Abstract
Recent studies have demonstrated that volatile anesthetic postconditioning confers myocardial protection against ischemia-reperfusion (IR) injury through activation of the reperfusion injury salvage kinase (RISK) pathway. As RISK has been shown to be impaired in hypercholesterolemia. Therefore, we investigate whether anesthetic-induced cardiac protection was maintained in hypercholesterolemic rats. In the present study, normocholesteolemic or hypercholesterolemic rat hearts were subjected to 30 min of ischemia and 2 h of reperfusion. Animals received 2.4% sevoflurane for 5 min or 3 cycles of 10-s ischemia/10-s reperfusion. The hemodynamic parameters, including left ventricular developed pressure, left ventricular end-diastolic pressure and heart rate, were continuously monitored. The infarct size, apoptosis, p-Akt, p-ERK1/2, p-GSK3β were determined. We found that both sevoflurane and ischemic postconditioning significantly improved heart pump function, reduced infarct size and increased the phosphorylation of Akt, ERK1/2 and their downstream target of GSK3β in the healthy rats. In the hypercholesterolemic rats, neither sevoflurane nor ischemic postconditioning improved left ventricular hemodynamics, reduced infarct size and increased the phosphorylated Akt, ERK1/2 and GSK3β. In contrast, GSK inhibitor SB216763 conferred cardioprotection against IR injury in healthy and hypercholesterolemic hearts. In conclusions, hyperchoesterolemia abrogated sevoflurane-induced cardioprotection against IR injury by alteration of upstream signaling of GSK3β and acute GSK inhibition may provide a novel therapeutic strategy to protect hypercholesterolemic hearts against IR injury.
Introduction
Myocardial ischemia reperfusion injury can be reduced by multiple interventions, such as ischemic postconditioning [1] and volatile anesthetic postconditioning [2], in animal hearts and human hearts. Activation of reperfusion injury salvage kinase (RISK) pathway (mainly PI3K-Akt-GSK3β axis and ERK1/2) contributes to ischemic/anesthetic-induced myocardial protection [1,2]. Multiple prosurvival signaling pathways, including PI3K-Akt and ERK1/2, converge on glycogen synthase kinase 3β (GSK3β) [3]. In addition, the phosphorylation of GSK3β inhibits the opening of mitochondrial permeability transition pore (mPTP) and reduces mitochondria-dependent apoptosis and necrosis [4]. Recent studies have demonstrated that MG53, a newly identified tripartite motif-containing (TRIM) family protein, is indispensable for ischemic postconditioning-induced cardioprotection through the activation of PI3K-Akt-GSK3β axis and ERK1/2 pathway [5]. Although anesthetic and ischemic postconditioning can activate overlapping signal events, whether MG53 is related to the cardiac sevoflurane postconditioning remains elusive.
In recent years, anesthetic postconditioning has mainly been documented in healthy subjects, and the effect of sevoflurane postconditioning on hypercholesterolemic rat heart remains unclear. A number of prospective clinical studies have shown that both coronary artery disease (CAD) and the risk factor for cardiac death after acute myocardial infarct (AMI) are directly related to hypercholesterolemia [6,7]. Furthermore, hypercholesterolemia is associated with alterations of Akt and ERK1/2 phosphorylation and abrogates ischemic postconditioning-induced cardioprotection by interfering with nitric oxide synthase signaling [8,9]. These studies indicate that hypercholesterolemia may adversely affect sevoflurane-induced cardioprotection. Therefore, the aim of the current study was to investigate whether sevoflurane-induced cardioprotection was maintained in hypercholesterolemic rats.
Materials and Methods
1. Animals
All of the animals were treated according to the guidelines of the Guide for the Care and Use of Laboratory Animals (United States National Institutes of Health). The Laboratory Animal Care Committee of Zhejiang University approved all experimental procedures and protocols. All efforts were made to minimize the number of animals used and their suffering. The rats were housed in polypropylene cages, and the room temperature was maintained at 22 °C, with a 12-hour light-dark cycle. Six-week-old male Sprague-Dawley rats, weighing 130-180 g, were used for all experiments.
2. Study Groups and Experimental Protocol
To investigate whether sevoflurane-induced cardioprotection was maintained in hypercholesterolemic rats, the experiments were conducted as follows: 1) normocholesterolemic ischemia reperfusion group (IR): rats were fed standard pellet chow for 8 weeks and received no further treatment before myocardial ischemia; 2) normocholesterolemic sevoflurane postconditioning group (IR + SPO): rats were fed standard pellet chow for 8 weeks and then treated with 2.4% sevoflurane (Maruishi Pharmaceutical Co, Ltd, Osaka, Japan) via sevoflurane vaporizer (Sevotec 5; Datex-Ohmeda, Tewksbury, MA, USA) for 5-min immediately after reperfusion [10]; 3) normocholesterolemic ischemic postconditioning group (IR + IPO): rats were fed standard pellet chow for 8 weeks and then treated with three episode of 10-s of ischemia/10-s reperfusion immediately after reperfusion [11]; 4) hypercholesterolemic ischemia reperfusion group (HC + IR): rats were fed 2% cholesterol pellet chow for 8 weeks and received no further treatment before myocardial ischemia; 5) hypercholesterolemic sevoflurane postconditioning group (HC + IR + SPO): rats were fed 2% cholesterol pellet chow for 8 weeks and then treated with 2.4% sevoflurane for 5-min immediately after reperfusion. 6) hypercholesterolemic ischemic postconditioning group (HC + IR + IPO): rats were fed standard pellet chow for 8 weeks and then treated with three episode of 10-s of ischemia/10-s reperfusion immediately after reperfusion; To explore the cardioprotective role of GSK3β phosphorylation in hypercholesterolemic rat hearts, we conducted a second experiment. The selective GSK inhibitor SB216763 (0.6 mg/kg i.v., 5 min before reperfusion) [12] were administered to normocholesterolemic and hypercholesterolemic rats. All inhibitors were from Sigma-Aldrich Inc (St Louis, Mo, USA).
3. Serum Lipid Assay
Blood was harvested from the caudal vein and centrifuged (3000 rpm, 10 min, 4°C) to obtain serum. Serum lipid levels were measured by spectrophotometry using commercial assay kits for total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (Beijing BHKT Clinical Reagent Co., Ltd., Beijing, China) according to the manuals and as described by Ballantyne et al [13].
4. Hematoxylin-eosin Staining
To determine that chronic treatment with a high-cholesterol diet for 8 weeks does not result in the development of coronary atherosclerosis in rats, hematoxylin-eosin staining for thoracic aorta and coronary artery was conducted. The small segments of thoracic aorta and the heart were harvested from rats fed with either high cholesterol or normal chow for 8 weeks for histologic examination. The samples were fixed in 4% paraformaldehyde and embedded in paraffin. Hematoxylin-eosin staining was conducted as Iliodromitis et al [14] have described elsewhere.
