Abstract
Ferric uptake regulator (Fur) is a global regulator that controls bacterial iron homeostasis. In this study, a fur deletion mutant of the deep-sea bacterium Shewanella piezotolerans WP3 was constructed. Physiological studies revealed that the growth rate of this mutant under aerobic conditions was only slightly lower than that of wild type (WT), but severe growth defects were observed under anaerobic conditions when different electron acceptors (EAs) were provided. Comparative transcriptomic analysis demonstrated that Fur is involved not only in classical iron homeostasis but also in anaerobic respiration. Fur exerted pleiotropic effects on the regulation of anaerobic respiration by controlling anaerobic electron transport, the heme biosynthesis system, and the cytochrome c maturation system. Biochemical assays demonstrated that levels of c-type cytochromes were lower in the fur mutant, consistent with the transcriptional profiling. Transcriptomic analysis and electrophoretic mobility shift assays revealed a primary regulation network for Fur in WP3. These results suggest that Fur may act as a sensor for anoxic conditions to trigger and influence the anaerobic respiratory system.
Introduction
Iron is one of the most important micronutrients for bacterial growth and an essential cofactor for several proteins that participate in major cellular processes [1]. Due to the scarcity of available iron under aerobic conditions, as well as the toxicity of free iron at elevated concentrations via the Fenton reaction [2], bacteria employ a number of strategies by which to regulate intracellular iron concentrations, such as the synthesis and export of chelators [3], reduction by ferric reductases [4], and the expression of oxidative stress genes [5].
In most bacterial species, iron homeostasis is controlled by the ferric uptake regulator (Fur). Generally, Fur can act as both a positive and negative regulator of transcription. Fur senses excess intracellular Fe2+ and binds to the promoter regions of genes that participate in cellular processes, thereby directly obstructing or activating the transcription of these genes [6]–[8]. Even in its iron-free (apo) form, Fur can act as a transcriptional repressor [9]. Most indirect Fur regulation occurs at the posttranscriptional level in the presence of iron through the repression of a non-coding regulatory RNA (ryhB), to allow its target genes to be expressed [10]–[12]. In addition to its major role in the regulation of gene expression in the iron homeostasis system, Fur also functions as a pleiotropic transcriptional regulator and is involved in the control of diverse cellular processes, such as acid tolerance, redox-stress responses, flagellar chemotaxis, and virulence factor production [13]–[16]. Recent studies on the effects of iron concentration or fur inactivation have provided some evidence for the regulation of anaerobic respiration by Fur/iron in different bacterial species. In Salmonella enterica, nitrate respiration is controlled by Fur through the regulators NarP and NarL [17]. In Shewanella oneidensis MR-1, a fur mutation results in the reduced expression of genes encoding proteins that are involved in electron transport and cytochrome systems, such as cymA (tetraheme cytochrome c), omcA/B (decaheme cytochrome c), and ccmH/E (cytochrome c biogenesis protein), under anaerobic conditions [18]. In Bacillus subtilis, several cytochrome systems (e.g., cydABCD) have been reported to be repressed by iron limitation [19], and in Pasteurella multocida, the expression of genes that are involved in energy metabolism and electron transport (e.g., fumarate reductase, dimethyl sulfoxide reductase, and NapF) is decreased in response to iron restriction [20]. Moreover, FurA can act as a heme sensor protein [21] and directly control the tetrapyrrole biosynthesis pathway, which is involved in many metabolic processes, including anaerobic respiration, in Anabaena sp. PCC 7120 [22]. These studies have indicated a close relationship between iron regulation (primarily by Fur) and anaerobic respiration.
The Shewanella genus is known for its ability to use a broad range of electron acceptors, such as Fe (III), Mn (IV), trimethylamine N-oxide (TMAO), dimethyl sulfoxide (DMSO), sulfur, nitrate, and fumarate [23]. Members of this genus are ubiquitous in many environments and have been proposed as candidates for the bioremediation of metal and organic contaminants [24]. The majority of isolated Shewanella species are capable of iron respiration, in which iron plays essential roles as a both a protein cofactor and an EA [25]. Furthermore, the electron transfer chain in anaerobic respiration in Shewanella is predominantly composed of cytochrome c proteins, which contain heme groups that use iron as a cofactor [25], [26], indicating a relationship between iron regulation by Fur and anaerobic respiration in Shewanella.
The Fur protein is well conserved in the Shewanella genus [27]. In the model Shewanella species Shewanella oneidensis MR-1 (hereafter abbreviated as MR-1), which was originally isolated from Oneida Lake [28], Fur has been suggested to coordinate the regulation of energy metabolism. This conclusion was reached because mutations in fur in MR-1 affected the transcription of several genes that are involved in the electron transport system, energy metabolism, and regulation [15], [18], [29]. However, only a small number of physiological studies have compared the WT and fur mutant of MR-1, and these studies did not reveal any substantial differences in the growth or utilization of different EAs under anaerobic conditions [15], [18], [29]. Consequently, the role of Fur in the anaerobic respiration of Shewanella remains elusive.
Here, the role of Fur in anaerobic respiration was investigated in Shewanella piezotolerans WP3 (hereafter abbreviated as WP3), which was isolated from deep-sea sediments of the west Pacific [23], [30]. Deep-sea sediments contain high levels of authigenic ferric oxides [31] and low levels of oxygen. WP3 can use various external EAs under anaerobic conditions [32], and it is able to reduce hydrous ferric oxide to produce superparamagnetic magnetite particles with an average grain size of 4–6 nm [33]. The WP3 genome includes 55 putative cytochrome c genes, explaining the versatile respiratory capabilities of this strain [23].
To investigate the role of Fur in anaerobic respiration of the deep-sea bacterium WP3, a comparative transcriptomic analysis of WT WP3 and its fur mutant under anaerobic conditions was performed. In addition, physiological studies, cytochrome c content measurements, and DNA binding experiments were performed to verify the role of Fur in anaerobic respiration. Fur is shown to have important roles in regulating anaerobic respiration in WP3. This work calls for more attention on elucidating the general roles and molecular mechanisms of Fur regulation in deep-sea bacteria.
Materials and Methods
Bacterial Strains, Culture Conditions, and Physiological Studies
All bacterial strains and plasmids used in the present study are listed in Table S1. Cultures of Escherichia coli (E. coli) were grown aerobically in Luria–Bertani medium at 37°C. The Shewanella strains were cultured at 20°C under aerobic and anaerobic conditions. For aerobic cultivation, a modified 2216E culture (5 g tryptone, 1 g yeast extract, 34 g sodium chloride, and 50 mg FePO4 per liter) was used; for anaerobic cultivation, an oligotrophic medium (0.1 g tryptone, 0.2 g yeast extract, 34 g sodium chloride, 4.8 g HEPES, and 3.4 ml sodium lactate per liter) was dispensed into serum bottles gassed with O2-free nitrogen. After the media were autoclaved, the EAs were added at the required concentrations (2 mM nitrate; 20 mM dimethyl sulfoxide (DMSO); 20 mM fumarate, and 15 mM hydrous ferric oxide (HFO)) [34]. Chloramphenicol (25 µg ml−1 for E. coli; 12.5 µg ml−1 for WP3) was added to the medium when required. Siderophores were detected under anaerobic conditions (Coy anaerobic glove box) on solid culture medium via the application of chrome azurol-S (CAS)-based techniques. CAS screening plates were prepared using a previously described procedure [35], [36]. The HFO solution was prepared according to a previously described procedure [37]. The Fe2+ concentration was determined by measuring the absorbance at 562 nm on a SHIMADZU UV-2550 spectrophotometer (SHIMADZU CO., Kyoto, Japan) following the ferrozine method [33] after extraction with 1 N HCl. The OD600 was measured with a SHIMADZU UV-2550 spectrophotometer to obtain a growth curve. All of the physiological studies were performed in triplicate, and the average values and standard deviations were calculated.
Generation of Mutant and Complementation Strains
The genes fur (Ferric uptake regulator), ccmC (cytochrome c biogenesis protein), and fccA (flavocytochrome c) were deleted in-frame using a fusion PCR method with the pRE112 plasmid, as previously described [38]. Chromosomal mutants were selected by resistance to chloramphenicol and sucrose, and deletions were confirmed using PCR sequencing.
For complementation, we used the Shewanella–E. coli shuttle plasmid vector pSW2, which was developed from the WP3 filamentous phage SW1 (unpublished data). The complete fur gene was ligated into the phage-based vector pSW2 to generate the pSW2-Fur plasmid. The plasmid was introduced into WM3064 by calcium chloride transformation and then mobilized into the fur mutant by mating. Complementation of the fur locus in the fur mutant strain was confirmed using PCR. The primers that were used to generate the PCR products are listed in Table S2.
RNA Isolation and RNA Sequencing
Total RNA from WP3 WT and fur mutant strain at mid-log phase under anaerobic conditions using 20 mM fumarate as the EA were extracted in triplicate using Trizol reagent, respectively. The triplicate samples were mixed for RNA sequencing. Ribosomal RNA was removed using the RiboMinus™ Transcriptome Isolation Kit (Invitrogen, Carlsbad, CA, USA). RNA sequencing was performed on the Illumina HiSeq 2000 (Illumina, San Diego, CA, USA) at the Beijing Genomics Institute (BGI, China), following the manufacturer’s instructions. The accession code of our RNA-Seq dataset is GSE47773.
