Abstract
2-aminophenol (2-AP) is a toxic nitrogen-containing aromatic pollutant. Burkholderia xenovorans LB400 possess an amn gene cluster that encodes the 2-AP catabolic pathway. In this report, the functionality of the 2-aminophenol pathway of B. xenovorans strain LB400 was analyzed. The amnRJBACDFEHG cluster located at chromosome 1 encodes the enzymes for the degradation of 2-aminophenol. The absence of habA and habB genes in LB400 genome correlates with its no growth on nitrobenzene. RT-PCR analyses in strain LB400 showed the co-expression of amnJB, amnBAC, amnACD, amnDFE and amnEHG genes, suggesting that the amn cluster is an operon. RT-qPCR showed that the amnB gene expression was highly induced by 2-AP, whereas a basal constitutive expression was observed in glucose, indicating that these amn genes are regulated. We propose that the predicted MarR-type transcriptional regulator encoded by the amnR gene acts as repressor of the amn gene cluster using a MarR-type regulatory binding sequence. This report showed that LB400 resting cells degrade completely 2-AP. The amn gene cluster from strain LB400 is highly identical to the amn gene cluster from P. knackmussi strain B13, which could not grow on 2-AP. However, we demonstrate that B. xenovorans LB400 is able to grow using 2-AP as sole nitrogen source and glucose as sole carbon source. An amnBA − mutant of strain LB400 was unable to grow with 2-AP as nitrogen source and glucose as carbon source and to degrade 2-AP. This study showed that during LB400 growth on 2-AP this substrate was partially converted into picolinic acid (PA), a well-known antibiotic. The addition of PA at lag or mid-exponential phase inhibited LB400 growth. The MIC of PA for strain LB400 is 2 mM. Overall, these results demonstrate that B. xenovorans strain LB400 posses a functional 2-AP catabolic central pathway, which could lead to the production of picolinic acid.
Introduction
Several aromatic compounds with nitro and amino groups such as 2-aminophenol (2-AP) are toxic and persistent organic pollutants (POPs) in the environment. 2-AP is a compound used in the production of dyes, plastics and pharmaceuticals, which could be released in industrial wastewaters, polluting the environment [1], [2]. 2-AP is cytotoxic [3] and has carcinogenic potential [4]. Therefore, the removal of 2-AP is required for the clean-up of polluted sites. Some microorganisms are able to degrade aerobically 2-AP [1], [5].
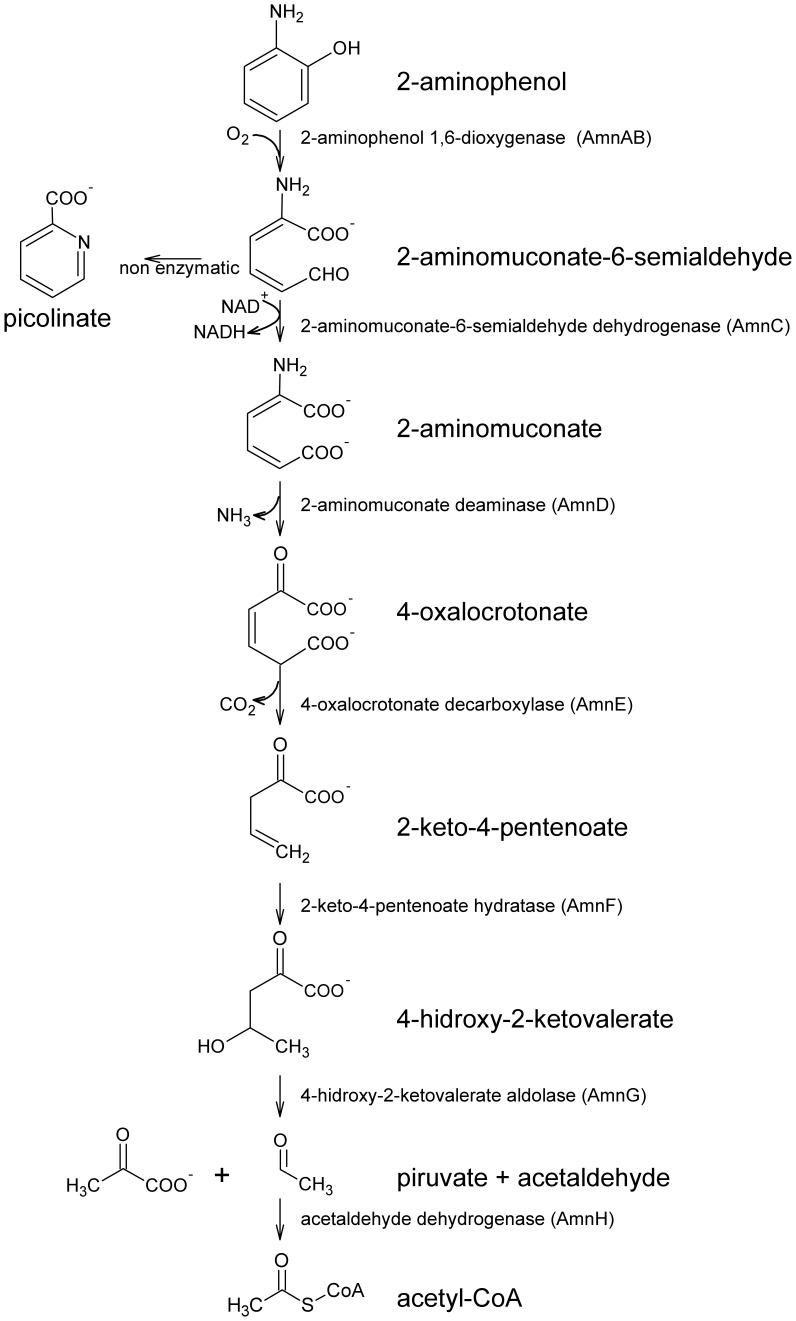
The aerobic 2-AP metabolic pathway (Fig. 1) has been described in Pseudomonas pseudoalcaligenes JS45, Pseudomonas putida HS12, Pseudomonas sp. AP-3 and Pseudomonas knackmussii B13 [6]–[9]. The enzymes AmnBA, AmnC, AmnE, AmnD, AmnF, AmnG, AmnH are involved in the degradation of 2-AP into pyruvate and acetyl-CoA [1], [8], [10]–[12]. 2-aminophenol-1,6-dioxygenase (AmnAB) converts 2-AP through an extradiol meta cleavage into 2-aminomuconic 6-semialdehyde (2-AMS). 2-AMS is further oxidized by the AmnC dehydrogenase into 2-aminomuconic acid (2-AM), which is converted by AmnD into 4-oxalocrotonic acid with a concomitant release of ammonium. 4-oxalocrotonic acid is further degraded by the enzymes AmnE, AmnF, AmnG and AmnH into pyruvate and acetyl-CoA.
Figure 1. Model of the aerobic metabolism of 2-aminophenol in bacteria.
The substrate and product(s) of each enzyme are indicated. The non-enzymatic production of picolinic acid during 2-AP catabolism in B. xenovorans LB400 is depicted.
B. xenovorans LB400 is a model bacterium for the degradation of polychlorobiphenyls (PCBs) and other aromatic compounds [13]–[15]. Its genome comprises a major chromosome (C1), a minor chromosome (C2) and one megaplasmid (MP) [15]. A wide range of aromatic compounds including PCBs, hydroxyphenylacetates and hydroxybenzoates are metabolized by a number of peripheral catabolic pathways into eleven central catabolic pathways [14]–[17]. Strain LB400 possesses the amn genes that encode the enzymes from the 2-AP central catabolic pathway (Fig. 1), but the functionality of this pathway has not been studied. The aim of this study was to determine the functionality of the 2-AP catabolic pathway from B. xenovorans LB400.
Materials and Methods
Chemicals
2-Aminophenol (99% purity), nitrobenzene (>98% purity) and picolinic acid (99% purity), were obtained from Sigma-Aldrich (Saint Louis, MO, USA).
Bacterial Strain and Culture Conditions
B. xenovorans LB400 was cultivated in Luria Bertani (LB) medium and in nitrogen-free BLKN medium [18] using glucose (10 mM) as sole carbon source at 30°C. For selective growth of strain LB400, OF basal medium agar (Oxoid) with glucose 1% (w/v) and supplemented with bacitracin (0.2 U/mL) and polymyxin B (0.3 U/mL), and BLKN minimal medium agar with NH4Cl (1 mM) and crystal of biphenyl (1 mg) were used.
To study growth using 2-AP as carbon and nitrogen source in liquid media, LB400 cells previously cultured in BLKN medium using biphenyl (10 mM) as sole carbon source and NH4Cl (10 mM) as sole nitrogen source, harvested by centrifugation and washed twice with sodium phosphate buffer (0.02 M, pH 7.0), were incubated in BLKN minimal medium with benzoate (10 mM) and NH4Cl (10 mM) for 24 h at 30°C on a rotary shaker (150 rpm). The cells were harvested by centrifugation, washed twice with sodium phosphate buffer and suspended in BLKN medium supplemented with glucose (10 mM), 2-AP (1 mM) or NH4Cl (1 mM) in darkness. Growth was determined by measuring turbidity at 525 nm and by counting colony-forming units (CFU). The CFU were determined using a microdot method in LB plates and were calculated as the mean ± SD of at least three independent experiments. To study the growth of strain LB400 using 2-AP as nitrogen and carbon source on solid media, BLKN agar with and without glucose (10 mM) was used. 2-AP crystals (1 mg) and/or an aliquot (5 µL) of nitrobenzene (1 M) were added. The plates were inoculated with LB400 cells and incubated at 30°C for 48 h.