5. Surgical Preparation
The IR surgery was performed according to the methods of Zhang et al [15] with modifications. Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.), plus additional doses (25 mg/kg i.p.) every 60 min to maintain effective anesthesia [16] and were mechanically ventilated with room air to maintain arterial pH, PCO2 and PO2 within the normal physiological range. The pericardium was opened and a 6-0 silk suture was passed under the left atrial appendage for 2-3 mm through a small polytetrafluoroethylene tube. The tube was pulled to occlude the coronary artery for 30 min and the occlusion was confirmed by epicardial cyanosis in the area at risk, while successful reperfusion (for 120 min) was verified by epicardial hyperemia. Left ventricular developed pressure (LVDP), left ventricular end-diastolic pressure (LVEDP) and heart rate (HR) were measured throughout the experimental period by a polyethylene catheter placed into the left ventricle connecting a pressure transducer connected to a data acquisition system (PowerLab; ADInstruments, Shanghai Trading Co, Ltd, Shanghai, China).
6. Determination of Infarct Size
At the end of reperfusion, the coronary artery was reoccluded and perfused with 1% Evans blue dye to identify the unstained area as the area at risk. The left ventricle was separated, frozen, cut into transverse slices, and incubated in 1% triphenyltetrazolium chloride solution at 37°C for 10 min. The area of infarct (pale) and risk (red) was measured by planimetry using ImageJ 1.37 from the National Institutes of Health (Bethesda, MD, USA). The infarct size was expressed as a percentage of the area at risk. Samples with an area at risk <15% or >45% of the left ventricle were excluded [17].
7. Detection of Myocardial Apoptosis
Apoptosis was assessed using the TUNEL method. At the end of reperfusion, the hearts were fixed in 4% paraformaldehyde and embedded in paraffin for TUNEL staining. The heart tissue sections were stained using an in situ cell death detection kit (POD; Roche Diagnostics Corp, Indianapolis, IN, USA), following the manufacturer’s protocol. Ten microscopic fields (400×) from each section were assayed by counting brown nuclei. The percentage of TUNEL-positive nuclei (brown nuclei) was calculated.
8. Immunoblotting
Fifteen minutes following reperfusion, the samples were taken from ischemic zone. The expression of myocardial Akt, phosphorylated-Akt (Ser473) (p-Akt), ERK1/2 and phosphorylated-ERK1/2 (Thr202/Tyr204) (p-ERK1/2), GSK3β, phosphorylated-GSK-3β (Ser9) (p-GSK3β) (Cell Signaling Technology, Beverly, MA, USA) were determined by Western blotting as we described elsewhere [18]. At the end of reperfusion, the expression of MG53 (Gene Tex, San Antonio, TX, USA) and PI3K-p85 (Cell Signaling Technology, Beverly, MA, USA) was determined by Western blotting as we described elsewhere [18]. The quantitative protein band density was assayed by ImageJ 1.37.
9. Statistical Analysis
Data are shown as mean ± SD. Lipid levels were analyzed using the unpaired Student’s t test. All other data were analyzed by one-way ANOVA following Newman-Keuls post hoc test. A value of P <0.05 was considered to be statistically significant. All statistical analyses were performed using GraphPad Prism Version 4.0 (GraphPad Prism Software, San Diego, CA, USA).
Results
Seventy rats were used for myocardial infarction experiments (2 were excluded as a result of area at risk <15% and 4 died of refractory ventricular arrhythmias during the 30-min occlusion) and 79 animals were used for immunoblotting (7 died of refractory ventricular arrhythmias during the 30-min occlusion). An additional 53 rats were used for TUNEL staining (5 died of refractory ventricular arrhythmias during the 30-min occlusion). 10 rats were used for hematoxylin-eosin staining.
1. High-cholesterol Diet Induced a Moderate and Steady Increase in Serum Cholesterol without Substantial Development of Coronary Atherosclerosis in rats
Levels of TC (136.5 ± 12.8 mg/dl), LDL-C (57.9 ± 7.2 mg/dl), and TG (128.2 ± 7.1 mg/dl) were increased in rats fed high-cholesterol chow compared with those (68.3 ± 6.6 mg/dl, 26.1 ± 4.8 mg/dl, and 59.4 ± 8.9 mg/dl) fed normal chow (P < 0.01). The level of HDL-C (29.6 ± 3.9 vs. 28.3 ± 4.8) was not significantly different between normocholesterolemic and hypercholesterolemic rats.
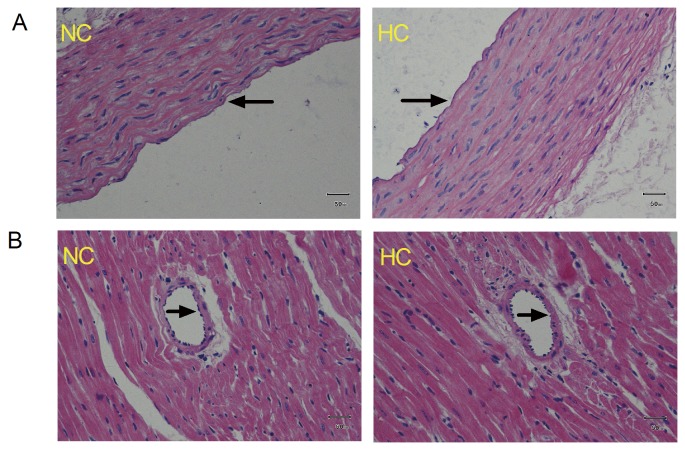
Atherogenesis was detected in the form of subintimal accumulation of lipids and foamy macrophages. There was no deposition of lipids and foamy macrophages in the subintimal area in the cross-section of the aortic wall (Figure 1A) and the myocardial branch of coronary artery from rats fed with either high cholesterol or normal chow for 8 weeks (Figure 1B).
Figure 1. Representative cross-sections of the thoracic aorta (A) and the left myocardial branch of the coronary artery (B) from normocholesterolemic (NC) and hypercholesterolemic (HC) rats (fed 2% cholesterol-enriched chow for 8 weeks) by hematoxylin-eosin (HE) staining (400×).
The arterial lumen is indicated by the arrow, n = 5 hearts/group.
2. Hypercholesterolemia Abrogates the Left Ventricular Hemodynamic Improvement Induced by Sevoflurane and Ischemic Postconditioning
As shown in Table 1, there was no significant difference in baseline left ventricular hemodynamic parameters among all groups (P > 0.05). After 60 min of reperfusion, LVDP and LVEDP were significantly increased in IR + SPO and IR + IPO compared with that in IR (P < 0.05). However, such improving effect was not observed in HC + SPO and HC + IPO compared with that in HC + IR (P > 0.05).
