Abstract
Psychiatric neurosurgery teams in the United States and Europe have studied deep brain stimulation (DBS) of the ventral anterior limb of the internal capsule and adjacent ventral striatum (VC/VS) for severe and highly treatment-resistant obsessive-compulsive disorder. Four groups have collaborated most closely, in small-scale studies, over the past 8 years. First to begin was Leuven/Antwerp, followed by Butler Hospital/Brown Medical School, the Cleveland Clinic and most recently the University of Florida. These centers used comparable patient selection criteria and surgical targeting. Targeting, but not selection, evolved during this period. Here, we present combined long-term results of those studies, which reveal clinically significant symptom reductions and functional improvement in about two-thirds of patients. DBS was well tolerated overall and adverse effects were overwhelmingly transient. Results generally improved for patients implanted more recently, suggesting a ‘learning curve’ both within and across centers. This is well known from the development of DBS for movement disorders. The main factor accounting for these gains appears to be the refinement of the implantation site. Initially, an anterior–posterior location based on anterior capsulotomy lesions was used. In an attempt to improve results, more posterior sites were investigated resulting in the current target, at the junction of the anterior capsule, anterior commissure and posterior ventral striatum. Clinical results suggest that neural networks relevant to therapeutic improvement might be modulated more effectively at a more posterior target. Taken together, these data show that the procedure can be successfully implemented by dedicated interdisciplinary teams, and support its therapeutic promise.
Keywords: deep brain stimulation, obsessive-compulsive disorder, internal capsule, ventral striatum, learning curve
Introduction
In its most severe and treatment-resistant form, obsessive-compulsive disorder (OCD) results in marked suffering and impairment in self-care, education, work and social life. Some patients who are severely affected fail to obtain adequate relief despite years of conventional behavioral and drug therapies. After exhausting these treatments, the remaining therapeutic option was ablative surgery, including anterior capsulotomy (review Greenberg et al.1) and anterior cingulotomy, Dougherty et al.2 used for over 40 years.
Deep brain stimulation (DBS) has emerged as a well-accepted alternative to ablative procedures for movement disorders such as Parkinson’s disease and dystonia. It is currently being investigated for highly resistant OCD. Small-scale use in controlled3–5 or open studies6–9 have suggested therapeutic promise. Although DBS has been applied at several locations along the rostral–caudal extent of the anterior limb of the internal capsule and/or the adjacent striatum (VC/VS), one surgical approach has been used most frequently. The target includes the ventral anterior limb of the internal capsule and adjacent ventral striatum. We refer to this site at the junction of the ventral capsule/ventral striatum as the ‘VC/VS’.
The VC/VS site was initially based on anterior capsulotomy, a technique introduced by Talairach and later refined by Leksell.10 Capsulotomy lesions were originally located over a centimeter rostral to the anterior commissure (AC). The target tissue was heated via a probe inserted via craniotomy, so the procedure is referred to as ‘thermo-’ or ‘open’ capsulotomy. Gamma knife capsulotomy developed later, targeting progressively smaller and more ventral tissue volumes than thermocapsulotomy.11 For both thermo and gamma capsulotomy, it has been suggested that relatively more posterior lesions might be associated with better clinical results.12,13
DBS for OCD has undergone a parallel development in that the VC/VS target site has become more posterior as experience was gained, representing the greatest technical evolution from the beginning of this work in 1998. One hypothesis influencing this work is that more posterior locations may be more effective. Fibers within the cortico-striatal-thalamo-cortical (CSTC) networks hypothesized as central to the therapeutic effects of lesions or DBS14 become more compact as they course posteriorly toward the thalamus, to which they connect via the inferior thalamic peduncle.15,16 The VC/VS may thus represent a node of CSTC circuits that is readily targeted for modulation by DBS.
Here we present data on therapeutic outcomes and adverse effects from the four centers that have collaborated most closely using this technique, beginning with the work of Nuttin and colleagues.17 We were particularly interested in assessing whether the results of more recent VC/VS implantations may be superior to the earlier attempts, and if so, why. Our approach is a systematic comparison of three patient cohorts, divided into the early, middle and latest groups. The outcomes of greatest interest are Yale–Brown Obsessive Compulsive Scale (YBOCS) of OCD severity, functioning, adverse effect burden and effects on co-occurring non-OCD symptomatology. Data on longer-term outcomes of VC/VS DBS will be emphasized where possible, because the viability of DBS as a therapy for otherwise intractable OCD (which is overwhelmingly a chronic illness) will depend upon the durability of benefit and its tolerability. Thus far, long-term (1 year or more) effects of VC/VS DBS have been the subject of two reports, totaling fourteen patients.3,18
Methods
Overview
Data were collected by four groups, which worked in collaboration over 8 years, starting with the Leuven/Antwerp group in 1998. Long-term results from four patients of that initial cohort have been reported.3,4 Work at the first US center, Butler Hospital (BH) began in 2000, followed by the Cleveland Clinic (CC) in 2001. Combined 3-year follow-up data for ten patients from BH and CC have been reported.18 The fourth center, the University of Florida (UF), began a National Institute of Mental Health funded pilot study in 2003. Case reports addressing notable observations in that study have appeared,19,20 but not long-term outcomes. Data from the four sites are reported here as of 1 July 2005. Local ethics committee approval was obtained for the Leuven/Antwerp study. IRB and FDA Investigational Device Exemption approvals were obtained at each US site. Patient selection was based on criteria developed earlier to determine eligibility for neurosurgery for otherwise intractable OCD2 and followed the guidelines of the DBS for OCD Collaborative Group.21 Like patient selection, the DBS devices used surgical technique, and postoperative patient management were comparable across centers. The common elements of those protocols are detailed below. Methodological differences across centers are noted afterwards.
Patient selection
Evaluation procedures and entry criteria were comparable across centers and remained essentially unchanged over the period of data collection. All patients were adults.
OCD diagnosis and severity
Detailed patient screening, record review, interviews with treating clinicians and baseline assessments, including the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition,22 were used to assure that OCD was the primary diagnosis (the disorder judged by clinicians and patients as imposing the greatest burdens of symptom and functional impairment). OCD had to be of at least 5 year’s duration. YBOCS symptom intensity in the ‘severe’ range was required (score ≥28 at US sites, ≥30 at Leuven). OCD had to be judged to cause marked functional impairment.