Minimum inhibitory concentration (MIC) of PA for strain LB400 was determined by Mueller-Hinton broth (Difco, Detroit, USA) microdilution at 5×105 CFU/mL according to Clinical and Laboratory Standards Institute (CLSI) guidelines [19]. Mueller-Hinton medium was supplemented with PA in the concentration range from 0.032 mM (4 µg/ml) to 4.159 mM (512 µg/ml): 0.032, 0.065, 0.130, 0.260, 0.520, 1.040, 2.079 and 4.159 mM. MIC for PA was the lowest concentration at which no LB400 growth was observed. MIC analysis was done in triplicate.
Generation of an amnBA − Mutant Strain by Gene Disruption
A B. xenovorans LB400 mutant defective for 2-aminophenol 1,6-dioxygenase encoded by the amnB and amnA genes was constructed by gene disruption through homologous recombination. For this purpose, an amnBA fragment (430 bp), which was amplified using the primers AR1 y BF2, was cloned in the pCR2.1-TOPO cloning vector. Resulting plasmid pCR2.1amnBA was digested with EcoRI, and the EcoRI fragment of pCR2.1amnBA was cloned into EcoRI site of pK18mobsacB and introduced into E. coli CC118 [λpir] prior to selection for Km-resistance clones carrying amnBA fragment. Resulting plasmid pK18mobsacBamnBA was transferred into B. xenovorans LB400 by triparental mating with E. coli HBH101 (pRK600) as helper strain [20]. Km-resistance colonies able to utilize biphenyl as sole carbon source were selected. The amnBA − mutant was verified by PCR amplification with primer sets M13R and AR1, M13R and CR, M13R and DR, JF and CR.
RNA Isolation
Total RNA was isolated from LB400 cells using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturers’ recommendations. DNase I treatment was carried out using the RNase-Free DNase set (Qiagen, Hilden, Germany) to degrade any residual DNA. Amplification of 16S rRNA gene and amnB gene using specific primer sets (Table 1) were used as controls to exclude DNA contamination. The RNA concentration was quantified using a Qubit fluorometer (Invitrogen) and a Nanodrop spectrophotometer (Thermo Scientific). RNA integrity was tested by agarose (1%) gel electrophoresis.
Table 1. Primers used in this study.
| Gene | Primer | Sequence (5′–3′) | Reference | |
| amnR | RF | ACCTACCGCTACATACGACTG | This study (*) | |
| RR | TCAAACCACTGCTGCTCATC | * | ||
| amnJ | JF | CTCGGATGTCTATGTCTCGG | * | |
| amnA | AF1 | GTGGTGGCGCTATTGTCCAG | * | |
| AR1 | CAGCAAGTGCTTTGCCGGCT | |||
| amnB | BF1 | CGCATCTGGTCTATGGGGA | * | |
| BF2 | CTACGACGGCTACTTGTGGGA | * | ||
| BR1 | GTTGCCAGTGCCGATGAC | * | ||
| BR2 | GACCTCGGTTCGTTCTGA | * | ||
| amnC | CR | GACAGCCTCATCAACCATCTC | * | |
| amnD | DF | TTTTTGTCTCAGGCACCAG | * | |
| DR | ATCGGGTCAGGCAGCTGA | * | ||
| amnE | EF | AGGTCGGCTCCACGGCGGC | * | |
| ER | GATGACATCGGACAGCCCCAT | * | ||
| amnG | GR | CCTGAGGCACAGAGTATTGATGGC | * | |
| 16S rRNA | 27f | AGAGTTTGATCMTGGCTCAG | 16 | |
| 1492r | TACGGYTACCTTGTTACGACT | 16 | ||
| ftsZ | FZF1 | CGATTACGGTGCGCTGGATA | * | |
| FZR1 | ATGCCGGAATGTCGTACGTG | * | ||
| internal sequence (pK18mobsacB) | M13R | AACAGCTATGACCATG | Life Technologies | |
RT-PCR and Real-Time RT-PCR
Reverse transcription-PCR (RT-PCR) was carried out with sequence-specific primers design in this study (Table 1) by using SuperScript One-step RT-PCR with Platinum Taq (Invitrogen, Carlsbad, USA). The co-expression assays were performed using the primers indicated in Table 1. The cycles of amplification (35 cycles) was carried outs as follows: 95°C for 1 min, 60°C for 0.5 min, 72°C for 2 min, after an initial denaturation at 95°C for 5 min. Negative and positive controls were included in each RT-PCR assay. At least two independent RT-PCR reactions for each condition were done to assess reproducibility.
For RT-qPCR, total RNA (100 ng) was transcribed with Verso cDNA kit (Thermo Scientific, Lafayette, USA). The RT-qPCR was performed according to the MIQE guidelines [21]. Real-time PCR was performed on Mx3000P qPCR system (Stratagene), using the Kapa Sybr fast qPCR master mix (2×) universal kit (Kapabiosystems, Boston, USA) and 0.3 µM of each primer. Samples were initially denatured at 95°C for 5 min. A 40-cycle amplification and quantification protocol (95°C for 15 s, 62°C for 15 s and 72°C for 15 s) with a single fluorescence measurement per cycle followed by a melting-curve program (95°C for 15 s, 25°C for 0.1 s, 70°C for 15 s and 95°C for 0.1 s) were used according to manufacturer’s recommendations. The PCR melting curves confirm the amplification of a single product for each primer pair. The BF2 and BR1 primers were used for amnB gene amplification, yielding a product of 208 bp. The ftsZ (BxeA0491) gene was amplified as a reference gene, yielding an amplicon of 113 bp. A standard curve in triplicate was made with 5 serial dilutions (10 fold) for each amplicon in a linear range (9.2 ng –0.09 pg) of LB400 genomic DNA. qPCR efficiencies were calculated from the slopes of the log-linear portion of the calibration curves, using the equation E = 10(−1/slope). The qPCR efficiency rate was 1.95 for both amnB and ftsZ genes. The ftsZ gene was stably expressed according to the algorithms of BestKeeper [22]. Relative amnB gene expression ratios were determined by the Pfaffl method [23], normalizing the gene expression to LB400 cells grown in BLKN medium with glucose as sole carbon source and NH4Cl as sole nitrogen source.
Degradation Assay
Resting cells (turbidity 525 nm = 0.6) were incubated in sodium phosphate buffer (50 mM, pH 7.2) with 2-AP (1 mM). Aliquots of cell suspensions were taken at different incubation times and centrifuged (19,283×g for 2 min). Assays with boiled cells and without cells were used as controls. Cell-free supernatants were analyzed, using a Waters liquid chromatograph model 515 equipped with a UV detector and a RP-C18/Lichrospher 5-mm column (Supelco, Bellefonte, USA). The aqueous mobile phase contained 20% methanol, 10% acetonitrile and 70% sodium acetate (0.1 M) solution. The flow rate was 1 mL min−1. 2-AP was quantified using calibration curves with authentic standards. Resting cells experiments were performed in triplicate.
Metabolism of 2-AP
LB400 cells grown in BLKN medium were incubated in presence of 2-AP (1 mM) in darkness at 30°C. Aliquots (2 mL) were collected by centrifuged (19,283×g for 2 min) at different times and cell-free supernatants were analyzed by reverse phase chromatography with a Jasco liquid chromatograph equipped with a diode array detector and a Whatman C-18 column (25 cm by 4.6 mm ID). The aqueous mobile phase contained 20% methanol, 20% acetonitrile and 0.1% phosphoric acid and the flow rate was 1.0 mL min−1. The compounds were monitored at 278 nm. Under these conditions, 2-AP and picolinic acid have retention times of 4.56 and 3.73 min, respectively. 2-AP and picolinic acid were quantified using commercial authentic standards.
Bioinformatics Analysis
The amn genes and the neighborhood in the genome of strain LB400 was analyzed using the Vector NTI suite 9.0 software. For the sequence alignment the database of the National Center for Biotechnology Information (NCBI/BLAST Home) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used. Clustal W software was used for analysis of the sequence of amnB gene with selected sequences retrieved from GenBank. The software package MEGA 5.0 [24] was used for phylogenetic analyses. A phylogenetic tree was built by using the Neighbor-Joining method with a bootstrap analysis of 5000.
