Abstract
Chromosomal translocations juxtaposing the androgen-responsive TMPRSS2 promoter with the ETS-family transcription factor ERG result in aberrant ERG up-regulation in approximately 50% of prostate cancers. Studies to date have demonstrated important roles of ERG in inducing oncogenic properties of prostate cancer. Its molecular mechanisms of action, however, are yet to be fully understood. Here we report that ERG activates Wnt/LEF1 signaling cascade through multiple mechanisms. ERG bound to the promoters of various Wntgenes to directly increase ligand expression. Consequently, ERG overexpression increased active β-catenin level in the cells and enhancedTCF/LEF1 luciferase reporter activity, which could be partially blocked by WNT-3A inhibitor IWP-2. Most importantly, our data defined LEF1 as a direct target of ERG and that LEF1 inhibitionfully abolished ERG-induced Wnt signaling and target gene expression. Further, functional assays demonstrated that Wnt/LEF1 activation phenocopiedthat of ERG in inducing cell growth, epithelial-to-mesenchymal transition, and cellinvasion, while blockade of Wnt signaling attenuated these effects. Concordantly, LEF1 expression is significantly up-regulated in ERG-high human prostate cancers. Overall, this study provides an important mechanism of activation of Wnt signaling in prostate cancer and nominates LEF1 as a critical mediator of ERG-induced tumorigenesis. Wnt/LEF1 pathway might provide novel targets for therapeutic management of patients with fusion-positive prostate cancer.
Introduction
Prostate cancer is the most commonly diagnosed non-skin cancer and a leading cause of cancer-related death in men of the industrialized world. Over the past years, genetic aberrations such as chromosomal translocations have been reported in a majority of prostate cancers (1). In particular, approximately 50% of human prostate cancers were found to contain chromosomal rearrangements that juxtapose the androgen-responsive TMPRSS2 promoter to the coding region of the oncogenic ETS family transcription factor ERG, resulting in abnormally high expression of ERG protein (2). Mechanistically, TMPRSS2-ERG gene fusions were found caused by double-strand DNA breaks and repair that are induced by androgen and/or genomic stress (3–5). Due to the high specificity of TMPRSS2-ERG gene fusion and its being an early molecular event in PCa (6), it has been shown to providea valuable tool for PCa diagnosis (7, 8). In addition, numerous studies have investigated the potential of TMPRSS2-ERG gene fusions in predicting PCa aggressiveness in various patient cohorts. While controversial reports exist (9–11), a majority of such studies have suggested that TMPRSS2-ERG gene fusions are associated with aggressive or fatal types of PCa (12–15). Consequently, studies have also begun to evaluate drug sensitivities of this molecular sub-type of PCa with an ultimate goal to design therapeutics that specifically target TMPRSS2-ERG gene fusions (16, 17).
Moreover, there are many studies that have attempted to characterize the roles of ERG in prostate tumorigenesis and to decipher the underlying molecular mechanisms. Studies have shown that ERG plays important roles in epithelial-to-mesenchymal transition (EMT) and in increasing cell invasion (18–20). Besides these most prominent functions, ERG has also been shown to increase PCa cell proliferation in vitro; a function that becomes apparent upon stable or relatively long-term ERG dys-regulation (21, 22). In addition, transgenic studies have reported that mouse prostate with TMPRSS2-ERG gene fusion alone develops prostatic intraepithelial neoplasia (mPIN) (18), but when cooperated by other oncogenic pathways such as PTEN deletion or androgen receptor (AR) overexpression, itleads to the development of prostatic adenocarcinoma (3, 23, 24). Mechanistically, ERG is able to regulate multiple oncogenic pathways such as c-Myc, AR and EZH2, resulting in the abrogation of epithelial differentiation and promotion of cancerous de-differentiation (21, 22, 25). Bioinformatics analyses have also revealed increased expression of Wnt-associated pathways in TMPRSS2-ERG fusion-positive human PCa (26) and the expression of Wnt receptor frizzled-4 (FZD4) was experimentally shown to positively correlate with that of ERG (27). Whether and how ERG directly regulates components of the Wnt pathway to induce prostate tumorigenesis, however, have not been carefully examined.
Wnt signaling plays major roles in embryonic development, organogenesis as well as in human diseases including malignancy such as PCa (28). In the canonical Wnt/β-catenin pathway, the binding of Wnts such as WNT3A to the receptors activates a signaling cascade that prevents phosphorylation of cytoplasmic β-catenin by GSK-3β and results in its cytoplasmic accumulation followed by nuclear translocation. Once in the nucleus, β-catenin is recruited by the TCF/LEF1 transcription factors to activate target genes such as c-Myc, MMPs, AXIN2, and LEF1 itself. Upregulation of WNT1, nuclear β-catenin, and LEF1 are strongly correlated with advanced, hormone-refractory PCa (29, 30). Mutations that constitutively stabilize β-catenin and thus activate Wnt signaling has been reported in approximately 5% of PCa (31, 32). For example, a study has reported stabilizing β-catenin mutation in AR-negative PCa cell line, potentially contributing to bone metastasis (33). Moreover, using the state-of-the-art exome-sequencing, Kumar et al. has recently reported mutations in the Wnt pathway molecules specifically in castration-resistant PCa cells (34).
The function of Wnt/TCF signaling in PCa has been extensively explored in various cell lines and mouse models. Activation of β-catenin in mouse prostate has been shown to result in high-grade prostatic intraepithelial neoplasia (PIN) and continuous prostatic growth after castration (35). Using probasin promoter directed gene expression, Yu et al. has further shown that while the mouse prostate expressing nuclear β-catenin alone or SV40-large T-antigen alone developed mPIN, the activation of both pathways resulted in invasive prostate carcinoma (36). Similarly, Wnt signaling has also been shown to synergize with K-ras to accelerate prostate tumorigenesis in the mice (37). Interestingly, a recent study has reported that increased Wnt paracrine signaling in the surrounding stroma can also initiate mouse prostate cancer (38). These studies strongly support the prostate tumorigenic role of Wnt/TCF signaling in a physiological setting.
In this study, we report TMPRSS2-ERG as a critical activator ofWnt/LEF1 pathway through multiple mechanisms. We show that ERG directly induces the expression of various Wnt ligands as well as the LEF1 transcription factor. We nominate LEF1 as the most important ERG-targeted component of the Wnt pathway, as LEF1 knockdown completely abolished ERG-induced Wnt signaling, downstream gene expression, and oncogenic properties. Our study thus characterizes a critical signal transduction pathway downstream of TMPRSS2-ERG gene fusions and offers novel therapeutic opportunities for targeting fusion-positive prostate cancer.
Materials and Methods
Cell Lines and Treatments
Prostate cancer cell lines LNCaP, VCaP, 22Rv1, BPH1, RWPE-1, DU145, and human embryonic kidney cell line HEK293T cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). Immortalized PrEC cells were provided by Dr. William C. Hahn (Dana-Farber Cancer Institute). Control L cells and WNT3A-producing L cells were provide by Dr. Cara Gottardi (Northwestern University). All cell lines were maintained according to recommended conditions.
Plasmids and small interference RNAs
Human ERG and LEF1 cDNAwere amplified by reverse transcription-PCR from VCaP cells and cloned into pcDNA3.2 vector (Invitrogen, Carlsbad, CA). Dominant-negative LEF1 (dnLEF1) was provided by Dr. Marian Waterman (University of California, Irvine). For RNA interference assays, control luciferase GL2 Duplex siRNA (D-00110-01-20), LEF1 siRNA (L-015396-00-0005) and ERG siRNA (D-003886-01) were from Dharmacon (Lafayette, CO). Lentivirus mediated stable ERG knockdown in VCaP cells was performed with ERG shRNA construct (Open Biosystem, Lafayette, CO). Lentivirus particles were generated according to manufacturer’s instruction and stable clones were selected. For ERG stable overexpression, PCa cells were infected with lentivirus particle containing ERG overexpression construct, and selected with puromycin for 7 days after viral infection.
