Abstract
Background and aim
The use of transdermal therapeutic systems has spread worldwide since they allow effective local drug delivery. In the present study, we investigated the efficacy and safety of a new betamethasone valerate medicated plaster (Betesil®) to manage facial swelling, edema, inflammation, ecchymosis, and hematoma, when applied immediately after a facial rejuvenation procedure.
Materials and methods
We applied the plaster to the skin of 20 healthy patients for 12 hours immediately after hyaluronic acid-based procedure performed with the aim of erasing facial wrinkles of perioral and nasolabial folds and improving chin and eye contour. A further 20 patients underwent the same cosmetic procedure, but they were treated with an aescin 10% cream (applied immediately after the procedure, in the evening, and the morning after) and served as control group.
Results
Betesil® application resulted in a significant improvement in swelling/edema/inflammation score, if compared with aescin 10% cream (P < 0.01). As for facial ecchymosis and hematoma around the needle injection track, only two patients in the active treatment group displayed minimal ecchymosis and hematoma. In the control group, two patients presented minimal ecchymosis and three slight hematoma. However, using the ecchymosis/hematoma score, no significant difference between Betesil® and aescin 10% cream groups was observed. Patients’ satisfaction was significantly higher among subjects receiving Betesil®, if compared to patients receiving aescin 10% cream (P < 0.01).
Conclusion
The present study supports the use of Betesil® plaster immediately after facial cosmetic procedures in order to safely control swelling, edema, and inflammation.
Keywords: aesthetic medicine, transdermal therapeutic system, betamethasone valerate, hyaluronic acid
Introduction
Corticosteroids are a versatile option for treatment of many pathologies, including dermatological conditions, due to their availability in a wide range of potencies and formulations.1–3 Betamethasone valerate (BMV) is a medium-potency corticosteroid without mineralocorticoid properties.4 BMV has been successfully used in a lotion formulation for treatment of seborrheic dermatitis and scalp psoriasis.5–7 Furthermore, the BMV foam formulation has been found effective and safe for treatment of several dermatological pathologies, such as moderate-to-severe scalp psoriasis,4 scalp seborrheic dermatitis,8 mild-to-moderate plaque psoriasis (not affecting the scalp),9 alopecia areata,10 and stasis dermatitis.11 A ready-to-use medicated plaster for continuous and sustained transdermal delivery of BMV, Betesil® (IBSA Farmaceutici Italia, Lodi, Italy), has recently been developed to treat several cutaneous inflammatory conditions. The transdermal therapeutic system is a very popular modality of drug administration whose pharmacokinetics has gained wide acceptance by avoiding hepatic first-pass metabolism and fluctuation in plasma drug concentration observed following oral therapies. The administration is easier, and it is possible to interrupt treatment immediately, if necessary. Although the transdermal therapeutic system is an attractive alternative to oral administration, only a limited number of drugs are available in the form of transdermal plasters.12 Many studies have confirmed the superiority of BMV plasters over the cream formulation. In 2006, Pacifico et al13 enrolled 42 patients with mild-to-moderate psoriasis. The patients involved in this study were treated, in a half-side distribution, with a BMV 0.1% tape and BMV 0.12% cream for 30 days. After a 4-week therapy, both BMV 0.1% tape and BMV 0.12% cream were found effective in decreasing lesion size, but the tape caused a greater decrease in Psoriasis Area and Severity Index (PASI; 39.5% reduction), if compared with the BMV 0.12% cream (62% reduction), indicating a higher efficacy of the tape formulation. In a prospective, randomized, assessor-blind, parallel-group, active-controlled, multicenter Phase III study, Naldi et al14 evaluated the efficacy and safety of a new plaster containing BMV 0.1% and a BMV 0.1% cream, in patients affected by mild-to-moderate chronic plaque psoriasis. A total of 231 patients were randomized to receive BMV 0.1% plaster (116 patients) or BMV 0.1% cream (115 patients) for 3–5 weeks. Plasters were applied once daily, and they were worn for at least 20 consecutive hours. BMV 0.1% cream was applied twice a day, in the morning and evening. The plaster displayed a greater efficacy in decreasing the total size of target plaques, if compared with the cream. BMV plaster was also used with positive results in subjects with relapses of chronic tendinopathies, as shown by Salini and Abate.15 In that study, 15 patients affected by this condition were treated with a BMV plaster applied to the affected tendon. After 28 days of treatment, pain significantly decreased either at rest or during activities, and a significant improvement in functional limitation was also observed. Saraceno et al investigated the effects of BMV 0.1% tape in patients with prurigo nodularis, a chronic skin condition characterized by pruriginous nodules.16 Twelve patients were treated with the BMV tape or a moisturizing itch-relief cream containing feverfew, over a period of 4 weeks. The tape was applied once a day for 24 hours to the nodular lesions in the left side of the body and changed every morning, while the cream was applied to the lesions present in the right side twice a day. Over the 4-week treatment period, lesions treated with the tape showed a greater reduction in itching, excoriation, and infiltration from baseline, if compared with those lesions treated with the cream. To date, no study has investigated the clinical efficacy of BMV medicated plaster in improving facial swelling, edema, inflammation, ecchymosis, and hematoma following facial rejuvenation procedures.
