Abstract
We examined the effects of LJM716, a HER3 (ERBB3) neutralizing antibody that inhibits ligand-induced and ligand-independent HER3 dimerization, as a single agent and in combination with BYL719, an ATP competitive, p110α-specific inhibitor against HER2-overexpressing breast and gastric cancers. Treatment with LJM716 reduced HER2-HER3 and HER3-p85 dimers, P-HER3 and P-AKT both in vitro and in vivo. Treatment with LJM716 alone markedly reduced growth of BT474 xenografts. The combination of LJM716/lapatinib/trastuzumab significantly improved survival of mice with BT474 xenografts compared to lapatinib/trastuzumab (p=0.0012). LJM716 and BYL719 synergistically inhibited growth in a panel of HER2+ and PIK3CA mutant cell lines. The combination also inhibited P-AKT in HER2-overexpressing breast cancer cells and growth of HER2+ NCI-N87 gastric cancer xenografts more potently than LJM716 or BYL719 alone. Trastuzumab-resistant, HER2+/PIK3CA mutant MDA453 xenografts regressed completely after three weeks of therapy with LJM716 and BYL719 whereas either single agent inhibited growth only partially. Finally, mice with BT474 xenografts treated with trastuzumab/LJM716, trastuzumab/BYL719, LJM716/BYL719 or trastuzumab/LJM716/BYL719 exhibited similar rates of tumor regression after three weeks of treatment. Thirty weeks after treatment discontinuation, 14% of mice treated with trastuzumab/LJM716/BYL719 whereas >80% in all other treatment groups were sacrificed due to a recurrent large tumor burden (p=0.0066). These data suggest that dual blockade of the HER2 signaling network with a HER3 antibody that inhibits HER2-HER3 dimers in combination with a p110α-specific inhibitor in the absence of a direct HER2 antagonist is an effective treatment approach against HER2-overexpressing cancers.
Keywords: PI3K, HER2, HER3, breast cancer
Introduction
The phosphatidylinositol-3 kinase (PI3K) pathway is an important regulator in cell survival, proliferation, and apoptosis. PI3K is a major signaling hub downstream of HER2 and other receptor tyrosine kinases (RTKs) amplified in cancer cells. PI3K activates AKT, SGK, PDK1, mTOR and other signaling molecules involved in cell cycle progression and survival. PI3K is arguably the most frequently somatically altered pathway in cancer (1), with mutation and/or amplification of the genes encoding the PI3K catalytic subunits p110α (PIK3CA) and p110β (PIK3CB), the PI3K regulatory subunit p85α (PIK3R1), the PI3K effectors AKT1-3 and PDK1, RTKs such as HER2 (ERBB2), MET and FGFR1, and loss of the lipid phosphatases PTEN and INPP4B. PI3K is activated by growth factor RTKs and G-protein-coupled receptors (GPCRs). PI3K phosphorylates phosphatidylinositol 4,5-bisphosphate (PIP2) to produce the second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3) (2, 3). Upon formation of PIP3, the pleckstrin homology (PH) domain of AKT and PDK1 colocalize at the plasma membrane, resulting in phosphorylation of AKT at T308 and its activation. Negative regulation of this pathway is conferred by PTEN and INPP4B, which cleave phosphate groups in PIP3 and PIP2, respectively. AKT activates the mTOR-containing complex 1 (TORC1) which, via S6K and 4E-BP1, regulates mRNA translation and protein synthesis. mTOR is part of another complex (TORC2), which phosphorlylates AKT at S473 and fully induces its catalytic activity.
HER2 is amplified in about 25% of breast cancers and is associated with high cancer virulence and poor patient prognosis (4, 5). In HER2+ breast cancer cells, HER2 couples to and phosphorylates the kinase deficient HER3 receptor which, in turn, potently activates PI3K/AKT. As a result, HER3 is required for HER2 and PI3K-mediated tumorigenesis. For example, HER3 is activated in breast cancers with HER2 overexpression (6) and co-expression of HER2 and HER3 is associated with decreased patient survival (7). Mammary tumors occurring in mice overexpressing the Neu transgene, the rat homolog of human HER2, exhibit increased expression and phosphorylation of HER3 (8). HER3 is as essential as HER2 for maintaining cell viability in a panel of HER2-overexpressing breast cancer cells (9). In addition, loss of HER3 prevents HER2-mediated transformation of mammary epithelium (10). HER2 is unable to directly bind to and activate p85/PI3K. Conversely, HER3 contains six p85-binding motifs and, when dimerized with and activated by HER2, can potently activate PI3K (9). Expression of the H1047R PIK3CA mutation in cells that overexpress HER2 upregulates the HER3/HER4 ligand heregulin (HRG) and knockdown of HER3 inhibits growth of HER2+/PI3K mutant cells (11). Finally, genetic ablation of HER3 significantly delays tumor formation and reduces metastases in transgenic mice expressing the Polyomavirus T antigen in the mammary gland (12), a mouse tumor model where PI3K is also required for transformation (13).