Results
Bioinformatic Analysis of the amn Genes in B. xenovorans Strain LB400
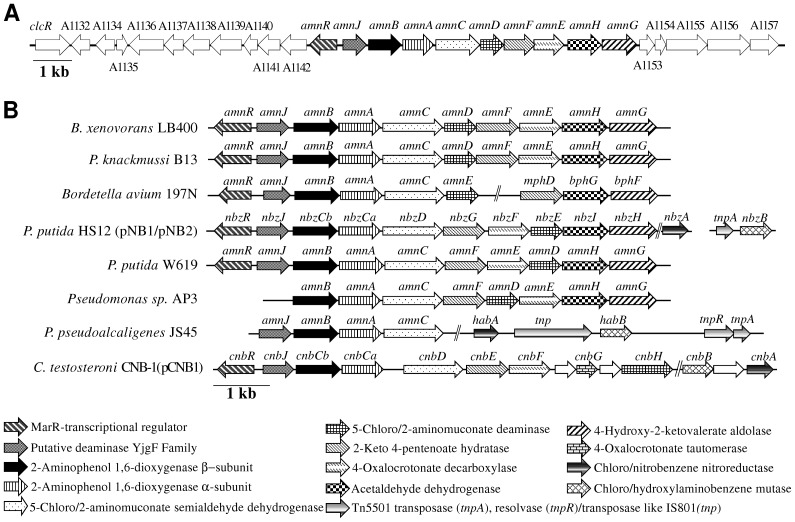
The amn genes from B. xenovorans LB400 are clustered at C1 (Fig. 2). The amn gene cluster includes the BxeA1143, BxeA1144, BxeA1145, BxeA1146, BxeA1147, BxeA1148, BxeA1149, BxeA1150, BxeA1151 and BxeA1152 genes (hereafter amnR, amnJ, amnB, amnA, amnC, amnD, amnF, amnE, amnH and amnG genes, respectively) and has an extension of 8,529 bp. The predicted amn genes of strain LB400 are listed in Table 2. The amnRJBACDFEHG gene cluster encodes the enzymes of the catabolic pathway for the conversion of 2-aminophenol into pyruvate and acetyl-CoA (Fig. 1). The amnBA genes encode the 2-aminophenol-1,6-dioxygenase that open the 2-aminophenol ring via meta-cleavage, producing 2-AMS. The predicted amnC gene encodes 2-aminomuconic-6-semialdehyde dehydrogenase that oxidizes 2-AMS into 2-AM. The amnD gene encodes the enzyme 2-aminomuconic deaminase that converts 2-AM into 4-oxalocrotonic acid and ammonium, which could be used as nitrogen source for bacterial growth. The predicted amnE encodes the 4-oxalocrotonate decarboxylase, which converts 4-oxalocrotonate into 2-keto-4-pentenoate. The amnF gene encodes 2-keto-4-pentenoate hydratase that oxidizes 2-keto-4-pentenoate into 4-hidroxy-2-ketopentanoate. The predicted amnG gene encodes 4-hidroxy-2-ketovalerate aldolase that degrades 4-hidroxy-2-ketopentanoate into pyruvate and acetaldehyde. The amnH gene encodes the enzyme acetaldehyde dehydrogenase for the final conversion of acetaldehyde into acetyl-CoA. The amnR and amnJ genes are also present in the amn gene cluster from strain LB400. The amnR gene encodes a MarR-type transcriptional regulator, which probably acts as transcriptional repressor of the amn gene cluster. The amnJ gene is present in diverse bacterial amn gene clusters, suggesting that its product is required for the 2-AP biochemical pathway. Gaillard et al. [9] proposed that AmnJ is a ferredoxin-like protein. We have observed that the AmnJ protein has a conserved domain from the YjgF super family proteins that have enamine/imine deaminase activity [25]–[26].
Figure 2. Organization of the predicted genes encoding the 2-aminophenol central pathway in B. xenovorans LB400 and comparison of bacterial amn gene clusters.
A, The amn genes encoding the 2-aminophenol central pathway located at the major chromosome (C1). Genes of the amn gene cluster neighborhood are represented with white arrows. The genes located upstream of the amn gene cluster encode the following proteins: clcR, LysR-type transcriptional regulator of the 3-chlorocatechol pathway; BxeA1132, pseudogene; BxeA1134, LysR-type transcriptional regulator; BxeA1135, XRE-type transcriptional regulator; BxeA1136 LysR-type transcriptional regulator; BxeA1137, MarR-type transcriptional regulator; BxeA1138, TetR-type transcriptional regulator; BxeA1139, hypothetical protein; BxeA1140, DoxX family protein; BxeA1141, TetR-type transcriptional regulator; BxeA1142, DoxX family protein. The genes located downstream of amn gene cluster encode the following proteins: BxeA1153, putative esterase; BxeA1154, TraG conjugal transfer coupling protein; BxeA1155, outer membrane protein from MFS multidrug efflux system; BxeA1156, inner membrane protein from MFS multidrug efflux system; BxeA1157, transmembrane protein from MFS multidrug efflux system. The sizes of the genes and the intergenic regions are on scale. B, Comparison of bacterial amn gene clusters. The amn sequences (accession number) are: B. xenovorans LB400 (NC007951), P. knackmussii B13 (AJ617740), Bordetella avium 197N (NC010645), P. putida HS12 plasmid pNB1 (AF319593) and plasmid pNB2 (AF319592), P. putida W619 (CP000949), Pseudomonas sp. AP3 (AB020521), P. pseudoalcaligenes JS45 (PPU93363), Comamonas testosteroni CNB-1 plasmid pCNB1 (EF079106).
Table 2. Identification of genes involved in the 2-aminophenol degradation pathway in B. xenovorans LB400.
| ORF | Gene | Orientation | Size (aa) | Function | Homologya (source) | Accession number | E valueb | Amino acid identity | |
| % | aa range | ||||||||
| BxeA1143 | amnR | R | 205 | MarR-family transcriptional regulator | MarR-family transcriptional regulator(Bordetella avium 197N) | ABE31805.1 | 1.0 E-110 | 79 | 154 |
| BxeA1144 | amnJ | F | 137 | Putative imino/enamina deaminase | Putative ferredoxin (Pseudomonas putida pNB1) | ABE31804.1 | 1.0 E-27 | 49 | 136 |
| BxeA1145 | amnB | F | 304 | 2-Aminophenol 1,6-dioxygenase, β subunit | 2-Aminophenol 1,6-dioxygenase β subunit(Bordetella avium 197N) | ABE31803.1 | 1.0 E-162 | 89 | 300 |
| BxeA1146 | amnA | F | 270 | 2-Aminophenol 1,6-dioxygenase, α subunit | 2-Aminophenol 1,6-dioxygenase α subunit(Bordetella avium 197N) | ABE31802.1 | 1.0 E-119 | 74 | 202 |
| BxeA1147 | amnC | F | 492 | 2-Aminomuconate-6-semialdehyde dehydrogenase | 2-Aminomuconate-6-semialdehyde dehydrogenase(Bordetella avium 197N) | ABE31801.1 | 0.0 | 88 | 434 |
| BxeA1148 | amnD | F | 145 | 2-Aminomuconate deaminase | 2-Aminomuconate deaminase(Bordetella avium 197N) | ABE31800.1 | 1.0 E-62 | 83 | 121 |
| BxeA1149 | amnF | F | 269 | 2-Keto-4-pentenoate hydratase | 2-Keto 4-pentenoate hydratase(Alcaligenes sp. O-1) | ABE31799.1 | 7.0 E-95 | 63 | 166 |
| BxeA1150 | amnE | F | 254 | 4-Oxalocrotonate decarboxylase | 4-Oxalocrotonate decarboxylase(Burkholderia multivorans CGD2M) | ABE31798.1 | 6.0 E-94 | 71 | 174 |
| BxeA1151 | amnH | F | 313 | Acetaldehyde dehydrogenase | Acetaldehyde dehydrogenase (acylating)(Comamonas testosteroni CNB1 pCNB1) | ABE31797.1 | 1.0 E-143 | 83 | 262 |
| BxeA1152 | amnG | F | 345 | 4-Hydroxy 2- ketovalerate aldolase | 4-Hydroxy-2-ketovalerate aldolase(Comamonas testosteroni CNB1 pCNB1) | ABE31796.1 | 1.0 E-164 | 87 | 290 |
The amn genes from B. xenovorans LB400 and P. knackmussii B13 are almost identical; therefore the identity between them are not listed.
E values are based on BLASTP results of the non-redundant NCBI database.
The genetic neighborhood of the amn gene cluster in strain LB400 was analyzed. The genes located upstream (between BxeA1142 and BxeA1131 (clcR) genes) and downstream (between BxeA1153 and BxeA1157) of the amn gene cluster were studied (Fig. 2A). Diverse transcriptional regulator genes are located upstream of the amn gene cluster. The BxeA1141, BxeA1138, BxeA1137, BxeA1136, BxeA1135 and BxeA1134 genes encode a TetR-type transcriptional regulator closely related to a TetR-type regulator from Rhizobium leguminosarum bv. viciae USDA 2370 (accession number EJB01162.1; 56% identity), a TetR-type transcriptional regulator closely related to a TetR-type regulator protein from Acidovorax sp. JS42 (ABM43064.1; 93% identity), a MarR-type transcriptional regulator closely related to a MarR-type regulator protein from Acidovorax sp. JS42 (ABM43065.1; 77% identity), a LysR-type transcriptional regulator closely related to a LysR-type regulator protein from Bordetella petrii DSM 12804 (CAP41606.1; 81% identity), a XRE-type transcriptional regulator closely related to a XRE regulator protein from Alcaligenes sp. HPC1271 (EKU29625.1; 86% identity), and a LysR-type transcriptional regulator closely related to a LysR-type regulator protein from Acidovorax delafieldii 2AN (EER60975.1; 67% identity), respectively (Fig. 2). Three genes located downstream of the amn gene cluster encode membrane transporter proteins. The BxeA1155, BxeA1156 and BxeA1157 genes encode three components (an outer membrane protein, an inner membrane protein and a transmembrane protein, respectively) of a major facilitator superfamily (MFS) multidrug efflux system. This multidrug efflux system possesses high identity with a multidrug efflux transporter pump from Ralstonia solanacearum GMI1000 [27], including the channel-forming component (CAD17593.1; 54% identity), the inner membrane protein (CAD17592.1; 58% identity) and the transmembrane protein (CAD17591.1; 57% identity), respectively. The BxeA1154 gene encodes a TraG conjugal transfer coupling protein that has 33% identity with the TraG protein (accession number AFT71063.1) from Alcanivorax dieselolei B5 [28].