Treatment resistance
The threshold for entry was failure to obtain meaningful OCD improvement after adequate conventional treatment. Although the LV and UF centers used formal definitions of treatment resistance that differed slightly from the criteria at BH and CC (which were identical), in essence all required adequate trials (≥3 months, with doses at or if tolerated, beyond the FDA maximum recommended dose) of serotonin reuptake inhibitors (SRIs), one of which had to be clomipramine. Trials combining an SRI with additional medications (including a neuroleptic and a benzodiazepine) were also required. In the group as a whole, 23 of 26 patients had had three or more SRI trials (including clomipramine). In total, 22 of 26 had had three or more trials combining an SRI with different augmenting agents (mainly first- or second-generation neuroleptics). All patients were required to have had behavior therapy, defined as a minimum of 20 sessions of therapist-guided exposure and response prevention. Patients who attempted behavioral therapy but who demonstrated marked intolerance to it (in the therapist’s judgment) were eligible. In none of the patients enrolled did sustained efforts at behavior therapy plus pharmacotherapy reduce symptoms to a tolerable level on a long-term basis.
Exclusion criteria
Patients were excluded if there was a history of a current or past psychotic disorder, a manic episode within the preceding 3 years, any current clinically significant neurological disorder or medical illness (except for tic disorders), any clinical significant abnormality on preoperative magnetic resonance imaging (MRI), any labeled DBS contraindication and/or inability to undergo presurgical MRI, current or unstably remitted substance abuse or dependence, pregnancy or lack of use of effective contraception in women of childbearing age, a clinical history of severe personality disorder, inability to adhere to the operational requirements of the study or imminent risk of suicide (in the investigators’ judgment).
Independent review
At each center, committees including psychiatrists not connected with the research made final eligibility determinations after reviewing clinical histories, baseline evaluations and the consent process. For LV, a regional body, the Committee for Neurosurgery for Psychiatric Disorders, determined eligibility, after detailed review of records, according to strict criteria. The Committee, a collaborative effort among all Flemish Universities, has played this role since 1975.
Patient characteristics
Demographic and clinical features of patients are detailed in Table 1. OCD onset ranged from ages 7 to 34 years (15.1±1.6; mean±s.e.m.); 20 of the 26 patients developed OCD by age 18. Illness duration ranged from 8 to 41 years (22±1.5 years) and was of 10 or more years duration for 25 of 26 patients. Average YBOCS OCD severity at presurgical baseline was 34.0±0.5; all patients scored at least 30, four points above an accepted threshold for severe OCD. Global assessment of functioning (GAF) scores (available for 21 of the 26 patients as the GAF was not administered at UF) showed a mean baseline score of 34.8±1.1, reflective of major functional impairment. GAF scores were 40 or less in 20 of the 21 patients administered that scale. Anxiety not specific to OCD was notable; the overall mean baseline Hamilton Anxiety Rating Scale (HAM-A) score was 22.1±1.9. The most common associated diagnoses were current or past depression. In total, 23 of the 26 patients met severe combined immunodeficiency (SCID) criteria for either current or lifetime depressive episodes. One met depressive episode criteria in the context of bipolar II mood disorder. Mean depression severity was in the clinically significant range for patients at each center, as documented by different Hamilton Depression Rating Scale (HAM-D) versions. Baseline HAM-D means were 25.2±1.3 at BH (24-item HAM-D), 17.0±0.6 at CC (24-item HAM-D), 12.2±1.5 at UF (21-item HAM-D) and 23.8±2.3 at LV (21-item HAM-D).
Table 1.
Patient characteristics: demographic and clinical features of subjects
| Patient (center) | Age | M/F | OCD onset | OCD duration | Axis I comorbid | Axis II | Follow-up duration |
|---|---|---|---|---|---|---|---|
| (years) | (years) | (months) | |||||
| Butler (BH) | |||||||
| BH1 | 32 | M | 10 | 22 | MDD | OCPD | 36 |
| BH2 | 40 | F | 16 | 24 | BPII | 36 | |
| BH3 | 39 | M | 12 | 27 | Dysthymia | 36 | |
| BH4 | 26 | F | 15 | 11 | MDD | 36 | |
| BH5 | 32 | M | 10 | 22 | MDD | 24 | |
| Cleveland Clinic (CC) | |||||||
| CC1 | 59 | F | 19 | 40 | None | 6* | |
| CC2 | 35 | F | 12 | 23 | MDD | 36 | |
| CC3 | 22 | M | 8 | 14 | MDD | STYP(tr) | 36 |
| CC4 | 23 | M | 7 | 16 | MDD | 36 | |
| CC5 | 45 | M | 19 | 26 | None | 24 | |
| U of Florida (UF) | |||||||
| UF1 | 32 | F | 24 | 8 | MDD | 24 | |
| UF2 | 50 | M | 34 | 16 | MDD | 12 | |
| UF3 | 38 | M | 22 | 16 | MDD | 12 | |
| UF4 | 32 | M | 10 | 22 | MDD | 6 | |
| UF5 | 32 | F | 15 | 17 | MDD | 3 | |
| Leuven (LV) | |||||||
| LV1 | 35 | M | 12 | 23 | MDD | HST(tr); NAR(tr) | 12* |
| LV2 | 52 | F | 24 | 28 | MDD; GAD | 36 | |
| LV3 | 39 | F | 16 | 23 | MDD; PD | DEP PD | 36 |
| LV4 | 35 | M | 12 | 23 | MDD | 36 | |
| LV5 | 40 | F | 14 | 26 | MDD | 36 | |
| LV6 | 37 | M | 16 | 21 | MDD | 36 | |
| LV7 | 39 | F | 15 | 24 | MDD | 24 | |
| LV8 | 40 | M | 14 | 26 | MDD; PD | 24 | |
| LV9 | 23 | M | 12 | 11 | MDD | 12 | |
| LV10 | 30 | F | 9 | 21 | None | 6 | |
| LV11 | 57 | F | 16 | 41 | MDD | 3 | |
| Mean | 37.1 | 15.1 | 22.0 | 31.4 | |||
| s.e.m. | 1.9 | 1.6 | 1.5 | 4.1 | |||
Abbreviations: BPII, bipolar II mood disorder; DEP PD, dependent personality disorder; F, female; GAD, generalized anxiety disorder; HST(tr), histrionic traits; M, male; MDD, major depressive disorder; NAR(tr), narcissistic traits; OCD, obsessive-compulsive disorder; OCPD, obsessive-compulsive personality disorder; PD, panic disorder; STYP(tr), schizotypal traits.
Details in text.
Surgical procedure
DBS device implantation
DBS was implemented similarly at all centers. Because capsulotomy lesions have historically encompassed much of the anterior capsule, impinging upon the adjacent ventral striatum, a lead design was selected that allowed stimulation along the dorsal–ventral extent of the capsule, extending into ventral striatum. All centers used essentially identical stimulating leads (US Centers: Model 3387IES; LV: Pisces Quad Compact Model 3887; Medtronic Inc., Minneapolis, MN, USA). The leads were 1.27mm in diameter, with four cylindrical electrode contacts 3mm in length, spaced 4mm apart. Each electrode contact could be set independently as positive, negative or off. Following convention, contacts were numbered from 0 (most distal) to 3 (most proximal).