In this study, we used cancer cells and xenografts with different modes of aberrant PI3K pathway activation to examine the effects of LJM716, a HER3 monoclonal antibody that inhibits ligand-induced and ligand-independent HER3 dimerization and activation (14) as a single agent or in combination with BYL719, a p110α-specific PI3K inhibitor (15). Treatment with the LJM716 antibody reduced HER2-HER3 and HER3-p85 dimers, P-HER3 and P-AKT and, as a single agent, delayed growth of HER2+ xenografts. LJM716 and BYL719 synergistically inhibited growth and PI3K in a panel of HER2+ and PIK3CA mutant cell lines. HER2+/PIK3CA mutant, trastuzumab-resistant MDA453 xenografts regressed completely after only three weeks of therapy with LJM716 and BYL719 whereas either single agent inhibited growth partially. The combination of trastuzumab/LJM716/BYL719 induced striking regression of large trastuzumab-sensitive BT474 tumors and prolonged survival of mice after discontinuation of treatment. Overall, these data suggest that dual blockade of the HER2 network with a HER3 antibody that inhibits HER2-HER3 dimers in combination with a p110α-specific inhibitor in the absence of a direct HER2 antagonist is an effective treatment approach against HER2-overexpressing cancer.
Materials and Methods
Cells and reagents
All cell lines were obtained from ATCC, maintained in ATCC-recommended media plus 10% FBS (Gibco) and authenticated by short tandem repeat profiling using Sanger sequencing (March 2011). HR6 cells were derived from a trastuzumab-resistant BT474 xenograft in our laboratory and have been described previously (16). The following drugs were used: lapatinib (GW-572016, LC Laboratories), trastuzumab (Vanderbilt University Hospital Pharmacy), LJM716 and BYL719 (both from Novartis).
Immunoprecipitation and immunoblot assays
Cells were prepared as described (17). Immunoprecipitation was performed by incubating 500 μg of protein extract with 1 μg of a HER3 C-terminus antibody (Neomarkers) conjugated to biotin and incubated with streptavidin-coupled Dynabeads (Life Technologies) overnight at 4°C. The mixture was washed 5 times in NP-40 lysis buffer and boiled for 5 min in 2x loading buffer before being subjected to SDS-PAGE. Lysates were separated by 7% SDS-PAGE and proteins were transferred onto nitrocellulose membranes (Bio-Rad). Primary antibodies included: Y1197 and Y1289 P-HER3, S473 and T308 P-Akt, total Akt, T202/Y204 P-Erk, total Erk, P-GSK3α/β, P-S6 (all from Cell Signaling), HER3 (Santa Cruz Biotechnology), HER2 (Neomarkers) and β-actin (Sigma). Immunoreative bands were detected by enhanced chemiluminescence after incubation with horseradish peroxidase-conjugated secondary antibodies (Promega).
Fluorescent proximity–based antibody-dependent detection (VeraTag) assay
VeraTag assays were performed on formalin-fixed, paraffin-embedded (FFPE) tumor sections as described previously (18, 19), with modifications (20).
Monolayer and three-dimensional growth assays
The CellTiterGlo Assay was performed per manufacturer's instructions (Promega) and details are provided in Supplementary Materials and Methods. For monolayer assay with crystal violet staining, cells were seeded in 6-well plates (5×104/well) in 10% FBS-containing medium followed by treatment with inhibitors. Media and inhibitors were replenished every 2–3 days until 60–80% confluence was achieved in untreated wells. Cells were then stained with crystal violet and quantified as described (21). For growth in 3D, cells were seeded on growth factor-reduced Matrigel (BD Biosciences) in 48-well plates following published protocols (22). Inhibitors were added to the medium at the time of cell seeding; 12 to 16 days later, the plates were scanned and colonies measuring ≥25 μm were counted using GelCount software (Oxford Optronix). Colonies were photographed using an Olympus DP10 camera mounted in an inverted microscope.
Xenograft studies
All mouse experiments were approved by the Institutional Animal Care Committee of Vanderbilt University. Details are provided in Supplementary Materials and Methods.