Western blotting and antibodies
Western blotting was carried out using a standard protocol as previously described (22). Antibodies used in this study are listed in Table S1.
Luciferase reporter assay
WNT pathway luciferase reporter assay was performed as previously described (39). Briefly, cells were seeded in 24-well plate and co-transfected with the SuperTOPFlash reporter, the Renilla expression plasmid pRL-TK, the ERG expression plasmid pcDNA3-ERG, and pcDNA3-β-catenin or stimulated with WNT3A conditional medium. To confirm the role of LEF1 in the activation of WNT signaling, dnLEF1 was co-transfected with the plasmids as indicated. X-tremeGeneHP (Roche Diagnostics, Indianapolis, IN) was utilized to increase transfection efficiency in LNCaP cells, while lipofectamine 2000 (Invitrogen) was used to transfect other cell lines. Luciferase activities were determined 48 h post-transfection and normalized against Renilla internal control values.
ChIP, ChIP-qPCR and quantitative qRT-PCR Assays
ChIPwas carried out as previously described (22, 40). Quantitative PCR was performed with GoTaqqPCR Master Mix 2X (Promega, Madison, WI) using an Applied BiosystemsStepOnePlus Real Time PCR System. All primers were designed using Primer 3 and synthesized by Integrated DNA Technologies (IDT, Coralville, IA) and are listed in Table S2.
Gene expression microarray and bioinformatics analysis
Microarray profiling was performed using HumanHT-12 v 4.0 Expression BeadChip (Illumina, San Diego, CA) as previously described (41). The bead-level data were preprocessed using GenomeStudio (Illumina, San Diego, CA), and the expression data were analyzed and normalized using the beadarray package in Bioconductor. Differential and coordinated expression of a gene set between microarray experimentalconditions was performed using Gene Set Enrichment Analysis (GSEA) tools.
Cell proliferation and invasion assay
Cell proliferation assay was carried out using the WST-1 kit according to the manufacturer’s instruction (Clontech Laboratories, Mountain View, CA). For IWP2 treatment, LNCaP cells were with 5nM of IWP2 or DMSO as control for 24–72 h prior to WST-1 incubation and measurement. Cell invasion was examined as previously described using Boydem chamber assay (42).
Accession numbers
The microarray data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus database under the accession number GSE47423.
Results
ERG regulates WNT pathway genes in prostate cancer
To evaluate the link between ERG and Wnt signaling, we examined ERG occupancy on a set of 168Wnt pathway genes defined by KEGG functional annotation. ERGChIP-Seq data obtained from the TMPRSS2-ERG fusion-positive VCaP cells revealed at least one ERG binding event at the regulatory elements of 72 out of 168 KEGG WNT pathway genes (Fig. 1A). As we have previously shown that AR is a critical mediator of ERG function (22), we also examined AR ChIP-Seq data around these regions. Our results demonstrated that AR did not co-occupy these regions, suggesting Wnt signaling as a potentially novel, AR-independent, pathway downstream of ERG. Out of these genes, many are Wnt ligands (Fig. 1B). To confirm these observations, we carried out ERG and IgGChIP-qPCR in VCaP cells using primers flanking the binding sites near a number of Wnt ligand genes. Our data confirmed significant ERG enrichment at the promoters of known target genes such as PSA and TMPRSS2 as well as at the binding sites of various Wnt ligands (Fig. 1C). Next, we asked whether these binding events result in ERG-mediated regulation of Wnt pathway gene expression. We carried out RNA interference experiments to knockdown ERG expression in VCaP cells. QRT-PCR analysis showed that stable ERG knockdown led to significant inhibition of WNT2, WNT3A and WNT11 gene expression, while WNT1 expression was not detectable (Fig. 1D). To obtain a global view of gene expression changes, expression microarrays were then carried out in the control and ERG-knockdown VCaP cells (Fig. 1E). Using Gene Set Enrichment Analysis (GSEA) (43), we found that Wnt pathway genes were significantly (P=0.012) enriched for down-regulation by ERG knockdown, suggesting that ERG may induceWntsignaling in PCa (Fig. 1F). We next sought to confirm this in another independent prostate cancer cell line and further examine how ERG regulates Wnt signaling in prostate cancer.
Figure 1. ERG regulates Wnt pathway genes in prostate cancer.
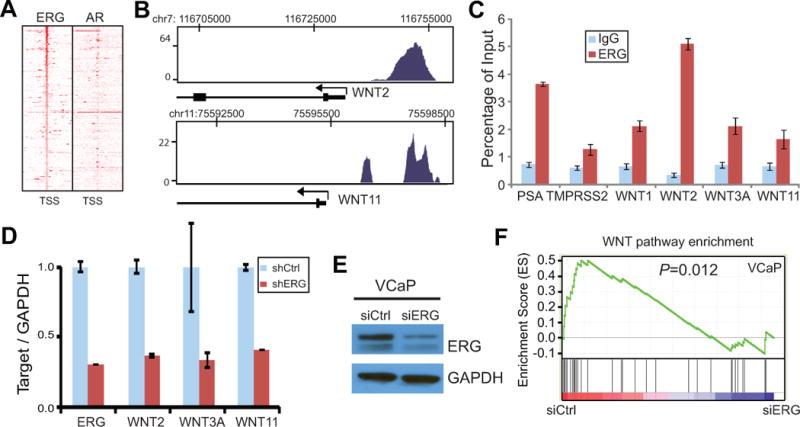
A. Heatmap of ERG binding sites around Wnt pathway gene promoters. ERG and AR ChIP-Seq was carried out in VCaP cells as previously described (22). Heatmap shows ChIP-Seq read intensity around the transcription start site of Wnt pathway genes. Genes were ranked by the height of ChIP-Seq peaks.
B. ERG occupancy near the promoters of representative Wnt pathway genes. ERG ChIP-Seqwas done in VCaP cells as in A. Chromosomal positions are shown at the top and the gene structure at the bottom. Black arrow indicates transcription start site and direction.
C. ERG directly bindsWnt ligand gene promoters. ERG and IgGChIP was carried out in VCaP cells. ChIP-qPCR was done using gene-specific primers. PSA and TMPRSS2 are known target genes of ERG. Error bars indicate triplicate experiments, mean ± standard error of the mean (SEM).
D. Wnt ligand expression is reduced following ERG knockdown. VCaP cells were infected with ERG shRNAlentivirus and selected for clones with stable knockdown. Gene expression was assayed using qRT-PCR and normalized to GAPDH.
E. Western blot confirms ERG knockdown by siRNA. VCaP cells were transfected with siRNA duplex targeting control luciferase or ERG gene.
F. Wnt pathway genes are enriched for down-regulation upon ERG knockdown. VCaP control (siCtrl) and ERG knockdown (siERG) cells as confirmed in D were analyzed by expression microarray. GESA was utilized to determine whether the expression of Wnt pathway genes was at significantly different between siCtrl and siERG cells.