RNA-seq Data Analysis and RT-PCR Validation
The raw sequencing data were trimmed of linker sequences, and a set of unique sequences was created by combining all of the reads with identical sequences. Unique sequences were mapped to the WP3 genome with SOAPaligner (soap2) [39]. The uniquely mapped reads were collected and analyzed with the DEG-seq package [40] to identify differentially expressed genes (estimation of gene expression based on RPKM values). The results of this analysis yield P- and Q-values for each gene to denote the difference in its expression between libraries [40]. In order to validate the data generated by RNA-seq, the expression levels of 8 randomly selected genes (swp0429, swp1175, swp1055, swp3209, swp3979, swp3980, swp3981 and swp4950) were quantified using RT-PCR. The RT-PCR log2 ratio values were plotted against the RNA-seq data log2 values.
Fur Box Analysis and Logo Graph
The Fur Box was analyzed using the web-based tool RegPredict, which is available at http://regpredict.lbl.gov [41]. The Fur Box was identified by searching the 5′ regions of the genes in WP3 using the MR-1 information matrix for Fur. The information matrix for the generation of the Fur Logo was produced by aligning the WP3 Fur binding sequences predicted by the RegPredict web server. A graphical representation of the matrix through a Logo graph was obtained with Weblogo software, which is available at http://weblogo.berkeley.edu.
RT-PCR Analysis
RT-PCR was performed using 7500 System SDS software and 20 µl reaction mixtures containing 10 µl SYBR Green-I Universal PCR Master Mix (Applied Biosystems, Warrington, UK), 0.5 µM of each primer, and 1 µl cDNA template. The primer pairs for the selected genes were designed using Primer Express software (Applied Biosystems, Foster City, CA, USA) (Table S2). In this method, pepN, which exhibits stable expression under various conditions, was used as the reference gene. The gene transcription levels of the targets were normalized to pepN in both the WT and fur mutant WP3 strains under anaerobic conditions [23]. RT-PCR assays were performed in triplicate for each sample. The mean value and standard deviation of the relative RNA expression levels were calculated.
Cytochrome c Content Measurement
The WP3 WT and fur mutant strain were incubated at 20°C under aerobic and anaerobic conditions using 20 mM fumarate as the EA. The cells were harvested at mid-log phase by centrifugation and resuspended in the phosphate buffered saline (PBS). After the cells were lysed with an ultrasonic cell disruptor, the soluble protein was measured by Bradford protein assay. For equal part (50 µg), reduce the heme iron from Fe3+ to Fe2+ by adding a few grains of sodium dithionite to the sample, cover, and mix by slowly inverting until a color change upon reduction of the sample was observed. The cytochrome c content was evaluated in a spectrophotometer (Amersham Ultrospec 3100, GE Healthcare, USA), recording from 400 to 600 nm using the untreated protein as blank.
Expression and Purification of the Fur Protein
The entire fur ORF (a 429-bp DNA fragment) containing an EcoRI site (5′-end) and an XholI site (3′-end) was PCR-amplified and then cloned into the EcoRI/XholI sites of the plasmid pET28a which carries an N-terminal His-tag. The resulting fur recombinant expression plasmid, pET28a-fur, was transformed into E. coli BL21 (DE3) cells. The cell cultures were incubated at 37°C in LB medium until an OD600 of 1.0 was reached. Protein expression was then induced by adding 0.1 mM IPTG (final concentration), and the cells were subsequently grown at 37°C for 4 h. The cells were then harvested by centrifugation and resuspended in 20 ml PBS. After the cells were lysed with an ultrasonic cell disruptor, the cell lysate was purified using a nickel-nitrilotriacetic acid (Ni-NTA) agarose column as directed by the manufacturer (GE Healthcare). Recombinant Fur was eluted with elution buffer containing 500 mM imidazole.
Purified Fur from the elution buffer was concentrated in the phosphate buffered saline using Amicon Ultra-15 Centrifugal Filter Unit with Ultracel-10 membrane, according to the manufacturer’s protocol (Millipore Corporation, Bedford, MA). The concentration of the protein was determined by the Bradford assay.
Electrophoretic Mobility Shift Assay (EMSA)
Double-stranded DNA probes were generated by PCR with DIG-labeled dNTPs using the primers listed in Table S2 and purified with the Cycle Pure Kit (Omega). The binding reaction was performed with ∼0.2 pmol DIG-labeled probes and ∼200 pmol purified Fur protein in 20 µl binding buffer containing 40 mM KCl, 12.5 mM Tris (pH 7.5), 125 µM MnCl2, 1.25 mM MgCl2, 5% glycerol, 0.5 mM DTT, 0.01% BSA and 50 µg/ml Salmon Sperm DNA. Specific competitors (2 pmol and 20 pmol unlabeled probes) were added when necessary. The reaction mixtures were incubated at 20°C for 30 min and then loaded onto 6% non-denaturing polyacrylamide gels. Following non-denaturing polyacrylamide gel electrophoresis, gel was transferred onto positively charged nylon membrane (Amersham, GE Healthcare, USA), and UV-cross linked. The membrane was then subjected to detection by chemiluminescent EMSA kit (Pierce, Thermo Scientific, USA) following the manufacturer’s protocol.
Results and Discussion
Generation and Physiological Evaluation of the WP3 fur Mutant
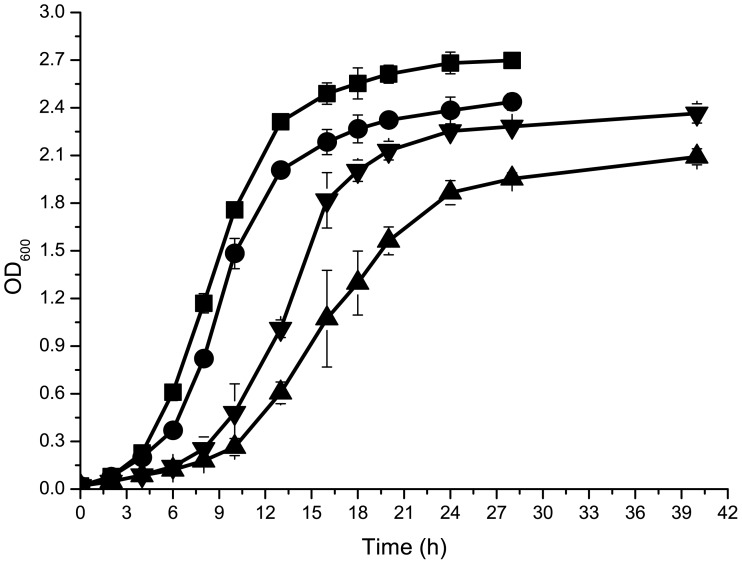
A fur deletion mutant was constructed in WT WP3. When grown aerobically at 20°C on 2216E agar medium, the fur mutant formed smaller colonies than the WT strain. Similarly, the mutant exhibited a lower growth rate than the WT strain when cultivated in liquid 2216E medium (Figure 1), indicating that fur inactivation resulted in a slight growth deficiency under aerobic conditions. The colonies of the fur mutant also appeared paler in color than WT WP3 colonies, potentially indicating the presence of lower heme levels because the pink pigmentation of WT WP3 cells has been attributed to high heme content [26]. To further investigate the behavior of the fur mutant, an iron chelator (2, 2′-dipyridyl) was added to the liquid 2216E medium to mimic iron depletion. In the presence of 60 µM iron chelator, both strains displayed clear growth inhibition. Notably, the fur mutant displayed a much shorter lag phase than the WT strain and achieved a higher cell density at stationary phase (Figure 1), suggesting that the fur mutant had a higher tolerance to the stress of iron depletion, consistent with the findings in MR-1 [15]. Anaerobic incubations on CAS screening plates revealed that the fur mutant produced a larger yellow halo around the colony periphery than the WT strain, indicating that the fur mutant possessed an enhanced ability to produce a diffusible, Fe(III)-chelating compound that outcompeted the WT strain for Fe(III) (Figure S1). This enhancement also explains the better growth of the fur mutant under iron-depleted conditions compared to the WT strain.
Figure 1. Growth curves of WT WP3 and the fur mutant in liquid 2216E with or without 60 µM iron chelator under aerobic conditions.
(▪) WT WP3, (•) fur mutant, (▴) WT WP3 with 60 µM iron chelator, and (▾) fur mutant with 60 µM iron chelator. The data represent averages of triplicate cultures.