Aim
In the present study we tested the hypothesis that a new BMV medicated plaster can safely reduce facial swelling, edema, inflammation, ecchymosis, and hematoma in a series of healthy patients who underwent an intradermal hyaluronic acid-based facial rejuvenation procedure with the aim of erasing facial wrinkles of the perioral and nasolabial folds and improving chin and eye contour. Addressing the above mentioned complications is crucial due to the increase in the clinical use of hyaluronic acid, not only in the aesthetic medicine clinic,17,18,19 but also in restorative medicine20 and for the treatment of other pathologies, such as lower-leg telangiectasia,21 premature ejaculation,22 and osteoarthritis.23,24,25
Materials and methods
The present study was performed at the Poliambulatorio del Secondo Parere (Modena, Italy). This study was designed and performed according to the Declaration of Helsinki and approved by the local Institutional Review Board.
Patients and study design
All patients signed the informed consent before the beginning of the procedure. Forty patients (8 males and 32 females), aged between 37 and 65 (mean 52.04 ± 1.4 years), participated in the study. The patients underwent a rejuvenation procedure to erase facial wrinkles of the perioral and nasolabial folds and improve chin and eye contour. After this procedure, the patients were randomized to receive either a BMV plaster (Betesil®; n=20) applied immediately after the procedure for 12 hours or an aescin 10% cream (Nédema® Crema Gel; Agave Farmaceutici, Prato, Italy; n=20) applied immediately after the procedure, in the evening and the morning after (control group). Aescin cream was chosen since this compound possesses well-documented anti-edematous and anti-inflammatory properties.26
Treatment and assessment of results
After cooling the skin, the subjects were injected (total of 5.21 ± 0.2 injections varying according to the individual patient’s medical needs) with 2 mL of cross-linked hyaluronic acid (Aliaxin® Global Performance; IBSA Farmaceutici Italia; 25 mg/mL; molecular weight 1000–2000 kDa; extrusion force 23 ± 3 Newton) with free contents of 1,4-butanediol diglycidyl ether (BDDE) (<0.1 ppm), using a 27 G needle and cannula, in the deep malar areas, followed by a single mesotherapy injection, using a 27 G needle, into the cheeks, chin, and mandible line, with 2 mL of a compound composed of 32 mg non-cross-linked hyaluronic acid (700–800 kDa) plus a complex including ammonium molybdate, ammonium metavanadate, calcium chloride, iron sulphate, potassium chloride, copper sulphate, magnesium chloride, manganese sulphate, sodium acetate, sodium hydrogen carbonate, sodium chloride, sodium hydrogen phosphate, sodium metasilicate, sodium selenite, stannous chloride, zinc sulphate, alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glutamic acid, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine, adenine (vitamin B4), biotin (vitamin B8), calcium pantothenate (vitamin B5), choline chloride, folic acid (vitamin B9), inositol (vitamin B7), nicotinammide (vitamin B3), pyridoxine (vitamin B6), riboflavin (vitamin B2), thiamine (vitamin B1), vitamin B12, deoxythymidine, glucose, putrescine, sodium pyruvate, and lipoic acid (Viscoderm® Skinko E; IBSA Farmaceutici Italia). A mesotherapy injection of the same compound was repeated once a month for 2 months with the aim of prolonging the effect of the first injection of cross-linked hyaluronic acid. This particular combination of the two different hyaluronic acid products had produced successful results in previous unpublished rejuvenation procedures performed at the Poliambulatorio del Secondo Parere (Modena, Italy). At the end of the procedure, a Betesil® (IBSA Farmaceutici Italia) plaster was applied to the face over the treated areas, and cut to shape to adequately cover the injected surfaces (Figures 1 and 2), and no other oral or topical medication was allowed. Betesil® is a 75 × 100 mm transparent medical plaster, with an adhesive layer containing 30 μg/cm2 BMV. The plaster is self-adhesive and waterproof and can be cut in order to be properly adapted to the treated skin area.
