Abstract
IMPORTANCE
Increased glutamate levels in the right associative striatum have been described in patients during a first episode of psychosis. Whether this increase would persist after effective antipsychotic treatment is unknown.
OBJECTIVES
To compare the glutamate levels in antipsychotic-naive patients with first-episode psychosis in the right associative striatum and right cerebellar cortex using proton magnetic resonance spectroscopy before and 4 weeks after antipsychotic treatment and to compare these results with normative data from sex-matched healthy control subjects.
DESIGN, SETTING, AND PARTICIPANTS
Before-after trial in an inpatient psychiatric research unit among 24 antipsychotic-naive patients with first-episode psychosis and 18 healthy controls matched for age, sex, handedness, and cigarette smoking.
INTERVENTIONS
Participants underwent 2 proton magnetic resonance spectroscopy studies: patients were imaged at baseline and after 4 weeks of antipsychotic treatment, while controls were imaged at baseline and at 4 weeks after the baseline measurement. Patients were treated with oral risperidone (open label) for 4 weeks with dosages that were titrated on the basis of clinical judgment.
MAIN OUTCOMES AND MEASURES
Glutamate levels were estimated using LCModel (version 6.2-1T) and were corrected for the cerebrospinal fluid proportion within the voxel.
RESULTS
Patients with first-episode psychosis had higher levels of glutamate in the associative striatum and the cerebellum during the antipsychotic-naive condition compared with controls. After clinically effective antipsychotic treatment, glutamate levels significantly decreased in the associative striatum, with no significant change in the cerebellum. No differences in glutamate levels were observed between groups at 4 weeks.
CONCLUSIONS AND RELEVANCE
Increased glutamate levels observed at baseline in patients with first-episode psychosis normalized after 4 weeks of clinically effective antipsychotic treatment. These results provide support for the hypothesis that improvement in clinical symptoms might be related to a decrease in glutamate levels.
The current hypothesis for the origin of schizophrenia proposes that the disorder stems from neurodevelopmental deficits that result in a disturbance of glutamatergic neurotransmission, especially for N-methyl-D-aspartate receptor–mediated signaling.1 The deteriorating course of the disease may be partially explained by cortical neuronal toxic effects secondary to enhanced glutamatergic exposure,2 and dopaminergic dysregulation may be the final common pathway that results from altered glutamatergic neurotransmission.3
Neuroimaging studies4–8 using proton magnetic resonance spectroscopy (1H-MRS) have shown increased levels of the metabolites glutamate, glutamine, glutamate + glutamine (Glx), and glutamine/glutamate in patients with first-episode psychosis (FEP) who are antipsychotic-naive or who have been minimally exposed to antipsychotic medication. On the other hand, studies9–12 in medicated patients have demonstrated the same levels or decreased levels of these metabolites compared with control subjects.
Recently, our group reported a cross-sectional study13 in antipsychotic-naive patients with FEP that showed increased glutamate levels in the right associative striatum (ie, precommissural dorsal caudate), a region characterized by a high density of dopamine receptors and dopamine afferents. Conversely, glutamate levels were similar in patients with FEP and controls in the cerebellum, a brain region with a negligible quantity of dopamine receptors and an absence of dopamine afferents, suggesting that the glutamate level anomalies may be restricted to dopamine-rich regions of the brain.
The objectives of this study were to compare the striatal and cerebellar glutamate levels in the right associative striatum and the right cerebellar cortex in antipsychotic-naive patients with FEP before and 4 weeks after antipsychotic treatment and to compare these results with normative data from age- and sex-matched healthy control subjects.
Methods
Participants
The study was approved by the ethics and scientific committees of the Instituto Nacional de Neurología y Neurocirugía. Participants were included in the study after successful completion of an informed consent procedure in which written consent was obtained from both parents for individuals younger than 18 years.
Twenty-eight patients were recruited from the Emergency Department, the Neuropsychiatry Department, and the Adolescent Program of Neuropsychiatric and Imaging Study at the Instituto Nacional de Neurología y Neurocirugía during their first nonaffective psychosis episode. For inclusion in the study, individuals were assessed using the Structured Clinical Interview for DSM-IV. Patients were deemed eligible if they were antipsychotic naive and had less than 2 years of psychotic symptoms. Exclusion criteria included high risk for suicide, psychomotor agitation, comorbidity with other Axis I disorders, concomitant medical or neurological illness, and current substance abuse or a history of substance dependence (excluding nicotine). Twenty-four of 28 patients initially recruited completed the study. Two patients did not respond to treatment, the third patient demonstrated excessive movement during the baseline 1H-MRS, and the fourth patient withdrew consent. Only patients who responded to treatment were scheduled for follow-up 1H-MRS.
Eighteen right-handed, age- and sex-matched healthy control subjects were also recruited. The controls were assessed in the same manner as the patients, and any individual with a history of psychiatric illness or a family history of schizophrenia was excluded. All participants (patients and controls) were screened for drugs of abuse (cannabis, cocaine, heroin, opioids, and benzodiazepines) at the time of study inclusion and 1 hour before 1H-MRS.
After baseline 1H-MRS, patients began treatment with oral risperidone (open label) that was titrated over 4 weeks using a flexible dosing schedule starting at 2 mg/d up to a maximum of 5 mg/d. The risperidone dosage was titrated according to clinical judgment and treatment response. Treatment response was defined clinically as a reduction of at least 30% on the total score of the Positive and Negative Syndrome Scale (PANSS) after 4 weeks of treatment. Patients were hospitalized at the Neuropsychiatry Department of the Instituto Na-cional de Neurología y Neurocirugía for the duration of the study. The use of other concomitant medications (eg, benzo-diazepines, mood stabilizers, and antidepressants) was not allowed for the duration of the study. Participants underwent two 1H-MRS studies: patients were imaged at baseline (anti-psychotic free) and after 4 weeks of antipsychotic treatment, and controls were imaged at baseline and at 4 weeks after the baseline measurement.