DBS leads were placed bilaterally using standard stereotactic procedures established in DBS for movement disorders.23 Surgical trajectory planning used structural neuroimaging (MRI and computed tomography (CT)) and application of a stereotactic frame to acquire data for computerized targeting platforms. Leads were implanted relative to a set of standard anatomical landmarks (AC and posterior (PC) commissures, AC–PC plane), and the anterior limb of the internal capsule. Intraoperative testing was performed to evaluate effects of acute stimulation, particularly untoward effects. Results were consistent with observations made during surveys of electrode contacts (described below) conducted in the first weeks after implantation (see Okun et al.24).
The final stage of surgery was implantation of programmable implantable neurostimulators (INSs; Soletra, Synergy, or Kinetra model, Medtronic Inc.) in the pectoral or abdominal region, usually on the same day of surgery (US centers) or about 1 week later (LV). One INS was connected to each brain stimulating lead by extension wires tunneled subcutaneously, while patients received general anesthesia.
DBS target and target evolution during the trials
The DBS lead implantation site became systematically more posterior during these studies, based on the clinical results observed, other empirical results (see Introduction) and theoretical considerations. In the first patient in this series, using a target based most closely on thermocapsulotomy (approximately 15mm anterior to the AC), improvement in OCD severity was minimal. Outcomes improved in subsequent patients in whom progressively more posterior targets were used. In addition, animal model data25 and a review of clinical, anatomical and neuroimaging findings8 suggested that stimulation of a more posterior and inferior target (the caudal nucleus accumbens) might be advantageous in OCD. Finally, electric field modeling suggested that the effective stimulation volumes during DBS were small relative to those of lesions (R Testerman, personal communication). These considerations contributed to the hypothesis that the neural systems implicated in OCD might be more efficiently modulated by stimulation at the more posterior/inferior VC/VS location, where cortico-basal fibers become more compact. The collective experience with intraoperative test stimulation also supported this view, as it appeared that effects on mood, anxiety and OCD symptoms could be elicited using lower stimulation energies with more posterior targets.
Investigators from the collaborating centers were present especially for the first implantations at each study site, which helped ensure comparability and consistency of targeting. Leads were implanted to follow the trajectory of the anterior capsule in the coronal plane, extending ventrally so that the most distal contact was below the capsule white matter. In the later patients, with more posterior targeting, electrode contact 0 was placed in the ventral striatum just below the axial plane defined by the anterior and posterior commissures, contacts 1 and 2 in the ventral half of the capsule, and contact 3 at the dorsal margin of the capsule. This is illustrated in Figure 1 that shows the surgical planning and lead positions on postoperative CT co-registered with the preoperative MRI from a representative patient in the later cohort.
Figure 1.
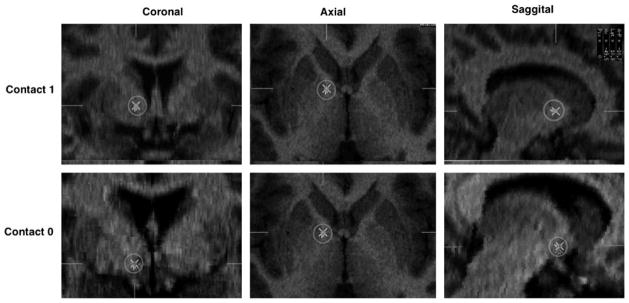
Post-implant locations (X within circles) of the two lower electrode contacts in a representative patient from the third cohort. The centers of contacts 0 (bottom) and 1 (top) in the coronal, axial and sagittal planes are shown for the right side (all implants were bilateral).
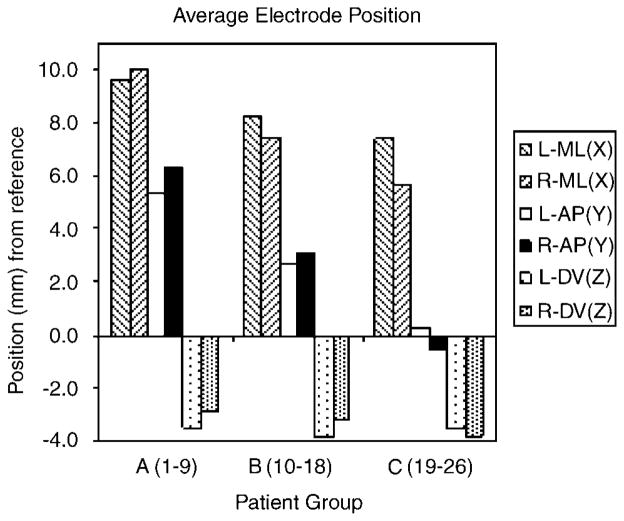
Postoperative imaging (CT for BH and UF patients; MRI for LV and CC patients) was co-registered with the preoperative stereotactic MRI to localize the lead locations. Figure 2 illustrates the shift in average lead tip locations in three subgroups of patients based on date of implantation: group A included patients 1–9 (LV1-5; BH1-3; CC1); group B included patients 10–18 (CC2-4, BH4-5, LV6-8, UF1) and group C, patients 19–26 (UF2-5, CC5, LV9-11). These three groups of patients represent the early, midterm and most-recently implanted patients from the overall sample. The last patient in group A was implanted on 15 January 2002; in group B, 16 April 2003 and in group C, 23 March 2005. At least three of the four centers contributed one or more patients to each of these three groups. The first group of patients implanted (mainly at LV and BH) had more anterior lead positions. Targeting for second and third group of patients was progressively more posterior (and slightly medial due to the trajectory of the capsule in this region). For the latter patients, the target was the junction of the anterior capsule and the ventral striatum, within 1–2mm of the posterior border of the AC. Distal contact 0 was most often in the caudal nucleus accumbens in these patients and with the most posterior lead placements, the lead traversed the rostral bed nucleus of the stria terminalis.
Figure 2.
Average electrode positions in patient groups (see text for details). Position plotted is location of tip of distal electrode contact obtained from post-implant imaging. ML position is relative to the midline; anterior–posterior (AP) position is relative to the anterior commissure (AC); dorso–ventral (DV) position is relative to the AC–PC (posterior commissure) line; (n = 23). Two patients from group A and one patient from group B did not have immediate post-operative images that allowed for reasonable estimation of electrode position due to issues related to patient care, and/or image resolution.