Table 1. The effect of sevoflurane and ischemic postconditioning on left ventricular hemodynamic parameters in rat hearts exposed to ischemia-reperfusion.
| Group | Baseline | Ischemia | Reperfusion |
|||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |||
| LVDP, mmHg | ||||||
| IR | 127 ± 8 | 81 ± 5 | 108 ± 11 | 89 ± 9 | 84 ± 6 | 77 ± 10 |
| IR + SPO | 130 ± 9 | 83 ± 5 | 111 ± 11 | 106 ± 5* | 101 ± 5* | 97± 6* |
| IR + IPO | 123 ± 7 | 80 ± 7 | 115 ± 15 | 107 ± 11* | 102 ± 5* | 96 ± 7* |
| HC + IR | 130 ± 12 | 85 ± 11 | 115 ±10 | 98 ± 9 | 88 ± 12 | 80 ± 13 |
| HC + IR + SPO | 134 ± 8 | 80 ± 7 | 110 ± 7 | 94 ± 12 | 87 ± 13 | 83 ± 12 |
| HC + IR + IPO | 127 ± 7 | 84 ± 5 | 107 ± 10 | 92 ± 7 | 86 ± 7 | 84 ± 8 |
| LVEDP, mmHg | ||||||
| IR | 4.1 ± 1.5 | 10.1 ± 1.6 | 5.3 ± 1.8 | 7.6 ± 1.8 | 7.9 ± 1.4 | 8.2 ± 1.5 |
| IR + SPO | 3.9 ± 1.0 | 9.4 ± 1.7 | 4.5 ± 1.4 | 5.1 ± 1.2* | 5.9 ± 1.1* | 5.7 ± 1.5* |
| IR + IPO | 4.0 ± 1.4 | 9.5 ± 2.1 | 5.0 ± 1.1 | 5.6 ± 1.2* | 5.5 ± 1.0* | 5.9 ± 1.1* |
| HC + IR | 4.1 ± 1.0 | 9.9 ± 1.2 | 5.4 ± 1.0 | 6.9 ± 1.3 | 7.8 ± 1.0 | 8.4 ± 1.3 |
| HC + IR + SPO | 4.5 ± 1.2 | 10.4 ± 1.1 | 6.3 ± 1.0 | 7.2 ± 1.2 | 7.4 ± 1.2 | 7.8 ± 1.5 |
| HC + IR + IPO | 3.9 ± 1.4 | 10.0 ± 1.8 | 6.4 ± 1.6 | 7.0 ± 1.8 | 7.3 ± 1.5 | 7.7 ± 1.6 |
| HR, beats/min | ||||||
| IR | 345 ± 35 | 325 ± 41 | 312 ± 48 | 305 ± 39 | 315 ± 42 | 299 ± 41 |
| IR + SPO | 332 ± 32 | 312 ± 41 | 315 ± 39 | 321 ± 35 | 306 ± 40 | 313 ± 39 |
| IR + IPO | 350 ± 34 | 310 ± 34 | 318 ± 40 | 309 ± 37 | 310 ± 39 | 300 ± 41 |
| HC + IR | 328 ± 31 | 308 ± 43 | 303 ± 39 | 300 ± 34 | 298 ± 41 | 288 ± 39 |
| HC + IR + SPO | 340 ± 39 | 318 ± 39 | 300 ± 39 | 310 ± 30 | 313 ± 44 | 304 ± 41 |
| HC + IR + IPO | 330 ± 38 | 320 ± 38 | 310 ± 32 | 308 ± 36 | 310 ± 33 | 300 ± 37 |
Effects of sevoflurane and ischemic postconditioning on left ventricular hemodynamic parameters in rat hearts exposed to ischemia-reperfusion. IR: ischemia reperfusion; SPO: sevoflurane posconditioning; IPO: ischemic posconditioning; HC: hypercholesterolemia. Data are mean ± SD, n = 8 hearts/group. *P < 0.05 vs. IR.
3. Hypercholesterolemia Abrogates the Myocardial Infarct-sparing Effect of Sevoflurane and Ischemic Postconditioning
The area at risk was not significantly different among all groups (data not shown). As shown in Figure 2, the infarct size was larger by 18% in the HC + IR (52 ± 5%) compared with the IR group (44 ± 5%, P < 0.05). The infarct size was markedly decreased in the IR + SPO (27 ± 6%) and IR + IPO (26 ± 5%) over the IR group (44 ± 5%, P < 0.05). The reduced myocardial infarct conferred by sevoflurane and ischemic postconditioning was not found in hypercholesterolemic rats.
Figure 2. Effects of sevoflurane and ischemic postconditioning on infarct size expressed as a percentage of area at risk in rat hearts exposed to ischemia-reperfusion.
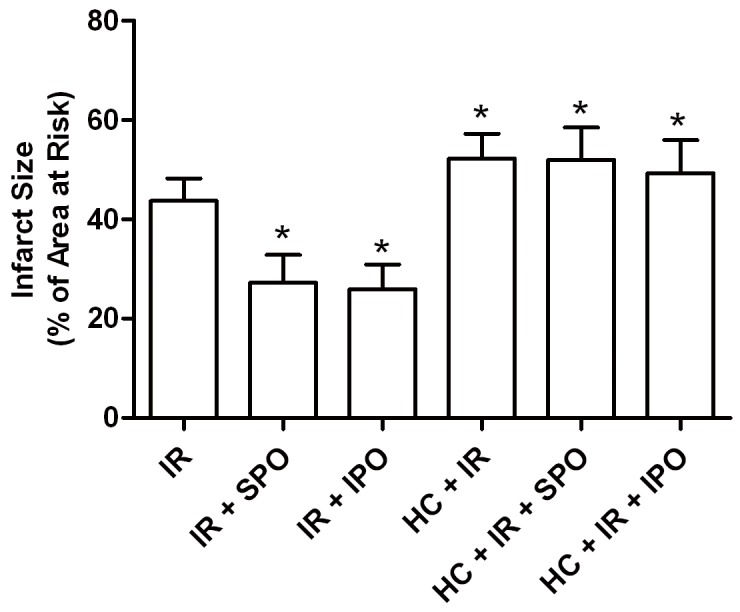
IR: ischemia reperfusion; SPO: sevoflurane posconditioning; IPO: ischemic posconditioning; HC: hypercholesterolemia. Data are mean ± SD, n = 8 hearts/group. *P < 0.05 vs. IR.