Results
HER3 antibody inhibits HER3-PI3K signaling
We first treated a panel of HER2 gene-amplified human breast cancer cells with LJM716 (14). HR6 cells, derived from BT474 xenografts are resistant to trastuzumab in vivo and overexpress EGFR and HER3 ligands (16). MDA453, HCC1954, and SUM190 cells contain a mutation in the catalytic domain (H1047R) and MDA361 cells contain a mutation in the helical domain (E545K) of PIK3CA. HCC1569 cells are PTEN-null (23). In all cell lines, the antibody potently inhibited phosphorylation at two of the six p85 binding sites in HER3, Tyr-1197 and Tyr-1289, starting at 1 h through 24 h (Fig. 1). This inhibition of P-HER3 translated into inhibition of downstream P-AKT. Notably, HR6 and HCC1954 cells did not show decreased S473 P-AKT but had modest inhibition of T308 P-AKT. In most cases there was a recovery of P-AKT at 24 h after the addition of LJM716. Treatment with LJM716 did not affect P-ERK, suggesting that HER3 signals mainly through the PI3K/AKT pathway. Some cell lines exhibited a reduction in total HER3 (MDA361, SUM190, HCC1954, and MDA453) but this reduction in receptor protein was not uniform and not necessary to see a reduction in P-HER3 and P-AKT levels upon treatment with LJM716. This suggests that the mechanism of action of LJM6716 does not solely involve receptor downregulation.
Figure 1. LJM716 inhibits HER3-PI3K signaling.

Breast cancer cell lines were treated with 10 μg/ml of LJM716 over a 24-h time course. Whole cell lysates were prepared and separated in a 7% SDS gel followed by immunoblot analysis with indicated antibodies.
LJM716 blocks HER2-HER3 heterodimers
The crystal structure of HER3 bound to the LJM716 Fab fragment reveals that LJM716 binds to a complex epitope distributed across domains II and IV of the HER3 ectodomain (14). This interaction locks HER3 in a tethered, inactive conformation, where it is unable to dimerize with other members of the ERBB receptor family. Thus, we next examined if LJM716 inhibits HER2/HER3 interactions employing a fluorescent antibody–based proximity assay (VeraTag). This assay can quantify protein–protein interactions in FFPE cell pellets or tissue sections and involves the use of two monoclonal antibodies, one conjugated via a cleavable tether to a fluorescent reporter tag, and the other linked to a photosensitizer molecule. Photoactivation with red light results in release of singlet oxygen which then cleaves the tether and releases the fluorescent tag on the second antibody. The area of influence of the singlet oxygen is limited by the proximity of receptors to which the antibodies are directed (18). The released fluorescent tag can then be collected and quantified using capillary electrophoresis and used as an indicator of levels of protein–protein interaction. Mice bearing BT474 and MDA453 xenografts were treated with two doses of 20 mg/kg LJM716 i.p. or vehicle over a 3-day period. Tumors were harvested 4 h after the second dose of antibody. FFPE tumor sections were subjected to VeraTag analysis of total HER2, total HER3, Y1289 P-HER3, HER2-HER3 dimers and HER3-p85 (PI3K) dimers. There was not a difference in total HER2 or HER3 between LJM716-treated tumors and controls. However, both tumor types treated with the antibody exhibited a significant reduction in HER2-HER3 dimers and Y1289 P-HER3 compared to untreated xenografts. BT474 but not MDA453 xenografts also showed a reduction in HER3-p85 (PI3K) complexes (Fig. 2A). We next confirmed that the HER3 antibody inhibits ligand-independent HER2-HER3 interactions by immunoprecipitating HER3 from lysates of cells grown in culture in the absence or presence of a saturating concentration of LJM716 for 1–4 h. HER2 immunoblot analysis of HER3 antibody pulldowns showed a clear reduction in HER2 in lysates from BT474 and SKBR3 cells treated with the antibody (Fig. 2B). Consistent with the above mentioned structural data (14), these results suggest that LJM716 disassembles constitutive HER2-HER3 dimers in intact cells.
Figure 2. HER3 antibody disrupts HER2/HER3 interactions.
A. Mice bearing BT474 or MDA453 xenografts were treated with two doses of 20 mg/kg LJM716 delivered i.p. over a period of 72 h and sacrificed 4 h after the last dose. Formalin fixed paraffin embedded tumor sections were subjected to VeraTag analysis as indicated in Methods. B. BT474 and SKBR3 cells treated with 10 μg/ml LJM716 for 0–4 h. Cell lysates were prepared and were precipitated with a C-terminus HER3 antibody. Antibody pulldowns were next subjected to immunoblot analysis with HER2 and HER3 antibodies.