DBS programming
Acute monopolar survey
At average of 3 weeks after implantation (at US centers), a monopolar survey was conducted to identify untoward, as well as positive, effects of acute stimulation at any given contact, for use primarily in determining which electrode configurations to use for chronic stimulation. This survey, and subsequent DBS adjustments, was carried out by a single investigator at each center. The programmer display was kept of sight of the patient, who remained masked to DBS status. Each of the four contacts on each side was activated singly and in turn, set negative with the INS case set positive. For the US centers, DBS was at 100–130 Hz, at pulse widths ranging from 90 to a maximum of 450 μs. DBS intensities generally ranged from 2 to 8V, resulting in currents ranging approximately from 2 to 15 mA, depending on electrode impedance (generally 500– 1000Ω). Stimulation at each configuration and setting was tested for 1–2 min, interspersed with no stimulation periods. Procedures at LV were similar, although amplitudes up to the INS maximum of 10.5V were tested, with correspondingly higher currents. The LV patients also underwent supplemental surveys, which included bipolar and monopolar stimulation. At all centers, patients were prompted to describe any adverse effects during the survey, including effects on sensation, motor function, mood or thinking. Similarly, they were asked to describe any changes in mood, anxiety or OCD symptoms during each test. If an untoward effect appeared at a certain stimulation threshold, the parameters were held stable until the effect disappeared or for a maximum of 1 min.
Chronic DBS
Similar to DBS therapies for movement disorders, stimulation adjustment was an iterative process, based on the physician programmer’s judgment of therapeutic improvement and tolerability over time. Parameters for chronic DBS were initially guided most by those used in the first patients in the LV series.3 That experience, and the results of intra- and postoperative testing converged to indicate that the best combination of therapeutic benefit and tolerability was generally achieved when the ventral contacts (0 and/or 1) were active and negative. Patients required close monitoring for deterioration in psychiatric status or stimulation-related adverse effects throughout.
As with target location, average DBS parameters changed from the first to last patient cohorts. The change in targeting to a more posterior position was associated with the use of lower mean stimulation energy (voltage, pulse width and number of active contacts) for the later subjects compared to the earlier ones. Figure 3 illustrates these changes in average stimulation parameters from the first patients (group A) to the later cohorts (groups B and C) irrespective of study center. Stimulation frequency ranged between 100 and 130 Hz for all patients, with the exception of one patient in group A (CC1), where a very atypical low frequency was selected.
Figure 3.
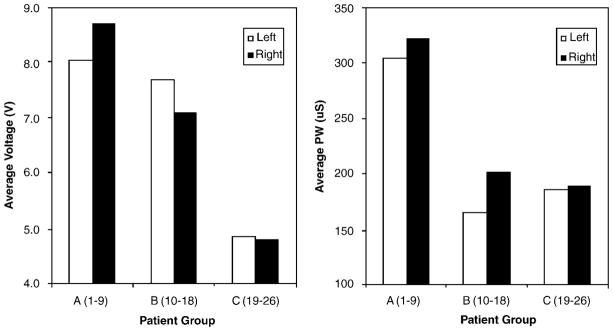
Average voltage (left) and pulse width (right) parameter settings for the three patient subgroups.
DBS was delivered continuously until interrupted by stimulator battery depletion, which occurred after 5–13 months across patients. After INS battery depletion, the devices were replaced in outpatient surgery under local anesthesia (except for one patient, LV8, who routinely requested general anesthesia), after which DBS was resumed.
Concomitant therapies
Because DBS was used as an adjunctive treatment in a severely affected group, concomitant pharmacotherapy was allowed throughout the trials. All subjects had been treated extensively with medications for OCD prior to beginning DBS, a key entry criterion in the four protocols. Most subjects continued on medications in conjunction with DBS. Medications were held constant for at least 3 months at each center after chronic DBS began, although adjustments necessary to manage adverse events were allowed. In the LV patients, medication was tapered off to a minimum 6 weeks before surgical intervention and was kept stable for 1 year after implantation. At the start of DBS, 18 patients were on an antidepressant (mainly SRIs), 18 were on benzodiazepines or other anxiolytics, 12 were on neuroleptics (first- or second-generation), 5 were on a mood stabilizer (anticonvulsants and/or lithium) and 1 was on a nonbenzodiazepine hypnotic.
Behavior therapy
Although supportive psychotherapy was allowed (even encouraged), behavior therapy specifically aimed at improving OCD (exposure and response prevention) was not initiated until either 6 months (BH, CC and UF) or 1 year (LV) after implantation. At BH, CC and UF patients could continue behavior therapy if established at least 3 months preimplantation.
Center-specific design features
Although DBS was administered openly during long-term treatment at all centers, controlled data were obtained at two centers (LV and UF), each of which used a different design. Protocol differences between centers are summarized below.
Leuven/Antwerp (LV)
This center began working in 1998, informed by preliminary work at the Karolinska Institute, Stockholm, Sweden. Two patients operated in Stockholm had no clear benefit. Data from the first 11 patients implanted at LV are described here. DBS was delivered openly during initial parameter optimization. After patients displayed stable improvement for at least 2 months (which took from 2 to 10 months (mean: 5.6 months), patients entered a double-masked crossover phase. Patients were assigned to 3-month blocks of masked active or sham DBS, and then crossed over to the other (masked) condition. As of the data cutoff for this report, three patients remained in the masked phase. Target coordinates (as lead tip position) evolved most over this first pilot study, from a maximum of 15mm anterior to AC in the first patient, to 0–2mm posterior to the commissure in the most recent patients. DBS was adjusted by a single unmasked investigator, in a different location from the rating psychiatrist, with the programmer display out of sight of the patient. Parameters were selected on the basis of acute responses to DBS, including improvements in mood, nonspecific anxiety and reduction in obsessions or compulsions.26 Ratings were made by a single clinician, who was unaware of DBS status or parameters in the masked phase, and by the same researcher during open stimulation.
Butler Hospital and Cleveland Clinic
These studies (described together as their protocols were essentially identical) each enrolled five patients. Treatment was open label; patients were told that DBS would occur most of the time, and in fact was on continuously except when interrupted by INS battery depletion. DBS was adjusted by a single unmasked investigator, with the programmer display not visible to patients. Ratings were made by one clinician at each site, who was unaware of DBS status or parameters.
University of Florida
This study used double-masked sham stimulation (for 1 or 2 months) and a staggered start of single-masked active DBS (after 1 or 2 months), followed by long-term open DBS. Five patients had been implanted as of the cutoff date of this report. One of two highly experienced raters completed the assessments. After a 1-month recovery (DBS off), a masked delayed start period began. Some patients began masked DBS the first month and the other half remained with DBS off until month 2. All subjects and evaluators were blinded to when stimulation actually began. Stimulation parameters were determined when outpatient DBS began.