Magnetic Resonance Studies
Proton magnetic resonance spectroscopy was performed in a 3-T whole-body imaging system (Signa Excite HDxt; GE Health-care) with a high-resolution 8-channel head coil at the Neuroimaging Department of the Instituto Nacional de Neurología y Neurocirugía. The participant’s head was positioned along the canthomeatal line and immobilized by a forehead strap. Participants were initially imaged with a T1-weighted spoiled gradient-echo 3-dimensional axial acquisition (SPGR) oriented above and parallel to the anterior commissure–posterior commissure line (echo time, 5.7 milliseconds; repetition time, 13.4 milliseconds; inversion time, 450 milliseconds; flip angle, 20°; field of view, 25.6 cm; 256 × 256–pixel matrix; and section thickness, 1.2 mm). These T1-weighted SPGR images were reformatted to sagittal and coronal views and were used for optimal 1H-MRS voxel placement.
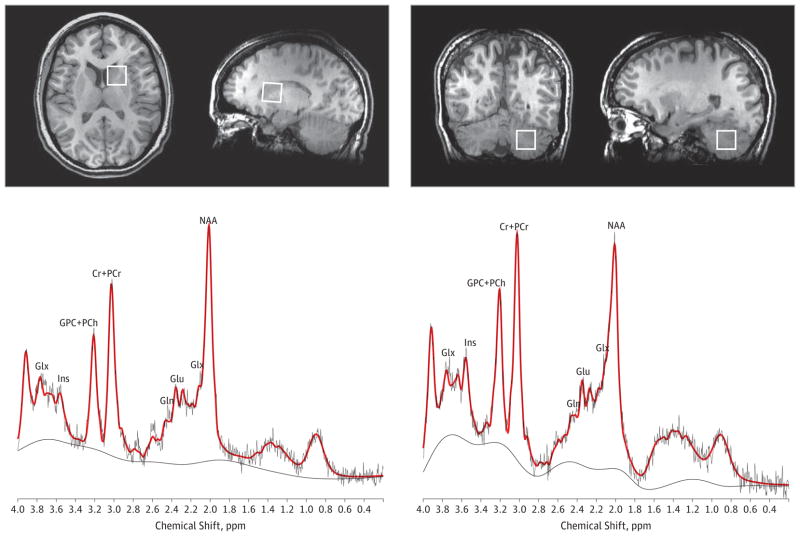
The 1H-MRS spectra were obtained using point-resolved spectroscopy centered on the right dorsal caudate nucleus and on the right cerebellar cortex (echo time, 35 milliseconds; repetition time, 2000 milliseconds; spectral width, 5000 Hz; 4096 data points used; and 128 water-suppressed averages and 16 water-unsuppressed averages) in volume elements (voxels) of 8 mL (2 × 2 × 2 cm). The lower end of the dorsal caudate voxel (ie, associative striatum) was located 3 mm dorsal to the anterior commissure to include the maximum amount of gray matter (GM) and with a dorsal extension (thickness) of 2 cm. The cerebellar voxel was located in the cerebellar cortex below the inferior cerebellar peduncle avoiding the midline (Figure 1). During acquisition, 1H-MRS spectra were shimmed to achieve full-width at half maximum (FWHM) of 12 Hz or less (measured on the unsuppressed water signal from the voxel). Spectra with larger FWHM were excluded from further analyses.
Figure 1. Voxel Placement in 2 Regions of Interest: the Right Associative Striatum and the Right Cerebellar Cortex.
Representative spectra with the raw data (in black) and fitted data (in red) are shown for each region. Cr + PCr indicates creatine-containing compounds; Gln, glutamine; Glu, glutamate; Glx, glutamate + glutamine; GPC + PCh, choline-containing compounds; Ins, myo-inositol; and NAA, N-acetylaspartate.
1H-MRS Data Analysis
Water-suppressed spectra were analyzed using LCModel (version 6.2-1T).14 Spectra were normalized to the unsuppressed water signal, yielding quantification of glutamate, creatine (Cr), phosphocreatine, myo-inositol (Ins), phosphocholine (PCh), N-acetylaspartate (NAA), and glycerophosphocholine (GPC). Spectroscopic values are expressed in institutional units.
The analysis used a standard basis set of metabolites, including Cr, Ins, NAA, PCh, GPC, taurine, L-lactate, aspartate, L-alanine, glucose, glutamate, glutamine, glutathione, scyllo-inositol, phosphocreatine, guanidinoacetate, γ-aminobutyric acid, Cr methylene group, and N-acetylaspartylgluta-mate acid, as well as lipids (Lip) and macromolecules (MMs) (Lip09, Lip13a, Lip13b, Lip20, MM09, MM12, MM14, MM17, and MM20). This basis set, which was included in LCModel, was acquired with the same sequence parameters used in our study. All metabolites with a Cramer-Rao lower bound (CRLB) exceeding 20% as reported by LCModel were considered poor quality and were excluded from further analyses.15
The SPGR images used for localization of the voxels were subsequently segmented into GM, white matter, and cerebro-spinal fluid using a computer program (Statistical Parametric Mapping 8; Wellcome Department of Imaging Neurosciences, University College London). To calculate the percentages of GM, white matter, and cerebrospinal fluid content within a voxel, the size and location of each area were extracted from the spectra file headers using in-house software that allowed for the correction of the cerebrospinal fluid fraction of the spectroscopic values.13
For the images at 4 weeks, the same acquisition parameters were used. The images from the baseline image session, corresponding to the axial, sagittal, and coronal projections of the spectroscopic voxels, were saved, including the location of the 1H-MRS voxels. These baseline images and the sulcal-gyral pattern assisted with relocalization of the 1H-MRS voxels in the images at 4 weeks.16
Statistical Analysis
The results are presented as means (SDs). Demographic and clinical characteristics and 1H-MRS metabolites were compared between FEP and control groups. Analyses of variance with repeated measures (2 [diagnostic groups] × 2 [regions] × 2 [times]) were conducted to investigate glutamate levels, the metabolite of interest. Independent-sample t tests and paired-sample t tests were used for comparisons within groups for the rest of the metabolites. Frequency data were analyzed using χ2 test or Fisher exact test. The percentages of GM in the 1H-MRS voxels of the associative striatum and cerebellum were included as covariates. The statistical comparisons were performed with a significance level set at P < .05.