4. Hypercholesterolemia Inhibits the Myocardial Anti-apoptotic Effect of Sevoflurane and Ischemic Postconditioning
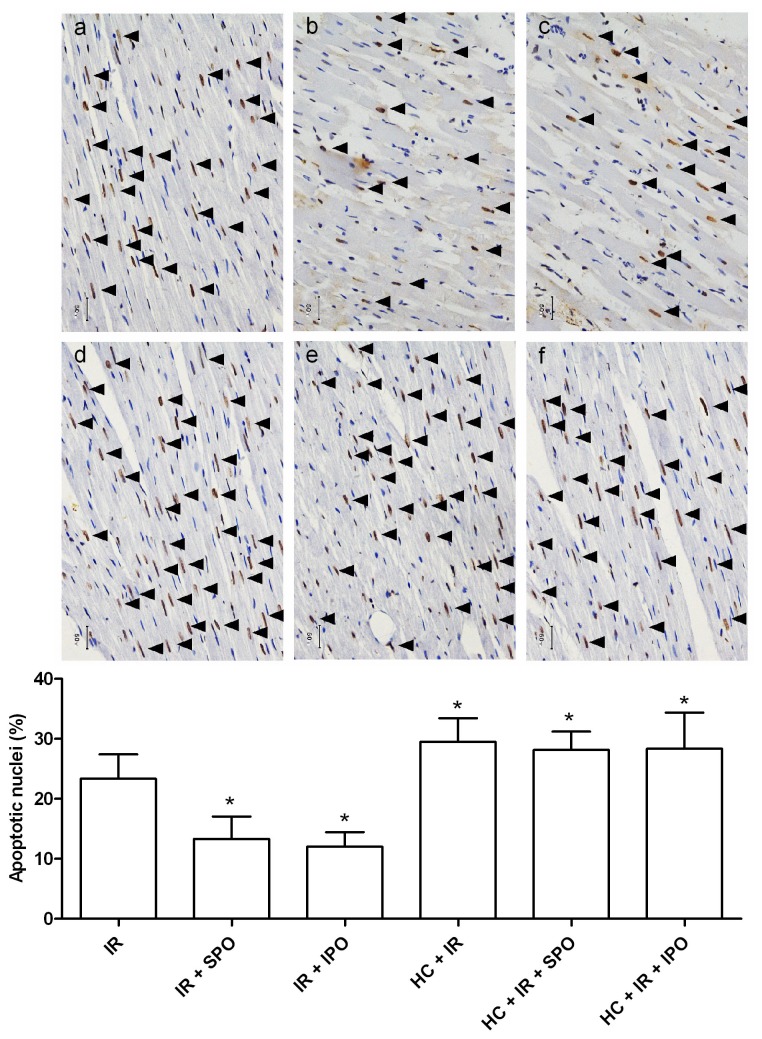
As shown in Figure 3, The number of TUNEL-positive nuclei expressed as a percentage of total nuclei was markedly decreased in the IR + SPO (13 ± 4%) and IR + IPO (12 ± 2%) over the IR group (23 ± 4%, P < 0.05), however, the reduced apoptotic nuclear conferred by sevoflurane and ischemic postconditioning was not found in hypercholesterolemic rats. Interestingly, the number of TUNEL-positive nuclei was increased by 30% in the HC + IR (30 ± 4%) compared to the IR group (23 ± 4%, P < 0.05).
Figure 3. Effects of sevoflurane and ischemic postconditioning on apoptosis expressed as a percent of total nuclei in tissue sections from rat hearts exposed to ischemia-reperfusion (TUNEL staining, 400×).
TUNEL-positive nuclei (brown nuclei) was indicated by the arrow. a normocholesterolemic ischemia reperfusion group (IR), b normocholesterolemic sevoflurane postconditioning group (IR + SPO), c normocholesterolemic ischemic postconditioning group (IR + IPO), d hypercholesterolemic ischemia reperfusion group (HC + IR), e hypercholesterolemic sevoflurane postconditioning group (HC + IR + SPO), f hypercholesterolemic ischemic postconditioning group (HC + IR + IPO). IR: ischemia reperfusion; SPO: sevoflurane postconditioning; IPO: ischemic postconditioning; HC: hypercholesterolemia. Data are mean ± SD, n = 6 hearts/group. *P < 0.05 vs. IR.
5. Hypercholesterolemia Abrogates the Upregulation of MG53 Expression Induced by Sevoflurane and Ischemic Postconditioning
Neither sevoflurane nor hypercholesterolemia alters the expression of MG53 in the absence of IR, which indicates that sevoflurane doesn’t have a direct effect on MG53 expression (Figure S1).
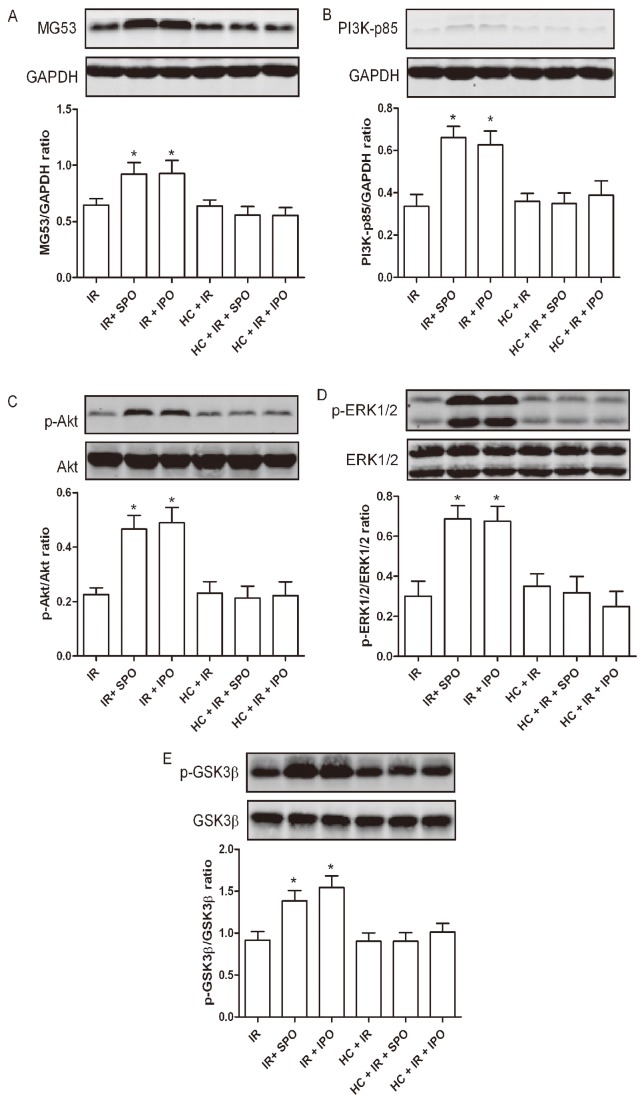
Sevoflurane and ischemic postconditioning upregulated the expression of MG53 in healthy rats (P < 0.05), but this was completely blocked by hypercholesterolemia (Figure 4A). The expression of MG53 was not significantly different between IR and HC + IR groups.
Figure 4. Effects of sevoflurane and ischemic postconditioning on the expression of MG53 (A), PI3K-p85 (B), p-Akt (C), p-ERK1/2 (D), p-GSK3β (E) in rat hearts exposed to ischemia-reperfusion (IR).