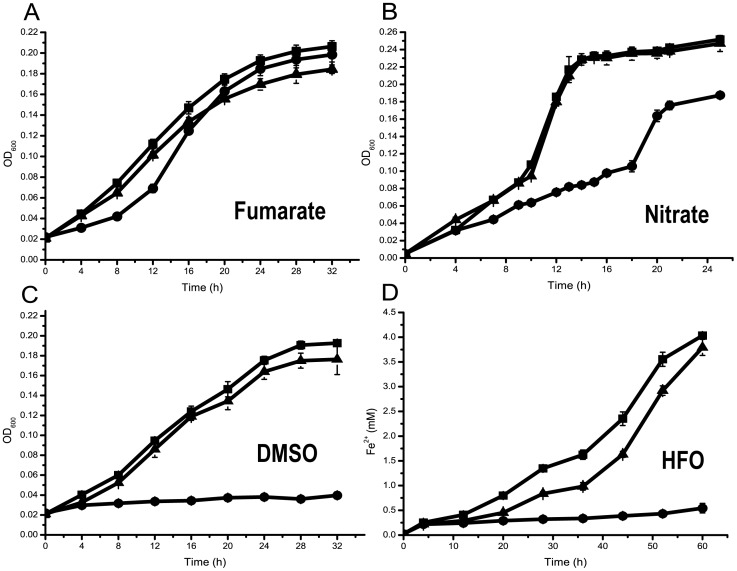
To elucidate the roles of Fur related to the anaerobic respiration in WP3 cells, the fur mutant WP3Δfur was cultivated in oligotrophic medium with fumarate, nitrate, DMSO, or HFO as the sole EA. The initial growth rate of the fur mutant on fumarate was lower than that of the WT strain; however, the growth rates of the strains were nearly identical at stationary phase (Figure 2A). In the presence of nitrate, the fur mutant exhibited a pronounced lag phase in growth, with a much lower growth rate and lower cell densities compared to the WT strain (Figure 2B). The most significant growth deficiency was observed for the mutant grown in the presence of DMSO (Figure 2C), and the initial reduction in HFO was decreased severely (Figure 2D). To confirm the casual relationship between the disruption of the fur gene and the differences in the growth of the fur mutant and the WT strain, a complementation assay was conducted by cloning and introducing the fur gene back into the mutant strain. As shown in Figure 2, the recovery of the fur gene in the mutant restored its respiratory ability when any of the EAs were provided. These results confirmed the role of Fur in the regulation of respiration, particularly in the anaerobic respiration of WP3 cells.
Figure 2. Growth curves of the WT WP3, fur mutant, and fur complement strains with different electron acceptors under anaerobic conditions.
(a–c) Growth on 20 mM fumarate, 2 mM nitrate, and 20 mM DMSO as the electron acceptor, respectively. (d) Time course of Fe2+ concentration with 15 mM HFO as an electron acceptor. The following abbreviations are used for all of the panels: (▪) WT WP3, (•) fur mutant, (▴) fur complement strain. The data represent averages of triplicate cultures.
Previous physiological studies of MR-1 demonstrated that the fur mutant resembled the parental strain in its ability to grow anaerobically on different EAs such as MnO2, Fe(OH)3, Fe(III) citrate, nitrate, nitrite, DMSO, TMAO, fumarate, thiosulfate, sulfite, and AQDS [29]. Recently, the growth rate of the fur mutant was also tested in Salmonella enterica serovar Typhimurium, Dichelobacter nodosus, and Desulfovibrio vulgaris; in these strains, the absence of fur did not cause any notable changes in growth under anaerobic conditions [42]–[44]. Here, a series of physiological experiments confirmed the roles of Fur in the regulation of anaerobic respiration in WP3. Iron is a cofactor of heme, an important component of cytochromes for electron delivery during the anaerobic respiration of Shewanella [45]. Fur is a major regulator in iron homeostasis, and it is thus likely that Fur is utilized in the regulation of the anaerobic respiration system. However, the mechanism by which Fur exerts its influence in anaerobic respiration remains to be elucidated.
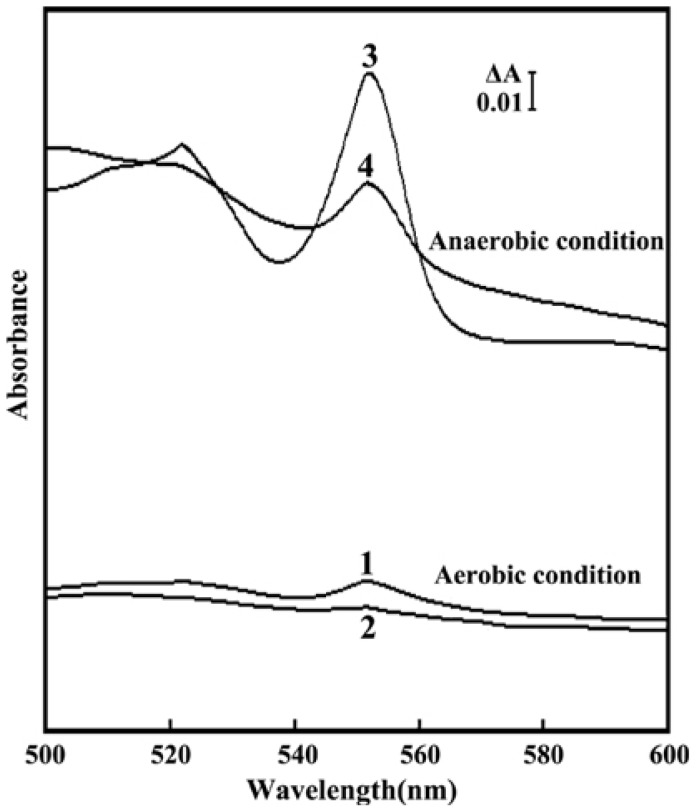
All sequenced Shewanella genomes include a large number of c-type cytochrome genes; for example, 55 c-type cytochrome-encoding genes were detected in WP3 [23], and the products of these genes are believed to transfer electrons to EAs [46]. To assess the impact of Fur on the cellular levels of c-type cytochromes, reduced-minus-oxidized difference spectra were obtained (Figure 3). The absorption maxima peak of c-type cytochromes occurred at ≈550 nm. The results revealed that (1) the amount of c-type cytochromes in each strain was higher under anaerobic conditions than aerobic conditions and (2) the fur mutant contained greatly reduced levels of c-type cytochromes compared to the WT strain, particularly under anaerobic conditions. These results suggest that the Fur protein might regulate anaerobic respiration by affecting the levels of c-type cytochromes.
Figure 3. Spectrum analysis of the cytochrome c content of WT and fur mutant strains under aerobic and anaerobic conditions.
The reduced-minus-oxidized difference spectra of equal amounts of total protein from the WT and fur mutant strains treated with sodium dithionite were recorded. The absorbance levels of the corresponding untreated strains were set as a control. Line 1 Absorbance of the aerobically grown WT strain. Line 2 Absorbance of the aerobically grown fur mutant strain. Line 3 Absorbance of the anaerobically grown WT strain. Line 4 Absorbance of the anaerobically grown fur mutant strain.
Genes Regulated by Fur
To examine the global impact of Fur in anaerobic respiration, comparative transcriptomic analysis was performed for the WP3Δfur and WT WP3 strains. The data generated using RNA-seq were validated by quantitative PCR, and a high correlation (r2 = 0.839, n = 8) was observed between the two transcriptional datasets.
In total, 1160 genes (approximately 23% of the WP3 genome) exhibited differential expression under anaerobic conditions (at least 2-fold difference), and the COG annotations of these genes are displayed in Table 1. The absence of fur resulted in the increased expression of 988 genes and the decreased expression of 172 genes, indicating that Fur acts primarily as a repressor in the global regulation in WP3.
Table 1. Number of differentially expressed genes in Δfur.
| Differentially Expressed Genes in Δfur | |||
| Cluster of Orthologous Groups | Number of “Fur Repressed”a Genes | Number of “Fur Activated”b Genes | Total |
| No COG Assigned | 174 | 57 | 231 |
| Energy production and conversion (C) | 76 | 14 | 90 |
| Cell cycle control, cell division, and chromosome partitioning (D) | 7 | 1 | 8 |
| Amino acid transport and metabolism (E) | 71 | 6 | 77 |
| Nucleotide transport and metabolism (F) | 27 | 1 | 28 |
| Carbohydrate transport and metabolism (G) | 20 | 1 | 21 |
| Coenzyme transport and metabolism (H) | 48 | 7 | 55 |
| Lipid transport and metabolism (I) | 33 | 4 | 37 |
| Translation, ribosomal structure, and biogenesis (J) | 99 | 5 | 104 |
| Transcription (K) | 39 | 27 | 66 |
| Replication, recombination, and repair | 43 | 1 | 44 |
| Cell wall/membrane/envelope biogenesis (M) | 60 | 4 | 64 |
| Cell motility (N) | 25 | 3 | 28 |
| Posttranslational modification, protein turnover, and chaperones (O) | 40 | 5 | 45 |
| Inorganic ion transport and metabolism (P) | 54 | 9 | 63 |
| Secondary metabolites biosynthesis, transport, and catabolism (Q) | 14 | 0 | 14 |
| General function prediction only (R) | 72 | 12 | 84 |
| Function unknown (S) | 62 | 10 | 72 |
| Signal transduction mechanisms (T) | 34 | 13 | 47 |
| Intracellular trafficking, secretion, and vesicular transport (U) | 49 | 3 | 52 |
| Defense mechanisms (V) | 17 | 3 | 20 |
| Total | 1064 | 186 | 1250 |
Categorized According to Cluster of Orthologous Groups (COGs).