Figure 1.
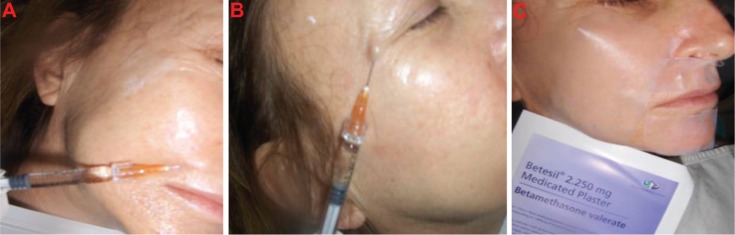
Aliaxin® Global Performance and Viscoderm® Skinko E injections (A and B) and application of Betesil® patch following cosmetic procedure (C).
Figure 2.
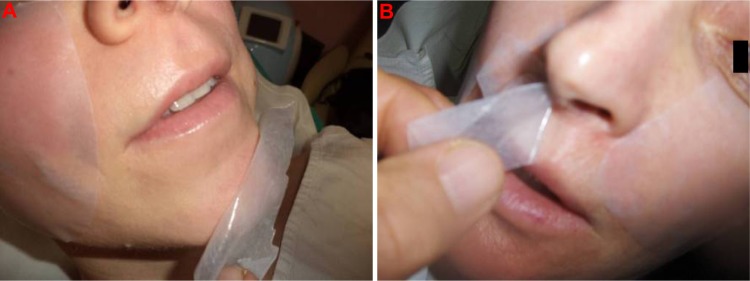
Good adhesiveness of Betesil® patch to the skin and ease of removal (A and B).
Each plaster contains 2.250 mg of BMV as active substance. The adhesive layer of the plaster contains sodium hyaluronate, 1,3-butylene glycol, glycerol, disodium edetate, tartaric acid, aluminum glycinate, polyacrylic acid, sodium polyacrylate, hydroxypropylcellulose, caramellose sodium, methyl parahydroxybenzoate, propyl parahydroxybenzoate, and purified water.
The day after the procedure, the patients were individually admitted to a follow-up examination where the following parameters were quantitatively evaluated:
Overall facial swelling/edema/inflammation (scored on a 0–4 scale): 0 = no swelling/edema/inflammation; 1 = minimal swelling/edema/inflammation; 2 = slight swelling/edema/inflammation; 3 = moderate swelling/edema/inflammation; and 4 = severe swelling/edema/inflammation.
Ecchymosis and hematoma around the needle injection track (scored on a 0–4 scale): 0 = no ecchymosis/hematoma; 1 = minimal ecchymosis/hematoma; 2 = slight ecchymosis/hematoma; 3 = moderate ecchymosis/hematoma; and 4 = severe ecchymosis/hematoma.
Subjective satisfaction of the patient after the procedure (scored on a 0–3 scale): 1 = not satisfied; 2 = quite satisfied; and 3 = very satisfied.
Time out from daily activities due to adverse reactions and patients’ compliance with the plaster, based on adhesiveness, skin reaction, and comfort, were also documented.