IR: ischemia reperfusion; SPO: sevoflurane postconditioning; IPO: ischemic postconditioning; HC: hypercholesterolemia. Data are mean ± SD, n = 6 hearts/group. *P < 0.05 vs. IR.
6. Hypercholesterolemia Abrogates the Upregulation of PI3K-p85 and p-Akt Expression Induced by Sevoflurane and Ischemic Postconditioning
The levels of total Akt were not significantly different among all groups. Therefore, the levels of p-Akt were expressed as the percentage of total protein. Sevoflurane and ischemic postconditioning significantly increased the expression of PI3K-p85 and p-Akt in healthy rats (P < 0.05), however, this didn’t occur in hypercholesterolemic ones. The expression of PI3K-p85 and p-Akt didn’t significantly differ between IR and HC + IR groups (Figure 4B and Figure 4C).
7. Hypercholesterolemia Abrogates the Upregulation of p-ERK1/2 Expression Induced by Sevoflurane and Ischemic Postconditioning
No significant differences were found in the expression of total ERK1/2 among any groups. Sevoflurane and ischemic postconditioning significantly increased the p-ERK1/2 in healthy rats (P < 0.05). Interestingly, this effect was inhibited in the hypercholesterolemic groups. No difference in the expression of p-ERK1/2 was observed between IR and HC + IR groups (Figure 4D).
8. Hypercholesterolemia Abrogates the Upregulation of p-GSK3β Expression Induced by Sevoflurane and Ischemic Postconditioning
Immunoblots of total GSK3β were not significantly different between groups. Sevoflurane and ischemic postconditioning significantly increased the p-GSK3β in healthy rats (P < 0.01), however, this effect was blocked by hypercholesterolemia. The expression of p-GSK3β didn’t significantly differ between IR and HC + IR groups (Figure 4E).
9. GSK3β Inhibitor was Cardioprotective in Hypercholesterolemic Hearts
To investigate whether the significant inhibition of sevoflurane and ischemia-induced GSK3β phosphorylation in hypercholesterolemic rat hearts was due to the inactivation of Akt and ERK1/2, we treated healthy and hypercholesterolmic rats with GSK3β inhibitor SB216763.
While sevoflurane and ischemic postconditioning did not improve the hemodynamic parameters of hypercholesterolemic rat hearts, SB216763 significantly improved LVDP and LVEDP in the hypercholeslerolemic group as well as in the healthy group after 60 min of reperfusion (Table 2).
Table 2. The effect of GSK3β inhibitor SB216763 on left ventricular hemodynamic parameters in rat hearts exposed to ischemia-reperfusion.
| Group | Baseline | Ischemia | Reperfusion |
|||
|---|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |||
| LVDP, mmHg | ||||||
| IR | 127 ± 8 | 81 ± 5 | 108 ± 11 | 89 ± 9 | 84 ± 6 | 77 ± 10 |
| IR + SB | 125 ± 9 | 83 ± 9 | 110 ± 12 | 105 ± 8* | 102 ± 7* | 95 ± 7* |
| HC + IR | 130 ± 12 | 85 ± 11 | 115 ±10 | 98 ± 9 | 88 ± 12 | 80 ± 13 |
| HC + IR + SB | 127 ± 7 | 84 ± 8 | 117 ± 10 | 110 ± 7# | 100 ± 7# | 93 ± 8# |
| LVEDP, mmHg | ||||||
| IR | 4.1 ± 1.5 | 10.1 ± 1.6 | 5.3 ± 1.8 | 7.6 ± 1.8 | 7.9 ± 1.4 | 8.2 ± 1.5 |
| IR + SB | 3.8 ± 1.1 | 9.8 ± 1.5 | 5.0 ± 1.4 | 5.3 ± 1.2* | 5.7 ± 1.3* | 5.9 ± 1.2* |
| HC + IR | 4.1 ± 1.0 | 9.9 ± 1.2 | 5.4 ± 1.0 | 6.9 ± 1.3 | 7.8 ± 1.0 | 8.4 ± 1.3 |
| HC + IR + SB | 3.9 ± 1.4 | 9.6 ± 1.4 | 4.9 ± 1.2 | 5.6 ± 1.1 | 5.8 ± 1.3# | 6.0 ± 1.4# |
| HR, beats/min | ||||||
| IR | 345 ± 35 | 325 ± 41 | 312 ± 48 | 305 ± 39 | 315 ± 42 | 299 ± 41 |
| IR + SB | 322 ± 33 | 310 ± 36 | 320 ± 33 | 311 ± 39 | 315 ± 36 | 310 ± 33 |
| HC + IR | 328 ± 31 | 308 ± 43 | 303 ± 39 | 300 ± 34 | 298 ± 41 | 288 ± 39 |
| HC + IR + SB | 320 ± 38 | 310 ± 39 | 312 ± 30 | 318± 41 | 310 ± 37 | 302 ± 34 |
Effects of GSK3β inhibitor SB216763 on left ventricular hemodynamic parameters in rat hearts exposed to ischemia-reperfusion. IR: ischemia reperfusion; SB: SB216763; HC: hypercholesterolemia. Data are mean ± SD, n = 8 hearts/group. *P < 0.05 vs. IR, # P < 0.05 vs. HC + IR.
Interestingly, we found that SB216763 significantly reduced infarct size in both normal and hypercholesterolemic hearts (IR + SB, 29 ± 5% vs. IR, 44 ± 5%; HC+ IR + SB, 35 ± 8% vs. HC + IR, 52 ± 5%; P < 0.05) (Figure 5).
Figure 5. Effects of GSK3β inhibitor SB216763 on infarct size expressed as a percentage of area at risk in rat hearts exposed to ischemia-reperfusion.
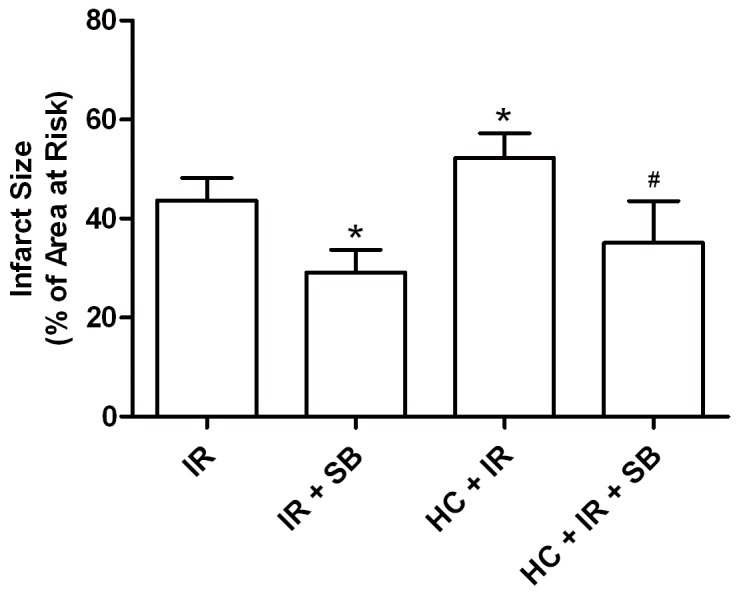
IR: ischemia reperfusion; SB: SB216763; HC: hypercholesterolemia. Data are mean ± SD, n = 8 hearts/group. *P < 0.05 vs. IR; # P < 0.05 vs. HC + IR.