Genes with increased expression in the absence of fur.
Genes with decreased expression in the absence of fur.
Among the genes with defined functions (Table 1), the following two groups were highly enriched with differentially expressed products: (1) genes involved in translation, ribosomal structure and biogenesis (2) genes related to energy production and conversion. Interestingly, the expression of genes encoding polar flagellum (swimming) and phages were also induced significantly in the fur mutant, indicating the role of Fur in motility and phage formation. Furthermore, 101 genes in 53 operons were identified having putative Fur binding sites in their corresponding upstream promoter regions (Table S3). The putative element with a 10-1-10 inverted repeat in WP3 (Figure S2) showed high sequence identity to the consensus sequence of MR-1 [18].
(1) Genes with functions in iron homeostasis
The largest gene module identified was composed nearly exclusively of an iron acquisition system, in agreement with the crucial role of Fur in iron homeostasis (Table 2). This result is also consistent with the results of the CAS-based analysis, which indicated increased iron absorption in the fur mutant under anaerobic conditions. Four homologous TonB systems have been annotated in the WP3 genome (swp3077–3080, swp3204–3207, swp3979–3981, and swp4948–4954). In gram-negative bacteria, TonB systems utilize the proton motive force across the cytoplasmic membrane to transduce the energy for delivering iron-siderophore complexes into the periplasmic space [47]. In the fur mutant WP3Δfur, the TonB1 (swp3979–3981) and TonB2 (swp4948–4954) transporting systems were significantly induced, while there were much smaller or no detectable changes in the mRNA levels of the other systems (TonB3 and TonB4 transport systems). Notably, Fur Box motifs were identified upstream of the two TonB operons but not upstream of the other two operons (Table S3). The differences in the expression and gene regulation of the four TonB systems suggest that they may possess different functions in facilitating the uptake of various iron sources. Similar findings in Vibrio spp. and MR-1 have been reported [18], [48].
Table 2. Fur-responsive modules for iron acquisition and storage systems with fumarate as the EA.
| Functional category | ORF | Gene product | WT/Δ furlog2 ratio | p-value | q-value | |
| Ferredoxin | swp0771 | Bacterioferritin-associated ferredoxin (Bfd) | −4.92 | 0 | 0 | |
| Ferrous iron transport system | swp3270 | Ferrous iron transport protein B (FeoB) | −2.10 | 6.5E-131 | 2.4E-129 | |
| swp3271 | Ferrous iron transport protein A (FeoA) | −1.93 | 7.87E-37 | 9.78E-36 | ||
| TonB-dependent receptor | swp0083 | TonB-dependent siderophore receptor | −3.84 | 1.62E-18 | 1.14E-17 | |
| swp3978 | TonB-dependent Heme/hemoglobin receptor (HmuA) | −6.45 | 0 | 0 | ||
| swp5150 | TonB-dependent siderophore receptor | −4.56 | 4.4E-277 | 3.6E-275 | ||
| Energy-transducing TonB system | swp3979 | TonB, C-terminal | −8.35 | 7.91E-37 | 9.79E-36 | |
| swp3980 | ExbB proton channel | −5.44 | 1.19E-44 | 1.78E-43 | ||
| swp3981 | Biopolymer transport protein ExbD | −5.13 | 3.61E-25 | 3.13E-24 | ||
| swp4950 | Biopolymer transport protein ExbD | −5.34 | 1.04E-93 | 2.95E-92 | ||
| swp4952 | Biopolymer transport protein | −5.31 | 1.31E-60 | 2.52E-59 | ||
| swp4953 | ExbB proton channel | −5.30 | 9.34E-61 | 1.81E-59 | ||
| Siderophore biosynthesis system | swp0084 | Siderophore biosynthesis protein | −6.34 | 3.01E-12 | 1.49E-11 | |
| ABC transporter system | swp3982 | ABC hemin transporter (HmuB) | −5.27 | 1.32E-64 | 2.72E-63 | |
| swp3983 | ABC hemin transporter (HmuC) | −6.07 | 1.46E-32 | 1.64E-31 | ||
| swp3984 | ABC hemin transporter (HmuD) | −6.18 | 8.24E-69 | 1.79E-67 | ||
| swp4105 | ABC iron (III) transporter (FbpA) | −3.73 | 8.08E-17 | 5.16E-16 | ||
| swp4106 | ABC iron (III) transporter (FbpB) | −3.16 | 2.5E-10 | 1.1E-09 | ||
| swp4107 | ABC iron (III) transporter (FbpC) | −4.08 | 0 | 0 | ||
| Iron storage system | swp0167 | Ferritin and Dps | 1.21 | 2.54E-07 | 8.82E-07 | |
| swp1175 | Bacterioferritin | 4.00 | 9.82E-52 | 1.65E-50 | ||
| swp1176 | Bacterioferritin | 2.31 | 8.25E-18 | 5.59E-17 | ||
Interestingly, the iron storage proteins (ferritin and bacterioferritin) that displayed increased expression in the MR-1 fur mutant [18] displayed repressed expression patterns in the WP3 fur mutant constructed in the present study (Table 2). Iron storage systems can sequester excess iron, decrease iron toxicity, and decrease the production of reactive oxygen species (ROS) via the Fenton reaction [49]. In E. coli, Fur indirectly regulates intracellular iron storage by repressing the expression of a the small RNA RyhB in the presence of iron [50]. In this study, Fur Box motifs were identified upstream of genes implicated in iron storage (Table S3), suggesting that these genes are directly regulated by Fur in WP3. This result is in accordance with the direct positive regulation reported in V. cholera, Neisseria meningitides, and E. coli [6]–[8].
(2) Genes encoding secondary regulators
The indirect expression pattern suggested that Fur may act with other regulators to coordinate anaerobic respiration. Several secondary regulators or regulatory proteins were observed to be regulated by the Fur protein. Among these regulators, ArcA is a global regulator that controls hundreds of genes involved in aerobic/anaerobic respiration in a few gram-negative bacterial species [51]–[54]. The arcA mutant of MR-1 exhibits impaired aerobic growth and defective utilization of DMSO in the absence of O2 [53], [55], and ArcA was previously shown to be required for the regulation of cytochrome c proteins [54]. The change in arcA expression (∼4-fold increase in Δfur) in WP3 suggests that Fur regulates anaerobic respiration indirectly through ArcA. The Crp/Fnr family transcriptional regulator swp3806, which is an ortholog of the V. cholerae cAMP-binding protein Crp (66% identity), was up-regulated (∼16-fold increase) in the fur mutant, with a Fur Box located upstream of the Crp-like regulator gene. Crp was previously reported to be a major regulator of anaerobic respiration; in MR-1, crp mutants are defective in using several EAs [56], [57]. It is very likely that Fur regulates anaerobic respiration in WP3 by interacting with the Crp-like protein directly, as there is a Fur Box in its promoter region. The TetR family transcriptional regulator SO1415 was characterized as a transcriptional factor that is involved in anaerobic energy metabolism in MR-1 [58]; its homolog in WP3, swp4152, displayed a ∼2-fold decrease in expression in the Δfur mutant, suggesting that it may be a novel transcriptional factor in anaerobic respiration. A relationship was also observed between Fur and histone-like nucleoid structuring protein (H-NS) (swp3473, ∼3-fold increase in Δfur). In S. typhimurium, Fur regulates HilA expression and virulence by negatively regulating H-NS [59]. In E. coli, Fur-mediated activation of ftnA transcription is due to Fur binding to the ftnA promoter region, resulting in competition for H-NS binding [7]. In WP3, Fur likely exerts its influence on anaerobic respiration at least partially through interaction with secondary regulators and regulatory proteins.
(3) Genes with functions in the anaerobic electron transport system
Eleven genes that are involved in anaerobic electron transport were significantly repressed in the fur mutant (Table 3); this finding may explain the deficient growth of the fur mutant under anaerobic conditions with a variety of EAs. The tetraheme c cytochrome CymA, a key protein that controls respiration in the presence of a variety of EAs, such as metals, DMSO, nitrate, and nitrite [60], [61], was repressed in the fur mutant. A cymA gene deletion mutant of WP3 (constructed in a previous study) displayed growth deficiencies when a variety of EAs were tested, including fumarate, HFO, DMSO, nitrate, and nitrite [34]. However, mutation of cymA only partially influenced fumarate respiration, indicating that other proteins are involved in receiving electrons from the menaquinone pool under fumarate-respiring conditions. No Fur-binding box motif was identified upstream of the cymA gene, suggesting potential indirect regulation by Fur.