ERG directly induces Wnt ligand expression in prostate cancer cells
To validate ERG occupancy on the promoter regions of Wnt ligands in an independent prostate cell line, we carried out ectopic ERG overexpression in the TMPRSS2-ERG fusion-negative LNCaP cells to generate stable cell lines. Western blot analysis demonstrated that ectopic ERG expression in LNCaP was at a level comparable with endogenous ERG in VCaP resulted from gene fusion (Fig. S1A). ERG ChIP was then carried out in the control and ERG-expressing LNCaP cells. ChIP-qPCR analysis confirmed highly significant ERG enrichment at the promoters of various Wnt ligands such as WNT1, WNT2, WNT3A, and WNT11 following ERG overexpression (Fig. 2A). Next, we asked whether these binding events result in the regulation of gene expression. Using qRT-PCR we compared Wntgene expression between the control and ERG-expressing LNCaP cells (Fig. 2B). Through analysis of previously reported ERG targets, we first validated that ERG indeed induced PLAT and PLAU but inhibited PSA and TMPRSS2 expression (18, 22). Interestingly, our data showed that ERG drastically induced Wnt ligand gene expression. Similar patterns of ERG-mediated regulation of target genes were also observed in another independent prostate cancer cell line, 22Rv1 (Fig. 2C). We next decided to focus on WNT3A, one of the classical and best understood Wnt ligands. Using western blot analysis, we demonstrated that the WNT3A protein was drastically induced by ERG overexpression while AR, a previously reported ERG-inhibited gene, was indeed repressed (Fig. 2D).
Figure 2. ERG directly induces Wnt ligand gene expression.
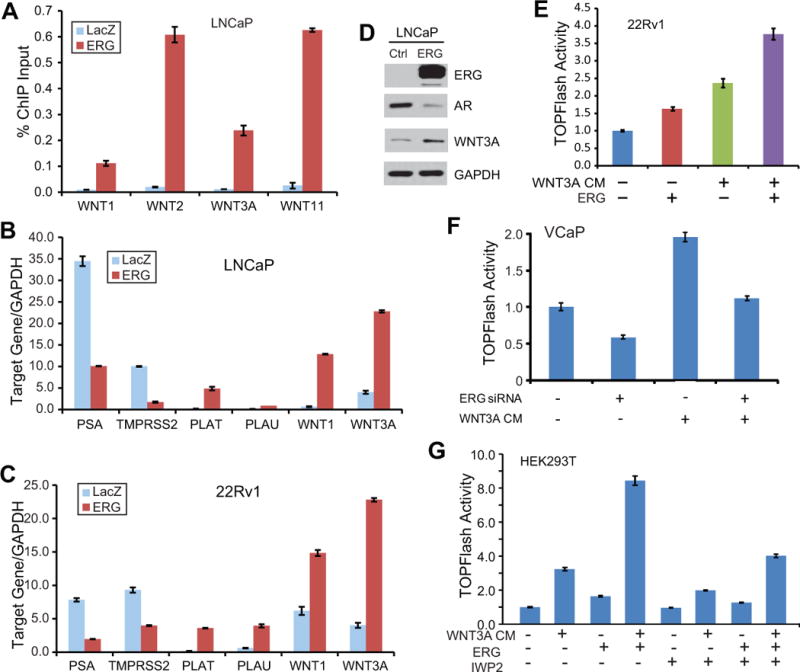
A. Ectopic ERG binds at the promoters of various Wnt ligand genes. LNCaP cells were infected with adenovirus expressing lacZcontrol or ERG gene and then analyzed by ChIP using an anti-ERG antibody. ChIP-qPCR was carried out using promoter-specific primers. Error bars indicate triplicate experiments, mean ± SEM.
B–C. Ectopic ERG induces Wnt ligand gene expression. QRT-PCR was carried out in LNCaP (B) or 22Rv1 (C) cells infected with lacZ- or ERG-expressing adenovirus. PSA and TMPRSS2 have been previously shown inhibited by ERG, while PLAT and PLAU induced by ERG.
D. WNT3A protein is increased by ectopic ERG. Western blot was carried out in LNCaP cells infected with lacZ- or ERG-expressing adenovirus. AR is a control gene that has been previously shown inhibited by ERG (22). GAPDH is the loading control.
E. ERG induces Wnt signaling. 22Rv1 prostate cancer cells were treated with WNT3A conditioned medium (CM) and/or transfected with ERG, along with SuperTOPFlash luciferase reporter construct and the control Renilla expression plasmid. Luciferase activities was assayed at 48 h post-transfection and normalized against Renilla internal control values. Error bars indicate triplicate experiments, mean ± SEM.
F. ERG knockdown inhibits Wnt signaling. VCaP cells were treated with WNT3A CM and/or ERG knockdown. Cells were analyzed for SuperTOPFlash luciferase activity.
G. ERG-induced Wnt signaling can be partially blocked by WNT inhibitor IWP-2. HEK293T cells were treated as described in E in the presence or absence of WNT inhibitor IWP-2, and analyzed for luciferase activities.
This regulation of gene expression has prompted us to examine whether ERG activates Wnt signaling in prostate cancer cells. We utilized theSuperTOPFlash luciferase reporter which contains a stretch of 16x TCF/LEF1 binding sites. HEK293 cell line, a frequently used system for the study of SuperTOPFlashactivity, was tested first. As a positive control WNT3A conditioned medium (CM) was obtained from L-cells with stable WNT3A overexpression and secretion (44). HEK293T cells were treated with WNT3A CM, ERG overexpression, or both. Our results first confirmed that WNT3A CM indeed induced Wnt signaling and further demonstrated that ERG overexpression also significantly increased Wnt signaling as indicated by SuperTOPFlash activity (Fig. S1B). In addition, we found that, in the presence of WNT3A CM, ERG was able to further increase Wnt signaling, exhibiting a strong synergistic effect. This suggests that in addition to inducing Wnt ligand expression, ERG may be able to activate Wnt pathway through additional mediators. To validate these findings in prostate cancer cells, we utilized the 22Rv1 cell line that is easy to transfect. Similarly, we found that WNT3A CM and ERG are able to significantly induce SuperTOPFlash activity in PCa cells (Fig. 2E). By contrast, via combining with ERG RNA interference we found that ERG knockdown in VCaP cells, on the other hand, significantly reduced endogenous and WNT3A CM-induced Wnt signaling (Fig. 2F).
We next asked whether disrupting Wnt signaling will block ERG-induced SuperTOPFlash reporter activity. We chose to use IWP-2, a Wnt inhibitor that prevents palmitylation of Wnt proteins thereby blocking Wnt secretion and activity and also blocks downstream Lrp6 receptor phosphorylation as well as Dvl2 and β-catenin accumulation (45). SuperTOPFlash reporter assays revealed that IWP-2 significantlyinhibited Wnt signaling that was induced by WNT3A CM, ERG overexpression, or both (Fig. 2G). Taken together, ERG induces Wnt signaling in the presence or absence of WNT3A CM, and this activity can be partially blocked by Wnt inhibitor IWP-2. We then took this study one step further and examined how ERG regulates other key components of the Wnt pathway.
ERG increases active β-catenin and stimulatesWnt signaling in prostate cancer cells
As Wnt ligand binding to receptors turns on a series of biochemical reactions ultimately leading to accumulation of unphosphorylated, active β-catenin, which subsequently associates with TCF/LEF1 family transcriptional factors to induce Wnt downstream genes, we carried out western blot analysis of β-catenin. Our analysis of control and ERG-expressing 293T cells revealed significantly increased active β-catenin level by ectopic ERG overexpression (Fig. 3A). This is especially prominent in cells that were co-transfected with ectopicwild type β-catenin and thus there were much more total β-catenin available. By contrast, ERG did not alter the level of total β-catenin. On the contrary, western blot analysis showed that ERG knockdown in VCaP cells resulted in dramatic decrease of active β-catenin level (Fig. 3B).