Outcome measures
The primary outcome measure was the YBOCS, analyzed as a continuous end point. All centers used the HAM-D ratings (see above for scale versions) to measure co-occurring depressive symptoms before and during DBS. As noted, 23 of 26 patients had a current or lifetime history of depression on the SCID. Nonspecific anxiety symptoms are common in this patient population. The HAM-A27 was used at three centers (LV, BH, CC, which enrolled 21 patients), to measure nonspecific anxiety, also common in this population. In the same 21 patients, global functional status was assessed with the GAF.28 For all 26 patients, functional outcomes were assessed descriptively in three categories: occupational or school functioning, capacity for independent living (including ADLs) and social engagement.
Safety and tolerability outcomes
Potential adverse effects in these and other domains were also evaluated during clinical interviews at each center (semistructured interviews were used at BH and CC). Formal cognitive testing was also preformed before and during DBS (see ‘Results’).
Data analysis
For all continuous outcomes (YBOCS, GAF, HAM-A% and HAM-D%), a repeated measures assessment was performed using generalized estimating equations (GEE), which is well suited to the evaluation of longitudinal studies with correlated data. The GEE analyses used an exchangeable working correlation matrix, normal distribution and identify link function. Model effects, for time and treatment, were evaluated conservatively using score statistics reported for type 3 results. Treatment effects were estimated as differences between baseline and the DBS treatment period. Analyses were performed using SAS (v9.1). OCD severity was also assessed categorically, as the number of patients at each rating point who were in each of three mutually exclusive categories: (1) those with a less than 25% YBOCS decrease from preimplantation baseline; (2) those with at least a 25%, but less than a 35%, reduction in OCD severity and (3) those with at least a 35% decrease.
Data included in this report
Across centers, data were collected at presurgical baseline after 3, 6, 12, 24 and 36 months of chronic DBS. In total, 17 patients had at least 24 months of follow-up, and 12 had reached 36 months (mean duration: 24.0±2.5 months). The two sites (LV and UF) where patients underwent masked sham DBS trials (using different designs) will report those data separately.
Of the 26 patients implanted, one (CC1) died (due to recurrence of breast cancer 1 year after implantation). Her data were not carried forward. Stimulation was discontinued in three patients after 12 months (LV1, BH2 and BH4) due to inadequate improvement. Patient LV1, first in the overall series whose device implantation was the most anterior, was judged a nonresponder after 12 months and chose to undergo thermocapsulotomy, after which he improved. Patients BH2 and BH4 continued treatment as usual, and were followed and rated with DBS off. As discontinuation of DBS in some proportion of patients is likely after long-term treatment, their data, including the later time points with DBS off, were included in the intent-to-treat analysis. An additional patient (LV4) underwent device removal and later capsulotomy (due to lack of stable improvement) after 3 years of follow-up had been completed.
Results
OCD severity
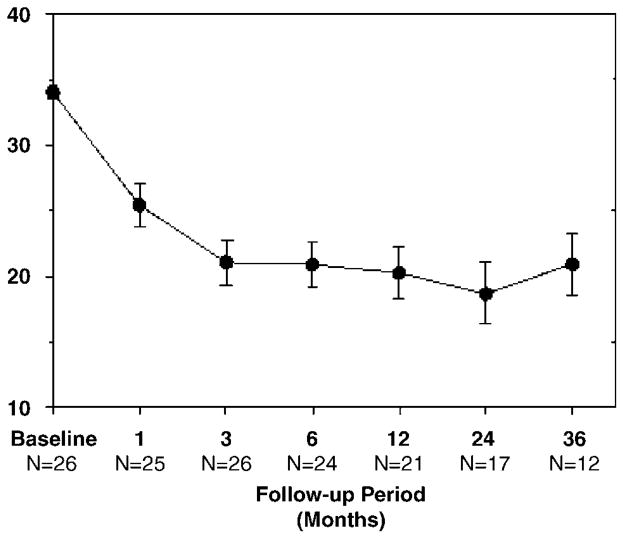
Figure 4 shows YBOCS severity scores before and during chronic DBS. The mean preimplantation baseline YBOCS score (±s.e.m.) was 34.0±0.5, indicating severe illness. Postoperative, pre-DBS ratings were available for a subset of 15 patients (not collected for LV patients) after device implantation but before stimulation began. Mean YBOCS scores remained in the severe range (31.50±1.2) at this time point. Only one patient (UF1) had a clinically significant pre-DBS score drop, from 37 to 21 points, which returned essentially to baseline (36 points) by the 1-month rating point. The score of 21 was obtained the day after surgery and may have reflected perioperative factors, including insertional edema, residual effects of anesthesia or intraoperative stimulation. Mean YBOCS scores decreased after stimulation onset, reaching 20.9±2.4 at 36 months (repeated measures overall GEE for time: c2 = 20.35; P = 0.002). This degree of improvement was apparent by the third month of active stimulation, when the mean YBOCS had declined to 21.0±1.8. On average, there was a 12.5±1.4 point decrease in YBOCS scores observed between baseline and treatment phases (c2 = 19.59; P < 0.001).
Figure 4.
Mean (±s.e.m.) Yale–Brown Obsessive Compulsive Scale (YBOCS) severity scores pretreatment and at each deep brain stimulation (DBS) treatment rating point.
We also examined changes in YBOCS during chronic stimulation categorically. Table 2 shows the number of study patients in each of three, mutually exclusive, categories from 1 to 36 months after the start of chronic DBS. The percentage of patients meeting the full response criterion (≥35% YBOCS decrease) increased from 28% at 1 month (7 of 25 patients—one of the total was not rated at 1 month) to 61.5% (16 of 26) at last follow-up. Conversely, the percentage with less than a 25% YBOCS decrease (nonresponse) declined from 68% (17 of 25) at 1 month to 27% (7 of 26) at last follow-up (Table 2). Overall, a total of 73% of patients had at least a 25% YOBCS improvement at last follow-up; a large majority of those improvements were a 35% or greater YBOCS reduction.
Table 2.