Table 3. Fur-responsive modules for anaerobic electron transport with fumarate as the EA.
| Functional category | ORF | Gene product | WT/Δ furlog2 ratio | p-value | q-value | |
| Anaerobic respiration system | swp4806 | Nitrate/Nitrite/TMAO/metal/DMSO/Fumarate reductases, membrane-bound tetrahemecytochrome c (CymA) | 1.25 | 2.18E-37 | 2.77E-36 | |
| Anaerobic metal reduction system | swp3277 | Decaheme cytochrome c (OcmA) | 1.43 | 7.05E-29 | 6.96E-28 | |
| swp3278 | Decaheme cytochrome c (MtrC) | 2.44 | 2.8E-182 | 1.4E-180 | ||
| swp3279 | Decaheme cytochrome c (MtrA) | 1.37 | 1.9E-55 | 3.47E-54 | ||
| swp3280 | Outer membrane protein precursor (MtrB) | 1.19 | 2.44E-16 | 1.53E-15 | ||
| Anaerobic nitrate respiration system | swp4456 | Periplasmic nitrate reductase (NapDβ) | 2.04 | 1.1E-168 | 4.7E-167 | |
| swp4457 | Anaerobic dehydrogenases (NapAβ) | 1.37 | 4.56E-17 | 2.95E-16 | ||
| swp4458 | Nitrate reductase (NapBβ) | 1.69 | 1.48E-07 | 3.8E-07 | ||
| swp2772 | Membrane-bound nitrate reductase (NapC) | −1.25 | 1.91E-13 | 1.01E-12 | ||
| swp2773 | Periplasmic nitrate reductase, (NapBα) | −1.81 | 1.19E-14 | 6.7E-14 | ||
| swp2774 | Anaerobic dehydrogenase (NapAα) | −2.34 | 4.14E-08 | 1.55E-07 | ||
| swp2775 | NapDα | −2.48 | 1.05E-15 | 6.34E-15 | ||
| Anaerobic nitrite respiration system | swp0613 | Cytochrome c nitrite reductase (NrfA) | −2.01 | 8.87E-06 | 2.54E-05 | |
| Anaerobic DMSO respiration system | swp0724 | Twin-arginine translocation pathwaysignal (DmsA) | 1.44 | 2.33E-06 | 7.19E-06 | |
| swp3456 | Conserved hypothetical protein (DmsH) | 1.80 | 0.000747 | 0.001609 | ||
| Anaerobic fumaraterespiration system | swp0220 | Fumarate reductase, flavoprotein subunit | −3.74 | 1.36E-23 | 1.12E-22 | |
| swp0429 | Fumarate reductase, transmembrane subunit | 4.67 | 8.1E-193 | 4.3E-191 | ||
| swp2950 | Fumarate reductase, iron-sulfur protein subunit | −2.45 | 1.17E-27 | 1.12E-26 | ||
| swp2952 | Fumarate reductase, flavoprotein subunit | −2.49 | 1.11E-17 | 7.46E-17 | ||
| Others | swp4579 | Cytochrome c, putative | −7.03 | 6.9E-211 | 4E-209 | |
| swp4577 | Cytochrome c family protein | −6.57 | 0 | 0 | ||
| swp4146 | Cytochrome, putative | −2.42 | 8.38E-05 | 0.000208 | ||
| swp3856 | Cytochrome c | −3.12 | 7.58E-06 | 2.19E-05 | ||
In addition to cymA, a variety of genes encoding c-type cytochromes were also regulated by Fur in WP3 (Table 3), including a periplasmic protein (MtrA), a cell-surface decaheme cytochrome c (MtrC/OmcA), and an integral outer-membrane protein (MtrB), which are all essential for metal reduction [46]. The repression of the omcA-mtrABC operon may have prevented the fur mutant from reducing HFO during the initial phase of growth. A Fur Box motif was identified within the upstream sequence in the putative promoter region of omcA, suggesting positive regulation through the direct binding of Fur.
There are two functional periplasmic dissimilatory (NAP) nitrate-reducing systems in WP3 (NAP-α and NAP-β), and deletion of either system has little effect on the ability of the cells to respire nitrate [34]. In this study, both of these NAP systems were regulated by Fur; the genes in the NAP-α system (swp2272–2275) were up-regulated in the fur mutant, while those of the NAP-β system (swp4456–4458) were down-regulated. Because our experiment was conducted at 20°C, the NAP-β system dominated the nitrate reduction. Moreover, a conserved Fur Box was identified upstream of napD (swp4456, NAP-β system). In conjunction with the growth deficiencies under nitrate-respiring conditions, these results indicate that Fur is involved in nitrate respiration. A similar involvement of Fur in nitrate/nitrite respiration was observed in Salmonella enterica [17].
The transcriptomic data revealed that a variety of putative fumarate reductase genes are differentially regulated by Fur. Among these genes, flavocytochrome c (swp4352) displayed the highest identity (63%) with the periplasmic fumarate reductase FccA in MR-1. FccA is the sole fumarate reductase given that the MR-1 FccA deletion mutant is unable to reduce fumarate [62]. Similarly, the fccA deletion mutant of WP3 did not grow under fumarate-respiring conditions. However, the expression of the flavocytochrome c was only slightly changed in WP3, indicating that other factors are involved in the clear physiological change at mid-log phase under fumarate-respiring conditions.
Overall, the results clearly demonstrate that Fur plays an important role in controlling the expression of genes that are involved in anaerobic electron transport. The identification of a conserved Fur Box motif in the promoter regions of the genes mentioned above suggests that these genes may be regulated through direct binding of Fur.
(4) Genes with functions in heme biosynthesis and transport
Heme is an iron-containing cofactor that is involved in redox reactions within cells [63]. Most heme-containing c-type cytochromes, such as CymA, NrfA, and MtrA, facilitate electron transport during anaerobic respiration [34], [46]. Heme can be obtained from external sources or produced by a dedicated biosynthetic pathway [63]. The expression of genes that are involved in heme biosynthesis, including hemA (swp3892), hemB (swp0440), hemC (swp0402), and hemK (swp0051 and swp4046), was significantly decreased in the fur mutant (Figure S3). The glutamyl tRNA reductase gene (hemA) is the first committed step in heme biosynthesis [64]. Meanwhile, putative genes for heme transport, such as the ABC-type heme transport system (hmuUTV, swp3982-swp3984) and the TonB-dependent heme/hemoglobin receptor (hugA, swp3978), were largely up-regulated (>20-fold, Table 2) in the fur mutant. The deficiency in heme biosynthesis was also reflected by the paler color of mutant cells. These data demonstrate that both the heme biosynthesis and electron transport systems are regulated by Fur, influencing the cytochrome c content and anaerobic respiration of cells.
(5) Genes with functions in the cytochrome c maturation system
The c-type cytochromes are ubiquitous hemoproteins that function primarily as electron carriers between enzymes involved in cellular energy transduction processes, such as photosynthesis and/or respiration [65]. In addition to its role in the maturation of c-type cytochromes, the cytochrome c maturation (CCM) system regulates cytochrome c content in bacterial cells [66]. The significant decrease in the c-type cytochrome content in the WP3 fur mutant indicates a relationship between the CCM system and the Fur regulation system. The complex CCM system is composed of ten components (CcmABCDEFGH, DsbA, and DsbD) and functions in transporting heme from the cytoplasm to the periplasm and to maturated apo-cytochrome c [66]. All of the CCM system genes were observed to be repressed in the fur mutant. To validate the transcriptomic results, RT-PCR assays were conducted to detect changes in expression levels (Figure S4). Among the CCM system genes, the ccmC gene displayed the most significant decrease in expression, by approximately 75% in the fur mutant compared to WT WP3. A ccmC gene deletion mutant was constructed to evaluate the potential role of the system in anaerobic respiration. The ccmC mutant cells exhibited a whitish color, similar to that of the fur mutant, and were unable to respire anaerobically when fumarate, Fe(III) and DMSO were provided as EAs (data not shown). These data suggest that repression of the CCM system may cause a significant loss in cytochrome c content in the fur mutant under anaerobic conditions.
Experimental Validation of the Predicted Fur Box by EMSA
A Fur Box motif was identified upstream of several genes/operons involved in anaerobic respiration, such as omcA, napD, and the Crp-like regulator gene (Table S3). In MR-1, omcA also possesses a potential Fur-binding site in its upstream region [18]. The omcA, napD, and Crp-like regulator genes were all repressed in the fur mutant under fumarate-respiring conditions. To evaluate the functionality of the predicted Fur-binding sites, the regulatory regions of these three genes were subjected to PCR amplification and a gel mobility shift assay with the WP3-purified Fur protein. The WP3 Fur protein was purified as a recombinant His-tagged protein expressed in E. coli, and its activity was confirmed by binding to a known Fur Box in the TonB receptor promoter region. Non-Fur Box DNA fragment (swp1869 promoter) with a size similar to that of each of the investigated fur-binding regions was used as a negative controls (Figure S5).