Figure 3. ERG increases activeβ-cateninin prostate cancer.
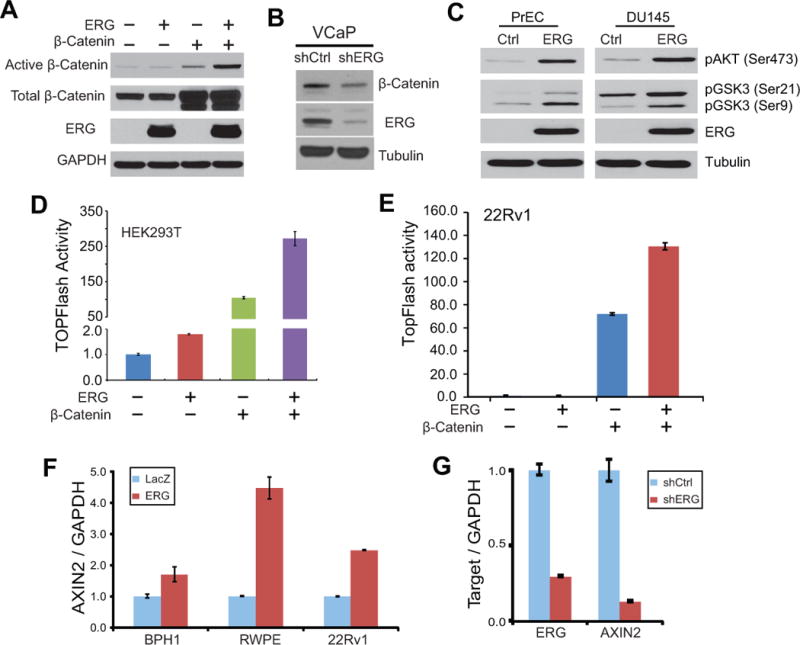
A. Active β-catenin is increased by ERG overexpression. HEK293T cells were transfected with ERG or β-catenin plasmid. The levels of total and active β-catenin were determined by western blot analysis.
B. ERG knockdown decreased active β-catenin level. VCaP cells were subjected to ERG knockdown using shRNAlentivirus to generate stable cells. Cells were analyzed by western blotting using active β-catenin antibody.
C. ERG overexpression induces AKT and GSK-3β phosphorylation. Prostate cell lines with stable expression of control or ERG gene were analyzed by immunoblotting.
D–E. ERG and β-catenin synergistically induce Wnt signaling. HEK293T (D) or 22Rv1 (E) cells were transfected with ERG and/or β-catenin, along with SuperTOPFlash luciferase reporter construct and the control Renilla expression plasmid. Luciferase activities was assayed at 48 h post-transfection and normalized against Renilla internal control values. Error bars indicate triplicate experiments, mean ± SEM.
F. AXIN2 is up-regulated by ERG. Prostate cell lines including BPH1, RWPE and 22Rv1 were infected with ERG-expressing construct and selected for stable overexpression. QRT-PCR was carried out to assay transcript levels.
G. AXIN2 is down-regulated following ERG knockdown. Stable ERG knockdown was generated in VCaP cells using shRNAlentivirus followed by colony selection.
Previous studies have shown that WNT3A is able to induce phosphorylation of AKT and the downstream GSK-3β, which in turn leads to accumulation of active β-catenin (46–48). In addition, ERG overexpression has been shown to cooperate with AKT up-regulation in producing adenocarcinoma in mice (23). We thus investigated the levels of these proteins in prostate cancer cells following ERG overexpression. Interestingly, western blot analysis revealed that AKT and GSK-3β phosphorylation were indeed drastically increased by ERG overexpression, being consistent with the accumulation of active β-catenin (Fig. 3C).
Active β-catenin is known to translocate to the nucleus where it interacts with TCF/LEF1 proteins for transcriptional activation of downstream genes. We thus examined the effect of β-catenin and/or ERG overexpression on SuperTOPFlash reporter activity in 293T cells. Our results confirmed that either ERG or ectopic β-catenin overexpression indeed dramatically increased SuperTOPFlash activity (Fig. 3D). Similar effects were also observed in prostate cancer cell lines such as 22RV1 andLNCaP (Fig. 3E & Fig. S2A). In addition, there is a strong synergistic effect between β-catenin and ERG overexpression, being consistent with the level of active β-catenin shown in Fig. 3A. It is also plausible that ERG may be able to induce additional components of the Wnt pathway that are downstream of β-catenin. To further test the role of ERG in activating Wnt signaling, we examined the expression of AXIN2, a direct target and also a negative feedback regulator of the Wnt/TCF pathway (49). ERG was over-expressed in a panel of prostate cell lines that do not harbor TMPRSS2-ERG gene fusions (Fig. S2B). QRT-PCR analysis demonstrated significant AXIN2 up-regulation following ectopic ERG overexpression in prostate cell lines such as BPH1, RWPE and 22Rv1 (Fig. 3F). Being consistent with this, ERG knockdown in VCaP cells, on the other hand, resulted in drastic decrease of AXIN2 expression (Fig. 3G).
LEF1 is a critical mediator of ERG-induced Wnt signaling
LEF1 is a member of the TCF family transcription factors that interact with active β-catenin to turn on downstream Wnt pathway genes. LEF1 has been implicated in promoting prostate cancer progression (50). Intriguingly, analysis of VCaP ERG ChIP-Seq data revealed several strong ERG binding events near the LEF1 promoter, suggesting that LEF1 may be a direct target of ERG in prostate cancer (Fig. 4A). To confirm this, LNCaP cells were infected with a series dilution of ERG-expressing adenovirus and subjected to ERG ChIP. ChIP-qPCR analysis revealed significant ERG occupancy on the LEF1 promoter in ERG-overexpressing LNCaP cells (Fig. 4B). Being concordant with this, LEF1 protein level was drastically induced following increasing amount of ERG overexpression, while that of AR, an ERG-inhibited gene, was gradually repressed (Fig. 4C). The ability of ERG to dramatically increase LEF1 expression was also confirmed in independent prostate cancer cell lines such as 22Rv1 and DU145 (Fig. 4D). By contrast, other members of TCF family transcription factors such as TCF1, TCF3 and TCF4 were not significantly alteredby ERG (Fig. S3A). Moreover, western blot analysis revealed that ERG knockdown in VCaPcells drastically reduced LEF1 expression (Fig. 4E). Taken together, out data suggest that LEF1 may be the most important TCF family transcription factor that conveys the effects of ERG on Wnt signaling in prostate cancer.
Figure 4. ERG directly regulates LEF1 expression and function.