HER3 antibody in combination with trastuzumab and lapatinib improves survival in vivo
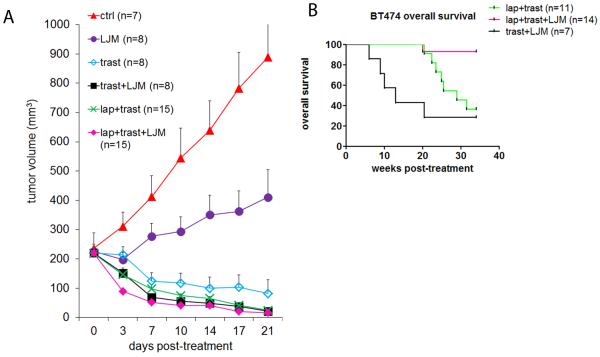
We next assessed the ability of LJM716 to inhibit tumor growth in vivo as a single agent or in combination with HER2 inhibitors. Mice bearing BT474 xenografts measuring ≥200 mm3 were treated with vehicle, LJM716, trastuzumab, trastuzumab/LJM716, lapatinib/trasuzumab or lapatinib/trastuzumab/LJM716. Treatment with LJM716 alone markedly delayed growth of BT474 xenografts (Fig. 3A). The combination of LJM716/trastuzumab was more active than each antibody alone. All treatment arms significantly inhibited xenograft growth, particularly lapatinib/trastuzumab, LJM716/trastuzumab and the 3-drug combination. Mice treated with any of these three combinations exhibited a close to complete response with tumors measuring <25 mm3 after 3 weeks of treatment (Fig. 3A). Treatment was stopped at this time and tumor regrowth was monitored. To assess the effect of treatment on survival, mice were followed until they reached a tumor burden of 2,000 mm3, time when they had to be humanely euthanized according to institutional guidelines. At 34 weeks of follow-up with no treatment, less than 40% of mice in the lapatinib/trastuzumab and the LJM716/trastuzumab arms were alive whereas 93% of mice in the lapatinib/trastuzumab/LJM716 group were so (p=0.0012, Log-rank test) (Fig. 3B).
Figure 3. HER3 antibody in combination with HER2 inhibitors improves survival.
A. Female athymic mice were injected with BT474 cells and randomized to vehicle or the indicated combinations of 20 mg/kg LJM716, 20 mg/kg trastuzumab and 100 mg/kg lapatinib. Treatment was administered for 3 weeks. Tumors were measured two to three times a week with calipers. Number of mice per treatment group is indicated in Figure. Each data point represents the mean tumor volume in mm3 + SEM. B. Therapy was stopped at 3 weeks; mice in the lapatinib/trastuzumab, lapatinib/trastuzumab/LJM716 and trastuzumab/LJM716 treatment groups were monitored for tumor re-growth. Plot of overall mouse survival is shown. The x-axis indicates the weeks after discontinuation of treatment. Per institutional guidelines, mice were sacrificed once tumor burden was ≥2000 mm3.
HER3 antibody synergizes with p110α inhibitor against HER2+ tumor cells
We speculated that a combination of a p110α-specific inhibitor and the HER3 antibody would be a potent inhibitor of PI3K signaling in HER2+ cells and, as such, induce significant growth inhibition in the absence of a direct antagonist of HER2. Thus, we tested the combination of LJM716 and the p110α inhibitor BYL719 in an 18-cancer cell line panel enriched with HER2 gene-amplified and PIK3CA mutant cells. Treatment with LJM716 alone inhibited proliferation, defined as >25% growth inhibition (GI) relative to control, in 6/18 (33%) cell lines as measured by the cell content of ATP (CellTiterGlo assay). Treatment with BYL719 induced >25% GI in 9/18 (50%) cell lines, particularly those with hotspot mutations (i.e. H1047R, E545K) in PIK3CA (Fig. 4A, cell lines marked red). In 12/18 (67%) cell lines, treatment with the combination of LJM716 with BYL719 resulted in >25% growth inhibition (Fig. 4A). Combination activity exceeded that enacted by either agent in isolation in 11/18 (61%) cell lines. Analysis using the Chalice software package confirmed that combination treatment resulted in synergistic action of the two compounds (Suppl. Fig 1). We confirmed these results in a second assay where cells are plated in monolayer followed by crystal violet staining. We observed a statistical decrease in growth of 4 of 5 HER2+ breast cancer cell lines treated with LJM716 and BYL719 compared to either single agent (Fig. 4B, Suppl. Fig 2). Similar results were observed in single cells plated in 3-D Matrigel and assessed for colony formation for 14–21 days, where 5/5 cell lines treated with LJM716 and BYL719 exhibited a statistically larger reduction in growth compared to either single agent (Fig. 4C, Suppl. Fig 3). Finally, we examined the effect of the combination and single drugs on cell signaling at 1–24 h. Treatment with BYL719 as a single agent increased P-HER3 in all four cell lines examined (Fig. 4D), consistent with the reported observation that inhibition of PI3K/AKT results in compensatory upregulation of active HER3 (24, 25). BYL719 reduced both S473 and T308 P-AKT, although in some cases this inhibition was partial. In BT474 and MDA361 cells, more potent inhibition of S473 P-AKT S473 was achieved with the combination of LJM716/BYL719 (at 24 h) than with either single agent. A similar result was observed with HCC1954 cells treated for 1 h with the combination (Fig. 4D). Treatment with the combination did not affect P-ERK in three of the four cell lines.
Figure 4. LJM716 and p110α inhibitor synergistically inhibit tumor cell growth and PI3K.