The analysis of the genetic organization of the amn genes in bacteria indicate a conserved amnJBACD gene cluster encoding enzymes for the conversion of 2-AP until 4-oxalocrotonate, and amnFEHG genes encoding enzymes involved in the transformation of 4-oxalocrotonate into acetyl-CoA. Regulation of the amn gene cluster is generally mediated by an amnR gene-encoded Mar-type family transcriptional regulator located upstream of the amn gene cluster. B. xenovorans LB400, P. knackmussii B13 [9] and Bordetella avium 197N [29] shared the amnRJBACD gene organization (Fig. 2). P. putida strains HS12 and W619 shared the amnRJBAC gene cluster, whereas the amnD gene is located after the amnFE genes. P. pseudoalcaligenes strain JS45 and Pseudomonas sp. strain AP-3 showed a similar amnBAC gene organization, but in these strains the amnR gene and amnRJ genes have not been reported (Fig. 2). In Pseudomonas sp. strain AP-3 the amnD gene is located between amnF and amnE genes. Comamonas testosteroni strain CNB-1 possesses a similar gene cluster for the 2-amino-5-chlorophenol catabolism [30]. The cnbJBACD gene cluster that encodes the enzymes for the conversion of 2-amino-5-cholorophenol into 4-oxalocrotonate has the same gene organization (Fig. 2). The genetic organization of amnFEHG genes, which encode the enzymes for the conversion of 4-oxolocrotonate into acetyl-CoA and is conserved in other bacteria, is located in B. xenovorans strain LB400 downstream of the amnRJBACD gene cluster. However, in Pseudomonas strains HS12, W619 and AP-3, the amnD gene is located within the amnFEGH cluster (Fig. 2). The amnFEGH region has not been reported in the amn gene cluster of P. pseudoalcaligenes strain JS45.
The nitrobenzene catabolic peripheral pathway funnels into the 2-aminophenol central pathway in bacteria. The habA and habB genes that encode enzymes involved in the conversion of nitrobenzene into 2-aminophenol are absent in the genome of B. xenovorans LB400. In the 2-AP-degraders P. putida HS12 and P. pseudoalcaligenes JS45 the hab genes are located distantly from the amn gene cluster (Fig. 2). In accordance with the absence of the hab genes, strain LB400 was unable to grow using nitrobenzene as sole nitrogen or carbon source.
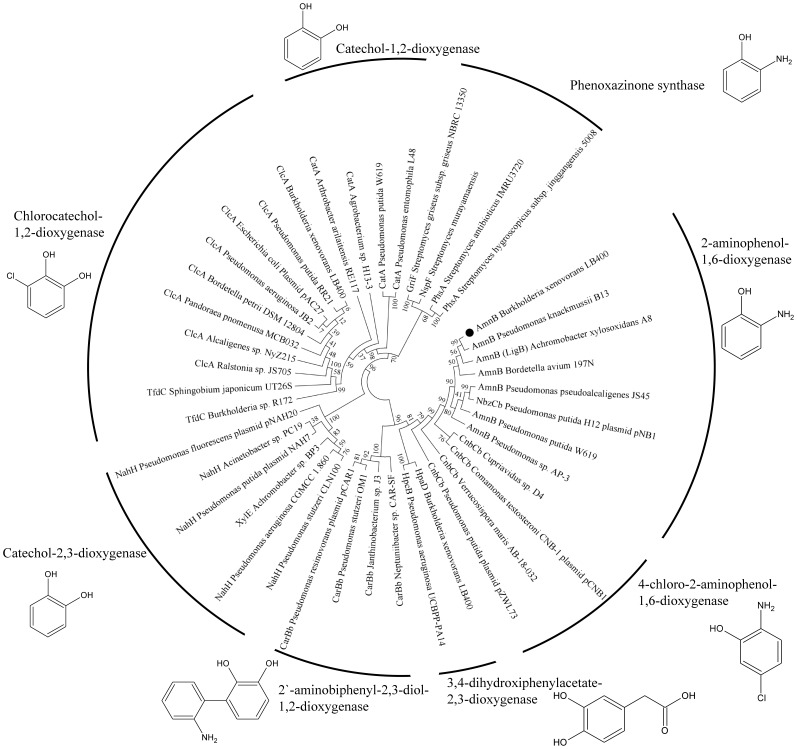
The phylogenetic sequence analysis of the 2-aminophenol 1,6-dioxygenase AmnB β-subunits and related class III ring-cleavage dioxygenases is illustrated in Figure 3. The 2-aminophenol 1,6-dioxygenase β-subunits were clustered in two branches. One branch includes the AmnB proteins of B. xenovorans LB400, P. knackmussii B13, B. avium 197N and Achromobacter xylosoxidans A8. The second branch comprises 2-aminophenol 1,6-dioxygenase β-subunits from Pseudomonas sp. AP-3, P. putida W619, P. putida HS12 and P. pseudoalcaligenes JS45. This sequence analyses suggest that AmnB protein of B. xenovorans LB400 evolved separately from AmnB protein of Pseudomonas sp AP-3 and P. pseudoalcaligenes strain JS45. This is in accordance with the genetic organization of amn gene clusters (Fig. 2). The gene organization of amnJRBACD cluster of B. xenovorans strain LB400, P. knackmussii B13, B. avium 197N is identical, and their AmnB enzymes were classified in the same branch in the phylogenetic tree. P. pseudoalcaligenes JS45 and Pseudomonas sp AP-3 showed a different amn gene cluster than B. xenovorans strain LB400 and also their AmnB proteins were classified in a different phylogenetic branch. The AmnB proteins are closely related to the 2-amino-5-chlorophenol-1,6-dioxygenase CnbCb proteins that oxidizes 4-chloro-2-aminophenol, but less related to dioxygenases subunits involved in the oxidation of 3,4-dihydroxyphenylacetate and 2′-aminobiphenyl-2,3-diol. All these dioxygenases shared a common ancestor with catechol 2,3-dioxygenase NahH and XylE proteins. Phenoxazinone synthases, which use the same substrate as AmnB proteins, are closely related to catechol 1,2-dioxygenases but not related to 2-aminophenol 1,6-dioxygenase AmnB β-subunits.
Figure 3. Phylogenetic tree showing the relatedness of AmnB and other class III ring-cleavage dioxygenases.
The dendrogram was constructed by the neighbor-joining method using MEGA 5.02 based on sequence alignments calculated by Clustal W. Sequence of deduced protein from amnB gene from B. xenovorans strain LB400 is highlighted (black circle). The proteins (accession number) are: AmnB B. xenovorans LB400 (YP559855.1); AmnB P. knackmussii B13 (CAE92875.1); AmnB (LigB) Achromobacter xylosoxidans A8 (YP003978676.1); AmnB Bordetella avium 197N (YP785083.1); AmnB P. pseudoalcaligenes JS45 (AAB71524.1); NbzCb P. putida HS12 plasmid pNB1 (AAK26519.1); AmnB P. putida W619 (YP001748883.1); AmnB Pseudomonas sp. AP-3 (O33477.1); CnbCb C. testosteroni CNB-1 plasmid pCNB1 (YP001967698.1); CnbCb Cupriavidus sp. D4 (AEI74584.1); CnbCb Verrucosispora maris AB-18-032 (YP004405267.1); CnbCb P. putida plasmid pZWL73 (DQ306889.1); HpaD B. xenovorans LB400 (YP553304.1); HpcB P. aeruginosa UCBPP-PA14 (ABJ13394.1); CarBb Neptuniibacter sp. CAR-SF (BAG30828.1); CarBb Janthinobacterium sp. J3 (BAC56744.1); CarBb P. stutzeri OM1 (BAA31269.1); CarBb P. resinovorans plasmid pCAR1 (BAC41547.1); NahH Acinetobacter sp. PC19 (AAW81679.1); NahH P. putida plasmid NAH7 (AAA98183.1); XylE Achromobacter sp. BP3 (ACF20629.1); NahH P. stutzeri CLN100 (CAD62376.1); NahH P. aeruginosa CGMCC 1.860 (ACV05014.1); NahH P. fluorescens plasmid pNAH20 (AAW81680.2); TfdC Sphingobium japonicum UT26S (YP_003544400.1); TfdC Burkholderia sp. R172 (Q7WYF8); ClcA Ralstonia sp. JS705 (CAA06968.1); ClcA Alcaligenes sp. NyZ215 (ABQ23434.1); ClcA Pandoraea pnomenusa MCB032 (ABS81346.1); ClcA B. xenovorans LB400 (ABE31816.1); ClcA E. coli plasmid pAC27 (AAA98281.1); ClcA P. aeruginosa JB2 (AAC69474.1); ClcA P. putida RR21 (CAE92861); ClcA Bordetella petrii DSM12804 (CAP41873.1); CatA Arthrobacter arilaitensis Re117 (YP_003918477.1); CatA P. putida W619 (ACA73195.1); CatA P. entomophila L48 (CAK15903.1); CatA Agrobacterium sp. H13-3 (YP_004279743.1); PhsA Streptomyces hygroscopicus subsp. jinggangensis 5008 (YP006248289.1); PhsA Streptomyces antibioticus IMRU3720 (AAA86668.1); GriF Streptomyces griseus subsp. griseus NBRC13350 (BAG21075.1); NspF Streptomyces murayamaensis (BAJ08174.1).