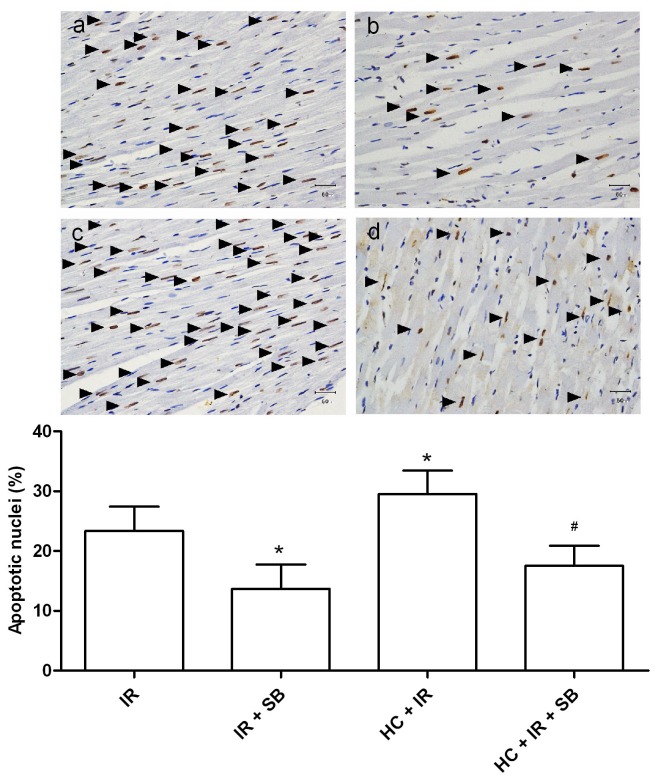
Moreover, SB216763 significantly decreased the number of TUNEL-positive nuclei in both healthy and hypercholesterolemic hearts (IR + SB, 14 ± 4% vs. IR, 23 ± 4%; HC+ IR + SB, 18 ± 3% vs. HC + IR, 30 ± 4%; P < 0.05) (Figure 6).
Figure 6. Effects of GSK3β inhibitor SB216763 on apoptosis expressed as a percent of total nuclei in tissue sections from rat hearts exposed to ischemia-reperfusion (TUNEL staining, 400×).
a normocholesterolemic ischemia reperfusion group (IR), b normocholesterolemic SB216763-treated group (IR + SB), c hypercholesterolemic ischemia reperfusion group (HC + IR), d hypercholesterolemic SB216763-treated group (HC + IR + SB). IR: ischemia reperfusion; SB: SB216763; HC: hypercholesterolemia. Data are mean ± SD, n = 6 hearts/group. *P < 0.05 vs. IR; # P < 0.05 vs. HC + IR.
Discussion
The current study suggested that sevoflurane and ischemic postconditioning-induced cardioprotection against IR was blunted in hypercholesterolemic rat hearts, with altered MG53/RISK signaling pathway that inhibit GSK3β. We found that inhibition of GSK3β may be a potential therapeutic intervention to reduce myocardial infarct and apoptosis in hypercholesterolemic subjects.
We used chronic treatment with a high-cholesterol diet for 8 weeks to induce hypercholesterolemia in rats [19,20]. We found that this diet induced a moderate and steady increase in serum cholesterol without substantial development of coronary atherosclerosis (Figure 1), which is consistent with previous studies and suggests that the factor influencing sevoflurane postconditioning in our study was hypercholesterolemia itself, and not subsequent atherosclerosis or other hypercholesterolemic complications [21,22]. Therefore, we used this hypercholesterolemic model to study the direct cardiac effects of hypercholesterolemia on sevoflurane postconditioning.
Effects of hypercholesterolemia on myocardial IR have yielded controversial results in rodents. A number of studies have shown that hypercholesterolemia increases myocardial infarct size in the setting of IR [8,19,20], but the underlying mechanism is poorly understood. In our study, the myocardial infarct size induced by 30-min occlusion and 120-min reperfusion was increased by 18% in hypercholesterolemic rat hearts than that in healthy ones and suggests that hypercholesterolemic rats are susceptible to IR injury. In contrast to our findings, Kupai et al [23] showed that the myocardial infarct size induced by 30-min left coronary artery occlusion and 120-min reperfusion was not significantly increased in hypercholesterolemic rats fed a 2% cholesterol-enriched diet for 12 weeks compared with that in normally fed rats, and Giricz et al [24] reported that the infarct size induced by 30-min global ischemia and 120-min reperfusion in hearts isolated from rats fed a 2% cholesterol diet for 12 weeks was not significantly increased than that from normally fed rats. The reasons for such diverse results are unknown but may be attributable to the different high-cholesterol diet treatment periods and the different IR models (in vivo or in vitro) [8,19,20,23,24].
Another principle finding of our current study was hypercholesterolemia alone increased myocardial apoptosis in the setting of IR. In the present study, the number of apoptotic nuclei was increased by 30% in hypercholesterolemic rat hearts than that in healthy ones. One reasonable explanation is increased free oxygen radicals and inflammation may prompt apoptotic signaling pathway [25]. Furthermore, hypercholesterolemia is associated with decreased expression of anti-apoptotic Bcl-2 [8], which plays a pivotal role in preventing apoptosis by inhibiting the activation of executioner caspases 3 [26] and the release of mitochondrial cytochrome c [27]. All of the above may contribute to the increased myocardial apoptosis in the setting of hypercholesterolemia during IR.