A. Heatmap representing percent growth inhibition for the listed cell lines 5 days after treatment with 33 nM (5 μg/ml) of LJM716, 330 nM BYL719 or the combination, relative to untreated cells as assessed by the CellTiterGlo Assay. Values for LJM716 were the average of two independent dose-titration curves. Synergistic inhibition (synergy score ≥2.0) was observed for the following cell lines: EFM192A, AU565, SKBR-3, BT474, MDA361 and MDA453 (all with HER2 gene amplification). Percent inhibition relative to IgG-treated (control) cells is visualized in the form of a heat map colored from blue (0% inhibition) to red (100% inhibition). Cell lines harboring PIK3CA hotspot mutations are highlighted in red. B. Cells were plated (1–5×104 cells/well) in 6-well plates and treated in triplicate with DMSO, 10 μg/ml LJM716 ± 1 μM BYL719. Media and drugs were replenished every 3–4 days. Cells were stained with crystal violet when the DMSO-treated (control) monolayers became confluent, ranging from 14–21 days. Quantification of integrated intensity (% control) is shown (*, p<0.05, t test). C. Cells were seeded in Matrigel and allowed to grow in the absence or presence of 10 μg/ml LJM716 and/or 1 μM BYL719 as indicated. Cell media and drugs were replenished every 3 days. Images shown were recorded 15–19 days after seeding. D. Cells were treated with 10 μg/ml LJM716 ± 1 μM BYL719 for 1 or 24 h. Whole cell lysates were prepared and separated in a 7% SDS gel followed by immunoblot analysis with the indicated antibodies.
Combination of PI3Kα inhibitor and HER3 antibody inhibits growth of HER2+ xenografts
Herein, we extended the in vitro observations (Fig. 4) to established tumors in mice. We initially investigated the effect short-term treatment of the HER3 antibody LJM716 and the PI3Kα inhibitor BYL719 had on xenografts to examine if synergistic changes in biomarkers of PI3/AKT pathway activity occurred in vivo. We treated athymic mice with established MDA453 and HCC1954 xenografts with vehicle, LJM716, BYL719 or the combination for three days. Tumors were harvested and subjected to immunoblot analysis. In HCC1954 xenografts treated with the combination, there was a more pronounced inhibition of T308 and S473 P-AKT, P-GSK3α/β and P-S6 compared to tumors in mice treated with either single agent (Fig. 5A).
Figure 5. Combination of LJM716 and BYL719 inhibits growth of HER2+ xenografts.
A. Mice with established HCC1954 or MDA453 xenografts were treated over a 72-h period with two doses of 20 mg/kg LJM716 and three daily doses of 30 mg/kg BYL719. Mice received BYL719 and LJM716 one and 24 h before sacrifice, respectively. Tumor cell lysates were prepared and separated in a 7% SDS gel followed by immunoblot analysis with the indicated antibodies. B. NCI-N87 tumor xenografts were grown in nude mice and treated with IgG (20 mg/kg q 2 days), LJM716 (20 mg/kg q 2 days), BYL719 (12.5 mg/kg daily) or LJM716/BYL719. Treatment of mice in the control and LJM716-treated groups was terminated at 34 days. The remaining groups were kept on treatment for an additional 14 days before the study was terminated. C. Female athymic mice were injected with MDA453 cells and randomized to vehicle or 20 mg/kg LJM716 three times per week and/or 30 mg/kg BYL719 p.o. daily. Treatment was administered for 21 days. Tumors were measured two to three times a week with calipers. Each data point represents the mean tumor volume in mm3 + SEM.
Next, we determined the effect of treatment in mice bearing either NCI-N87 gastric or MDA453 breast cancer xenografts. Athymic mice bearing NCI-N87 xenografts of ≥250 mm3 were randomized to therapy with vehicle, BYL719, LJM716 or the combination of both inhibitors. BYL719 and the combination of BYL719/LJM716 but not LJM716 alone inhibited growth of NCI-N87 tumors. After 48 days of continuous dosing, tumor volume in the group treated with both inhibitors was significantly smaller than in mice treated with the p110α inhibitor (p=0.038 Mann Whitney Rank Sum Test; Fig. 5B). NCI-N87 tumors in mice treated with LJM716, BYL719, or the combination of the two exhibited significantly reduced S473 P-AKT levels compared to tumors in control mice (Supplementary Fig. 4). Similar results were obtained in mice with MDA453 xenografts (≥250 mm3). These tumors are resistant to trastuzumab and harbor HER2 gene amplification, H1047R PIK3CA and a hemizygous deletion of PTEN. Both LJM716 and BYL719 as single agents inhibited MDA453 tumor growth whereas the combination induced complete tumor regression in 10/10 mice after only three weeks of therapy (Fig. 5C). After achieving a complete response, treatment was stopped. After 15 weeks of follow up, no mice exhibited a tumor recurrence.