Transcription of the amn Genes
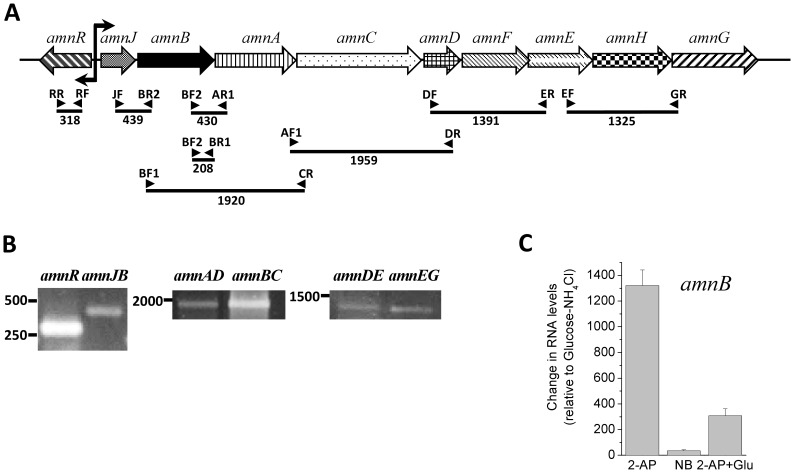
The transcription of the amn genes in strain LB400 was studied using specific primer sets (Fig. 4A). To study the co-expression of the amn genes, diverse primers sets were designed to amplify adjacent genes. RT-PCR of mRNA from LB400 cells grown until exponential phase in BLKN medium with glucose and 2-AP as sole carbon and nitrogen sources were performed. The expression of the amnR gene in strain LB400 was observed (Fig. 4B). Two divergent σ70-type promoters were identified in the amn gene cluster, which are located between the amnR gene and the amnJ gene. RT-PCR analyses showed the co-expression for amnJB, amnBAC, amnACD, amnDFE and amnEHG genes (Fig. 4B), indicating that the amn gene cluster is an operon in strain LB400. The co-expression of the amn genes strongly suggests that the promoter located upstream of the amnJ gene permits the expression of an amnJBACDFEHG single transcript. A putative MarR-type regulatory binding sequence (TAGTACGTATACGTTTCA) was identified upstream of the amnJBACDFEHG gene cluster, which showed a consensus MarR-type regulator binding sequence (5′-TAG(G/T)ACGTATACGTACTA-3′) located upstream of the amnJ gene from diverse 2-AP-degrading bacteria: P. putida W619, C. testosteroni CNB1, P. pseudoalcaligenes JS45, P. knackmussii B13 and Bordetella avium 197N. The amnB gene encodes the 2-aminophenol 1,6-dioxygenase beta subunit, which is the key enzyme of 2-AP catabolic pathway. The expression of amnB gene in LB400 cells grown in glucose in presence or absence of 2-AP and nitrobenzene was studied. Real time RT-PCR analysis showed that the amnB gene has a basal expression during growth in glucose. The amnB gene expression was highly induced by 2-AP, whereas lower induction was observed in cells during exposure to 2-AP and glucose (Fig. 4C). The lowest induction of amnB gene expression was observed in cells during exposure to nitrobenzene. Overall these results indicate a regulated expression of the amn gene cluster in strain LB400.
Figure 4. Transcriptional analysis of the amn genes.
A, Organization of the amn genes and schematic representation of the location and size of the amplicons obtained by RT-PCR experiments. Predicted promoters are shown as break arrows, bent in the directions of transcription. Primers are represented by arrowheads. B, PCR products from RT-PCR experiments using as templates mRNA obtained from LB400 cells collected at early exponential growth phase in liquid BLKN minimal medium with glucose (10 mM) and 2-AP (1 mM) as sole carbon and nitrogen source, respectively. The primer pairs used are listed in Table 1. The amplification products are amnR (318 bp), amnJB (439 bp), amnACD (1959 bp), amnBAC (1920 bp), amnDFE (1391 bp) and amnEHG (1325 bp). C, Transcriptional analysis of the amnB gene. RT-qPCR assays were performed using mRNA from LB400 cells grown in BLKN minimal medium supplemented with glucose (10 mM) and NH4Cl (1 mM) until exponential phase (turbidity at 600 nm of 0.5) and further incubated for 1 h in BLKN medium in presence of glucose (10 mM) and NH4Cl (1 mM), 2-aminophenol (1 mM) (2-AP), nitrobenzene (1 mM) (NB) or glucose (10 mM) and 2-AP (1 mM) (Glu+2-AP). The primer pairs used are listed in Table 1. The ftsZ gene was used as a reference gene.
2-aminophenol Degradation
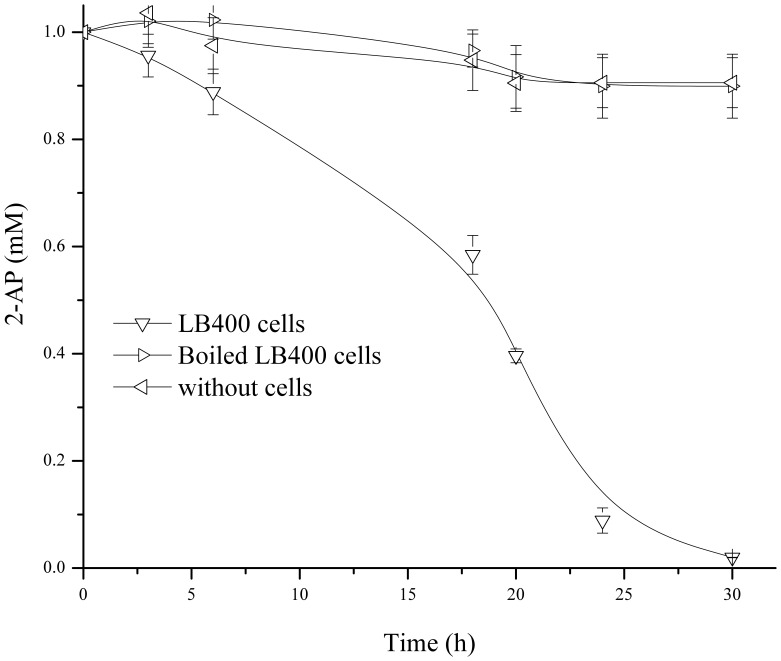
To further characterize the 2-AP catabolic pathway, 2-aminophenol degradation by strain LB400 was studied. Resting LB400 cells were incubated with 2-AP. LB400 cells degrade 15% of 2-AP after 6 h of incubation, whereas complete degradation was observed after 30 h (Fig. 5). In contrast, 2-AP was not degraded by boiled LB400 cells. The amnBA − mutant of strain LB400 is not able to degrade 2-AP, indicating that 2-aminophenol-1,6-dioxygenase is the sole enzyme involved in 2-AP degradation in strain LB400.
Figure 5. 2-aminophenol degradation by B. xenovorans LB400.
Glucose-grown LB400 cells were incubated in phosphate buffer with 2-AP (1 mM). Control assays with boiled cells or without cells are depicted. Each point is an average ± SDs of results from, at least, three independent assays.
Growth of Strain LB400 on 2-aminophenol
The expression of the amn genes and the degradation of 2-AP, suggest a functional 2-AP catabolic pathway in B. xenovorans strain LB400. In a further analysis of this metabolic pathway, the growth of strain LB400 in minimal medium with 2-AP as nitrogen or carbon source was studied. In a first approach, the growth of strain LB400 on BLKN agar plates in presence of 2-aminophenol crystals in absence or presence of glucose was studied. Small colonies of LB400 cells grown around the 2-AP crystals used as sole nitrogen source or as sole carbon and nitrogen source on BLKN plates were observed (data not shown). An increased number of small colonies around the 2-AP crystals on plates with 2-AP and supplemented with nitrobenzene was observed, suggesting that nitrobenzene stimulate growth probably by the induction of the 2-AP catabolic pathway genes. These small colonies were phenotypically confirmed as B. xenovorans LB400. The bacteria of the colonies were able to grow in minimal medium supplemented with biphenyl as sole carbon and energy source and show typical colonies on OFBPL medium agar (data not shown). In contrast, no colonies were observed on plates with nitrobenzene as sole carbon and nitrogen source (data not shown).