Partial Spearman rank correlations controlling for the effect of GM were performed to examine the relationship between clinical scale scores and glutamate levels for each region. The statistical threshold was established at P ≤ .016 (P = 0.5 ÷ 3 to control for multiple comparisons) to control for multiple comparisons for the PANSS subscales in the FEP group.
Outlier participants were detected using Cook distance criteria.17 Outliers were defined based on the absolute change in glutamate levels between baseline and 4 weeks. This method identified 2 outliers in the FEP group. The results are presented with inclusion and exclusion of the outliers.
Test-retest reliability for the control group was calculated as follows: (glutamate 1 level minus glutamate 2 level) divided by (glutamate 1 level plus glutamate 2 level divided by 2) times 100 for absolute (unsigned value) percentage difference and (glutamate 1 level minus glutamate 2 level) divided by glutamate 2 level times 100 for absolute percentage error. Coefficient of variation was defined as follows: the SD of glutamate 1 level and glutamate 2 level divided by the mean of glutamate 1 level and glutamate 2 level. The values for the percentage difference, percentage error, and coefficient of variation are presented as an individual’s mean (SD). Intra-class correlation coefficients were estimated using a 2-way random-effects model (average measure).
Results
Demographic and Clinical Characteristics
The DSM-IV diagnoses of the patients with FEP included in the study were as follows: schizophrenia (n = 9), brief psychotic disorder (n = 8), and schizophreniform disorder (n = 7). The educational level was higher in the control group compared with the FEP group (t40 = 4.57, P < .001). The FEP group had a mean (SD) duration of untreated psychosis of 20.33 (25.84) weeks (range, 1–104 weeks). The FEP and control groups were similar in age, sex, handedness, and cannabis or tobacco use (Table 1). The mean (SD) PANSS total scores for the FEP group were 94.5 (13.6) at baseline and 58.7 (9.6) after 4 weeks of antipsychotic treatment (t23 = 12.35, P < .001; mean [SD] decrease, 39% [9%]). The mean (SD) daily dose of risperidone used during the study was 3.42 (1.14) mg.
Table 1.
Demographic and Clinical Characteristics of the Study Participants at Baseline
| Variable | FEP Group | Control Group |
|---|---|---|
| Age, mean (SD) [range], y | 26.58 (8.49) [18–47] | 24.56 (5.07) [18–35] |
| Educational level, mean (SD), y | 10.96 (3.44) | 15.61 (2.99)a |
| Sex, No. | ||
| Male | 13 | 8 |
| Female | 11 | 10 |
| Handedness, No. | ||
| Right | 24 | 18 |
| Left | 0 | 0 |
| Ever used, No./total No. | ||
| Tobacco | 8/24 | 2/18 |
| Cannabis | 3/24 | 0/18 |
| Duration of untreated bxpsychosis, mean (SD), wk | 20.33 (25.84) | NA |
| PANSS subscale score | ||
| Positive | 23.33 (4.99) | NA |
| Negative | 24.08 (6.03) | NA |
| General psychopathology | 47.92 (8.75) | NA |
Abbreviations: FEP, first-episode psychosis; NA, not applicable; PANSS, Positive and Negative Syndrome Scale.
P < .05.
Glutamate Levels
Analyses were performed with inclusion of the full sample (24 patients and 18 controls) and with exclusion of the 2 outliers as identified by Cook distance criteria. The time × group × region (multivariate) interaction was not significant within the full sample (F = 2.45, P = .10), but this interaction was significant after exclusion of the 2 outliers (F = 3.41, P = .04). Univariate analysis showed that the time × group interaction was significant for the associative striatum but not for the cerebellum within the entire sample (F = 4.73, P = .04 vs F = 0.22, P = .64) and after exclusion of the 2 outliers (F = 6.83, P = .01 vs F = 1.04, P = .31).
Post hoc pairwise comparisons revealed that glutamate levels in the associative striatum were higher in patients compared with controls (t40 = 2.61, P = .01). This difference was maintained after exclusion of the 2 outliers (t38 = 2.89, P = .008). The FEP group had a decrease in their glutamate levels in the associative striatum after 4 weeks of antipsychotic treatment (t23 = 2.21, P = .04). This finding remained significant after exclusion of the 2 outliers (t21 = 3.00, P = .007). After treatment, patients did not differ from controls at 4 weeks (t40 = 1.36, P = .18). This finding remained nonsignificant after exclusion of the 2 outliers (t38 = 1.15, P = .25) (Figure 2).
Figure 2. Glutamate Levels in the Right Associative Striatum of Patients With First-Episode Psychosis (FEP) and Healthy Control Subjects at Baseline and at 4 Weeks.
Horizontal bars represent the mean for the groups. *P < .05 after the removal of 2 outlying participants indicated within the circles.
Pairwise analysis also revealed differences in glutamate levels in the cerebellum between patients and controls at base-line(t39 = 2.05, P = .047)and no difference at 4 weeks (t40 = 0.89, P = .37). After 4 weeks of antipsychotic treatment, patients (t22 = 1.00, P = .32) and controls (t17 = 1.70, P = .11) showed no significant glutamate level changes in the cerebellum.
Test-retest mean (SD) reliability for glutamate levels between baseline and 4 weeks in 18 controls showed an absolute percentage difference of 5.35% (4.77%), an absolute percentage error of 5.32% (4.75%), and a coefficient of variation of 0.038 (0.034) for the associative striatum, as well as an absolute percentage difference of 13.72% (10.64%), an absolute percentage error of 14.66% (11.83%), and a coefficient of variation of 0.097 (0.075) for the cerebellum. The intraclass correlation coefficients were 0.857 (95% CI, 0.619–0.947, df = 17, P < .001) for the associative striatum and 0.637 (95% CI, 0.031–0.864, df = 17, P = .02) for the cerebellum. Test-retest reliability measures for all the metabolites are given in the eTable in the Supplement.