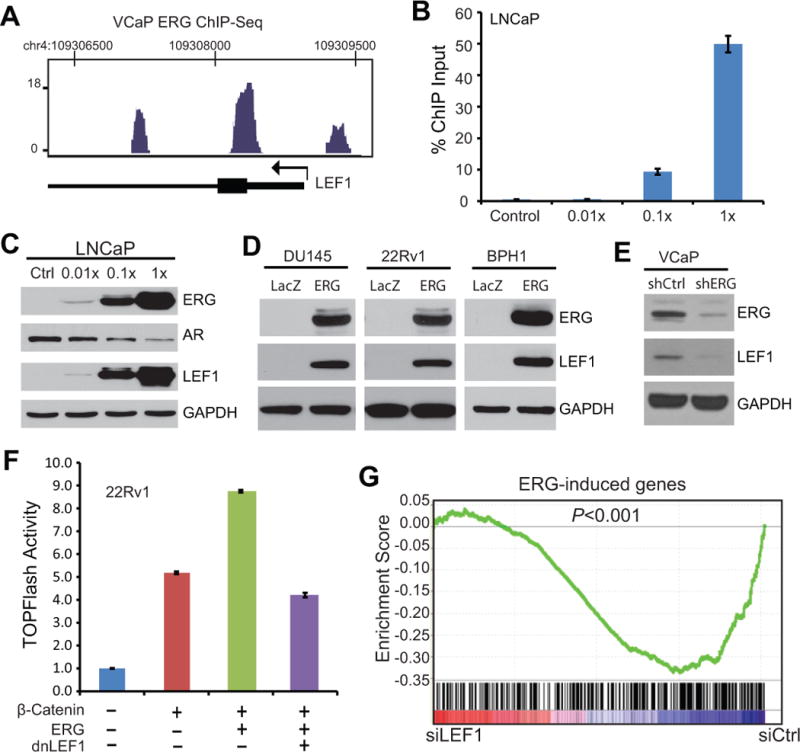
A. ERG occupancy near the promoters of the LEF1 gene. ERG ChIP-Seq was done in VCaP cells as described in Figure 1B.
B. Ectopic ERG binds to the LEF1 promoter. LNCaP cells were infected with increasing amount of ERG adenovirus over 4 doses and were analyzed by ChIP using anti-ERG antibody. ChIP-qPCR was carried out using LEF1 promoter primers. Error bars indicate triplicate experiments, mean ± SEM.
C. LEF1 expression is up-regulated following ERG overexpression. LNCaP cells were infected cell ERG adenovirus as described in B. AR was previously shown inhibited by ERG and GAPDH is a loading control.
D. ERG overexpression induces LEF1 protein. Prostate cancer cell lines were infected with control and ERG adenovirus for 48 h and then analyzed by western blotting.
E. ERG knockdown decreased LEF1 protein level. VCaP cells were subjected to ERG knockdown using shRNAlentivirus. Stable clones were selected and cells were analyzed by western blotting.
F. dnLEF1 abolishes ERG-induced SuperTOPFlash activity. 22Rv1 prostate cancer cells were transfected with ERG, β-catenin, and dnLEF1, along with luciferase reporter constructs. Cells were subjected to luciferase assay at 48 h after transfection.
G. ERG-induced genes are significantly down-regulated following LEF1 knockdown. LNCaP cells with stable ERG overexpression were subjected to siRNA-mediated knockdown targeting control or LEF1 gene. These control (siCtrl) or LEF1 (siLEF1) knockdown cells were analyzed for global gene expression using microarrays. ERG-induced genes were derived from microarray data profiling LNCaP cells with control or stable ERG overexpression. GSEA was utilized to determine whether ERG-induced genes were significantly differentially and concordantly expressed between siCtrl and siLEF1 LNCaP cells.
We next attempted to determine whether LEF1 inactivation is able to attenuate ERG-induced Wnt signaling using SuperTOPFlash reporter assays. A dominant-negative LEF1 (dnLEF1) was used to block LEF1 activity as previously reported (51). Importantly, luciferase reporter assays showed that while ERG substantially increased SuperTOPFlash activities in addition to that mediated by β-catenin overexpression, dnLEF1 fully blocked ERG-induced Wnt signaling. This result was observed in both 22Rv1 (Fig. 4F) and HEK293T cells (Fig. S3B). In addition, we used siRNA to target LEF1 in ERG-expressing cells (Fig. S3C) and examined the effect on SuperTOPFlash activity. Our results confirmed that siLEF1 significantly reduced Wnt signaling mediated by β-catenin and fully blocked ERG-induced SuperTOPFlash activities, suggesting LEF1 as a predominant mediator of ERG regulation of Wnt signaling (Fig. S3D). We thus asked whether LEF1 knockdown is able to reverse ERG regulation of downstream gene expression.
To address this, we carried out microarray profiling of LNCaP cells treated with control, ERG overexpression, or ERG overexpression with concomitant LEF1 knockdown (Fig. S3C). Analysis of the microarray data revealed 359 and 449 genes that were respectively induced and repressed by ERG with a 2-fd cutoff. GSEA analysis demonstrated that ERG-induced genes were significantly enriched for repression upon LEF1 knockdown (Fig. 4G). By contrast, ERG-repressed gene expression was restoredfollowing LEF1 depletion (Fig. S3E). Together, these data strongly suggest that LEF1 is a critical modulator of ERG regulation of downstream gene expression. This promoted us to examine whether Wnt/LEF1 signaling may also be critical in mediating ERG oncogenic function.
Wnt/LEF1 signaling mediates the oncogenic properties of ERG
ERG has been previously shown to regulate cell proliferation (21, 22). We first confirmed this using WST-1 cell growth assay in LNCaP cells treated with control or ERG overexpression (Fig. 5A). Next we treated these cells with IWP-2 to block Wntligands production. Interestingly, WST-1 assays showed that IWP-2 significantly reduced the proliferation of control and ERG-expressing LNCaP cells, likely due to its suppression of both endogenous and ERG-stimulated Wntligands secretion. We also noted that ERG continued to significantly increase cell growth even in the presence of IWP-2, albeit at a much lower level. This is likely due to its up-regulation of other downstream components of Wnt pathway such as LEF1. To test this, we carried out LEF1 knockdown in control and ERG-expressing LNCaP cells. WST-1 cell proliferation assays showed that LEF1 knockdown not only significantly reduced the growth of control cells, but also fully blocked ERG-mediatedLNCaP prostate cancer cell growth (Fig. 5B).
Figure 5. WNT/LEF1 signaling mediates oncogenic properties of ERG.
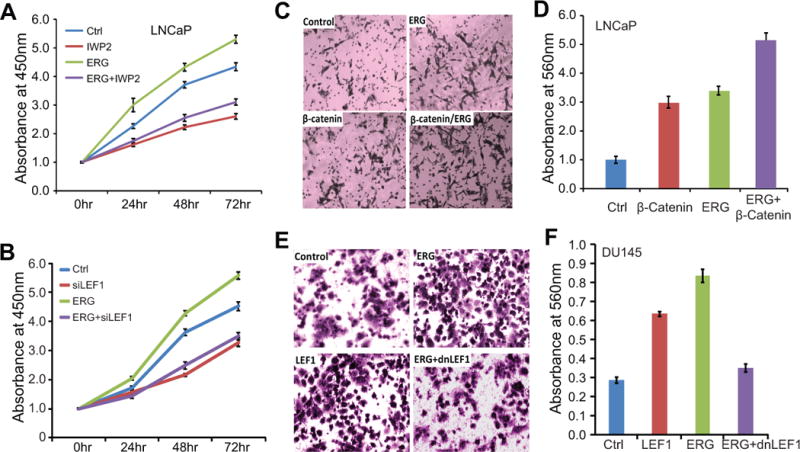
A. IWP-2 partially blocks ERG-induced cell growth. Stable control and ERG-expressing LNCaP cells were treated with Wnt inhibitor IWP-2. Cell growth was monitored using WST-1 assay at 0, 24, 48 and 72 h after treatment.
B. LEF1 knockdown abolishes ERG-induced cell growth. Stable control and ERG-expressing LNCaP cells were transfected with control and LEF1-targeting siRNA. Cells were subjected to WST-1 growth assay at 0, 24, 48 and 72 h after transfection.
C–D. ERG and β-catenin induce prostate cancer cell invasion. LNCaP cells with stable overexpression of control, ERG or β-catenin gene were analyzed for cell invasion using Boyden Chamber Assay. Cells invaded through the basement membrane were stained and a representative field is shown (C). The number of invaded cells was quantified using colormetry with absorbance at 560nm (D).