Statistical analysis
All data are represented as the means ± standard error of the mean (SEM) and were checked for normality using the Anderson–Darling test, using Minitab® 15 (Minitab Inc, State College, PA, USA). Data were then analyzed using GraphPad Prism 5 software (GraphPad Software Inc, San Diego, CA, USA). Facial swelling/edema/inflammation, ecchymosis/hematoma, and patient satisfaction score data were analyzed using a two-sample unpaired Student’s t-test.
Results
No drop-outs were observed in the present study. Betesil® application resulted in a significant improvement in the swelling/edema/inflammation score (0.3 ± 0.17), if compared with aescin 10% cream (1.3 ± 0.28) (P < 0.01) (Figure 3). Among the 20 patients receiving Betesil®, a patient presented minimal inflammation, a patient showed slight edema, and a patient displayed moderate swelling. Among the 20 patients receiving aescin 10% cream, seven reported no swelling, edema, or inflammation, five reported minimal edema and inflammation, four patients showed slight swelling, three had moderate swelling and inflammation, and a patient reported a severe degree of inflammation that resolved with ice application within 24 hours. When analyzing ecchymosis and hematoma around the needle injection track, using the ecchymosis/hematoma score, no difference was observed between the groups (Figure 4). In the group treated with Betesil® plaster, 18 patients did not show any ecchymosis or hematoma around the needle injection track a day after treatment, while two patients had minimal ecchymosis and hematoma (0.1 ± 0.06) (Figure 4). In the control group, 15 patients did not show any ecchymosis or hematoma, while two patients had minimal ecchymosis and three patients slight hematoma (0.4 ± 0.16) (Figure 4). Patient satisfaction score was significantly higher in patients receiving Betesil® (2.9 ± 0.06) versus subjects receiving aescin 10% cream (2.4 ± 0.16) (P < 0.01) (Figure 5). Among 20 patients from the Betesil® group, 18 were very satisfied with the procedure and two were quite satisfied. In the control group, eleven patients were very satisfied with the procedure, six were quite satisfied, and three were not satisfied (Figure 5).
Figure 3.
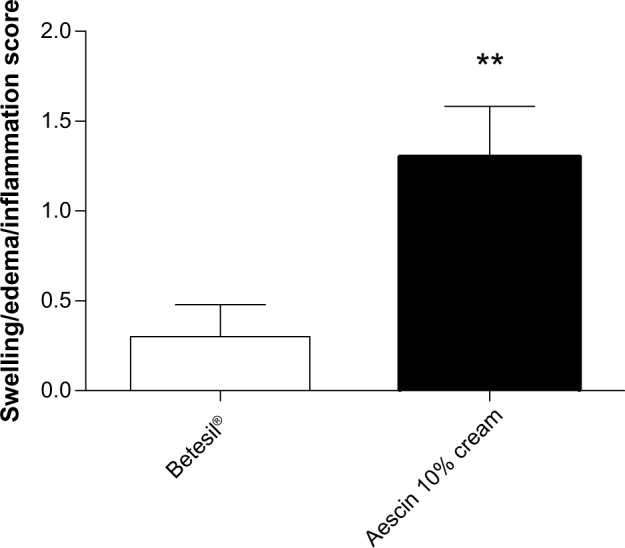
Facial swelling/edema/inflammation score in the Betesil®-treated group versus the control group, as assessed the day after the cosmetic procedure. Data are presented as the means ± SEM.
Note: **P < 0.01.
Abbreviation: SEM, standard error of the mean.
Figure 4.