We finally examined the activity of the combination of the HER3 antibody and the PI3Kα inhibitor with or without the HER2 antibody trastuzumab in a HER2-dependent, trastuzumab-sensitive xenograft. Mice bearing large BT474 xenografts (≥400 mm3) were treated with vehicle, BYL719, trastuzumab/BYL719, trastuzumab/LJM716, LJM716/BYL719 or BYL719/trastuzumab/LJM716. Mice in all these four groups exhibited rapid tumor regressions within 24 days of therapy (Fig. 6A). Thirty weeks after treatment discontinuation, 5/8 (63%) mice in the BYL719/trastuzumab/LJM716 exhibited tumor recurrences (≥200 mm3) whereas >85% of mice in all other groups did so (p=0.0160, Log-rank test; Fig. 6B). Moreover, 6/7 (86%) mice treated with trastuzumab/BYL719, 5/6 (83%) mice treated with trastuzumab/LJM716 and 6/6 (100%) mice treated with LJM716/BYL719 whereas only 1/7 (14%) mice treated with the triple drug combination had to be euthanized due to tumors reaching ≥2000 mm3 in volume This translated to a significant increase in survival in mice treated with the triple therapy compared to mice treated with various dual therapies (Fig. 6C).
Figure 6. Combination of p110α inhibitor, HER3 antibody and trastuzumab improves survival.
A. Female athymic mice were injected with BT474 cells as indicated in Methods. Once tumors reached a volume of ≥400 mm3, there were randomized to treatment with vehicle (controls) or the indicated combinations of 20 mg/kg LJM716, 20 mg/kg trastuzumab and 30 mg/kg BYL719. Treatment was administered for 24 days. Tumor diameters were measured two to three times a week with calipers and volume in mm3 was calculated. Each data point represents the mean tumor volume in mm3 + SEM. B, C. After 24 days, treatment was discontinued and mice were monitored for tumor recurrence. The x-axis indicates the number of weeks after treatment discontinuation. B. Plot of tumor-free mice over time after termination of therapy; tumors ≥200 mm3 in volume were scored as recurrences. C. Plot of overall mouse survival. Per institutional guidelines, mice were sacrificed once tumor burden was ≥2000 mm3.
Discussion
The HER2 receptor does not have a known activating ligand. Its tyrosine kinase activity can be induced by ligand-induced dimerization with the ERBB co-receptors EGFR and HER3 and/or by ligand-independent homo and hetero-oligomerization as a result of gene amplification and protein overexpression (26). Heregulin binds to HER3 causing a change from a closed to an open conformation exposing the dimerization loop in subdomain II of the receptor ectodomain which, in turn, leads to the formation of HER2-HER3 dimers. In HER2-overexpressing cells and tumors, HER2 and HER3 are constitutively phosphorylated in the absence of added ligands (9). HER3 neutralizing antibodies screened for their ability to inhibit ligand binding to HER3 are in clinical development in patients with solid tumors (27–29). As a single therapy, these antibodies have been shown to inhibit heregulin-dependent cancer cells but are less effective at inhibiting growth of HER2+ xenografts (17, 28). This supports the notion that HER2-overexpressing cancer cells also rely on constitutive, ligand-independent HER2-HER3 and/or HER2-EGFR dimers for tumor progression. LJM716 was designed to inhibit both ligand-independent and ligand-induced HER3 signaling. The crystal structural of the Fab fragment of LJM716 bound to the HER3 ectodomain reveals that the antibody binds a complex epitope on subdomains II and IV of HER3 that locks the receptor's extracellular domain in the tethered, closed conformation (14). We show herein that LJM716 inhibits dimerization between HER2 and HER3 in vivo (Fig. 2) and, as a single agent, inhibits the growth of HER2-overexpressing breast and gastric cancer xenografts (Fig. 3A, Fig. 5B). To our knowledge, this is the first report of a HER3 neutralizing antibody with preclinical activity as a single drug against HER2 gene-amplified tumors in vivo. We speculate LJM716 should be able to disrupt other PI3K-activating HER3-containing dimers where HER2 is not the kinase that phosphorylates HER3. Examples include EGFR, MET, IGF-IR, FGFR, Src and others kinases which can be investigated in future studies.