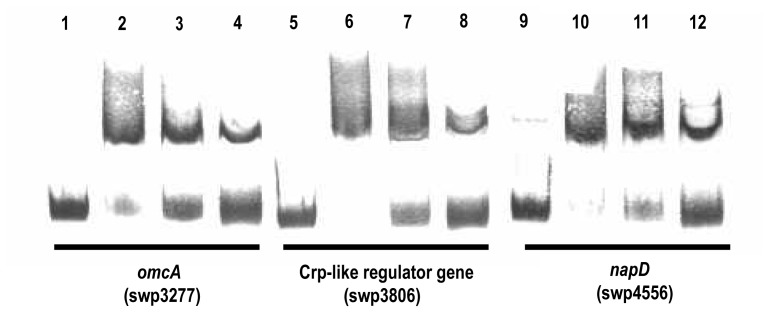
All three tested fragments were shifted in the presence of the purified Fur protein (Figure 4). The binding of Fur to the target promoters were not influenced by addition of the nonspecific competitor salmon sperm DNA, but were outcompeted by adding excess unlabeled probes (Figure 4). These results demonstrated the in vitro specific binding of Fur to all three of the DNA fragments with predicted Fur-binding sites. The interaction between Fur and the Crp-like regulator confirmed the hypothesis that Fur indirectly regulates anaerobic respiration through secondary regulators. Furthermore, the transcriptomic data showed that the napD operon, which is involved in nitrate reduction, and the omcA gene, which is involved in iron reduction, were both down-regulated in the fur mutant. Together with the confirmed Fur Box in the promoter regions, expression patterns of these two genes had indicated a Fur-dependent activation of each under anaerobic conditions.
Figure 4. Fur binding to selected promoters (omcA, Crp-like regulator gene and napD) by EMSA.
The binding assays were performed in the presence of 200(lanes 2–4, lanes 6–8, lanes 10–12) and 0.2 pmol DIG-labeled (lanes 1–12) promoter DNA. Non-specific competitor DNA (50 µg/ml Salmon Sperm DNA) was used in all these binding reactions to control for the presence of unspecific binding. Specific competitors (2 pmol and 20 pmol unlabeled probes) were added respectively in lane 3, 4; lane 7, 8; lane 11, 12 to verify the specificity of a band resulting from protein-binding to the labeled probe.
Fur Regulation Model in WP3
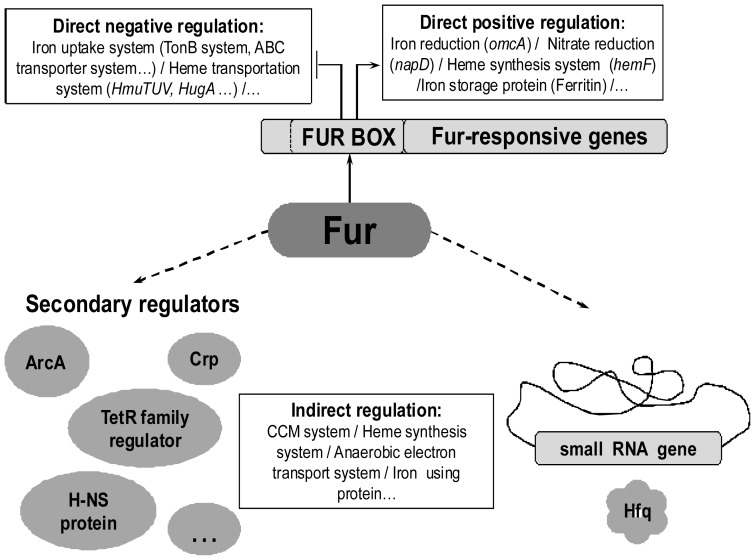
Based on our results, we have proposed a model for Fur-related regulation of iron homeostasis and anaerobic respiration in WP3 (Figure 5). According to the model, Fur acts primarily as a negative regulator in the iron uptake system, where gene expression is regulated by the direct binding of Fur and Fur Box sequences. Alternatively, Fur could function as a positive regulator in the iron storage system. Fur regulates anaerobic respiration through various mechanisms. First, Fur may regulate anaerobic respiration indirectly by regulating the expression of secondary regulators such as the ArcA regulator, a transcriptional factor that is involved in aerobic/anaerobic respiration [53], [56], [67]; the TetR family transcriptional regulator [27]; the H-NS protein, which is involved in iron storage and virulence; and the Crp-like regulator. A conserved Fur Box was identified in the promoter region of the Crp-like regulator (as shown by EMSA), suggesting that Fur directly controls the expression of the Crp-like regulator gene. Notably, some genes in WP3 contain both Fur Box and Crp Box in their promoter regions. For example, the gene cluster involved in ferrous iron transport (swp3271-swp3270), gene cluster encoding fumarate reductase (swp0428-swp0431), and gene cluster involved in iron reduction (swp3272-swp3275). It is thus possible that dual regulatory role occurred between the Crp and Fur transcriptional regulators. The detail regulatory interplay between Fur and CRP warrants further investigation. Second, as reported for other bacteria [10], [50], non-coding small RNAs are involved in the regulation of iron-containing proteins, such as Fe-S proteins, that provide iron for central metabolic processes. Using MR-1 ryhB as a seed against the WP3 genome revealed a region of strong conservation, suggesting that this WP3 sequence may be a ryhB-like gene (5199865–5200032). The RNA chaperone Hfq was also identified in the WP3 genome (swp0789). Further studies are required to determine the role of Hfq in Fur regulation of anaerobic respiration in WP3. Third, Fur could directly regulate genes that are involved in anaerobic respiration, such as napD and omcA, and positively regulate genes that are closely related to anaerobic respiration, such as those involved in the heme biosynthesis system and the CCM system. Because only a small number of genes that are involved in anaerobic respiration possess upstream Fur Box motifs, indirect regulation likely plays a predominant role in the Fur regulation system.
Figure 5. A model of the Fur regulatory system in WP3.
In this model, Fur acts as both a direct and indirect regulator of iron homeostasis and anaerobic respiration. As a direct regulator, the Fur protein generally binds to a Fur Box region to down- or up-regulate the expression of genes. As an indirect regulator, the Fur protein represses an antisense non-coding regulatory RNA or controls secondary regulators, such as Crp, ArcA, the H-NS protein, and the TetR family regulator. These proteins are also capable of regulating genes in the anaerobic respiration system as well as those that encode iron using proteins. The genes regulated by Fur are involved in iron homeostasis, the anaerobic electron transport system, the heme biosynthesis and transport systems, and the CCM system. Direct regulation is depicted with a solid arrow, and indirect regulation is depicted with a dotted arrow.
In the deep-sea iron-reducing bacterium WP3, the anaerobic iron respiration pathway produces iron in ferrous form (Fe2+), which can be taken up easily by WP3 itself [33]. Under these conditions, WP3 has a stable iron source, and iron can be used as a stable environmental signal molecule. Oxygen has the highest redox potential of the EAs [68] and is consumed at a low level in deep-sea sediments. In such cases, sensitive sensors would be produced by microorganisms to detect oxygen concentrations. Oxygenation of ferrous iron occurs immediately in the presence of O2 inside of the cell, and the Fur-Fe2+ complex, which contains the remaining free Fe2+, is able to bind to the Fur-box. Therefore, the Fur protein appears to act as a sensor for anoxic conditions by responding to environmental redox changes and regulating various metabolic pathways, including the anaerobic respiration system. Previously, two different pathways (Fnr and ArcA) were known to control anaerobic metabolism and were associated with either the cellular oxidation or reduction (redox) status [69], [70]. The ability of Fur to function as a sensor of anoxic conditions in association with free Fe2+ suggests that the Fur sensor is more sensitive to and responds more rapidly to changes in the cellular redox status than to the cellular oxidation status, which is characterized by the [4Fe-4S] cluster or the oxidation state of membrane-bound quinines. In Salmonella enterica, Fur is involved in the control of nitrate/nitrite respiration by sensing the cellular redox status [17]. In summary, Fur could play important roles, such as an iron sensor, in response to environmental redox changes and in the regulation of various metabolic pathways, including anaerobic respiration.
Supporting Information
Anaerobic incubation of the WT WP3 and fur mutant strains on a CAS screening plate.
(TIF)
The identification of a predicted consensus of the Fur-binding motif in WP3 using the web-based tool RegPredict ( http://regpredict.lbl.gov ). A sequence logo representation of a palindromic-motif model was derived based on those sites located upstream of the genes listed in Table S3. The error bars indicate the standard deviations of the sequence conservation.
(TIF)
The heme biosynthesis pathway in WP3. The pathway begins from L-Glutamate and proceeds through the formation of porphobilinogen, hydroxymethylbilane, and uroporphyrinogen III to coproporphyrinogen III, aided by five distinct enzymes (HemA-HemE). Next, HemF catalyzes the conversion of coproporphyrinogen III to protoporphyrinogen IX, and HemK catalyzes the subsequent formation of protoporphyrin IX. Lastly, heme is formed by HemH. The genes exhibiting attenuated expression in the fur mutant are highlighted in grey. Adapted from the KEGG database (http://www.genome.jp/kegg).