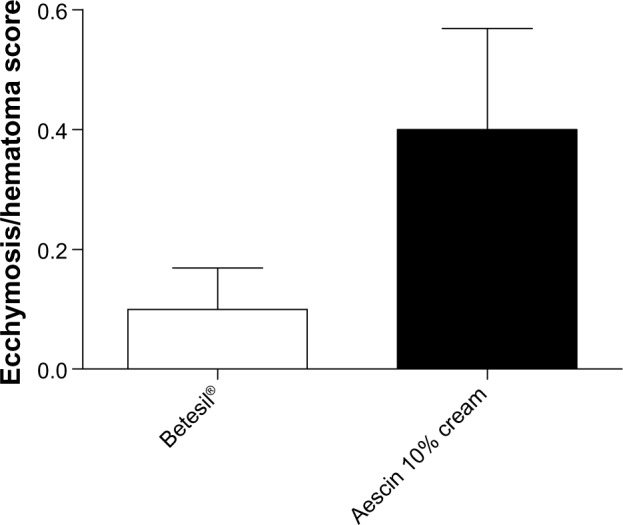
Facial ecchymosis/hematoma score in the Betesil®-treated group versus the control group, as assessed the day after the cosmetic procedure. Data are presented as the means ± SEM.
Abbreviation: SEM, standard error of the mean.
Figure 5.
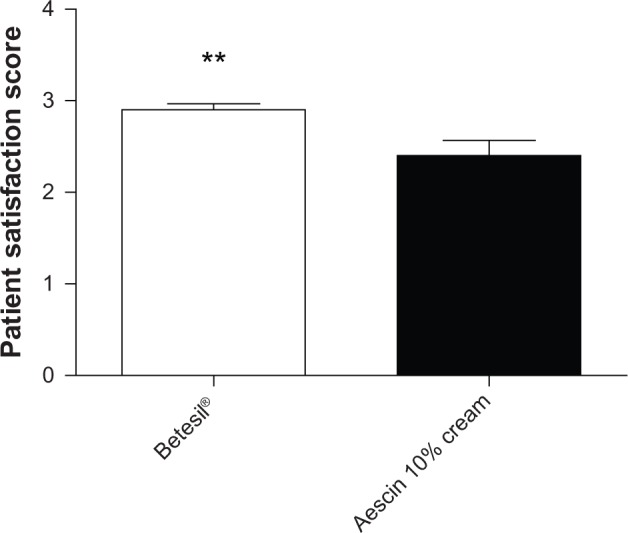
Patient satisfaction score in the Betesil®-treated group versus the control group, as assessed the day after the cosmetic procedure. Data are presented as the means ± SEM.
Note: **P < 0.01.
Abbreviation: SEM, standard error of the mean.
In the Betesil®-treated group, there was no withdrawal from daily activities, while in the control group, treatment caused absence from work activity for a day in two patients due to a severe degree of skin inflammation (first case) and ecchymosis (second case). The plaster was well tolerated by all patients except for a case of skin rash, which resolved without any treatment within 24 hours. All the patients declared that the plaster was comfortable to wear and showed good adhesiveness to the skin.
Discussion and conclusion
The present study shows that Betesil® plaster can be safely applied to the skin of patients undergoing facial rejuvenation procedures. Its application significantly reduced facial swelling, edema, and inflammation compared with aescin 10% cream. In the Betesil® group, only two patients showed minimal ecchymosis and hematoma, while in the control group, two patients had minimal ecchymosis and three slight hematoma, with no significant difference between groups. The few cases presenting ecchymosis and hematoma in both groups are explained by the safety profile of Aliaxin® Global Performance and Viscoderm® Skinko E that we previously observed in our clinic (unpublished observation). In the present study, patients’ satisfaction was higher in the active treatment group compared with the control group. This is the first study testing the clinical effectiveness of the Betesil® plaster in cosmetic medicine. The scientific interest in transdermal therapeutic systems has significantly increased in the last two decades because it represents a valid alternative to oral and hypodermic administration. However, currently, only a limited number of drugs are available as transdermal plasters. Indeed, the necessity for the drug released from a transdermal plaster to permeate through the stratum corneum and epidermis, in order to be taken up by capillary loops in the dermal papillae for systemic distribution, represents a rate-limiting step.27 Furthermore, previous studies also reported adverse effects caused by transdermal plasters, such as contact dermatitis, sensitization, and delayed hypersensitivity.28–44 As far as BMV plaster safety is concerned, Pacifico et al13 reported no serious local or systemic treatment-related adverse effects during 30 days of treatment in patients affected by mild-to-moderate psoriasis. No adverse effects were also reported by Saraceno et al16 in patients affected by prurigo nodularis treated for 4 weeks with BMV plasters, and by Naldi et al14 in patients affected by mild-to-moderate chronic plaque psoriasis treated with BMV plaster for 3–5 weeks. In our experience, Betesil® medicated plaster was well tolerated and contributed to the prevention of swelling, edema, and inflammation after hyaluronic acid-based facial rejuvenation procedure. BMV plaster has the advantages of an occlusive therapy, such as increased local hydration and rapid therapeutic response. Therefore, it increases patients’ compliance if compared with conventional occlusive bandages. BMV can also be applied to areas where the use of conventional occlusive bandages would not be possible. The plaster can be cut to fit specific areas that need pharmacological treatment. In this way, it is possible to avoid the use of cosmetically unappealing ointments that may also stain clothes. Furthermore, the high adherence of the plaster to the skin results in a longer persistence of the active ingredient in this location, without the need for continuous application of cream or ointment that can also be absorbed by clothing. In conclusion, the present study supports the use of Betesil® plaster immediately after facial cosmetic procedures in order to safely control facial swelling, edema, and inflammation.