HER2-overexpressing cells rely on HER2-HER3 dimers for potent activation of PI3K/AKT. In turn, PI3K signaling is critical for the viability and progression of HER2+ cancer cells. Indeed, HER2-directed therapies should inhibit PI3K downstream HER2 in order to inhibit growth of HER2-dependent cancer cells (30, 31). In tumor cells driven by HER2 or the rat homolog Neu, PIP3 formation is mediated by the catalytic activity of the p110α isozyme, encoded by the PIK3CA gene. Genetic ablation of p110α blocks tumor formation in transgenic mice expressing the Neu oncogene in the mammary gland (32). Consistent with these data, HER2+ tumor cells in culture are highly sensitive to PI3K inhibitors (33–35). Further supporting the reliance of HER2+ cancers on PI3K signaling downstream, a significant fraction of HER2 enriched breast tumors in the TCGA database also harbor activating mutations in PIK3CA (36). It is unclear if HER2+ tumor cells with PI3K pathway mutations have a more virulent phenotype than HER2+ tumors without those alterations. However, PI3K pathway mutations, including loss of PTEN, have been associated with resistance to anti-HER2 therapies (37–40). Although PI3K pathway antagonists are potent inhibitors of HER2-overexpressing cancer cells (34, 35), feedback upregulation of total and activated HER3 has been shown to dampen the full antitumor action of these inhibitors (24). Thus, in this work we examined dual blockade of the HER2/HER3/PI3K axis with an antibody targeting HER3 and a PI3Kα inhibitor. The combination of LJM716 with BYL719 was highly active against several HER2 gene-amplified cell lines irrespective of their PI3K mutation status (Fig. 4). As a single agent, LJM716 effectively inhibited P-HER3 and P-AKT in HER2+ cancer cell lines. In some instances, we observed a recovery of P-AKT 24 h after treatment with LJM716 (Fig. 1). However, LJM716 in combination with BYL719 induced more sustained inhibition of P-AKT and downstream effectors than either drug alone (Fig. 4D, Fig 5C), suggesting this approach overcomes the reactivation of HER3 triggered by inhibition of PI3K/AKT (17, 24, 25). Using this combination of HER3 and PI3Kα antagonists in the absence of a direct HER2 inhibitor, we observed complete elimination of trastuzumab-resistant MDA453 xenografts (Fig. 5B). Whether this combination has similar activity in patients with HER2+ breast cancer progressing on trastuzumab will require further clinical investigation.
It is increasingly accepted that for optimal inhibition of HER2 function in HER2+ breast cancer cells, treatment with at least two anti-HER2 drugs is required. Currently, the combinations of trastuzumab and lapatinib and of trastuzumab and pertuzumab are approved by the FDA for use in patients with metastatic HER2+ breast cancer. These three drugs interact with HER2 directly. Other plausible approaches for dual blockade of HER2 are concurrent use of a direct inhibitor of HER2 with a second drug targeted to a different component of the HER2/HER3/PI3K axis. Such combinations would include trastuzumab and a HER3 antibody or trastuzumab and a PI3K inhibitor, for example. Support for the first combination is shown in Fig. 3A where the combination of trastuzumab/LJM716 was clearly superior to each antibody alone against BT474 xenografts. Recent preclinical reports support efficacy of the combination of trastuzumab (or lapatinib) with PI3K inhibitors (24, 41–43), which are currently being tested in clinical trials. Based on the data presented herein, we propose that the combination of a HER3 antibody that eliminates both ligand-dependent and –independent HER3 dimerization and a p110α inhibitor is another strategy for dual blockade of the HER2/HER3/PI3K axis in HER2-overexpressing breast cancer. This dual approach should be explored clinically in patients with this breast cancer subtype that have progressed on trastuzumab.
Supplementary Material
Acknowledgements
The authors would like to thank John Winslow and Ahmed Chenna for their experimental and analytical contributions to VeraTag HER3-PI3K and VeraTag phospho-HER3 experiments. We also thank MorphoSys for their contributions in isolation and characterization of the LJM716 antibody.
Financial support: This work was supported in part by ACS 118813-PF-10-070-01-TBG (JTG) and DOD BC093376 (JTG) postdoctoral fellowship awards, R01 Grant CA80195 (CLA), American Cancer Society (ACS) Clinical Research Professorship Grant CRP-07-234 (CLA), Breast Cancer Specialized Program of Research Excellence (SPORE) P50 CA98131, Stand Up to Cancer Dream Team Translational Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0209) and Susan G. Komen for the Cure Foundation grant SAC100013 (CLA)
Footnotes
Conflicts of Interest: CUB, SAE, SDC, and QS are employees of Novartis Corporation. JW and LDE are employees of Integrated Oncology/LabCorp and Monogram Biosciences/LabCorp.