Number (and percentage) of patients by categorical response designation at each treatment rating point during chronic DBS stimulation
| DBS duration | < 25% YBOCS ↓ (no. of patients) | ≥25 < 35% YBOCS ↓ (no. of patients) | ≥35% YBOCS ↓ (no. of patients) | Total N |
|---|---|---|---|---|
| 1 Month | 17 (68%) | 1 (4%) | 7 (28%) | 25 |
| 3 Months | 11 (42%) | 2 (8%) | 13 (50%) | 26 |
| 6 Months | 9 (37.5%) | 4 (16.5%) | 11 (46%) | 24 |
| 12 Months | 7 (33%) | 4 (19%) | 10 (48%) | 21 |
| 24 Months | 3 (17.5%) | 3 (17.5%) | 11 (65%) | 17 |
| 36 Months | 3 (25%) | 2 (25%) | 7 (58%) | 12 |
| Last follow-up | 7 (27%) | 3 (11.5%) | 16 (61.5%) | 26 |
Abbreviations: DBS, deep brain stimulation; YBOCS, Yale–Brown Obsessive Compulsive Scale.
In addition, categorical YBOCS outcomes were examined in light of patients’ primary OCD symptoms (that is, those symptoms identified by patients and study physicians as causing the greatest distress and impairment). Symptom categories followed an influential four-subtype model derived from factor analysis. 29 The proportions of patients with at least a 35% YBOCS score reduction in each primary symptom category during stimulation were as follows: Obsessions and Checking: six of six patients (100%); Symmetry and Ordering: five of nine patients (55.6%); Cleanliness and Washing: five of eleven patients (45.5%). There were no patients enrolled for whom compulsive hoarding was the primary symptom subtype.
Outcome differences vs date of implantation
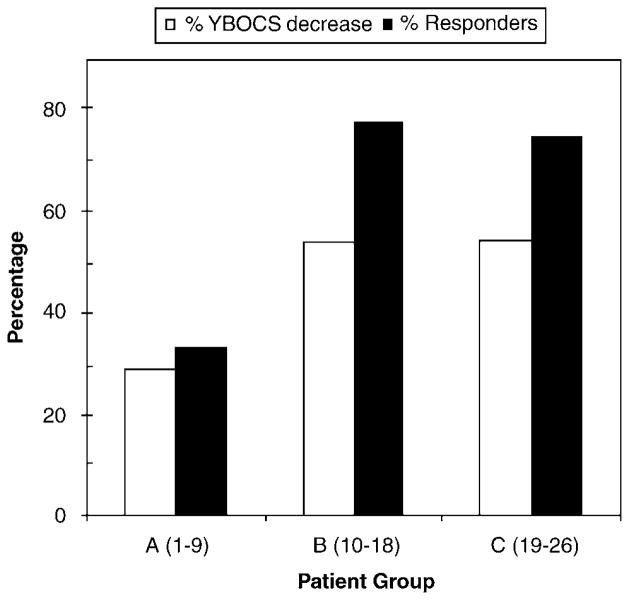
Across centers, the data show better OCD outcomes for patients implanted more recently. Figure 5 illustrates the relationship between implant date and clinical response at last follow-up in the three subgroups of patients for both continuous (%YBOCS reduction) and categorical (percent responders)measures. YBOCS decreases were 29.0±6.6% (range: 6–70%); 53.9±8.3% (range: 12–81%) and 54.3±12.7% (range: 6–94%) in groups A, B and C, respectively. The percentage of patients meeting the 35 percent YBOCS reduction (full response) criterion in each group were 33.3%, 77.8 and 75.0% of patients in groups A, B and C, respectively (see Figure 2 for lead location differences). The degree of clinical improvement in the latter two groups was nearly identical, using either a continuous decrease in YBOCS severity or the percentage meeting the 35% YBOCS decrease response criterion.
Figure 5.
Clinical response for the three patient subgroups: average percent Yale–Brown Obsessive Compulsive Scale (YBOCS) decrease and percentage of patients meeting responder criterion (35% reduction in YBOCS).
Relationship of YBOCS change to medication changes
Across centers, OCD severity reductions at last follow-up were greater in the subgroup whose medications remained unchanged (N= 15; mean YBOCS decrease: 53.8%) compared to those whose medications changed (N= 11; mean YBOCS decrease: 34%).
Global functioning
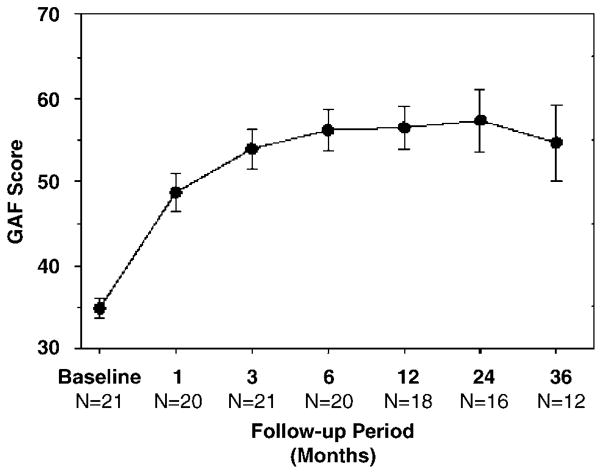
Figure 6 shows that mean scores on the GAF improved significantly over time during long-term DBS. At presurgical baseline, the mean GAF was 34.8±1.1 (for the 21 patients administered the scale, which was not used at UF). IN total 20 of 21 patients scored 40 or less (consistent with major functional impairment) at presurgical baseline. After 3 months of stimulation, the mean GAF had risen to 53.9±2.4, and was 59.0±3.3 at last follow-up (repeated measures overall GEE for Time: c2 = 18.05; P = 0.006). On average, a 19.8±2.3 point increase in GAF scores was observed between baseline and treatment phases (c2 = 16.46; P < 0.001). At last follow-up, only two patients continued to score in that range; 61.9% of the patients scored 51 or higher, a score demonstrated by none of the patients at baseline.
Figure 6.
Average (±s.e.m.) Global Assessment of Functioning (GAF) scores over time.
To represent global outcomes in the entire sample, study physicians at each site also provided an impression of the overall condition of the patients after chronic DBS, including symptom severity, functioning and quality of life, using a five-point rating (from ‘worse’ to ‘much better’). None of the 26 patients were rated as globally ‘worse’ or ‘slightly worse’ after stimulation; 4 (15.4%) were rated ‘unchanged’; 5 (19.2%) were rated ‘slightly better’ and 17 (65.4%) were rated ‘much better’. In addition, detailed clinical narratives were used to describe patient functioning before and after stimulation in three categories: (1) work, school or homemaking functioning; (2) independent living and activities of daily living and (3) social engagement. At last followup, work, school or homemaking functioning was described as fair or good in 21 of the 25 patients. Capacity for independent living was considered fair or good in 20 of 25 patients. Social engagement was considered fair or good in 21 patients, and minimal or poor in the remaining 4. Thus, social engagement was judged to have improved in all but four patients, to varying degrees ranging from greater social contact to getting married.