Author contributions
The authors contributed equally to this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Buys LM. Treatment options for atopic dermatitis. Am Fam Physician. 2007;75(4):523–528. [PubMed] [Google Scholar]
- 2.Lipozencić J, Wolf R. Atopic dermatitis: an update and review of the literature. Dermatol Clin. 2007;25(4):605–612. doi: 10.1016/j.det.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007;370(9583):272–284. doi: 10.1016/S0140-6736(07)61129-5. [DOI] [PubMed] [Google Scholar]
- 4.Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. Int J Dermatol. 1999;38(8):628–632. doi: 10.1046/j.1365-4362.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 5.Harris JJ. A national double-blind clinical trial of a new corticosteroid lotion: a 12-investigator cooperative analysis. Curr Ther Res Clin Exp. 1972;14(9):638–646. [PubMed] [Google Scholar]
- 6.Marks R, Bhogal B, Wilson L. The effect of betamethasone valerate on seborrhoeic dermatitis of the scalp. A clinical, histopathological cell kinetic study. Acta Derm Venereol. 1974;54(5):373–375. [PubMed] [Google Scholar]
- 7.Medansky RS, Handler RM. Treating psoriasis of the scalp with a new corticosteroid lotion. IMJ Ill Med J. 1974;145(6):503–504. [PubMed] [Google Scholar]
- 8.Milani M, Di Molfetta AS, Gramazio R, et al. Efficacy of betamethasone valerate 0.1% thermophobic foam in seborrhoeic dermatitis of the scalp: an open-label, multicentre, prospective trial on 180 patients. Curr Med Res Opin. 2003;19(4):342–345. doi: 10.1185/030079903125001875. [DOI] [PubMed] [Google Scholar]
- 9.Stein LF, Sherr A, Solodkina G, Gotlieb AB, Chaudhari U. Betamethasone valerate foam for treatment of nonscalp psoriasis. J Cutan Med Surg. 2001;5(4):303–307. doi: 10.1007/s10227-001-0006-0. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso G, Balducci A, Casadio C, et al. Efficacy of betamethasone valerate foam formulation in comparison with betamethasone dipropionate lotion in the treatment of mild-to-moderate alopecia areata: a multicenter, prospective, randomized, controlled, investigator-blinded trial. Int J Dermatol. 2003;42(7):572–575. doi: 10.1046/j.1365-4362.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiss SC, Nguyen J, Chon S, Kimball AB. A randomized controlled clinical trial assessing the effect of betamethasone valerate 0.12% foam on the short-term treatment of stasis dermatitis. J Drugs Dermatol. 2005;4(3):339–345. [PubMed] [Google Scholar]
- 12.Subedi RK, Oh SY, Chun MK, Choi HK. Recent advances in transdermal drug delivery. Arch Pharm Res. 2010;33(3):339–351. doi: 10.1007/s12272-010-0301-7. [DOI] [PubMed] [Google Scholar]
- 13.Pacifico A, Daidone R, Peris K. A new formulation of an occlusive dressing containing betamethasone valerate 0.1% in the treatment of mild to moderate psoriasis. J Eur Acad Dermatol Venereol. 2006;20(2):153–157. doi: 10.1111/j.1468-3083.2006.01387.x. [DOI] [PubMed] [Google Scholar]
- 14.Naldi L, Yawalkar N, Kaszuba A, et al. Efficacy and safety of the Betamethasone valerate 0.1% plaster in mild-to-moderate chronic plaque psoriasis: a randomized, parallel-group, active-controlled, phase III study. Am J Clin Dermatol. 2011;12(3):191–201. doi: 10.2165/11539780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Salini V, Abate M. Percutaneous steroidal treatment in relapses of chronic tendinopathies: a pilot study. Int J Immunopathol Pharmacol. 2011;24(1):211–216. doi: 10.1177/039463201102400125. [DOI] [PubMed] [Google Scholar]
