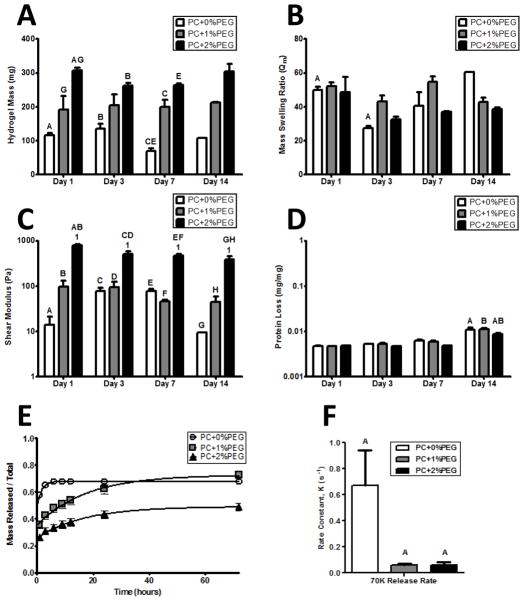
Figure 2. The physical properties of hydrogel formulations are stable in the absence of cells and can be tuned by exogenous PEGDAm incorporation.
A) Wet masses of swollen hydrogels of each formulation demonstrated a significantly increased degree of swelling with increasing PEG content. The masses of the gels were stable and did not significantly change over time. B) The mass swelling ratio was also stable and did not show any significant trends with neither the gel formulation nor time in buffer significantly contributing to the variance. C) The shear moduli of the PC+0% & 1% PEG gels did not change over time while the PC+2%PEG showed a slight decrease after day 1. At each day, the shear modulus of the PC+2%PEG gels was significantly different from the PC+0% & 1% PEG gels. D) Protein release from the hydrogels was not significant until day 14 when a negligible amount (0.01% w/w) was detected form all gel formulations. For panels A–D, significance was determined by 2-way ANOVA and Bonferroni post-tests with p < 0.05 and denoted by matched symbols on the graphs. To assess the bulk transport properties of the gels, release of encapsulated 70 kDa dextran (E) was monitored and the rate constants were compared (F) with a significantly higher rate for PC+0%PEG and comparable between the other two formulations.