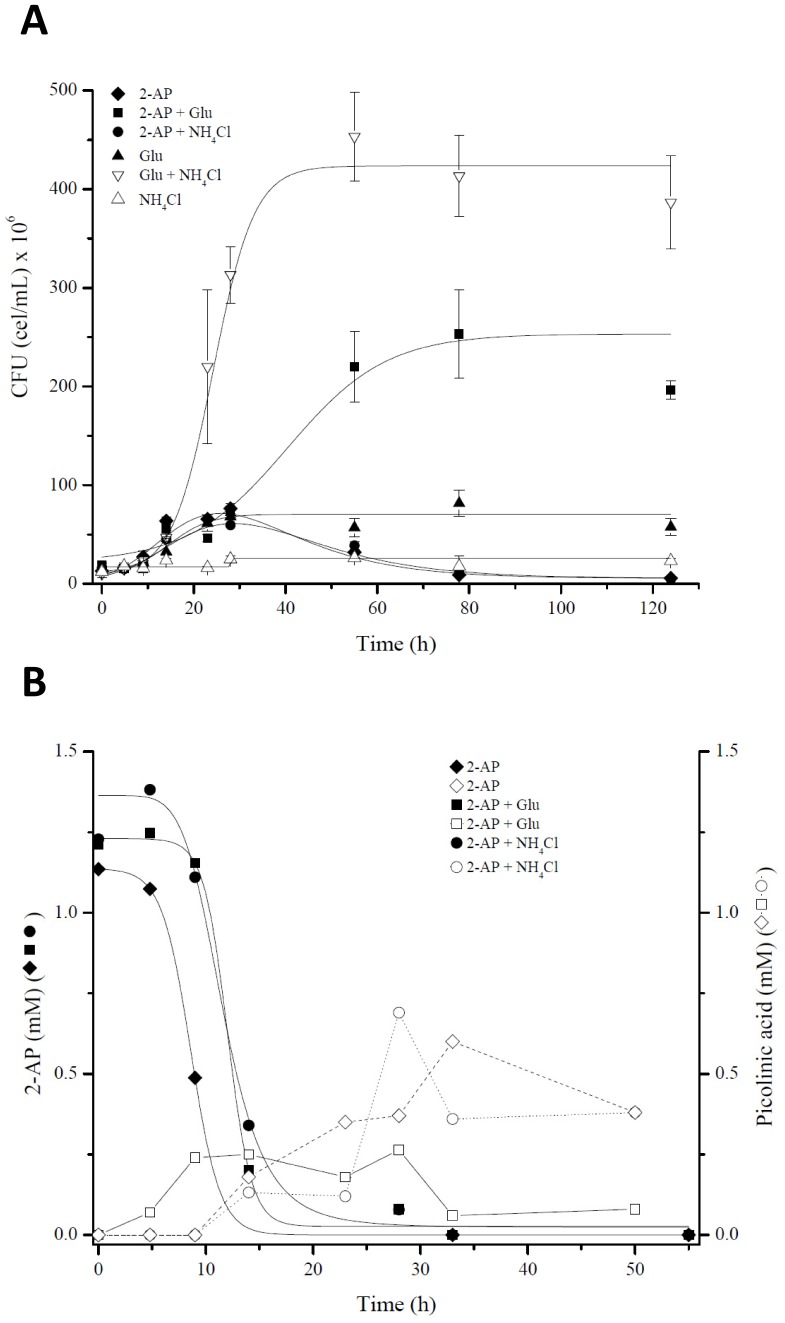
In a second approach, the growth of strain LB400 in liquid BLKN medium with 2-AP (1 mM) as sole nitrogen source or as sole nitrogen and carbon source was analyzed. Growth of LB400 cells in BLKN medium with 2-AP as sole nitrogen source and glucose as carbon source was observed (Fig. 6A). However, the cells reached lower biomass than cells incubated with NH4Cl as sole nitrogen source and glucose as sole carbon source. These results suggest that an inhibitory metabolite was produced during LB400 growth on 2-AP and glucose. LB400 cells showed a minimal growth using 2-AP as sole carbon and nitrogen source, which was similar to growth on glucose in absence of a nitrogen source, suggesting that in absence of other carbon and nitrogen source, 2-AP could be used as carbon source, but not as nitrogen source. No LB400 growth was observed in presence of NH4Cl and absence of a carbon source (Fig. 6A). To link the LB400 growth on 2-AP with the amn-encoded catabolic pathway, the growth on 2-AP of an amnBA − mutant derivative of B. xenovorans LB400 generated by homologous recombination was studied. The amnBA − mutant was unable to grow in BLKN medium with 2-AP as sole nitrogen source and glucose as carbon source, which correlates with no degradation of 2-AP (data not shown).
Figure 6. Growth of strain LB400 on 2-aminophenol.
A, Growth curve of strain LB400 in liquid BLKN minimal medium supplemented with 2-AP (1 mM), glucose (10 mM) and NH4Cl (1 mM). Growth was monitored measuring CFU. Each point is an average ± SDs of results from, at least, three independent assays. B, Degradation of 2-AP (filled symbols) and production of picolinic acid (empty symbols) during LB400 growth under these conditions were quantified by HPLC.
To study if during the degradation of 2-AP by strain LB400 a toxic metabolite was produced, the supernatants of cultures mentioned above were analyzed by HPLC. 2-AP was degraded during the first 15 h, independently if 2-AP was used as nitrogen or as carbon source (Fig. 6B). Interestingly, 2-AP degradation correlates with the production of a metabolite. This metabolite was identified as picolinic acid (PA) by comparison of its absorption spectrum and HPLC retention time with an authentic standard. Picolinic acid could be produced by a spontaneous non-enzymatic reaction than convert 2-AMS into PA. 2-AMS is produced from 2-AP by the 2-aminophenol-1,6-dioxygenase. During 2-AP degradation only partial conversion into PA was observed. The maximal PA concentration reached during growth using 2-AP (1 mM) as carbon source or as carbon and nitrogen source was approximately 0.5 mM, which correlates with a low cell growth. When 2-AP was used as sole nitrogen source a higher biomass was reached whereas a lower PA concentration (0.25 mM) was observed.
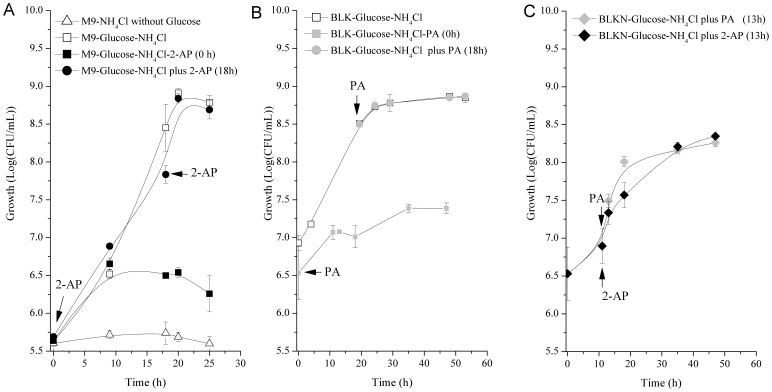
Figure 7 illustrate the effects of 2-AP and PA on B. xenovorans strain LB400 growth. LB400 growth on glucose and NH4Cl was not affected by the addition of 2-AP at late exponential phase (Fig. 7A). In contrast, when 2-AP was present since lag phase, a low cell density was reached at stationary phase. These results suggest that 2-AP has to be metabolized to exert an inhibitory effect on LB400 growth. On the other side, the effect of the addition of PA on LB400 growth was studied (Fig. 7B). The addition of PA at the lag phase reduced significantly the biomass reached at exponential phase of LB400 culture growing with glucose and NH4Cl. However, the addition of PA at late exponential phase did not affect the cell growth. The addition of 2-AP or PA at mid-exponential phase of LB400 cultures growing with glucose and NH4Cl inhibited also the cell growth (Fig. 7C). Finally, the minimal inhibitory concentration (MIC) of PA for B. xenovorans strain LB400 was measured. The MIC of PA that prevented LB400 growth was 2 mM.
Figure 7. Effects of 2-aminophenol and picolinic acid on B. xenovorans LB400 growth.
A, LB400 cells were cultured in M9 minimal medium with glucose (10 mM) as sole carbon source and NH4Cl (1 mM) as sole nitrogen source. Two cultures were supplemented with 2-AP (1 mM) at time 0 or at late exponential phase (18 h). As controls, LB400 cells were cultured in M9 minimal medium without a carbon source or cultured with glucose. B, LB400 cells were cultured in BLKN minimal medium with glucose (10 mM) and NH4Cl (1 mM) as sole carbon and nitrogen source, respectively. Two cultures were supplemented with picolinic acid (1 mM) at time 0 or at late exponential phase (18 h). C, LB400 cells cultured in BLKN minimal medium with glucose (10 mM) and NH4Cl (1 mM), were supplemented after 13 h with 2-AP (1 mM) or PA (1 mM).
Discussion
This study revealed that the 2-aminophenol catabolic central pathway is functional in the model aromatic-degrading bacterium B. xenovorans LB400. Strain LB400 is able to use 2-AP as sole nitrogen source for growth, indicating an active 2-AP catabolic central pathway. Only few bacterial strains capable to grow on 2-AP have yet been reported [5], [6]. Pseudomonas sp. strain AP-3 is able to grow in liquid medium using 2-AP as sole carbon and nitrogen source [5]. P. pseudoalcaligens strain JS45 grow in liquid medium using 2-AP as sole carbon and nitrogen source but only when the cells were previously induced with NB [6]. In contrast, the nitrobenzene-degrader Pseudomonas putida strain HS12 did not grow on 2-AP [7]. Interestingly, P. knackmussi strain B13 that share a highly identical amn gene cluster with B. xenovorans LB400 is not able to grow on 2-AP [9]. In our study, strain LB400 reached lower cell numbers in liquid medium using as sole nitrogen source for growth 2-AP (2.5×108 CFU mL−1) than ammonium chloride (4.5×108 CFU mL−1). On the other side, only minimal growth of strain LB400 was measured in liquid medium with 2-AP as sole nitrogen and carbon source. In addition, small colonies of strain LB400 were observed on agar plates using 2-AP crystals as sole nitrogen and carbon source. All these results indicate a reduced growth of strain LB400 on 2-AP, suggesting an inhibition of cell growth during the metabolism of 2-AP. The growth inhibition suggests the production of a toxic metabolite. In this study, we were able to demonstrate that during the degradation of 2-AP by strain LB400 this substrate is partially converted into the antibiotic picolinic acid. When 2-AP (1 mM) was used as sole carbon and nitrogen source for strain LB400 growth, high levels of PA (0.5 mM) were produced. In this report we showed that PA inhibited the growth of B. xenovorans strain LB400. The MIC of PA for strain LB400 is 2 mM. PA is a well known antibiotic for Gram-negative bacteria such as P. aeruginosa and Staphylococcus aureus, Mycobacterium avium complex and fungi such as Candida albicans [31]–[33]. PA is a chelating agent for metals ions such as Fe2+ and Zn2+. Interestingly, it has been reported that PA induces antitumoral activity of macrophages and chromium picolinate has been used as a dietary supplement for obese persons to promote weight loss [31]–[36]. Nishino and Spain [6] reported that crude extracts of P. pseudoalcaligenes strain JS45 transformed 2-AP through 2-AMS into PA. The addition of NAD to the extracts of strain JS45 prevented the formation of PA by favoring the formation of 2-aminomuconate by the 2-aminomuconic-6-semialdehide dehydrogenase AmnC. For the production of PA, a recombinant E. coli strain harboring only the genes for 2-aminophenol 1,6-dioxygenase from strain JS45 was used for the biotransformation of 2-AP into PA [37]. P. knackmussi strain B13 that has a highly identical 2-AP catabolic pathway as strain LB400 did not grow on 2-AP [9]. To explain the failure of strain B13 growth on 2-AP, Gaillard et al. proposed the production of a toxic intermediate from 2-AP or alternatively a metabolic misrouting. Our study demonstrates the conversion of 2-AP into PA by the amn-encoded catabolic pathway in B. xenovorans strain LB400. These results suggest that strain B13 also produces the toxic PA during 2-AP degradation, impeding its growth on 2-AP. The biotransformation of aromatic compounds into toxic metabolites and antibiotics has been reported [38]–[41]. Cámara et al. [40] reported that PCBs were converted by enzymes of the biphenyl catabolic pathway into highly toxic biphenyl dihydrodiols and dihydroxylated biphenyls. Blasco et al. [38], [39] demonstrated that chlorobenzoates were metabolized by bacteria through 4-chlorocatechol into the antibiotic protoanemonin.