The present study has shown that hypercholesterolemia induced by high-cholesterol diet abrogated sevoflurane and ischemic postconditioning-induced cardioprotection and modified cardioprotective signaling pathways. Here, we demonstrated that the myocardial p-Akt and p-ERK1/2 expression was upregulated by sevoflurane and ischemic postconditioning. Interestingly, such cardioprotection and upregulation of myocardial p-Akt and p-ERK1/2 expression were both lost in hypercholesterolemic rats. Furthermore, previous studies have demonstrated that both PI3k-Akt and MEK-ERK1/2 signals play a pivotal role in the cardioprotection of sevoflurane or ischemic postconditioning [1,2]. These results at least indicate that the loss of upregulation of p-Akt and p-ERK1/2 expression is closely associated with the loss of cardioprotection of sevoflurane postconditioning in hypercholesterolemic rats. MG53, an upstream signaling cascade of Akt, forms a functional complex with the p85 subunit of PI3K and contributes to acute membrane repair in cardiomyocytes [5,28]. In addition, ischemic postconditioning-induced cardioprotection is lost in MG53-deficient mice and overexpression of MG53 attenuates hypoxia- and oxidative-induced cardiomyocytes death through the activation of PI3K-Akt-GSK3β and ERK1/2 signaling pathways [5,29]. In healthy rats, sevoflurane significantly increased the levels of MG53 and PI3k-p85, however, this didn’t occur in hypercholesterolemic ones with sevoflurane postconditioning. Our results indicate that the dysfunction of the upstream protein kinases MG53 may in part contribute to the observed deficit in Akt and ERK1/2 phosphorylation in hypercholesterolemic rat hearts. Furthermore, the deficit in GSK3β phophorylation in hypercholesterolemic rat hearts may in part be attributed to the observed deficit in Akt and ERK1/2 phosphorylation. If, as mentioned above, upregulation of phosphorylated Akt, ERK1/2 and GSK3β plays a pivotal role in the infarct-sparing effect of sevoflurane postconditioning, we reasoned that the loss of cardioprotection in hypercholesterolemic myocardium, at least in part, be attributed to the dysfunction of Akt, ERK1/2 and GSK3β phosphorylation.
Our current study has shown that the cardioprotective effect of postconditioning was lost in hypercholesterolemic subjects, which suggests that hypercholesterolemic patients may not benefit from therapeutic application of sevoflurane in the clinical setting. Therefore, it is translationally important to explore novel cardioprotective strategies. We found that the GSK3β inhibitor SB216763 significantly improved heart pump function, reduced myocardial infarct size and apoptosis in hypercholesterolemic rats, which suggests that the downstream signal mechanisms of GSK3β in hypercholesterolemic myocardium is preserved. Together, these data indicate that inhibition of GSK3β would be a more promising therapeutic target to protect hypercholesterolemic hearts against IR injury.
Our findings are translationally important in that they determined whether the myocardial protection induced by sevoflurane occurs in hypercholesterolemic animals. However, there are several limitations in our current study. First, the loss of cardioprotection of sevoflurane postconditioning in hypercholesterolemic rats might also be due to changes in cardiovascular oxidative/nitrosative stress [23,30], which were not explored in the current study. Second, patients with hypercholesterolemia are usually also have coronary atherosclerosis, and our hypercholesterolemic rats did not, which indicates that the hypercholesterolemic rat model we used may not fully simulate the complex clinical setting of hypercholesterolemic patients, so our results may only apply to effect of hypercholesterolemia on cardioprotection induced by sevoflurane in a limited clinical setting.
In summary, we report that sevoflurane-induced cardioprotection against IR injury was abrogated in hypercholesterolemic rats. Hypercholesterolemia blocked the ability of sevoflurane to phosphorylate components of RISK pathway and consequently, the phosphorylation of the downstream target GSK3β. These data indicate that direct inhibition of GSK3β before myocardial infarct may be a potential therapeutic approach to prevent IR injury in the presence of hypercholesterolemia.
Supporting Information
Effects of sevoflurane on the expression of MG53 in sham-operated rat hearts. Normocholesterolemic and hypercholesterolemic sham-operated rats were treated with 2.4% sevoflurane via sevoflurane vaporizer for 5-min. Then hearts were harvested for immunoblotting. NC: normocholesterolemia; HC: hypercholesterolemia. Sev: sevoflurane. Data are mean ± SD, n = 6 hearts/group.
(TIF)
Funding Statement
This work was supported by the Natural Science Foundation of China (numbers 81170118 and 81201496), the Natural Science Foundation of Zhejiang Province (number R2090259), Medicine Administration Bureau of Zhejiang Province (number 2011ZZ009), and the Foundation from Science and Technology Department of Zhejiang Province (numbers 2009C13G2010218 and 2012C33088). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ferdinandy P, Schulz R, Baxter GF (2007) Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59: 418-458. doi: 10.1124/pr.107.06002. PubMed: 18048761. [DOI] [PubMed] [Google Scholar]
- 2. Pagel PS (2008) Postconditioning by volatile anesthetics: salvaging ischemic myocardium at reperfusion by activation of prosurvival signaling. J Cardiothorac Vasc Anesth 22: 753-765. doi: 10.1053/j.jvca.2008.03.005. PubMed: 18922439. [DOI] [PubMed] [Google Scholar]
- 3. Lecour S (2009) Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J Mol Cell Cardiol 47: 32-40. doi: 10.1016/j.yjmcc.2009.03.019. PubMed: 19344728. [DOI] [PubMed] [Google Scholar]
- 4. Juhaszova M, Zorov DB, Yaniv Y, Nuss HB, Wang S et al. (2009) Role of glycogen synthase kinase-3beta in cardioprotection. Circ Res 104: 1240-1252. doi: 10.1161/CIRCRESAHA.109.197996. PubMed: 19498210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Lv F, Jin L, Peng W, Song R et al. (2011) MG53 participates in ischaemic postconditioning through the RISK signalling pathway. Cardiovasc Res 91: 108-115. doi: 10.1093/cvr/cvr029. PubMed: 21285295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corti R, Fuster V, Badimon JJ, Hutter R, Fayad ZA (2001) New understanding of atherosclerosis (clinically and experimentally) with evolving MRI technology in vivo. Ann N Y Acad Sci 947: 181-195. PubMed: 11795266. [DOI] [PubMed] [Google Scholar]
- 7. Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR et al. (2001) Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation 104: 3046-3051. doi: 10.1161/hc5001.100624. PubMed: 11748098. [DOI] [PubMed] [Google Scholar]