(TIF)
Gene transcription levels of the 10 (CcmABCDEFGH, DsbA and DsbD) components involved in the cytochrome c maturation system in the WT WP3 and fur mutant strains under anaerobic conditions using fumarate as an EA. The ATP-hydrolyzing CcmA subunits (swp2043) and their membrane integral partners CcmB (swp2042), CcmC (swp2041), and CcmD (swp2040) load heme onto the heme chaperone CcmE (swp2039). Meanwhile, apocytochrome c (apocyt c) translocates through the secretion system (signal sequence cleavage) and is oxidized by DsbA (swp2175). The electron transport complex (DsbD, swp 4520, and CcmG, swp2047) then reduces the disulfide bond of apocyt c. Lastly, the CcmF (swp2046) and CcmH (swp2048) complex ligate heme to apocyt c, and holocytochrome c is produced. The transcription level of WT WP3 was set as 1. The WP3 pepN gene was used to normalize the RNA concentration of each sample. The data shown represent 3 independent experiments, and the error bars indicate standard deviations.
(TIF)
Fur binding to target promoters of napD , omcA and the Crp-like regulator gene. Fur binding to the TonB receptor promoter and swp_1869 promoter (not predicted to be bound by Fur) were used as the positive and negative control, respectively. The DNA probe was pre-incubated with the purified Fur protein at the indicated molar ratios. The amount of DNA is 1 pmol, and 0, 4, 8, 16 pmol purified His tag fusion Fur were used in the DNA binding assays. The probes remained unbound in the absence of Fur binding, and reduced mobility was observed with increasing Fur concentration for all three of the Fur targets.
(TIF)
Bacterial strains and plasmids used in the present study.
(PDF)
Primers used in this study.
(PDF)
Genes containing a putative Fur binding site in WP3.
(XLSX)
Acknowledgments
We thank Huahua Jian and Ping Sun for assistance with Cytochrome c content measurement and data analysis.
Funding Statement
This work was supported by National Science Foundation of China (Grant No. 31290232, 41076078), National Basic Research Program of China (Grant No.2011CB808800), China Ocean Mineral Resources R & D Association (Grant No.DY125-15-T-04), “ShuGuang” Project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Andrews SC, Robinson AK, Rodriguez-Quinones F (2003) Bacterial iron homeostasis. FEMS Microbiol Rev 27: 215–237. [DOI] [PubMed] [Google Scholar]
- 2. Touati D (2000) Iron and oxidative stress in bacteria. Arch Biochem Biophys 373: 1–6. [DOI] [PubMed] [Google Scholar]
- 3. Wandersman C, Delepelaire P (2004) Bacterial iron sources: from siderophores to hemophores. Annual Review of Microbiology 58: 611–647. [DOI] [PubMed] [Google Scholar]
- 4. Schroder I, Johnson E, de Vries S (2003) Microbial ferric iron reductases. FEMS Microbiol Rev 27: 427–447. [DOI] [PubMed] [Google Scholar]
- 5. Cornelis P, Wei Q, Andrews SC, Vinckx T (2011) Iron homeostasis and management of oxidative stress response in bacteria. Metallomics 3: 540–549. [DOI] [PubMed] [Google Scholar]
- 6. Delany I, Rappuoli R, Scarlato V (2004) Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis . Mol Microbiol 52: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 7. Nandal A, Huggins CC, Woodhall MR, McHugh J, Rodriguez-Quinones F, et al. (2010) Induction of the ferritin gene (ftnA) of Escherichia coli by Fe(2+)-Fur is mediated by reversal of H-NS silencing and is RyhB independent. Mol Microbiol 75: 637–657. [DOI] [PubMed] [Google Scholar]
- 8. Craig SA, Carpenter CD, Mey AR, Wyckoff EE, Payne SM (2011) Positive Regulation of the Vibrio cholerae Porin OmpT by Iron and Fur. J Bacteriol 193: 6505–6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miles S, Carpenter BM, Gancz H, Merrell DS (2010) Helicobacter pylori apo-Fur regulation appears unconserved across species. J Microbiol 48: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaballa A, Antelmann H, Aguilar C, Khakh SK, Song KB, et al. (2008) The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins. Proc Natl Acad Sci U S A 105: 11927–11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masse E, Salvail H, Desnoyers G, Arguin M (2007) Small RNAs controlling iron metabolism. Curr Opin Microbiol 10: 140–145. [DOI] [PubMed] [Google Scholar]
- 12. Masse E, Vanderpool CK, Gottesman S (2005) Effect of RyhB small RNA on global iron use in Escherichia coli . J Bacteriol 187: 6962–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escolar L, Perez-Martin J, de Lorenzo V (1999) Opening the iron box: transcriptional metalloregulation by the Fur protein. J Bacteriol 181: 6223–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ratledge C, Dover LG (2000) Iron metabolism in pathogenic bacteria. Annual Review of Microbiology 54: 881–941. [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Harris DP, Luo F, Wu L, Parsons AB, et al. (2008) Characterization of the Shewanella oneidensis Fur gene: roles in iron and acid tolerance response. BMC Genomics 9 Suppl 1S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ellermeier JR, Slauch JM (2008) Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190: 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teixido L, Cortes P, Bigas A, Alvarez G, Barbe J, et al. (2010) Control by Fur of the nitrate respiration regulators NarP and NarL in Salmonella enterica . Int Microbiol 13: 33–39. [DOI] [PubMed] [Google Scholar]
- 18. Wan XF, Verberkmoes NC, McCue LA, Stanek D, Connelly H, et al. (2004) Transcriptomic and proteomic characterization of the Fur modulon in the metal-reducing bacterium Shewanella oneidensis . J Bacteriol 186: 8385–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baichoo N, Wang T, Ye R, Helmann JD (2002) Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol Microbiol 45: 1613–1629. [DOI] [PubMed] [Google Scholar]
- 20. Paustian ML, May BJ, Kapur V (2001) Pasteurella multocida gene expression in response to iron limitation. Infect Immun 69: 4109–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pellicer S, Gonzalez A, Peleato ML, Martinez JI, Fillat MF, et al. (2012) Site-directed mutagenesis and spectral studies suggest a putative role of FurA from Anabaena sp. PCC 7120 as a heme sensor protein. FEBS J 279: 2231–2246. [DOI] [PubMed] [Google Scholar]
- 22. Gonzalez A, Bes MT, Valladares A, Peleato ML, Fillat MF (2012) FurA is the master regulator of iron homeostasis and modulates the expression of tetrapyrrole biosynthesis genes in Anabaena sp. PCC 7120. Environ Microbiol 14: 3175–3187. [DOI] [PubMed] [Google Scholar]
- 23. Wang F, Wang J, Jian H, Zhang B, Li S, et al. (2008) Environmental adaptation: genomic analysis of the piezotolerant and psychrotolerant deep-sea iron reducing bacterium Shewanella piezotolerans WP3. PLoS One 3: e1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, et al. (1999) Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 49 Pt 2: 705–724. [DOI] [PubMed] [Google Scholar]
- 25. Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, et al. (2008) Towards environmental systems biology of Shewanella . Nature Reviews Microbiology 6: 592–603. [DOI] [PubMed] [Google Scholar]
- 26. Meyer TE, Tsapin AI, Vandenberghe I, de Smet L, Frishman D, et al. (2004) Identification of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes. OMICS 8: 57–77. [DOI] [PubMed] [Google Scholar]
- 27. Rodionov DA, Novichkov PS, Stavrovskaya ED, Rodionova IA, Li X, et al. (2011) Comparative genomic reconstruction of transcriptional networks controlling central metabolism in the Shewanella genus. BMC Genomics 12 Suppl 1S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Myers CR, Nealson KH (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 29. Thompson DK, Beliaev AS, Giometti CS, Tollaksen SL, Khare T, et al. (2002) Transcriptional and proteomic analysis of a ferric uptake regulator (fur) mutant of Shewanella oneidensis: possible involvement of fur in energy metabolism, transcriptional regulation, and oxidative stress. Appl Environ Microbiol 68: 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang F, Wang P, Chen M, Xiao X (2004) Isolation of extremophiles with the detection and retrieval of Shewanella strains in deep-sea sediments from the west Pacific. Extremophiles 8: 165–168. [DOI] [PubMed] [Google Scholar]
- 31. Chen S-Y, Ambe S, Takematsu N, Ambe F (1996) The Chemical States of Iron in Marine Sediments by Means of Mössbauer Spectroscopy in Combination with Chemical Leachings. J Oceanography 52: 705–715. [Google Scholar]
- 32. Xiao X, Wang P, Zeng X, Bartlett DH, Wang F (2007) Shewanella psychrophila sp. nov. and Shewanella piezotolerans sp. nov., isolated from west Pacific deep-sea sediment. Int J Syst Evol Microbiol 57: 60–65. [DOI] [PubMed] [Google Scholar]
- 33. Wu W, Li B, Hu J, Li J, Wang F, et al. (2011) Iron reduction and magnetite biomineralization mediated by a deep-sea iron reducing bacterium Shewanella piezotolerans WP3. J Geophys Res 116: G04034. [Google Scholar]
- 34. Chen Y, Wang F, Xu J, Mehmood MA, Xiao X (2010) Physiological and evolutionary studies of NAP systems in Shewanella piezotolerans WP3. ISME J 5: 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160: 47–56. [DOI] [PubMed] [Google Scholar]
- 36. Fennessey CM, Jones ME, Taillefert M, DiChristina TJ (2010) Siderophores are not involved in Fe(III) solubilization during anaerobic Fe(III) respiration by Shewanella oneidensis MR-1. Appl Environ Microbiol 76: 2425–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49: 219–286. [DOI] [PubMed] [Google Scholar]