Effects on Comorbid Depressive Symptoms and on non-OCD-specific Anxiety
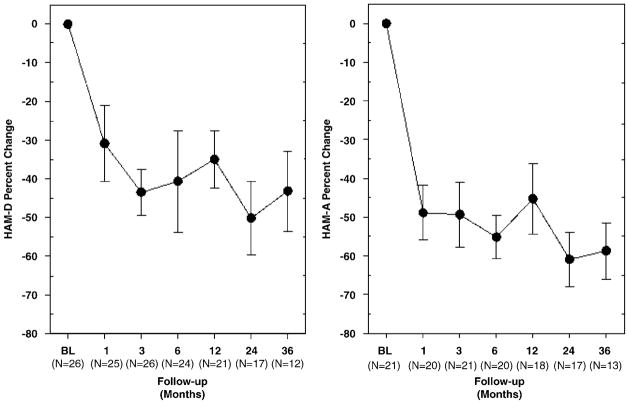
Comorbid depressive and non-OCD anxiety symptoms were common in the patients studied. Although different HAM-D versions were used across centers, all but 1 of the 26 patients had a HAM-D score of 11 or higher at baseline, scores which are above a commonly proposed threshold for depression remission (a HAM-D score of 7).30 Figure 7 (left) shows the percent change from baseline in depression ratings over time. After 36 months of DBS, average scores were progressively reduced by 43.2% (repeated measures overall GEE for time: c2 = 20.36; P = 0.002). At last follow-up for each patient, HAM-D scores were < 7 in 14 of the 26 patients (overall mean percent reduction: 52.8±6.2%). On average, there was a 40.0±5.9% decrease in HAM-D scores between baseline and treatment phases (c2 = 16.76; P < 0.001).
Figure 7.
Average (±s.e.m.) change in depression (left) and anxiety (right) measures over time.
Similarly, patients commonly had significant anxiety at baseline. Of the 21 patients who were administered the HAM-A (not used at UF), 19 patients scored 13 or higher, above the proposed HAM-A remission threshold (≤10) for non-OCD anxiety disorders.31 Figure 7 (right) shows percent change from preimplantation baseline in HAM-A scores (N= 21). After 36 months of DBS, average scores were progressively reduced by 58.7% (repeated measures overall GEE for time: c2 = 18.33; P = 0.006). Assessment of the last follow-up scores indicated that 14 of the 21 patients scored < 10 on the HAM-A (overall mean percent reduction: 50.0±6.5%). On average, a 52.6±4.5% decrease in HAM-A scores was observed between baseline and treatment phases (c2 = 18.23; P < 0.001).
Serious adverse effects
Potential complications of DBS can arise (1) as a result of surgery (‘procedure related’), (2) due to the implanted device (‘device related’) and (3) due to stimulation, or cessation of stimulation, in patients for whom DBS was associated with benefit (‘therapy related’). Those events judged by investigators at each site as serious (based on FDA guidelines), and at least possibly related to one of the above three factors, are detailed below. Other untoward events that were judged to be related to preexisting OCD or other comorbid psychopathology (‘disorder related’) are also noted. There were a total of 23 serious adverse events reported in eleven patients (42.3%) over a period equal to 52 patient years of experience in this cohort.
Procedure-related complications
In total, 2 of the 26 patients (7.7%) had small intracerebral hemorrhages after lead insertion. In one case, blood from a ruptured superficial bridging vein appeared to track down the insertion cannula. This hemorrhage remained asymptomatic and resolved on serial CT scans within days without treatment. In the other case, the hemorrhage appeared to be intracerebral in origin and was associated with apathy, which resolved within 3 months without other clinical sequelae. Another patient had a single generalized tonic-clonic seizure after lead implantation in the operating room, which prompted prophylactic phenytoin treatment after surgery, which was discontinued after 1 month, without seizure recurrence. One patient (3.8%) developed a superficial wound infection, which was successfully treated with antibiotics.
Device-related complications
A break in a stimulating lead or an extension wire requiring a replacement occurred in one patient each (total 7.7%).
Therapy-related complications
There were a total of nine events associated with stimulation, including four incidents of increased depression/suicidal ideation in three patients (11.5%). All of these patients had had similar episodes during their course of illness prior to implantation. Three of these four events represented clinical worsening, after improvement during active stimulation, when patients were crossed over into the stimulation off phase of the one study protocol (LV) that used that design. Increased OCD severity was reported in three patients, which was related to the stimulation off condition in one patient. Interestingly, in one of these cases, DBS—induced mood elevation was associated with a marked increase in OCD symptoms: the patient got ‘stuck’ in a compulsive ritual to a degree that required hospitalization. This was in marked contrast with clinical experience that elevated mood states can be associated with reduced OCD intensity (for example, Keck et al.32). In addition, there was one case of hypomania that was considered serious, and one report of domestic problems/irritability associated with stimulation.
Disorder related
Investigators judged seven events in four patients to be associated with patients’ preexisting disorders including three occurrences of increased depression/suicidal ideation, and one incident each of aggression/violent behavior, pyelonephritis, compression fracture and a motor vehicle accident.
Other adverse effects
Effects on mood
Changes in mood were the most common effects noted both during acute stimulation and upon the cessation of stimulation. These mood alterations included elevation of mood toward normal in depressed patients, elevation of mood above what is considered normal (hypomania), lowering of mood toward baseline in an improved patient, and lowering of mood beyond baseline. Elevation of mood toward normal was a desired effect of DBS and therefore, not considered an adverse event. Elevation of mood above normal (nonserious) was seen in eight patients, often associated with noticeably increased energy and speech production, but not with any associated behavioral impulsivity. Other than DBS being the obvious etiology, these episodes would be consistent with a clinical diagnosis of hypomania. Patient descriptions of these feelings included ‘feeling a lot better’, ‘happy’ and ‘giddy’. Mood elevation would begin within seconds to minutes after the adjustment of stimulation parameters (typically an increase in pulse width or amplitude). If no further parameter changes were made, symptoms would typically peak within 5–30 min and then gradually subside. Some episodes would persist until parameter changes were made. A reduction in stimulation settings always resulted in rapid resolution of these symptoms.
A lowering of mood (depression) could also be seen during acute titration. Subjects would often experience improvement in their premorbid depressive symptoms with certain stimulation settings, but would quickly worsen again upon a change in settings or cessation of stimulation. This was particularly noticeable when chronic stimulation (which had improved depression) was abruptly halted due to inadvertent battery shutoff or battery depletion. A mood decline was typically the first symptom noted when either of these events would occur. Eight subjects noted worsening mood during acute stimulation or upon the abrupt cessation of therapy. In none of these individuals was the degree of depression or suicidality experienced greater than anything they had experienced during their pre-DBS course of illness. During chronic stimulation, only one patient exceeded a score of 2 (‘wishes he were dead or any thoughts of possible death to self’) on item 3 of the HAM-D, on a single occasion. That patient was judged not acutely suicidal and received no further intervention.