References
- 1.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–7. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 3.Bellacosa A, Testa JR, Staal SP, Tsichlis PN. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991;254:274–7. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–28. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 6.Naidu R, Yadav M, Nair S, Kutty MK. Expression of c-erbB3 protein in primary breast carcinomas. Br J Cancer. 1998;78:1385–90. doi: 10.1038/bjc.1998.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiseman SM, Makretsov N, Nielsen TO, Gilks B, Yorida E, Cheang M, et al. Coexpression of the type 1 growth factor receptor family members HER-1, HER-2, and HER-3 has a synergistic negative prognostic effect on breast carcinoma survival. Cancer. 2005;103:1770–7. doi: 10.1002/cncr.20970. [DOI] [PubMed] [Google Scholar]
- 8.Siegel PM, Ryan ED, Cardiff RD, Muller WJ. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. Embo J. 1999;18:2149–64. doi: 10.1093/emboj/18.8.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 10.Vaught DB, Stanford JC, Young C, Hicks DJ, Wheeler F, Rinehart C, et al. HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res. 2012;72:2672–82. doi: 10.1158/0008-5472.CAN-11-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook RS, Garrett JT, Sanchez V, Stanford JC, Young C, Chakrabarty A, et al. ErbB3 ablation impairs PI3K/Akt-dependent mammary tumorigenesis. Cancer Res. 2011;71:3941–51. doi: 10.1158/0008-5472.CAN-10-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster MA, Hutchinson JN, Rauh MJ, Muthuswamy SK, Anton M, Tortorice CG, et al. Requirement for both Shc and phosphatidylinositol 3' kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol. 1998;18:2344–59. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garner A, Sheng Q, Bialucha CU, Chen D, Chen Y, Das R, et al. Abstract 2733: LJM716: an anti- HER3 antibody that inhibits both HER2 and NRG driven tumor growth by trapping HER3 in the inactive conformation. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research.2012. [Google Scholar]
- 15.Huang A, Fritsch C, Wilson C, Reddy A, Liu M, Lehar J, et al. Abstract 3749: Single agent activity of PIK3CA inhibitor BYL719 in a broad cancer cell line panel. Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research.2012. [Google Scholar]
- 16.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 17.Garrett JT, Olivares MG, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108:5021–6. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Y, Huang W, Tan Y, Jin X, Dua R, Penuel E, et al. A novel proximity assay for the detection of proteins and protein complexes: quantitation of HER1 and HER2 total protein expression and homodimerization in formalin-fixed, paraffin-embedded cell lines and breast cancer tissue. Diagn Mol Pathol. 2009;18:11–21. doi: 10.1097/PDM.0b013e31818cbdb2. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee A, Badal Y, Nguyen XT, Miller J, Chenna A, Tahir H, et al. Profiling the HER3/PI3K pathway in breast tumors using proximity-directed assays identifies correlations between protein complexes and phosphoproteins. PLoS One. 2011;6:e16443. doi: 10.1371/journal.pone.0016443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallweber J, Chenna A, Ravanera R, Huang W, Stathas D, Marshall G, et al. Abstract 3029: Profiling HER3/ErbB3 activation in formalin-fixed, paraffin-embedded (FFPE) breast tumor samples that express high and low HER2/ErbB2 levels using proximity-based immunoassays. Proceedings of the 104th Annual Meeting of the American Association for Cancer Research.2013. [Google Scholar]
- 21.Garrett JT, Sutton CR, Kuba MG, Cook RS, Arteaga CL. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clin Cancer Res. 2013;19:610–9. doi: 10.1158/1078-0432.CCR-12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 23.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2011;109:2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–84. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Freeman D, Ogbagabriel S, Rothe M, Radinsky R, Treder M. Fully human Anti-HER3 monoclonal antibodies (mAbs) have unique in vitro and in vivo functional and antitumor activities versus other HER family inhibitors. AACR Meeting Abstracts. 2008;2008:LB-21. [Google Scholar]
- 28.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–94. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Treder M, Hartmann S, Ogbagabriel S, Borges E, Green L, Kang J, et al. Fully human Anti-HER3 monoclonal antibodies (mAbs) inhibit oncogenic signaling and tumor cell growth in vitro and in vivo. AACR Meeting Abstracts. 2008;2008:LB-20. [Google Scholar]
- 30.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–41. [PubMed] [Google Scholar]
- 31.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Utermark T, Rao T, Cheng H, Wang Q, Lee SH, Wang ZC, et al. The p110alpha and p110beta isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012;26:1573–86. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maira SM, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–28. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 35.Yao E, Zhou W, Lee-Hoeflich ST, Truong T, Haverty PM, Eastham-Anderson J, et al. Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res. 2009;15:4147–56. doi: 10.1158/1078-0432.CCR-08-2814. [DOI] [PubMed] [Google Scholar]
- 36.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–56. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Razis E, Bobos M, Kotoula V, Eleftheraki AG, Kalofonos HP, Pavlakis K, et al. Evaluation of the association of PIK3CA mutations and PTEN loss with efficacy of trastuzumab therapy in metastatic breast cancer. Breast Cancer Res Treat. 2011;128:447–56. doi: 10.1007/s10549-011-1572-5. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Zhang Q, Zhang J, Sun S, Guo H, Jia Z, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakrabarty A, Bhola NE, Sutton C, Ghosh R, Kuba MG, Dave B, et al. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013;73:1190–200. doi: 10.1158/0008-5472.CAN-12-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.