Genome analysis indicates that the amn genes for 2-aminophenol degradation are clustered in the clc genomic island at C1 from B. xenovorans LB400. LB400 clc genomic island shows 99% sequence identity with the clc element of P. knackmussii strain B13 and contains genes of the 2-aminophenol and chlorocatechol catabolic pathways and genes involved in the transport of aromatic compounds. The amn gene clusters from B. xenovorans LB400 and P. knackmussii strain B13 have the same amn gene organization and the amn genes of both strains have almost identical gene sequence [9], which strongly suggest horizontal gene transfer of the amn gene cluster between bacteria. Genome analysis of strain LB400 indicates the absence of nbz (hab) genes involved in the conversion of nitrobenzene into 2-aminophenol. In accordance with the genome analysis, strain LB400 was unable to grow using nitrobenzene as sole nitrogen or carbon source. In contrast, both 2-AP-degrading Pseudomonas strains JS45 and HS12 possess the nbzA (habA) and nbzB (habB) genes for nitrobenzene degradation and are able to grown on nitrobenzene as carbon and nitrogen energy source [6], [42].
The 2-aminophenol-1,6-dioxygenase AmnB subunit of B. xenovorans LB400 is closely related to AmnB of P. knackmussi strain B13 (100% identity) and LigB of B. avium 197N (89% identity). 2-aminophenol-1,6-dioxygenase belong to the extradiol dioxygenases [8], [43] that include also the 2-amino-5-chlorophenol-1,6-dioxigenase and the catechol-2,3-dioxygenase. The substrates of these related extradiol dioxygenases comprise catechol, 2′-aminobiphenyl-2,3-diol, 3,4-dihydroxybiphenylacetate, 2-amino-5-chlorophenol and 2-aminophenol, suggesting the evolution of these catabolic enzymes. The extradiol dioxygenases are classified in two branches. The enzymes of substrates with a catechol group such as 2′-aminobiphenyl-2,3-diol 1,2-dioxygenases and 3,4-dihydroxyphenylacetate 2,3-dioxygenases are closely related to catechol 2,3-dioxygenases. The extradiol dioxygenases that recognize substrate in which one hydroxyl group of the catechol moiety has been replaced by an amino group are most distantly related to the catechol 2,3-dioxygenases. The 2-aminophenol-1,6-dioxigenase AmnB of Pseudomonas sp. strain AP3 is closely related to 2-amino-5-chlorophenol-1,6-dioxigenase CnbCb subunit of C. testosteroni strain CNB-1 (70% identity). In addition, the organization of the cnb gene cluster of C. testosteroni CNB-1 and the amn gene cluster of P. putida HS12 is conserved, suggesting a common origin. The phenoxazinone synthase (GriF, PhsA or NspF) of several members of the Streptomyces genus that use also 2-AP as substrate are not related to 2-aminophenol-1,6-dioxygenase [44]. This is not surprising since phenoxazinone oxidase is not an extradiol dioxygenase.
In this study, we showed that B. xenovorans strain LB400 grew using 2-AP as sole nitrogen source and that LB400 cells degraded completely 2-AP. In contrast, an amnBA − mutant was unable to grow on 2-AP as nitrogen source and to degrade this compound, linking the amn-encoded central pathway with 2-AP catabolism in strain LB400. In addition, RT-PCR analysis showed that amnA, amnB, amnC, amnD, amnE, amnF, amnG, amnH, amnJ and amnR genes were expressed in LB400 cells. These results demonstrate that the amn gene cluster is functional. The co-expression of the amnJB, amnBAC, amnACD, amnDFE and amnEHG genes and the presence of a promoter upstream of the amnJ gene indicate that the amn genes are clustered in an operon. The expression of the amnB gene in LB400 cells grown in glucose indicates a basal expression of the amn gene. The amnB gene expression is highly induced by 2-AP, whereas lower induction was observed in presence of 2-AP and glucose. These results suggest an induction of the amnB gene transcription by 2-AP or its metabolic intermediates and a down regulation of its transcription by glucose. In accordance with the regulation of the amn genes observed in our study, transcriptional analysis with a DNA chip showed a higher expression of the amn genes of strain LB400 in benzoate than in succinate [45]. The predicted amnR gene-encoded MarR-type transcriptional regulator of strain LB400 is probably a transcriptional repressor of the amn gene cluster. MarR-type transcriptional regulators bind as dimmers to palindromic sequences within the promoter. A regulatory palindromic sequence was observed in the intergenic region of the amnR gene and the amnJBACDFEHG gene cluster from strain LB400. MarR regulators bind to DNA in absence of the specific ligand, causing generally transcriptional repression [46]. P. knackmussi strain B13 showed by dot blot hybridization a basal amnB gene expression in glucose and a 50-fold increase amnB gene expression in 2-AP (1 mM) and anthranilate (1 mM) [9]. P. putida HS12 down regulated the amn genes in succinate [7]. In contrast, Pseudomonas sp. strain AP3 expresses constitutively the amn genes [8], which correlates with the absence of the amnR gene in its amn gene cluster.
Figure 1 illustrates the predicted functional 2-AP catabolic pathway of the model bacterium B. xenovorans strain LB400. 2-Aminophenol-1,6-dioxygenase encoded by the amnBA genes meta-cleaved 2-aminophenol into 2-AMS. The amnBA − mutant is not able to degrade 2-AP, indicating that 2-aminophenol-1,6-dioxygenase is the sole enzyme involved in 2-AP degradation in strain LB400. 2-AMS is further oxidized by 2-aminomuconic-6-semialdehyde dehydrogenase AmnC into 2-AM. Alternatively, 2-AMS could chemically rearrange into PA (Fig. 1). The 2-AM is deaminated by 2-aminomuconic deaminase AmnD releasing 4-oxalocrotonic acid and ammonium. The ammonium released at this step could be used as nitrogen source for bacterial growth. 4-oxalocrotonate is converted into pyruvate, acetaldehyde and acetil-CoA, by catabolic enzymes that are also involved in the catechol metabolism.
In conclusion, this study indicates that B. xenovorans strain LB400 possess a functional 2-AP catabolic pathway, which channel the aromatic pollutant 2-AP into the Krebs cycle. Interestingly, the 2-AP degradation in strain LB400 could lead to the production of the antibiotic picolinic acid, which inhibits its growth. The versatile catabolic repertoire for aromatic compounds of B. xenovorans LB400 allows the degradation of aromatic pollutants with chloro or amino substituent’s such as PCBs and 2-AP, which could be useful for bioremediation studies.
Acknowledgments
The authors acknowledge Franco Cardenas and Luis Rojas for support with RT-qPCR analysis and anonymous reviewers for helpful comments.
Funding Statement
M.S. gratefully acknowledges financial support from FONDECYT (1020221 and 1070507) (http://www.fondecyt.cl), Center for Nanotechnology and Systems Biology (http://www.usm.cl) and USM (131109, 130948) (http://www.usm.cl) grants. The funders had no role in study design, data collection and analyses, decision to publish, or preparation of the manuscript.