Other Metabolites
At baseline, patients showed higher Glx levels in the associative striatum compared with controls (t40 = 2.03, P = .048). After 4 weeks of antipsychotic treatment, patients demonstrated no difference in Glx levels in the associative striatum compared with controls (t40 = 1.27, P = .14). The Glx levels in the cerebellum did not differ between patients and controls at baseline (t39 = 0.14, P = .88) or at 4 weeks (t39 = 0.38, P = .28). Glutamine was not analyzed because of poor spectra fitting in both regions. The NAA levels in the associative striatum showed a trend-level difference between patients and controls at baseline (t40 = 1.97, P = .06) and no difference at 4 weeks (t40 = 0.80, P = .49). Higher NAA levels in patients vs controls were shown in the cerebellum at baseline (t40 = 2.24, P = .03), as well as a trend after antipsychotic treatment (t40 = 1.74, P = .08).
The GPC + PCh levels in the associative striatum were higher in the FEP group compared with the control group at baseline (t40 = 3.33, P = .002) and after antipsychotic treatment (t40 = 2.64, P = .01). Trend-level elevations of GPC + PCh levels in the cerebellum were observed for the FEP group compared with the control group at baseline (t40 = 2.01, P = .05) and after 4 weeks (t40 = 1.76, P = .09). Higher Ins levels were also found in the associative striatum of patients compared with controls at baseline (t40 = 3.85, P < .001) and after antipsychotic treatment (t40 = 2.88, P = .006). No differences in Ins levels were found in the cerebellum at baseline (t40 = 0.63, P = .52) or after 4 weeks of antipsychotic treatment (t40 = 0.40, P = .68). No differences were observed in Cr + phosphocreatine levels between the FEP and control groups in any region or under any conditions.
A comparison between images at baseline and 4 weeks showed no difference in the control group and demonstrated trends in the associative striatum for Glx levels (t23 = 1.77, P = .09) and Ins levels (t23 = 1.85, P = .08) in the FEP group. These results are summarized in Table 2.
Table 2.
Each Metabolite in 2 Regions of Interest at Baseline and at 4 Weeks in the FEP Group and the Control Group
| Variable | Mean (SD)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Baseline
|
At 4 wk
|
|||||||||||
| Glu | Glx | NAA | GPC + PCh | Ins | Cr + PCr | Glu | Glx | NAA | GPC + PCh | Ins | Cr + PCr | |
| Right associative striatum | ||||||||||||
|
| ||||||||||||
| FEP group | 31.34 (4.29)a | 34.93 (5.53)a | 24.20 (3.16) | 5.13 (0.70)a | 11.20 (1.73)a | 18.49 (2.21) | 29.25 (3.08) | 33.03 (3.41) | 23.33 (2.97) | 5.05 (0.59)a | 10.45 (1.39)a | 17.95 (1.86) |
|
| ||||||||||||
| Control group | 28.23 (3.03) | 31.94 (3.25) | 22.43 (2.46) | 4.51 (0.37) | 9.32 (1.31) | 17.73 (1.16) | 27.97 (2.85) | 31.29 (4.22) | 22.68 (3.22) | 4.55 (0.59) | 9.16 (1.48) | 17.47 (1.72) |
|
| ||||||||||||
| Right cerebellar cortex | ||||||||||||
|
| ||||||||||||
| FEP group | 24.46 (3.57)a,b | 26.63 (4.48)b | 19.49 (3.05)a | 5.41 (0.95) | 14.38 (2.68) | 19.54 (2.73) | 25.19 (4.73) | 28.98 (5.68)a | 19.96 (4.24)b | 5.58 (1.14) | 15.07 (3.54) | 20.32 (3.89) |
|
| ||||||||||||
| Control group | 22.24 (3.24) | 26.42 (4.51) | 17.13 (3.78) | 4.75 (1.13) | 13.84 (2.71) | 19.05 (3.78) | 23.88 (4.58) | 27.20 (4.55) | 17.70 (4.02) | 4.93 (1.20) | 14.64 (3.10) | 19.95 (3.97) |
Abbreviations: Cr + PCr, creatine-containing compounds; FEP, first-episode psychosis; Glu, glutamate; Glx, glutamate + glutamine; GPC + PCh, choline-containing compounds; Ins, myo-inositol; NAA, N-acetylaspartate.
P < .05.
One spectrum was rejected because of a Cramer-Rao lower bound exceeding 20%.
Relationships With Clinical Measures
Exploratory partial correlations between changes in metabolites and changes in clinical measures (PANSS total score and positive, negative, and general psychopathology subscales) were performed with inclusion of the proportion of GM as a covariate. This exploratory analysis revealed significant correlations (P < .016) between glutamate level (r = −0.531, df = 19, P = .013) and Glx level (r = −0.585, df = 19, P = .005) changes in the associative striatum and the score change on the PANSS general psychopathology subscale. In addition, a correlation trend was found between glutamate level in the associative striatum at 4 weeks and the PANSS positive subscale score at 4 weeks (r = 0.451, df = 19, P = .04).
CRLB, FWHM, and Signal-to-Noise Ratios
The CRLB values for each metabolite in the associative striatum and the cerebellum are given in Table 3. Controls had a higher Ins CRLB compared with patients in the associative striatum at baseline (t40 = 1.28, P = .02) but not after 4 weeks (t40 = 0.22, P = .82). No differences in CRLBs were observed in the associative striatum for other metabolites at baseline or after 4 weeks. No differences in CRLBs were observed in the cerebellum for any condition. The FWHM values and signal-to-noise ratios are given in Table 4. Patients had lower FWHM than controls in the caudate at baseline (t40 = 2.24, P = .03). Patients also had lower signal-to-noise ratios compared with controls in the cerebellum at 4 weeks (t40 = 2.14, P = .04).