E–F. dnLEF1 abolishes ERG-induced cell invasion. DU145 cells with stable overexpression of control, LEF1, ERG, ERG and dnLEF1 were analyzed for cell invasion as described above.
We next examined how Wnt/LEF1 pathway regulates the effects of ERG on cell motility. We have earlier shown that ERG further induces SuperTOPFlash reporter activities in addition to β-catenin (Fig. 3D–E), demonstrating an additive/synergistic effect in enhancing WNT/LEF1 signaling. In concordance with this, Boyden Chamber assay illustrated that while ectopic β-catenin dramatically induced LNCaP cell invasion, ERG overexpression significantly increased the invasion capability of both control and β-catenin-overexpressing cells (Fig. 5C–D). To determine whether LEF1 is the key mediator of ERG-induced cell invasion as was in the case of SuperTOPFlashassay described in Fig. 4E, cell invasion assays were carried out in an independent cell line DU145 with stable LEF1, ERG, or ERG and dnLEF1overexpression (Fig. 5E–F). We confirmed that ERG overexpression increased cell invasion and that LEF1 overexpression led to a comparable level of induction of cell invasion. Moreover, inactivation of LEF1using dnLEF1 in the ERG-expression cells suppressed invasion of ERG-overexpressing cells. These results, along with the cell proliferation data shown in Fig. 5A–B, strongly highlight LEF1 as a critical modulator of tumorigenic roles of ERG in PCa. As increased cell invasion is often causally associated with EMT and ERG overexpression has been previously associated with EMT (19, 20, 27), we decided to re-examine this in the context of Wnt/LEF1 signaling.
ERG and LEF1 induces EMT and LEF1 is selectively up-regulated in TMPRSS2-ERG fusion-positive prostate cancer
We examined the morphology of DU145 cells with stable overexpression of vector control or ERG. Importantly, we observed that while the control cells tend to grow in clusters or patches that are characteristic of epithelial cells, ERG overexpression led to a phenotypical switch of the cells to a more spindle-like shape and the ERG-expressing cells grow in isolated form with much less cell-to-cell contact (Fig. 6A). Consistent with this morphological change, qRT-PCR analysis showed that epithelial markers such as E-cadherin and Claudin-1 were indeed dramatically down-regulated by ERG and that LEF1 overexpression demonstrated a similar effect (Fig. 6B). Immunoblot analysis confirmed the loss of E-cadherin and Claudin-1 protein in ERG-expressing cells and further showed that this effect was phenocopied by LEF1 overexpression (Fig. 6C). With this understanding of a primary role of LEF1 in ERG-mediated oncogenic properties, we analyzed LEF1 expression in human PCa to see if their expression levels are correlated in vivo.
Figure 6. ERG and LEF1 induces EMT and are dys-regulated in prostate cancer.
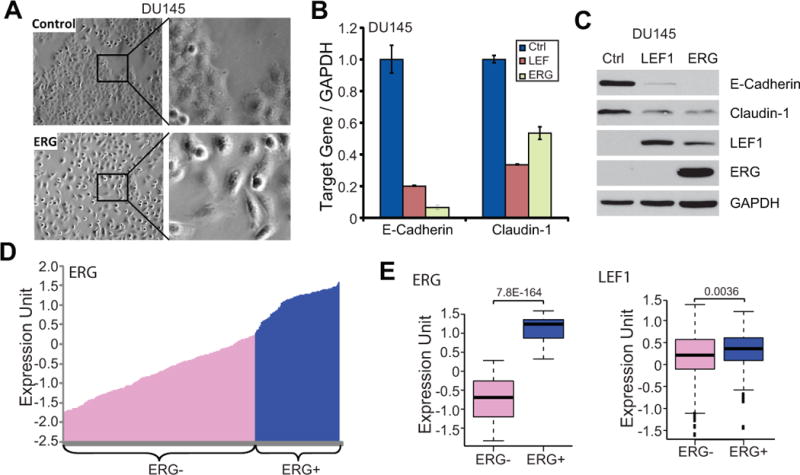
A. ERG overexpression induces EMT of prostate epithelial cells. Phase-contrast microscopy images of stable control and ERG-expressing DU145 cells are shown. ERG-expressing cells lost cell-to-cell contact and showed a spindle-like shape.
B. The expression of epithelial marksis decreased by ERG and LEF1. QRT-PCR analysis of E-cadherin and Claudin-1 were carried out in control and DU145 cells with ERG and LEF1 overexpression.
C. ERG and LEF1 inhibit the expression of epithelial marks. Stable control, ERG-expressing, and LEF1-expressing DU145 cells were analyzed by western blot.
D. Categorization of ERG- and ERG+ prostate cancer specimens. A total of 472 primary prostate cancer tissues were separated into ERG- and ERG+ groups based on the expression level of ERG gene (52).
E. LEF1 is significantly up-regulated in the ERG+ prostate cancers. Boxplot analysis shows the expression level of ERG and LEF1 in the ERG- and ERG+ prostate cancer specimens.
We re-analyzed a previously published microarray dataset that profiled global gene expression of 472 primary prostate cancer tissues (52). We rank ordered all samples by the level of ERG transcript and arbitrarily separated them intoTMPRSS2-ERG fusion-negative and –positive groups based on the expression profile (Fig. 6D). Statistical analysis confirmed that ERG level is substantially (P = 7.8E−164) different among these two groups and found that LEF1 is indeed significantly (P = 0.0036) over-expressed in the ERG-high prostate cancers (Fig. 6E).
Discussion
Over the past years, ERG has been shown to regulate various oncogenic pathways in prostate cancer. The first link to the Wnt pathway was suggested by bioinformatics analysis which revealed that Wnt pathway genes are up-regulated in TMPRSS2-ERG fusion-positive prostate cancer (26). Later studies have demonstrated that the Wnt receptor FZD4 was positively regulated by ERG and mediated its effect on regulating E-cadherin and β-intergrin expression (27). Another study further showed that integrin-linked kinase is a target of ERG and is important in ERG-induced EMT and invasive characteristics (20). This present study showed that ERG directly binds to and regulates various genes at different levels of the Wnt signaling cascade. Using TCF/LEF1 SuperTOPFlashreporter assays, we demonstratedhow inhibitors targeting various components of Wnt signaling pathway reduced or abolished ERG-induced signal transduction. As previous studies have found that TOPFlash assays usually do not work very well in PCa cell lines, especially LNCaP cells, we carried out several optimizations to overcome this hurdle. First, we adopted the SuperTOPFlash reporter which contains a stretch of 16x TCF/LEF1 binding sites and is thus much more sensitive to changes in Wnt signaling. Second, we found that LNCaP cells have relative low transfection efficiency with regular reagents and that X-tremeGeneHP (Roche) greatly augmented the transfection of the reporter plasmids and thus yielded acceptable luciferase activity. Moreover, we haveobserved that 22Rv1 PCa cell line is much easier to transfect, which was used a PCa model, in addition to the conventional HEK293 cell line forSuperTOPFlash assays. Therefore, this study is the firstto comprehensively analyze how ERG regulates Wnt signal transduction and how this process contributes to prostate tumorigenesis.