- 16.Saraceno R, Chiricozzi A, Nisticò SP, Tiberti S, Chimenti S. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21(6):363–366. doi: 10.3109/09546630903386606. [DOI] [PubMed] [Google Scholar]
- 17.Iannitti T, Capone S, Palmieri B. Short review on face rejuvenation procedures: focus on preoperative antiseptic and anesthetic delivery by JetPeel™-3 (a high pressure oxygen delivery device) Minerva Chir. 2011;66(3 Suppl 1):1–8. [PubMed] [Google Scholar]
- 18.Palmieri B, Capone S. Mesoterapia con acido ialuronico. Trattamento di rughe e cicatrici per il ringiovanimento cutaneo [Mesotherapy with hyaluronic acid. Treatment of wrinkles and scars for skin rejuvenation] Hi Tech Dermo. 2010;2:29–36. Italian. [Google Scholar]
- 19.Marusza W, Mlynarczyk G, Olszanski R, et al. Probable biofilm formation in the cheek as a complication of soft tissue filler resulting from improper endodontic treatment of tooth 16. Int J Nanomedicine. 2012;7:1441–1447. doi: 10.2147/IJN.S27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iannitti T, Bingöl AO, Rottigni V, Palmieri B. A new highly viscoelastic hyaluronic acid gel: rheological properties, biocompatibility and clinical investigation in esthetic and restorative surgery. Int J Pharm. July2013 doi: 10.1016/j.ijpharm.2013.06.066. Epub. [DOI] [PubMed] [Google Scholar]
- 21.Iannitti T, Rottigni V, Torricelli F, Palmieri B. Combination Therapy of Hyaluronic Acid Mesotherapic Injections and Sclerotherapy for Treatment of Lower Leg Telangiectasia Without Major Venous Insufficiency: A Preliminary Clinical Study. Clin Appl Thromb Hemost. 2013 Jan 2; doi: 10.1177/1076029612461844. Epub. [DOI] [PubMed] [Google Scholar]
- 22.Littara A, Palmieri B, Rottigni V, Iannitti T. A clinical study to assess the effectiveness of a hyaluronic acid-based procedure for treatment of premature ejaculation. Int J Impot Res. 2013;25(3):117–120. doi: 10.1038/ijir.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmieri B, Rottigni V, Iannitti T. Preliminary study of highly cross-linked hyaluronic acid-based combination therapy for management of knee osteoarthritis-related pain. Drug Des Devel Ther. 2013;7:7–12. doi: 10.2147/DDDT.S37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012;25(4):1093–1098. doi: 10.1177/039463201202500426. [DOI] [PubMed] [Google Scholar]
- 25.Iannitti T, Lodi D, Palmieri B. Intra-articular injections for the treatment of osteoarthritis: focus on the clinical use of hyaluronic acid. Drugs R D. 2011;11(1):13–27. doi: 10.2165/11539760-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res. 2001;44(3):183–193. doi: 10.1006/phrs.2001.0847. [DOI] [PubMed] [Google Scholar]