Table 3.
Cramer-Rao Lower Bound for Each Metabolite in 2 Regions of Interest at Baseline and at 4 Weeks in the FEP Group and the Control Group
| Variable | Mean (SD)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| At Baseline
|
At 4 wk
|
|||||||||||
| Glu | Glx | NAA | GPC + PCh | Ins | Cr + PCr | Glu | Glx | NAA | GPC + PCh | Ins | Cr + PCr | |
| Right associative striatum | ||||||||||||
|
| ||||||||||||
| FEP group | 6.67 (0.92) | 8.38 (1.28) | 3.38 (1.01) | 3.58 (0.72) | 8.33 (1.34) | 3.00 (0.51) | 7.17 (2.33) | 8.79 (2.00) | 4.08 (1.25) | 4.00 (0.93) | 9.58 (2.52) | 3.29 (0.95) |
|
| ||||||||||||
| Control group | 6.83 (1.04) | 8.33 (1.64) | 4.22 (1.86) | 4.00 (0.90) | 9.61 (2.06)a | 3.28 (0.57) | 6.56 (0.86) | 8.17 (1.76) | 3.33 (1.19) | 3.72 (0.46) | 9.78 (2.98) | 2.94 (0.42) |
|
| ||||||||||||
| Right cerebellar cortex | ||||||||||||
|
| ||||||||||||
| FEP group | 7.00 (1.13)b | 8.58 (1.81)b | 4.88 (1.30) | 3.42 (1.25) | 6.38 (2.31) | 2.58 (1.32) | 7.42 (1.71) | 8.88 (1.96)b | 4.88 (1.03) | 3.33 (0.56) | 6.29 (1.23) | 2.58 (0.72) |
|
| ||||||||||||
| Control group | 7.61 (1.50) | 9.39 (1.85) | 4.72 (1.32) | 3.44 (0.51) | 6.39 (0.98) | 2.39 (0.50) | 7.11 (1.08) | 8.33 (1.32) | 4.39 (0.85) | 3.33 (0.48) | 5.89 (1.08) | 2.33 (0.48) |
Abbreviations: Cr + PCr, creatine-containing compounds; FEP, first-episode psychosis; Glu, glutamate; Glx, glutamate + glutamine; GPC + PCh, choline-containing compounds; Ins, myo-inositol; NAA, N-acetylaspartate.
P < .05.
One spectrum was rejected because of a Cramer-Rao lower bound exceeding 20%.
Table 4.
The FWHM and SNR in 2 Regions of Interest at Baseline and at 4 Weeks in the FEP Group and the Control Group
| Variable | Mean (SD) | P Value | |
|---|---|---|---|
| FEP Group | Control Group | ||
| Right associative striatum at baseline | |||
| FWHM, ppm | 0.07 (0.01) | 0.08 (0.01) | .03 |
| SNR | 15.46 (2.39) | 14.78 (2.88) | .41 |
| Right associative striatum at 4 wk | |||
| FWHM, ppm | 0.08 (0.01) | 0.08 (0.01) | .38 |
| SNR | 13.83 (4.38) | 15.78 (2.26) | .10 |
| Right cerebellar cortex at baseline | |||
| FWHM, ppm | 0.07 (0.01) | 0.07 (0.01) | .51 |
| SNR | 17.69 (2.60) | 17.00 (3.06) | .44 |
| Right cerebellar cortex at 4 wk | |||
| FWHM, ppm | 0.07 (0.01) | 0.06 (0.01) | .29 |
| SNR | 16.54 (3.20) | 18.55 (2.72) | .04 |
Abbreviations: FEP, first-episode psychosis; FWHM, full-width at half maximum; SNR, signal-to-noise ratio.
Effects of Tissue Heterogeneity
The percentages of GM in the associative striatum did not differ between patients and controls at baseline (t40 = 0.53, P = .59) and at 4 weeks (t40 = 1.59, P = .11). The percentages of GM in the cerebellum were lower in the FEP group compared with the control group at baseline (t40 = 5.56, P < .001) and at 4 weeks (t40 = 4.65, P < .001). In addition to these differences in the percentages of GM, the results of the metabolite comparisons did not change regardless of whether the percentages of GM were included as a covariate (data not shown).
Discussion
To our knowledge, this is the first study to report using a within-participant design that clinically effective antipsychotic treatment normalizes glutamate levels in antipsychotic-naive patients with FEP. In the present study, we found that patients with FEP had increased levels of glutamate in both of the studied regions, the associative striatum and the cerebellum. The results are in agreement with a recent study18 finding that patients who had FEP and were clinically stable had lower glutamate levels compared with patients who were still symptomatic. Moreover, patients who are antipsychotic naive or minimally exposed to antipsychotic medication4–8 or are experiencing psychotic-state exacerbations19,20 have shown elevations in glutamate compounds, while studies9–12 in medicated patients have demonstrated the same levels or decreased levels of these metabolites compared with control subjects, suggesting that an improvement in clinical symptoms might relate to decreases in glutamate levels. Moreover, a recent study21 from our group found that antipsychotic-naive individuals who are initially at ultrahigh risk for psychosis and who later transitioned to psychosis had higher glutamate levels in the associative striatum but not in the cerebellum compared with those who did not develop psychosis and compared with healthy controls.
The associative striatum, or cognitive striatum,22 a region thought to be involved in the pathogenesis of schizophrenia,23,24 includes the rostral and dorsal part of the caudate nuclei or precommissural dorsal section, also known as the neostriatum. This structure is rich in dopamine afferents and dopamine receptor D2 and is frequently included in the quantification of in vivo occupancy studies of antipsychotics.25,26 In addition, the associative striatum establishes major connections with the medial prefrontal cortex, which has been implicated in the neurocognitive deficits observed in schizophrenia27 and in individuals at ultrahigh risk for psychosis who progressed to a psychotic disorder.28
The associative striatum, especially the precommissural dorsal caudate, has shown the highest dopamine receptor D2 availability after acute pharmacologically induced dopamine depletion in antipsychotic-free patients with schizophrenia.24 In the same study, no differences in receptor availability were observed in the other functional subdivisions of the striatum (limbic and sensorimotor striatum), suggesting that schizophrenia is associated with elevated dopamine function in the associative regions of the striatum.