LEF1 is the transcriptional effector of Wnt signaling in that it turns on the downstream transcriptional program downstream upon binding by nuclear β-catenin. Interestingly, LEF1 itself is also a Wnt target gene that is induced by Wnt activation. In this study we observed strong ERG protein binding events around the LEF1 promoter, suggesting its being a direct target of ERG. But being a Wnt target LEF1 can also be induced indirectly by ERG through Wnt signaling. Using immunoblot analysis we have repeatedly observed drastic up-regulation of LEF1 protein following ERG overexpression. It is plausible that ERG may also be able to stabilize LEF1 protein, which may be interesting lines for future studies. Nevertheless, our results illustrated that ERG exerts tight control of LEF1 protein and thus its transcriptional activity through multiple mechanisms. In addition, LEF1 knockdown is able to sufficiently abolish the effect of ERG in regulating target gene expression and inducing oncogenic properties. These results establish LEF1 as a critical target and mediator of ERG function in prostate cancer. Wnt/LEF1 signaling thus may provide readily targetable avenue for the treatment of TMPRSS2:ERG fusion-positive PCa. Gene therapies that deliver LEF1 siRNA or dnLEF1 vectors using approaches such as nanoparticles may be efficient in blocking ERG-induced oncogenesis and thus warrantee future investigation.
On the other hand, it is important to note that while Wnt/LEF1 signaling has been shown as an important oncogenic pathway in various cancer types, mostly through mutations that constitutively stabilize and activate β-catenin, although such mutations are only at low percentagein prostate cancer. The physiological importance of the Wnt/TCF signaling pathway in PCa is not as well documented as in breast, colorectal and other cancers. Nevertheless, this pathway is believed aberrantly activated in prostate cancer and therapeutics targeting Wnt signaling has been shown effective in reducing prostate cancer growth and progression. Using modern technologies, studies have recently sequenced the exomes of PCa tissues and have revealed mutations to the Wnt/TCF pathway genes as important contributors to castration resistance (34). In addition, using transgenic mice several groups have shown that Wnt/TCF pathway activation promotes prostate cancer initiation and progression (36, 38). Therefore, evidences are emerging that strongly support the physiological important of the Wnt/TCF pathway in PCa. However, it is important to note that, as AR can compete with TCF/LEF1 factors for elevated β-catenin as previously documented (53), increase of some components of the Wnt/TCF pathway may affect AR signaling rather than downstream Wnt target genes. Nonetheless, the strong up-regulation of LEF1 protein by ERG shall directly lead to the elevation of Wnt/LEF1 pathway, which would be highly significant, as LEF1 has been previously shown up-regulated and physiologically important in castration-resistant PCa (50). Although both Wnt/LEF1 pathway and ERG gene have been shown to promote prostate cancer in vivo using xenografts and transgenic mice, it will be extremely important in future studies to block Wnt/LEF1 signaling in ERG-increasing mouse models to further demonstrate the physiological importance of the ERG-Wnt-LEF1 axis in PCa.
Although Wnt/LEF1 pathway has been reported elevated in PCa, the underlying molecular mechanisms are not well understood. Our study showed that TMPRSS2-ERG gene fusions, the most common genetic rearrangements observed in PCa, is a critical activator of Wnt/LEF1 signaling. This tight control is mediated by its direct regulation of multiple components of the pathway. Indeed, analysis of gene expression in a cohort of 473 clinical specimens revealed that LEF1 is indeed up-regulated in ERG-high human prostate cancer. Moreover, through whole-transcriptome analysis a recent study has reported that Wnt ligands such as WNT2 and WNT11 are up-regulated by 3–4 folds in human PCa tissues that harbor the TMPRSS2-ERG gene fusions (54). In addition to TMPRSS2-ERG gene fusions, other fusions have been associated with ERG and other ETS family transcription factors. It would be very interesting to determine in future studies whether other ETS-family gene fusions also activate Wnt/LEF1 signaling.
Previous studies have shown that transgenic ERG expression in mouse prostate cooperates with PTEN/PI3K pathway inleading to the development of prostate adenocarcinoma in mice. The mechanism for this collaborative effect is unknown. In addition, various studies have already reportedthe crosstalk between PI3K/Akt pathway and Wnt signaling (46, 47). Future studies are thus warranted to examine whether PTEN loss and ERG overexpression converge at the Wnt/LEF pathway to cooperatively induce tumorigenesis. Our study further showed that ERG is also able to induce Akt phosphorylation and GSK-3β phosphorylation, which may further contribute to its ability to stabilize β-catenin and activate Wnt signaling. While this study is the first to report this regulation, future studies are needed to determine the underlying molecular mechanisms.
Moreover, in this study, we focused on ERG regulation of the Wnt/LEF1 pathway, we have previously shown that ERG plays major roles in disrupting AR signaling by direct inhibiting AR and a vast majority of its target genes such as PSA and TMPRSS2. We proposed that ERG suppression of AR signaling inhibits epithelial differentiation, thus contributing to prostate cancer progression. By contrast, in this study ERG activation of the Wnt/LEF1 pathway results in transition of the cells to more mesenchymal (thus poorly differentiated) cell types. Although the primary role of ERG in PCa is in regulating cell motility, studies have shown that ERG can also induce cell growth, especially upon stable or long-term overexpression (21, 22). In this study, we also found that Wnt/LEF1 pathway can also mediate this effect of ERG on cell growth. Moreover, LEF1 has been previously shown to up-regulate AR expression and transcriptional activity (50). These results strongly suggest a model wherein ERG inhibits prostatic differentiation by disrupting AR transcriptional regulation and induces mesenchymal mal-differentiation through activation of Wnt/LEF1 signaling. In addition, LEF1 up-regulation may compensate the anti-proliferative effect of ERG resulted from its inhibition of AR, thus leading to a net gain of EMT and cell invasion. Taken together, Wnt/LEF1 signaling is a critical downstream pathway that is important for ERG-mediated tumorigenesis.
Supplementary Material
Acknowledgments
Immortalized PrECcell line is a kind gift of Dr. William C. Hahn (DANA-FARBER Cancer institute). WNT3A L cells were kindly provided by Dr. Cara Gottardi (Northwestern University). We thank Dr. Marian Waterman (University of California, Irvine) for providing the dnLEF1 construct. This work was supported by funding from the NIH P50CA090386 pilot project (to J.Y.), K99/R00CA129565 (to J.Y.), R01CA172384 (to J.Y.), and the Research Scholar Award RSG-12-085-01 (to J.Y.) from the American Cancer Society.
Financial support:
This work was supported by funding from the NIH P50CA090386 pilot project (to J.Y.), K99/R00CA129565 (to J.Y.), R01CA172384 (to J.Y.), and the Research Scholar Award RSG-12-085-01 (to J.Y.) from the American Cancer Society.