References
- 1. Spain JC (1995) Biodegradation of nitroaromatic compounds. Annu Rev Microbiol 49: 523–555. [DOI] [PubMed] [Google Scholar]
- 2. Ye J, Singh A, Ward OP (2004) Biodegradation of nitroaromatics and other nitrogen-containing xenobiotics. World J Microbiol Biotechnol 20: 117–135. [Google Scholar]
- 3. Brennan RJ, Schiestl RH (1997) Aniline and its metabolites generate free radicals in yeast. Mutagenesis 12: 215–220. [DOI] [PubMed] [Google Scholar]
- 4. Ohkuma Y, Kawanishi S (2001) Oxidative DNA damage induced by a metabolite of carcinogenic o-anisidine: enhancement of DNA damage and alteration in its sequence specificity by superoxide dismutase. Arch Biochem Biophys 389: 49–56. [DOI] [PubMed] [Google Scholar]
- 5. Aoki K, Takenaka S, Murakami S, Shinke R (1997) Partial purification and characterization of a bacterial dioxygenase that catalyzes the ring fission of 2-aminophenol. Microbiol Res 152: 33–38. [Google Scholar]
- 6. Nishino SF, Spain JC (1993) Degradation of nitrobenzene by a Pseudomonas pseudoalcaligenes . Appl Environ Microbiol 59: 2520–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park HS, Kim HS (2000) Identification and characterization of the nitrobenzene catabolic plasmids pNB1 and pNB2 in Pseudomonas putida HS12. J Bacteriol 182: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takenaka S, Setyorini E, Kim Y-J, Murakami S, Aoki K (2005) Constitutive synthesis of enzymes involved in 2-aminophenol metabolism and inducible synthesis of enzymes involved in benzoate, p-hydroxybenzoate, and protocatechuate metabolism in Pseudomonas sp. strain AP-3. Biosci Biotechnol Biochem 69: 1033–1035. [DOI] [PubMed] [Google Scholar]
- 9. Gaillard M, Vallaeys T, Vorhölter FJ, Minoia M, Werlen C, et al. (2006) The clc element of Pseudomonas sp. strain B13, a genomic island with various catabolic properties. J Bacteriol 188: 1999–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis JK, He Z, Somerville CC, Spain JC (1999) Genetic and biochemical comparison of 2-aminophenol 1,6-dioxygenase of Pseudomonas pseudoalcaligenes JS45 to meta-cleavage dioxygenases: divergent evolution of 2-aminophenol meta-cleavage pathway. Arch Microbiol 172: 330–339. [DOI] [PubMed] [Google Scholar]
- 11. Park H, Kim H (2001) Genetic and structural organization of the aminophenol catabolic operon and its implication for evolutionary process. J Bacteriol 183: 5074–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spain JC, Nishino SF, Witholt B, Tan LS, Duetz WA (2003) Production of 6-phenylacetylene picolinic acid from diphenylacetylene by a toluene-degrading Acinetobacter strain. Appl Environ Microbiol 69: 4037–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seeger M, Zielinski M, Timmis KN, Hofer B (1999) Regiospecificity of dioxygenation of di- to pentachlorobiphenyls and their degradation to chlorobenzoates by the bph-encoded catabolic pathway of Burkholderia sp. strain LB400. Appl Environ Microbiol 65: 3614–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seeger M, Cámara B, Hofer B (2001) Dehalogenation, denitration, dehydroxylation, and angular attack on substituted biphenyls and related compounds by a biphenyl dioxygenase. J Bacteriol 183: 3548–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chain PSG, Denef VJ, Konstantinidis KT, Vergez LM, Agulló L, et al. (2006) Burkholderia xenovorans LB400 harbors a multi-replicon, 9.73-Mbp genome shaped for versatility. Proc Natl Acad Sci USA 103: 15280–15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Méndez V, Agulló L, González M, Seeger M (2011) The homogentisate and homoprotocatechuate central pathways are involved in 3- and 4-hydroxyphenylacetate degradation by Burkholderia xenovorans LB400. PloS ONE 6: e17583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Romero-Silva MJ, Méndez V, Agulló L, Seeger M (2013) Genomic and functional analyses of the gentisate and protocatechuate ring-cleavage pathways and related 3-hydroxybenzoate and 4-hydroxybenzoate peripheral pathways in Burkholderia xenovorans LB400. PloS ONE 8: e56038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruhn C, Lenke H, Knackmuss H (1987) Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol 53: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (2008) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard eighth edition. Document M07-A8 Wayne, PA: CLSI.
- 20. Beltrametti F, Reniero D, Backhaus S, Hofer B (2001) Analysis of transcription of the bph locus of Burkholderia sp. strain LB400 and evidence that the ORF0 gene product acts as a regulator of the bphA1 promoter. Microbiol 147: 2169–2182. [DOI] [PubMed] [Google Scholar]
- 21. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 22. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Best-Keeper Excel-Based tool using pair-wise correlation. Biotechnol Lett 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 23. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: 2002–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lambrecht JA, Flynn JM, Downs DM (2012) Conserved YjgF protein family deaminates reactive enamine/imine intermediates of pyridoxal 5′-phosphate (PLP)-dependent enzyme reactions. J Biol Chem 287: 3454–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burman JD, Stevenson CE, Sawers RG, Lawson DM (2007) The crystal structure of Escherichia coli TdcF, a member of the highly conserved YjgF/YER057c/UK114 family. BMC Struct Biol 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salanoubat M, Genin S, Artiguenave F, Gouzy J, Mangenot S, et al. (2002) Genome sequence of the plant pathogen Ralstonia solanacearum . Nature 415: 497–502. [DOI] [PubMed] [Google Scholar]
- 28. Lai Q, Li W, Shao Z (2012) Complete genome sequence of Alcanivorax dieselolei type strain B5. J Bacteriol 194: 6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sebaihia M, Preston A, Maskell DJ, Kuzmiak H, Connell TD, et al. (2006) Comparison of the genome sequence of the poultry pathogen Bordetella avium with those of B. bronchiseptica, B. pertussis, and B. parapertussis reveals extensive diversity in surface structures associated with host interaction. J Bacteriol 188: 6002–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu J, Jiang C, Wang B, Ma Y, Liu Z, et al. (2006) Novel partial reductive pathway for 4-chloronitrobenzene and nitrobenzene degradation in Comamonas sp. strain CNB-1. Appl Environ Microbiol 72: 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai S, Sato K, Shimizu T, Yamabe S, Hiraki M, et al. (2006) Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin and fluoroquinolones. J Antimicrob Chemoth 57: 85–93. [DOI] [PubMed] [Google Scholar]
- 32. Borawska MH, Czechowska SK, Markiewicz R, Pałka J, Świsłocka R (2008) Antimicrobial activity and cytotoxicity of picolinic acid and selected picolinates as new potential food preservatives. Pol J Food Nutr Sci 58: 415–418. [Google Scholar]
- 33. Musk DJ, Hergenrother PJ (2008) Chelated iron sources are inhibitors of Pseudomonas aeruginosa biofilms and distribute efficiently in an in vitro model of drug delivery to the human lung. J Appl Microbiol 105: 380–388. [DOI] [PubMed] [Google Scholar]
- 34. Fernandez-Pol JA, Bono VH, Johnson GS (1977) Control of growth by picolinic acid: differential response of normal and transformed cells. Proc Natl Acad Sci USA 74: 2889–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melillo G, Cox GW, Biragynn A, Sheffler A, Varesiot L (1994) Regulation of nitric-oxide synthase mRNA expression by interferon-gamma and picolinic acid. J Biol Chem 269: 8128–8133. [PubMed] [Google Scholar]
- 36. Vincent J (2003) The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med 33: 213–230. [DOI] [PubMed] [Google Scholar]
- 37. He Z, Spain JC (2000) One-step production of picolinic acids from 2-aminophenols catalyzed by 2-aminophenol 1,6-dioxygenase J Ind Microbiol Biotechnol. 25: 25–28. [Google Scholar]
- 38. Blasco R, Wittich RM, Mallavarapu M, Timmis KN, Pieper DH (1995) From xenobiotic to antibiotic, formation of protoanemonin from 4-chlorocatechol by enzymes of the 3-oxoadipate pathway. J Biol Chem 270: 29229–29235. [DOI] [PubMed] [Google Scholar]
- 39. Blasco R, Mallavarapu M, Wittich R, Timmis KN, Pieper DH (1997) Evidence that formation of protoanemonin from metabolites of 4-chlorobiphenyl degradation negatively affects the survival of 4-chlorobiphenyl-cometabolizing microorganisms. Appl Environ Microbiol 63: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cámara B, Herrera C, González M, Couve E, Hofer B, Seeger M (2004) From PCBs to highly toxic metabolites by the biphenyl pathway. Environ Microbiol 6: 842–850. [DOI] [PubMed] [Google Scholar]
- 41.Seeger M, Pieper DH (2009) Genetics of biphenyl biodegradation and co-metabolism of PCBs. In: Timmis KN, editor. Microbiology of hydrocarbons, oils, lipids, and derived compounds. Springer, Heidelberg, Germany. 1179–1199.
- 42. Park HS, Lim SJ, Chang YK, Livingston AG, Kim HS (1999) Degradation of chloronitrobenzenes by a coculture of Pseudomonas putida and a Rhodococcus sp. Appl Environ Microbiol 65: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lendenmann U, Spain JC (1996) 2-aminophenol 1,6-dioxygenase: a novel aromatic ring cleavage enzyme purified from Pseudomonas pseudoalcaligenes JS45. J Bacteriol 178: 6227–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Le Roes-Hill M, Goodwin C, Burton S (2009) Phenoxazinone synthase: what’s in a name? Trends Biotechnol 27: 248–258. [DOI] [PubMed] [Google Scholar]
- 45. Denef VJ, Park J, Tsoi TV, Rouillard JM, Zhang H, et al. (2004) Biphenyl and benzoate metabolism in a genomic context: outlining genome-wide metabolic networks in Burkholderia xenovorans LB400. Appl Environ Microbiol 70: 4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perera IC, Grove A (2011) MarR homologs with urate-binding signature. Protein Sci 20: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]