Although our main interest was to explore differences in glutamate levels associated with a dopamine-rich region, we included the cerebellar cortex for comparison because it has a negligible amount of dopamine receptors and has no dopamine afferents.29 On the other hand, the associative striatum and the cerebellar cortex have abundant glutamatergic cells and cortical afferents from the frontal cortex.30 In this sense, one of the differences between the associative striatum and the cerebellum is the dopaminergic afferents, which are restricted to the associative striatum. Although it is tempting to speculate that differences observed in glutamate levels in the associative striatum and not in the cerebellum may be related to dopaminergic tone, our study was not designed to address this question and would require direct quantification of dopamine neurotransmission.
Contrary to a previous report by our group,13 the present study found increased glutamate levels in the cerebellum during the baseline study but not after antipsychotic treatment. While the mean (SD) glutamate level in the associative striatum decreased from baseline to posttreatment (from 31.34 [4.29] to 29.25 [3.08] institutional units), the mean (SD) level in the cerebellum slightly increased (from 24.46 [3.57] to 25.19 [4.73] institutional units). The Glx levels in the cerebellum did not differ between patients with FEP and control subjects at baseline or at 4 weeks. Nevertheless, studies with larger samples are needed to confirm these results.
Preclinical investigations have shown that an elevation in striatal endogenous glutamate induces an increase in striatal dopamine release.31 The administration of the glutamate N-methyl-D-aspartate antagonist ketamine to human control subjects has been shown to enhance amphetamine-induced dopamine release32 and to decrease striatal 11C-raclopride binding, reflecting an increase in striatal synaptic dopamine even in the absence of an amphetamine challenge.33 Other studies34,35 in humans have documented the interaction between glutamate and dopamine. The conclusion of these studies is that glutamatergic antagonism is associated with an increase in dopamine release in the cerebral cortex and the striatum. In addition, N-methyl-D-aspartate receptor blockade in healthy humans has been shown to increase glutamine36 and glutamate37 levels in the anterior cingulate as measured by 1H-MRS.
Abnormalities in Metabolites Other Than Glutamate
In this study, we report a trend elevation in NAA levels in the associative striatum and higher NAA levels in the cerebellum of patients having FEP compared with control subjects at baseline. Although we replicated these findings from a previous study by our group,13 they are inconsistent with the results of a meta-analysis38 and of a study16 in patients with early schizophrenia. The reasons for these discrepant findings are unclear but may be related to spectroscopic acquisition, differences in patients’ clinical status, previous exposure to antipsychotic medication, and the use of ratios (NAA to Cr or PCh) instead of absolute metabolite levels. N-acetylaspartate, one of the prominent peaks shown consistently in 1H-MRS, is present almost exclusively in neurons and is thought to be a marker of neuronal functional integrity39 and of axonal mitochondrial metabolism.40 Higher NAA levels may be driven by increased axonal mitochondrial metabolism to maintain axonal conduction.41
Elevated levels of choline-containing compounds (represented collectively as GPC + PCh) have been interpreted as being supportive of the membrane hypothesis of schizophrenia,42 suggesting that phospholipid disturbances and increased myelin degradation support a generalized membrane disorder in patients with schizophrenia.43 Our results of elevated levels of GPC + PCh in the associative striatum of patients having FEP are in agreement with previous evidence in patients with schizophrenia.44 Glycerophosphocholine is one of the main products of membrane phospholipid breakdown. The increase in levels of GPC + PCh in the associative striatum observed in the FEP group suggests greater membrane turnover, which may result from changes in membrane mass or from proliferation of dendrites and synaptic connections in the associative striatum.45 We also found higher Ins levels in the associative striatum of patients with FEP at baseline and after treatment. These results are in agreement with previous findings in patients with FEP46 and in treated patients with schizophrenia.43 This increase in Ins levels could be related to astrocyte activation.47
Although we found no difference in the percentages of GM within the associative striatum voxel between patients having FEP and control subjects at baseline or at retest, we observed a decrease in the percentages of the cerebellar GM voxel in the FEP group compared with the control group at both imaging sessions. This finding is consistent with 2 studies13,48 in antipsychotic-naive patients having FEP.
Study Limitations
The limitations of this study need to be considered. First, we did not include cognitive evaluations. Therefore, we could not address the possible effect of cognition on glutamate levels. Second, we did not measure serum levels of risperidone in the FEP group to confirm treatment compliance; nevertheless, all patients studied were initially seen with a significant reduction of the PANSS total score as a primary measure of treatment efficacy, and patients were admitted to the hospital for supervised administration of the antipsychotic. Third, the groups could not be matched for education; educational levels were higher in the control group than in the FEP group. Parental education and socioeconomic status data were not obtained, but these would be better variables to use for matching given that the patients were young at their diagnosis of FEP and became ill before reaching their full academic potential. Fourth, direct measurement of glutamate neurotransmission is not possible with 1H-MRS. This technique measures both metabolic and vesicular glutamate. Fifth, Glx contamination of the NAA peak has been observed at short echo times, including the one used in this study (echo time, 35 milliseconds).49 Therefore, this potential contamination could be reflected by an artificial increase in NAA when glutamate is increased. Sixth, MMs were not measured, and they have a potential influence on Glx and glutamine quantification. Macromolecule contribution was modeled using the default LCModel function. Seventh, we only studied the right hemisphere for both regions to reduce imaging time in individuals with active psychosis. Nevertheless, during the course of a previous pilot study,50 our group studied both right and left associative striatum in patients with active psychotic symptoms without observing differences between the right and left sides in terms of glutamate quantification. Moreover, 1H-MRS studies that have assessed both hemispheres have failed to demonstrate distinct glutamatergic laterality in FEP,46 in individuals at high risk,51 in childhood-onset schizophrenia,52 and in patients with schizophrenia.11,53 However, we acknowledge that bilateral 1H-MRS examinations of the associative striatum are necessary to generalize these findings.