Footnotes
Disclosure of Potential Conflicts of Interest:
No potential conflicts of interest were disclosed
References
- 1.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–68. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 3.Carver BS, Tran J, Chen Z, Carracedo-Perez A, Alimonti A, Nardella C, et al. ETS rearrangements and prostate cancer initiation. Nature. 2009;457:E1. doi: 10.1038/nature07738. discussion E2–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin C, Yang L, Tanasa B, Hutt K, Ju BG, Ohgi K, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell. 2009;139:1069–83. doi: 10.1016/j.cell.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42:668–75. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perner S, Mosquera JM, Demichelis F, Hofer MD, Paris PL, Simko J, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–8. doi: 10.1097/01.pas.0000213424.38503.aa. [DOI] [PubMed] [Google Scholar]
- 7.Rice KR, Chen Y, Ali A, Whitman EJ, Blase A, Ibrahim M, et al. Evaluation of the ETS-related gene mRNA in urine for the detection of prostate cancer. Clin Cancer Res. 2010;16:1572–6. doi: 10.1158/1078-0432.CCR-09-2191. [DOI] [PubMed] [Google Scholar]
- 8.Tomlins SA, Aubin SM, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72. doi: 10.1126/scitranslmed.3001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saramaki OR, Harjula AE, Martikainen PM, Vessella RL, Tammela TL, Visakorpi T. TMPRSS2:ERG fusion identifies a subgroup of prostate cancers with a favorable prognosis. Clin Cancer Res. 2008;14:3395–400. doi: 10.1158/1078-0432.CCR-07-2051. [DOI] [PubMed] [Google Scholar]
- 10.Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, Fine SW, et al. TMPRSS2-ERG Gene Fusion Is Not Associated with Outcome in Patients Treated by Prostatectomy. Cancer Res. 2009;69:1400–6. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermans KG, Boormans JL, Gasi D, van Leenders GJ, Jenster G, Verhagen PC, et al. Overexpression of prostate-specific TMPRSS2(exon 0)-ERG fusion transcripts corresponds with favorable prognosis of prostate cancer. Clin Cancer Res. 2009;15:6398–403. doi: 10.1158/1078-0432.CCR-09-1176. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Cai Y, Ren C, Ittmann M. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 2006;66:8347–51. doi: 10.1158/0008-5472.CAN-06-1966. [DOI] [PubMed] [Google Scholar]
- 13.Demichelis F, Fall K, Perner S, Andren O, Schmidt F, Setlur SR, et al. TMPRSS2:ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26:4596–9. doi: 10.1038/sj.onc.1210237. [DOI] [PubMed] [Google Scholar]
- 14.Clark J, Attard G, Jhavar S, Flohr P, Reid A, De-Bono J, et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene. 2008;27:1993–2003. doi: 10.1038/sj.onc.1210843. [DOI] [PubMed] [Google Scholar]
- 15.Spencer ES, Johnston RB, Gordon RR, Lucas JM, Ussakli CH, Hurtado-Coll A, et al. Prognostic value of ERG oncoprotein in prostate cancer recurrence and cause-specific mortality. Prostate. 2013;73:905–12. doi: 10.1002/pros.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salami SS, Schmidt F, Laxman B, Regan MM, Rickman DS, Scherr D, et al. Combining urinary detection of TMPRSS2:ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2011.04.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao L, Tekedereli I, Wang J, Yuca E, Tsang S, Sood A, et al. Highly specific targeting of the TMPRSS2/ERG fusion gene using liposomal nanovectors. Clin Cancer Res. 2012;18:6648–57. doi: 10.1158/1078-0432.CCR-12-2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia. 2008;10:177–88. doi: 10.1593/neo.07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leshem O, Madar S, Kogan-Sakin I, Kamer I, Goldstein I, Brosh R, et al. TMPRSS2/ERG promotes epithelial to mesenchymal transition through the ZEB1/ZEB2 axis in a prostate cancer model. PLoS One. 2011;6:e21650. doi: 10.1371/journal.pone.0021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker-Santos DD, Guo Y, Ghaffari M, Vickers ED, Lehman M, Altamirano-Dimas M, et al. Integrin-linked kinase as a target for ERG-mediated invasive properties in prostate cancer models. Carcinogenesis. 2012;33:2558–67. doi: 10.1093/carcin/bgs285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, Furusato B, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–53. doi: 10.1038/onc.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–54. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zong Y, Xin L, Goldstein AS, Lawson DA, Teitell MA, Witte ON. ETS family transcription factors collaborate with alternative signaling pathways to induce carcinoma from adult murine prostate cells. Proc Natl Acad Sci U S A. 2009;106:12465–70. doi: 10.1073/pnas.0905931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King JC, Xu J, Wongvipat J, Hieronymus H, Carver BS, Leung DH, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–6. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunderfranco P, Mello-Grand M, Cangemi R, Pellini S, Mensah A, Albertini V, et al. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS One. 2010;5:e10547. doi: 10.1371/journal.pone.0010547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI, et al. TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res. 2006;66:10242–6. doi: 10.1158/0008-5472.CAN-06-1986. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–45. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 28.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 29.de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9:1801–7. [PubMed] [Google Scholar]
- 30.Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–56. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 31.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45:323–34. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 32.Gerstein AV, Almeida TA, Zhao G, Chess E, Shih Ie M, Buhler K, et al. APC/CTNNB1 (beta-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer. 2002;34:9–16. doi: 10.1002/gcc.10037. [DOI] [PubMed] [Google Scholar]
- 33.Wan X, Liu J, Lu JF, Tzelepi V, Yang J, Starbuck MW, et al. Activation of beta-catenin signaling in androgen receptor-negative prostate cancer cells. Clin Cancer Res. 2012;18:726–36. doi: 10.1158/1078-0432.CCR-11-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A, White TA, MacKenzie AP, Clegg N, Lee C, Dumpit RF, et al. Exome sequencing identifies a spectrum of mutation frequencies in advanced and lethal prostate cancers. Proc Natl Acad Sci U S A. 2011;108:17087–92. doi: 10.1073/pnas.1108745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, et al. Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate. 2009;69:249–62. doi: 10.1002/pros.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ. Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model. Oncogene. 2011;30:1868–79. doi: 10.1038/onc.2010.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearson HB, Phesse TJ, Clarke AR. K-ras and Wnt signaling synergize to accelerate prostate tumorigenesis in the mouse. Cancer Res. 2009;69:94–101. doi: 10.1158/0008-5472.CAN-08-2895. [DOI] [PubMed] [Google Scholar]
- 38.Zong Y, Huang J, Sankarasharma D, Morikawa T, Fukayama M, Epstein JI, et al. Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E3395–404. doi: 10.1073/pnas.1217982109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wang CY. TBL1-TBLR1 and beta-catenin recruit each other to Wnt target-gene promoter for transcription activation and oncogenesis. Nat Cell Biol. 2008;10:160–9. doi: 10.1038/ncb1684. [DOI] [PubMed] [Google Scholar]
- 40.Yu J, Rhodes DR, Tomlins SA, Cao X, Chen G, Mehra R, et al. A polycomb repression signature in metastatic prostate cancer predicts cancer outcome. Cancer Res. 2007;67:10657–63. doi: 10.1158/0008-5472.CAN-07-2498. [DOI] [PubMed] [Google Scholar]
- 41.Zhao JC, Yu J, Runkle C, Wu L, Hu M, Wu D, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22:322–31. doi: 10.1101/gr.131508.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu J, Cao Q, Mehra R, Laxman B, Yu J, Tomlins SA, et al. Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell. 2007;12:419–31. doi: 10.1016/j.ccr.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–52. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 45.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–7. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma M, Chuang WW, Sun Z. Phosphatidylinositol 3-kinase/Akt stimulates androgen pathway through GSK3beta inhibition and nuclear beta-catenin accumulation. J Biol Chem. 2002;277:30935–41. doi: 10.1074/jbc.M201919200. [DOI] [PubMed] [Google Scholar]
- 47.Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M. Inhibition of Wnt signaling downregulates Akt activity and induces chemosensitivity in PTEN-mutated prostate cancer cells. Prostate. 2005;62:61–8. doi: 10.1002/pros.20117. [DOI] [PubMed] [Google Scholar]
- 48.Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, et al. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151:211–20. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, et al. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 2009;69:3332–8. doi: 10.1158/0008-5472.CAN-08-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokoyama NN, Pate KT, Sprowl S, Waterman ML. A role for YY1 in repression of dominant negative LEF-1 expression in colon cancer. Nucleic Acids Res. 2010;38:6375–88. doi: 10.1093/nar/gkq492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, et al. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst. 2008;100:815–25. doi: 10.1093/jnci/djn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kypta RM, Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat Rev Urol. 2012;9:418–28. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 54.Chow A, Amemiya Y, Sugar L, Nam R, Seth A. Whole-transcriptome analysis reveals established and novel associations with TMPRSS2:ERG fusion in prostate cancer. Anticancer Res. 2012;32:3629–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


