [Google Scholar]
- Ratcliffe EM, D’Autreaux F, Gershon MD. Laminin terminates the Netrin/DCC mediated attraction of vagal sensory axons. Dev Neurobiol. 2008;68:960–971. doi: 10.1002/dneu.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickmann M, Fawcett JW, Keynes RJ. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J Embryol Exp Morphol. 1985;90:437–455. [PubMed] [Google Scholar]
- Rinaman L, Levitt P. Establishment of vagal sensorimotor circuits during fetal development in rats. J Neurobiol. 1993;24:641–659. doi: 10.1002/neu.480240509. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. The origin and development of the vagal and spinal innervation of the external muscle of the mouse esophagus. Brain Res. 1998;809:253–268. doi: 10.1016/s0006-8993(98)00893-2. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P, Lefebvre O, Bellissent-Waydelich A, Olsen J, Orian-Rousseau V, De Arcangelis A. The laminins: role in intestinal morphogenesis and differentiation. Ann NY Acad Sci. 1998;859:46–64. doi: 10.1111/j.1749-6632.1998.tb11110.x. [DOI] [PubMed] [Google Scholar]
- Spaziani R, Bayati A, Redmond K, Bajaj H, Mazzadi S, Bienenstock J, Collins SM, Kamath MV. Vagal dysfunction in irritable bowel syndrome assessed by rectal distension and baroreceptor sensitivity. Neurogastroenterol Motil. 2008;20:336–342. doi: 10.1111/j.1365-2982.2007.01042.x. [DOI] [PubMed] [Google Scholar]
- Stein E, Tessier-Lavigne M. Hierarchical organization of guidance receptors: silencing of netrin attraction by slit through a Robo/DCC receptor complex. Science. 2001;291:1928–1938. doi: 10.1126/science.1058445. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Tamamaki N, Furuta T, Ackerman SL, Ikenaka K, Ono K. Dorsally derived netrin 1 provides an inhibitory cue and elaborates the ‘waiting period’ for primary sensory axons in the developing spinal cord. Development (Cambridge, England) 2006;133:1379–1387. doi: 10.1242/dev.02312. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:3699–3704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Anderson RB, Anderson CR. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton Neurosci. 2004;112:1–14. doi: 10.1016/j.autneu.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ, Spencer NJ. Structure-function relationship of sensory endings in the gut and bladder. Auton Neurosci. 2010;153:3–11. doi: 10.1016/j.autneu.2009.07.018. [DOI] [PubMed] [Google Scholar]