- 38. Edwards RA, Keller LH, Schifferli DM (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207: 149–157. [DOI] [PubMed] [Google Scholar]
- 39. Li R, Li Y, Kristiansen K, Wang J (2008) SOAP: short oligonucleotide alignment program. Bioinformatics 24: 713–714. [DOI] [PubMed] [Google Scholar]
- 40. Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26: 136–138. [DOI] [PubMed] [Google Scholar]
- 41. Novichkov PS, Rodionov DA, Stavrovskaya ED, Novichkova ES, Kazakov AE, et al. (2010) RegPredict: an integrated system for regulon inference in prokaryotes by comparative genomics approach. Nucleic Acids Res 38: W299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bender KS, Yen HC, Hemme CL, Yang Z, He Z, et al. (2007) Analysis of a ferric uptake regulator (Fur) mutant of Desulfovibrio vulgaris Hildenborough. Appl Environ Microbiol 73: 5389–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Troxell B, Fink RC, Porwollik S, McClelland M, Hassan HM (2011) The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol 11: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parker D, Kennan RM, Myers GS, Paulsen IT, Rood JI (2005) Identification of a Dichelobacter nodosus ferric uptake regulator and determination of its regulatory targets. J Bacteriol 187: 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao H, Barua S, Liang Y, Wu L, Dong Y, et al. (2010) Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration. Microb Biotechnol 3: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shi L, Squier TC, Zachara JM, Fredrickson JK (2007) Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol 65: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Postle K, Kadner RJ (2003) Touch and go: tying TonB to transport. Mol Microbiol 49: 869–882. [DOI] [PubMed] [Google Scholar]
- 48. Kuehl CJ, Crosa JH (2010) The TonB energy transduction systems in Vibrio species. Future Microbiol 5: 1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beliaev AS, Klingeman DM, Klappenbach JA, Wu L, Romine MF, et al. (2005) Global transcriptome analysis of Shewanella oneidensis MR-1 exposed to different terminal electron acceptors. J Bacteriol 187: 7138–7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Masse E, Gottesman S (2002) A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli . Proc Natl Acad Sci U S A 99: 4620–4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iuchi S, Lin EC (1988) arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci U S A 85: 1888–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Evans MR, Fink RC, Vazquez-Torres A, Porwollik S, Jones-Carson J, et al. (2011) Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol 11: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gao H, Wang X, Yang ZK, Palzkill T, Zhou J (2008) Probing regulon of ArcA in Shewanella oneidensis MR-1 by integrated genomic analyses. BMC Genomics 9: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yuan J, Wei B, Lipton MS, Gao H (2012) Impact of ArcA loss in Shewanella oneidensis revealed by comparative proteomics under aerobic and anaerobic conditions. Proteomics 12: 1957–1969. [DOI] [PubMed] [Google Scholar]
- 55. Gralnick JA, Brown CT, Newman DK (2005) Anaerobic regulation by an atypical Arc system in Shewanella oneidensis . Mol Microbiol 56: 1347–1357. [DOI] [PubMed] [Google Scholar]
- 56. Gao H, Wang X, Yang ZK, Chen J, Liang Y, et al. (2010) Physiological roles of ArcA, Crp, and EtrA and their interactive control on aerobic and anaerobic respiration in Shewanella oneidensis . PLoS One 5: e15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saffarini DA, Schultz R, Beliaev A (2003) Involvement of cyclic AMP (cAMP) and cAMP receptor protein in anaerobic respiration of Shewanella oneidensis . J Bacteriol 185: 3668–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang Y, Harris DP, Luo F, Xiong W, Joachimiak M, et al. (2009) Snapshot of iron response in Shewanella oneidensis by gene network reconstruction. BMC Genomics 10: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Troxell B, Sikes ML, Fink RC, Vazquez-Torres A, Jones-Carson J, et al. (2011) Fur negatively regulates hns and is required for the expression of HilA and virulence in Salmonella enterica sv. Typhimurium. J Bacteriol 193: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Myers JM, Myers CR (2000) Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J Bacteriol 182: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Murphy JN, Saltikov CW (2007) The cymA gene, encoding a tetraheme c-type cytochrome, is required for arsenate respiration in Shewanella species. J Bacteriol 189: 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maier TM, Myers JM, Myers CR (2003) Identification of the gene encoding the sole physiological fumarate reductase in Shewanella oneidensis MR-1. J Basic Microbiol 43: 312–327. [DOI] [PubMed] [Google Scholar]
- 63. Cavallaro G, Decaria L, Rosato A (2008) Genome-based analysis of heme biosynthesis and uptake in prokaryotic systems. J Proteome Res 7: 4946–4954. [DOI] [PubMed] [Google Scholar]
- 64. Wang LY, Brown L, Elliott M, Elliott T (1997) Regulation of heme biosynthesis in Salmonella typhimurium: activity of glutamyl-tRNA reductase (HemA) is greatly elevated during heme limitation by a mechanism which increases abundance of the protein. J Bacteriol 179: 2907–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sanders C, Turkarslan S, Lee DW, Daldal F (2010) Cytochrome c biogenesis: the Ccm system. Trends Microbiol 18: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanders C, Turkarslan S, Lee DW, Onder O, Kranz RG, et al. (2008) The cytochrome c maturation components CcmF, CcmH, and CcmI form a membrane-integral multisubunit heme ligation complex. J Biol Chem 283: 29715–29722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shroff NP, Charania MA, Saffarini DA (2010) ArcB1, a Homolog of Escherichia coli ArcB, Regulates Dimethyl Sulfoxide Reduction in Shewanella oneidensis MR-1. J Bacteriol 192: 3227–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Orcutt BN, Sylvan JB, Knab NJ, Edwards KJ (2011) Microbial ecology of the dark ocean above, at, and below the seafloor. Microbiol Mol Biol Rev 75: 361–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spiro S, Guest JR (1990) FNR and its role in oxygen-regulated gene expression in Escherichia coli . FEMS Microbiol Rev 6: 399–428. [DOI] [PubMed] [Google Scholar]
- 70. Unden G, Bongaerts J (1997) Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320: 217–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anaerobic incubation of the WT WP3 and fur mutant strains on a CAS screening plate.
(TIF)
The identification of a predicted consensus of the Fur-binding motif in WP3 using the web-based tool RegPredict ( http://regpredict.lbl.gov ). A sequence logo representation of a palindromic-motif model was derived based on those sites located upstream of the genes listed in Table S3. The error bars indicate the standard deviations of the sequence conservation.
(TIF)
The heme biosynthesis pathway in WP3. The pathway begins from L-Glutamate and proceeds through the formation of porphobilinogen, hydroxymethylbilane, and uroporphyrinogen III to coproporphyrinogen III, aided by five distinct enzymes (HemA-HemE). Next, HemF catalyzes the conversion of coproporphyrinogen III to protoporphyrinogen IX, and HemK catalyzes the subsequent formation of protoporphyrin IX. Lastly, heme is formed by HemH. The genes exhibiting attenuated expression in the fur mutant are highlighted in grey. Adapted from the KEGG database (http://www.genome.jp/kegg).
(TIF)
Gene transcription levels of the 10 (CcmABCDEFGH, DsbA and DsbD) components involved in the cytochrome c maturation system in the WT WP3 and fur mutant strains under anaerobic conditions using fumarate as an EA. The ATP-hydrolyzing CcmA subunits (swp2043) and their membrane integral partners CcmB (swp2042), CcmC (swp2041), and CcmD (swp2040) load heme onto the heme chaperone CcmE (swp2039). Meanwhile, apocytochrome c (apocyt c) translocates through the secretion system (signal sequence cleavage) and is oxidized by DsbA (swp2175). The electron transport complex (DsbD, swp 4520, and CcmG, swp2047) then reduces the disulfide bond of apocyt c. Lastly, the CcmF (swp2046) and CcmH (swp2048) complex ligate heme to apocyt c, and holocytochrome c is produced. The transcription level of WT WP3 was set as 1. The WP3 pepN gene was used to normalize the RNA concentration of each sample. The data shown represent 3 independent experiments, and the error bars indicate standard deviations.
(TIF)
Fur binding to target promoters of napD , omcA and the Crp-like regulator gene. Fur binding to the TonB receptor promoter and swp_1869 promoter (not predicted to be bound by Fur) were used as the positive and negative control, respectively. The DNA probe was pre-incubated with the purified Fur protein at the indicated molar ratios. The amount of DNA is 1 pmol, and 0, 4, 8, 16 pmol purified His tag fusion Fur were used in the DNA binding assays. The probes remained unbound in the absence of Fur binding, and reduced mobility was observed with increasing Fur concentration for all three of the Fur targets.
(TIF)
Bacterial strains and plasmids used in the present study.
(PDF)
Primers used in this study.
(PDF)
Genes containing a putative Fur binding site in WP3.
(XLSX)