In conclusion, our results indicate initially increased glutamate levels in the associative striatum and in the cerebellum in antipsychotic-naive patients with FEP. After clinically effective antipsychotic treatment, glutamate levels significantly decreased in the associative striatum (Co-hen d effect size, 0.47), with minor increases in the cerebellum (Cohen d effect size, −0.13). Overall, the findings suggest that glutamate levels in the associative striatum normalized after effective antipsychotic treatment during the first episode of psychosis.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by an investigator-initiated grant from Janssen (Johnson & Johnson) (Drs de la Fuente-Sandoval and Graff-Guerrero), by Sistema Nacional de Investigadores (Drs de la Fuente-Sandoval, Ramírez-Bermúdez, and Graff-Guerrero), by a Consejo Nacional de Ciencia y Tecnología scholarship (Dr León-Ortiz), and by an Instituto Carlos Slim de la Salud scholarship (Dr Stephano).
Role of the Sponsors: Janssen (Johnson & Johnson), Sistema Nacional de Investigadores, Consejo Nacional de Ciencia y Tecnología, and Instituto Carlos Slim de la Salud had no further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the article for publication.
Footnotes
Previous Presentations: This study was presented at the 8th International Conference on Early Psychosis; October 11, 2012; San Francisco, California; at the 25th European College of Neuropsychopharmacology Congress; October 5, 2012; Vienna, Austria; and at the 51st Annual Meeting of American College of Neuropsychopharmacology; December 3, 2012; Hollywood, Florida.
Additional Contributions: Jesús Taboada, MD, and Oscar Marrufo, MD, provided facilities for the development of this study, and Wanna Mar contributed English-language and style revisions.
Conflict of Interest Disclosures: Dr de la Fuente-Sandoval has received grant support from Consejo Nacional de Ciencia y Technología, Instituto de Ciencia y Tecnología del Distrito Federal, and Janssen (Johnson & Johnson) and has served as a consultant or speaker for IMS Health, Carnot Laboratories, Eli Lilly, and Janssen. Dr Graff-Guerrero has received grant support from the National Institutes of Health, National Institute of Mental Health, Canadian Institutes of Health Research, Ontario Mental Health Foundation, Consejo Nacional de Ciencia y Technología, and Janssen (Johnson & Johnson) and has served as consultant or speaker for Abbott Laboratories, Gedeon Richter Plc, and Eli Lilly.
Author Contributions: Study concept and design: de la Fuente-Sandoval, Favila, Díaz-Galvis, Graff-Guerrero.
Acquisition of data: Léon-Ortiz, Azcárraga, Stephano, Favila, Alvarado-Alanis, Graff-Guerrero.
Analysis and interpretation of data: de la Fuente-Sandoval, Favila, Ramírez-Bermúdez.
Drafting of the manuscript: de la Fuente-Sandoval, Léon-Ortiz, Azcárraga, Stephano, Favila, Alvarado-Alanis, Graff-Guerrero.
Critical revision of the manuscript for important intellectual content: de la Fuente-Sandoval, Díaz-Galvis, Ramírez-Bermúdez, Graff-Guerrero.
Statistical analysis: de la Fuente-Sandoval, Favila, Graff-Guerrero.
Obtained funding: de la Fuente-Sandoval, Graff-Guerrero.
Administrative, technical, and material support: Léon-Ortiz, Azcárraga, Stephano, Favila, Alvarado-Alanis, Ramírez-Bermúdez, Graff-Guerrero.
Study supervision: de la Fuente-Sandoval.
References
- 1.Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S. Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci. 2001;24(6):330–334. doi: 10.1016/s0166-2236(00)01817-8. [DOI] [PubMed] [Google Scholar]
- 3.Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52(12):998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 4.Bartha R, Williamson PC, Drost DJ, et al. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1997;54(10):959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- 5.Théberge J, Bartha R, Drost DJ, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159(11):1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- 6.Bustillo JR, Rowland LM, Mullins P, et al. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Mol Psychiatry. 2010;15(6):629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goto N, Yoshimura R, Kakeda S, et al. Six-month treatment with atypical antipsychotic drugs decreased frontal-lobe levels of glutamate plus glutamine in early-stage first-episode schizophrenia. Neuropsychiatr Dis Treat. 2012;8:119–122. doi: 10.2147/NDT.S25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69(5):449–459. doi: 10.1001/archgenpsychiatry.2011.1519. [DOI] [PubMed] [Google Scholar]
- 9.Théberge J, Al-Semaan Y, Williamson PC, et al. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. Am J Psychiatry. 2003;160(12):2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- 10.Reid MA, Stoeckel LE, White DM, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68(7):625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bustillo JR, Chen H, Gasparovic C, et al. Glutamate as a marker of cognitive function in schizophrenia: a proton spectroscopic imaging study at 4 tesla. Biol Psychiatry. 2011;69(1):19–27. doi: 10.1016/j.biopsych.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowland LM, Kontson K, West J, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia [published online October 18, 2012] Schizophr Bull. doi: 10.1093/schbul/sbs092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Fuente-Sandoval C, León-Ortiz P, Favila R, et al. Higher levels of glutamate in the associative-striatum of subjects with prodromal symptoms of schizophrenia and patients with first-episode psychosis. Neuropsychopharmacology. 2011;36(9):1781–1791. doi: 10.1038/npp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 15.Jiru F, Skoch A, Klose U, Grodd W, Hajek M. Error images for spectroscopic imaging by LCModel using Cramer-Rao bounds. MAGMA. 2006;19(1):1–14. doi: 10.1007/s10334-005-0018-7. [DOI] [PubMed] [Google Scholar]
- 16.Bustillo JR, Rowland LM, Jung R, et al. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33(10):2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- 17.Cook R, Weisberg S. Residuals and Influence in Regression. New York, NY: Chapman & Hall; 1982. [Google Scholar]