- 27.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 28.Bircher AJ, Howald H, Rufli T. Adverse skin reactions to nicotine in a transdermal therapeutic system. Contact Dermatitis. 1991;25(4):230–236. doi: 10.1111/j.1600-0536.1991.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 29.Buckley DA, Wilkinson SM, Higgins EM. Contact allergy to a testosterone patch. Contact Dermatitis. 1998;39(2):91–92. doi: 10.1111/j.1600-0536.1998.tb05847.x. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael AJ, Foulds IS. Allergic contact dermatitis from oestradiol in oestrogen patches. Contact Dermatitis. 1992;26(3):194–195. doi: 10.1111/j.1600-0536.1992.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 31.Eichelberg D, Stolze P, Block M, Buchkremer G. Contact allergies induced by TTS-treatment. Methods Find Exp Clin Pharmacol. 1989;11(3):223–225. [PubMed] [Google Scholar]
- 32.Färm G. Contact allergy to nicotine from a nicotine patch. Contact Dermatitis. 1993;29(4):214–215. doi: 10.1111/j.1600-0536.1993.tb03545.x. [DOI] [PubMed] [Google Scholar]
- 33.Fisher AA. Dermatitis due to transdermal therapeutic systems. Cutis. 1984;34(6):526–527. 530–531. [PubMed] [Google Scholar]
- 34.Gordon CR, Shupak A, Doweck I, Spitzer O. Allergic contact dermatitis caused by transdermal hyoscine. BMJ. 1989;298(6682):1220–1221. doi: 10.1136/bmj.298.6682.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jordan WP. Clinical evaluation of the contact sensitization potential of a transdermal nicotine system (Nicoderm) J Fam Pract. 1992;34(6):709–712. [PubMed] [Google Scholar]
- 36.Machet L, Martin L, Toledano C, Jan V, Lorette G, Vaillant L. Allergic contact dermatitis from nitroglycerin contained in 2 transdermal systems. Dermatology. 1999;198(1):106–107. doi: 10.1159/000018082. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Calderón R, Gonzalo-Garijo MA, Rodriguez-Nevado I. Generalized allergic contact dermatitis from nitroglycerin in a transdermal therapeutic system. Contact Dermatitis. 2002;46(5):303. doi: 10.1034/j.1600-0536.2002.460513.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeld AS, White WB. Allergic contact dermatitis secondary to transdermal nitroglycerin. Am Heart J. 1984;108(4 Pt 1):1061–1062. doi: 10.1016/0002-8703(84)90488-5. [DOI] [PubMed] [Google Scholar]
- 39.Shouls J, Shum KW, Gadour M, Gawkrodger DJ. Contact allergy to testosterone in an androgen patch: control of symptoms by pre-application of topical corticosteroid. Contact Dermatitis. 2001;45(2):124–125. doi: 10.1034/j.1600-0536.2001.045002124.x. [DOI] [PubMed] [Google Scholar]
- 40.Topaz O, Abraham D. Severe allergic contact dermatitis secondary to nitroglycerin in a transdermal therapeutic system. Ann Allergy. 1987;59(5):365–366. [PubMed] [Google Scholar]
- 41.Torres V, Lopes JC, Leite L. Allergic contact dermatitis from nitroglycerin and estradiol transdermal therapeutic systems. Contact Dermatitis. 1992;26(1):53–54. doi: 10.1111/j.1600-0536.1992.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 42.Trozak DJ. Delayed hypersensitivity to scopolamine delivered by a transdermal device. J Am Acad Dermatol. 1985;13(2 Pt 1):247–251. doi: 10.1016/s0190-9622(85)70167-3. [DOI] [PubMed] [Google Scholar]
- 43.van der Willigen AH, Oranje AP, Stolz E, van Joost T. Delayed hypersensitivity to scopolamine in transdermal therapeutic systems. J Am Acad Dermatol. 1988;18(1 Pt 1):146–147. doi: 10.1016/s0190-9622(88)80056-2. [DOI] [PubMed] [Google Scholar]
- 44.Vincenzi C, Tosti A, Cirone M, Guarrera M, Cusano F. Allergic contact dermatitis from transdermal nicotine systems. Contact Dermatitis. 1993;29(2):104–105. doi: 10.1111/j.1600-0536.1993.tb03500.x. [DOI] [PubMed] [Google Scholar]