- 8. Osipov RM, Bianchi C, Feng J, Clements RT, Liu Y et al. (2009) Effect of hypercholesterolemia on myocardial necrosis and apoptosis in the setting of ischemia-reperfusion. Circulation 120: S22-S30. doi: 10.1161/CIRCULATIONAHA.108.842724. PubMed: 19752371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sack MN, Murphy E (2011) The role of comorbidities in cardioprotection. J Cardiovasc Pharmacol Ther 16: 267-272. doi: 10.1177/1074248411408313. PubMed: 21821527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drenger B, Ostrovsky IA, Barak M, Nechemia-Arbely Y, Ziv E et al. (2011) Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology 114: 1364-1372. doi: 10.1097/ALN.0b013e31820efafd. PubMed: 21368653. [DOI] [PubMed] [Google Scholar]
- 11. Zhao HX, Wang XL, Wang YH, Wu Y, Li XY et al. (2010) Attenuation of myocardial injury by postconditioning: role of hypoxia inducible factor-1alpha. Basic Res Cardiol 105: 109-118. doi: 10.1016/j.amjcard.2010.01.310. PubMed: 19597757. [DOI] [PubMed] [Google Scholar]
- 12. Gross ER, Hsu AK, Gross GJ (2004) Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res 94: 960-966. doi: 10.1161/01.RES.0000122392.33172.09. PubMed: 14976126. [DOI] [PubMed] [Google Scholar]
- 13. Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ et al. (2003) Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 107: 2409-2415. doi: 10.1161/01.CIR.0000068312.21969.C8. PubMed: 12719279. [DOI] [PubMed] [Google Scholar]
- 14. Iliodromitis EK, Zoga A, Vrettou A, Andreadou I, Paraskevaidis IA et al. (2006) The effectiveness of postconditioning and preconditioning on infarct size in hypercholesterolemic and normal anesthetized rabbits. Atherosclerosis 188: 356-362. doi: 10.1016/j.atherosclerosis.2005.11.023. PubMed: 16376892. [DOI] [PubMed] [Google Scholar]
- 15. Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH et al. (2006) Kappa-opioid receptors mediate cardioprotection by remote preconditioning. Anesthesiology 105: 550-556. doi: 10.1097/00000542-200609000-00019. PubMed: 16931988. [DOI] [PubMed] [Google Scholar]
- 16. Yu CK, Li YH, Wong GT, Wong TM, Irwin MG (2007) Remifentanil preconditioning confers delayed cardioprotection in the rat. Br J Anaesth 99: 632-638. doi: 10.1093/bja/aem261. PubMed: 17872933. [DOI] [PubMed] [Google Scholar]
- 17. Mykytenko J, Kerendi F, Reeves JG, Kin H, Zatta AJ et al. (2007) Long-term inhibition of myocardial infarction by postconditioning during reperfusion. Basic Res Cardiol 102: 90-100. doi: 10.1007/s00395-006-0625-0. PubMed: 17003965. [DOI] [PubMed] [Google Scholar]
- 18. Zheng Z, Yang M, Zhang F, Yu J, Wang J et al. (2011) Gender-related difference of sevoflurane postconditioning in isolated rat hearts: focus on phosphatidylinositol-3-kinase/Akt signaling. J Surg Res 170: e3-e9. doi: 10.1016/S0022-4804(11)00696-2. PubMed: 21704330. [DOI] [PubMed] [Google Scholar]
- 19. Zhao H, Wang Y, Wu Y, Li X, Yang G et al. (2009) Hyperlipidemia does not prevent the cardioprotection by postconditioning against myocardial ischemia/reperfusion injury and the involvement of hypoxia inducible factor-1alpha upregulation. Acta Biochim Biophys Sin (Shanghai) 41: 745-753. doi: 10.1093/abbs/gmp063. [DOI] [PubMed] [Google Scholar]
- 20. Penumathsa SV, Thirunavukkarasu M, Koneru S, Juhasz B, Zhan L et al. (2007) Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol 42: 508-516. doi: 10.1016/j.yjmcc.2006.10.018. PubMed: 17188708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roach PD, Balasubramaniam S, Hirata F, Abbey M, Szanto A et al. (1993) The low-density lipoprotein receptor and cholesterol synthesis are affected differently by dietary cholesterol in the rat. Biochim Biophys Acta 1170: 165-172. doi: 10.1016/0005-2760(93)90067-J. PubMed: 8399341. [DOI] [PubMed] [Google Scholar]
- 22. Horton JD, Cuthbert JA, Spady DK (1995) Regulation of hepatic 7 alpha-hydroxylase expression and response to dietary cholesterol in the rat and hamster. J Biol Chem 270: 5381-5387. doi: 10.1074/jbc.270.10.5381. PubMed: 7890651. [DOI] [PubMed] [Google Scholar]
- 23. Kupai K, Csonka C, Fekete V, Odendaal L, van Rooyen J et al. (2009) Cholesterol diet-induced hyperlipidemia impairs the cardioprotective effect of postconditioning: role of peroxynitrite. Am J Physiol Heart Circ Physiol 297: H1729-H1735. doi: 10.1152/ajpheart.00484.2009. PubMed: 19734363. [DOI] [PubMed] [Google Scholar]
- 24. Görbe A, Varga ZV, Kupai K, Bencsik P, Kocsis GF et al. (2011) Cholesterol diet leads to attenuation of ischemic preconditioning-induced cardiac protection: the role of connexin 43. Am J Physiol Heart Circ Physiol 300: H1907-H1913. doi: 10.1152/ajpheart.01242.2010. PubMed: 21398600. [DOI] [PubMed] [Google Scholar]
- 25. Wang TD, Chen WJ, Su SS, Lo SC, Lin WW et al. (2002) Increased cardiomyocyte apoptosis following ischemia and reperfusion in diet-induced hypercholesterolemia: relation to Bcl-2 and Bax proteins and caspase-3 activity. Lipids 37: 385-394. doi: 10.1007/s1145-002-0906-2. PubMed: 12030319. [DOI] [PubMed] [Google Scholar]
- 26. Okamura T, Miura T, Takemura G, Fujiwara H, Iwamoto H et al. (2000) Effect of caspase inhibitors on myocardial infarct size and myocyte DNA fragmentation in the ischemia-reperfused rat heart. Cardiovasc Res 45: 642-650. doi: 10.1016/S0008-6363(99)00271-0. PubMed: 10728385. [DOI] [PubMed] [Google Scholar]
- 27. Gustafsson AB, Gottlieb RA (2007) Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol 292: C45-C51. PubMed: 16943242. [DOI] [PubMed] [Google Scholar]
- 28. Wang X, Xie W, Zhang Y, Lin P, Han L et al. (2010) Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ Res 107: 76-83. doi: 10.1161/CIRCRESAHA.109.215822. PubMed: 20466981. [DOI] [PubMed] [Google Scholar]
- 29. Cao CM, Zhang Y, Weisleder N, Ferrante C, Wang X et al. (2010) MG53 constitutes a primary determinant of cardiac ischemic preconditioning. Circulation. 121: 2565-2574. doi: 10.1161/CIRCULATIONAHA.110.954628. PubMed: 20516375. [DOI] [PubMed] [Google Scholar]
- 30. Iliodromitis EK, Andreadou I, Prokovas E, Zoga A, Farmakis D et al. (2010) Simvastatin in contrast to postconditioning reduces infarct size in hyperlipidemic rabbits: possible role of oxidative/nitrosative stress attenuation. Basic Res Cardiol 105: 193-203. doi: 10.1007/s00395-009-0078-3. PubMed: 20066537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of sevoflurane on the expression of MG53 in sham-operated rat hearts. Normocholesterolemic and hypercholesterolemic sham-operated rats were treated with 2.4% sevoflurane via sevoflurane vaporizer for 5-min. Then hearts were harvested for immunoblotting. NC: normocholesterolemia; HC: hypercholesterolemia. Sev: sevoflurane. Data are mean ± SD, n = 6 hearts/group.
(TIF)