- 18.Egerton A, Brugger S, Raffin M, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology. 2012;37(11):2515–2521. doi: 10.1038/npp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ongür D, Jensen JE, Prescot AP, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64(8):718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ota M, Ishikawa M, Sato N, et al. Glutamatergic changes in the cerebral white matter associated with schizophrenic exacerbation. Acta Psychiatr Scand. 2012;126(1):72–78. doi: 10.1111/j.1600-0447.2012.01853.x. [DOI] [PubMed] [Google Scholar]
- 21.de la Fuente-Sandoval C, Léon-Ortiz P, Azcárraga M, Favila R, Stephano S, Graff-Guerrero A. Striatal glutamate and the conversion to psychosis: a prospective 1H-MRS imaging study. Int J Neuropsychopharmacol. 2013;16(2):471–475. doi: 10.1017/S1461145712000314. [DOI] [PubMed] [Google Scholar]
- 22.Mawlawi O, Martinez D, Slifstein M, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography, I: accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 24.Kegeles LS, Abi-Dargham A, Frankle WG, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 25.Graff-Guerrero A, Willeit M, Ginovart N, et al. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29(4):400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45(1):71–76. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- 27.Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 28.Seidman LJ, Giuliano AJ, Meyer EC, et al. North American Prodrome Longitudinal Study (NAPLS) Group. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camps M, Cortés R, Gueye B, Probst A, Palacios JM. Dopamine receptors in human brain: autoradiographic distribution of D2 sites. Neuroscience. 1989;28(2):275–290. doi: 10.1016/0306-4522(89)90179-6. [DOI] [PubMed] [Google Scholar]
- 30.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segovia G, Del Arco A, Mora F. Endogenous glutamate increases extracellular concentrations of dopamine, GABA, and taurine through NMDA and AMPA/kainate receptors in striatum of the freely moving rat: a microdialysis study. J Neurochem. 1997;69(4):1476–1483. doi: 10.1046/j.1471-4159.1997.69041476.x. [DOI] [PubMed] [Google Scholar]
- 32.Kegeles LS, Abi-Dargham A, Zea-Ponce Y, et al. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: implications for schizophrenia. Biol Psychiatry. 2000;48(7):627–640. doi: 10.1016/s0006-3223(00)00976-8. [DOI] [PubMed] [Google Scholar]
- 33.Breier A, Adler CM, Weisenfeld N, et al. Effects of NMDA antagonism on striatal dopamine release in healthy subjects: application of a novel PET approach. Synapse. 1998;29(2):142–147. doi: 10.1002/(SICI)1098-2396(199806)29:2<142::AID-SYN5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Aalto S, Ihalainen J, Hirvonen J, et al. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005;182(3):375–383. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- 35.Smith GS, Schloesser R, Brodie JD, et al. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1998;18(1):18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 36.Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry. 2005;162(2):394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 37.Stone JM, Dietrich C, Edden R, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17(7):664–665. doi: 10.1038/mp.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 39.Barker PB. N-acetyl aspartate: a neuronal marker? Ann Neurol. 2001;49(4):423–424. [PubMed] [Google Scholar]
- 40.Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7(8):1397–1400. [PubMed] [Google Scholar]
- 41.Ariyannur PS, Madhavarao CN, Namboodiri AM. N-acetylaspartate synthesis in the brain: mitochondria vs. microsomes. Brain Res. 2008;1227:34–41. doi: 10.1016/j.brainres.2008.06.040. [DOI] [PubMed] [Google Scholar]
- 42.Horrobin DF, Glen AI, Vaddadi K. The membrane hypothesis of schizophrenia. Schizophr Res. 1994;13(3):195–207. doi: 10.1016/0920-9964(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 43.Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophr Res. 2001;52(1–2):87–99. doi: 10.1016/s0920-9964(01)00155-4. [DOI] [PubMed] [Google Scholar]
- 44.Bustillo JR, Rowland LM, Lauriello J, et al. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry. 2002;159(1):130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- 45.Stanley JA, Kipp H, Greisenegger E, et al. Regionally specific alterations in membrane phospholipids in children with ADHD: an in vivo 31P spectroscopy study. Psychiatry Res. 2006;148(2–3):217–221. doi: 10.1016/j.pscychresns.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Wood SJ, Berger GE, Wellard RM, et al. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophr Res. 2008;102(1–3):163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Rothermundt M, Ohrmann P, Abel S, et al. Glial cell activation in a subgroup of patients with schizophrenia indicated by increased S100B serum concentrations and elevated myo-inositol. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):361–364. doi: 10.1016/j.pnpbp.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 48.Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and CSF in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89(1–3):12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Clementi V, Tonon C, Lodi R, Malucelli E, Barbiroli B, Iotti S. Assessment of glutamate and glutamine contribution to in vivo N-acetylaspartate quantification in human brain by 1H-magnetic resonance spectroscopy. Magn Reson Med. 2005;54(6):1333–1339. doi: 10.1002/mrm.20703. [DOI] [PubMed] [Google Scholar]
- 50.de la Fuente-Sandoval C, Favila R, Alvarado P, et al. Glutamate increase in the associative striatum in schizophrenia: a longitudinal magnetic resonance spectroscopy preliminary study [in Spanish] Gac Med Mex. 2009;145(2):109–113. [PubMed] [Google Scholar]
- 51.Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA. Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a 1H spectroscopy study. Schizophr Res. 2009;115(1):88–93. doi: 10.1016/j.schres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Seese RR, O’Neill J, Hudkins M, et al. Proton magnetic resonance spectroscopy and thought disorder in childhood schizophrenia. Schizophr Res. 2011;133(1–3):82–90. doi: 10.1016/j.schres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K. Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry. 2007;62(12):1396–1404. doi: 10.1016/j.biopsych.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.