Effects on anxiety
Improvement in anxiety was commonly seen during acute stimulation testing and continuing into the chronic phase of treatment. However, acute worsening of anxiety could also be elicited in certain patients during acute stimulation testing. Eleven subjects reported an acute increase in anxiety upon a change in stimulation parameters. Typically this was noted during stimulation of the most distal contact. One of these described a panic attack with symptoms including fear, flushing, tachycardia and hot flashes.20 In all of these instances, anxiety symptoms were quickly resolved by a change in parameter settings. A small number of subjects reported irritability during stimulation, which also resolved after parameters were changed.
Cognitive effects
A number of patients described changes in cognition associated with certain DBS parameters. These included acute cognitive ‘clouding’ or diminished concentration. One patient developed verbal perseveration when the most dorsal contact was activated intraoperatively. Another patient developed brief memory experiences (‘flashbacks’), recurring several times a day over several days, which ceased after parameters were changed.
Sensory, motor, and other effects
During acute testing or parameter changes, some patients reported stimulation-related olfactory and/or gustatory sensations, or paresthetic sensations, usually described as ‘tingling’ in orofacial locations, in the hands or feet or as ‘buzzing’ around the stimulators in the chest. Some patients also described DBS-related muscle contractions, mainly orofacial, including jaw muscle contraction (associated with dysarthria), facial muscle contractions (resulting in a ‘smile’ contralateral to the side of stimulation (see Nuttin et al.3 and Okun et al.19), or contractions of muscles in the neck or head. One patient reported an unwitnessed loss of consciousness during chronic stimulation, of unknown etiology (hypoglycemia due to insulin treatment for diabetes being one possibility).
The key variables associated with the occurrence of these acute or subacute effects were (1) the specific electrode contacts activated, (2) stimulation intensity and pulse width and (3) to a lesser extent, stimulation mode (unipolar or bipolar electrode configuration, with the former somewhat more associated with these effects). Most adverse effects (like most apparent benefit) were induced by stimulation of the ventral two contacts. As noted above, none of these effects were persistent, and no sensorimotor changes were noted on detailed postoperative neurological examinations.
Effects of DBS interruption
Some adverse events were associated with stimulation cessation: (1) as part of a study protocol as described above; (2) when the neurostimulator battery was depleted and (3) in one case where device shutoff apparently occurred after the magnetic switch on the Soletra INS was tripped by a metal detector. The impression of investigators at each site was that patients who experienced DBS interruption were most likely to report an acutely more depressed mood. This was most commonly associated with stimulator battery depletion, which was typically discovered after patients (who were unaware that the end of battery life had been reached) complained of clinical deterioration. A smaller number of patients described an acute worsening in OCD symptoms or increased nonspecific anxiety although patients generally reported that this was less marked than the change in their affective state. The clinical worsening typically abated somewhat over several days, and reversed after DBS was restarted. No patient became actively suicidal when DBS was interrupted. In response to such events, study centers generally began to estimate battery life based on stimulation parameters, and to attempt to replace devices before battery depletion. The battery life became substantially longer on average for patients in groups B and C as lower stimulation charge densities became possible based on improved efficacy (ranging from 6 months in a representative patient from group A to approximately 12–18 months in groups B and C), although this interval varied across patients depending on specific parameters.
Neuropsychological testing during long-term DBS
Patients completed neuropsychological assessments before implantation and again between 3 and 12 months of chronic open DBS. The same test battery was used at BH and CC.33 Although different neuropsychological test batteries were used at LV4 and UF (data to be published separately), the tests used sampled similar cognitive domains. There were no patterns of pervasive cognitive decline in any patient from the BH, CC and LV samples.
Adverse event frequency by order of implantation
We also examined the frequency of adverse events as experience was gained in the collaborative group as a whole. The frequencies of adverse events defined as serious by site investigators in the three subgroups of patients were tabulated. In group A, 15 serious adverse events (SAEs) were described in five of nine patients. In group B, there were five SAEs in three of nine patients. In group C, there were two SAEs in two of eight patients. Normalizing to account for different lengths of follow-up between groups, these results equate to 0.66 SAEs per patient year of DBS experience in group A, 0.22 in group B and 0.31 in group C.
Discussion
Data from these four centers, obtained collaboratively over 8 years, show positive outcomes of VC/VS stimulation in highly resistant OCD. Consistent with prior reports on part of this sample,3,18 clinically significant symptom reductions and functional improvements were seen in over 60% of these highly treatment-resistant patients, within and across study sites. DBS was well tolerated overall, and the vast majority of adverse effects were transient. On average, OCD improved from severe illness at baseline to moderate severity during chronic DBS. Although clinically significant symptoms persisted in most patients, 10 of 26 (38%) showed YBOCS severity declines to a score of 16, proposed as a remission threshold by some investigators, and which would be an extremely strict response definition in this population. Thirteen of the patients (50%) reached a YBOCS score of 20 or below. Recent data suggest that quality of life and psychosocial functioning begin to be more significantly affected at YBOCS scores higher than 20.34 By any of these metrics, the effectiveness outcomes for DBS compare favorably with those reported after lesion procedures, which have noted rates of response (defined variably) ranging from roughly 30 to 70% of patients.1
In addition to gains noted on global scales, at least 16 of the 25 patients moved toward more independent daily living and 20 of 25 were judged to have improved occupational or school functioning during DBS. Both changes suggest meaningful functional improvement in the group as a whole. How these real-world functional outcomes compare to what would be expected in a severely affected group without DBS requires controlled data, which are also necessary to address the other limitations above.
The combined data show that outcomes generally improved as patients were enrolled over the course of this work. Results were better on average for patients implanted more recently, both in terms of decrease in YBOCS severity, and in the proportions of patients meeting response criteria. Although the first nine patients implanted across centers had a mean YBOCS reduction of just < 30%, the second and third groups implanted in chronological order had mean YBOCS declines > 50%. Viewed categorically, the percentage of patients defined as having a full response by the 35% YBOCS criterion increased from approximately 30% in the first group, to approximately 70% in both the second and third groups implanted. The data also, more tentatively, suggest that the likelihood of serious adverse effects declined in the latter two groups.
This pattern of results is consistent with a ‘learning curve’, that is, improvement in outcomes as successive cohorts of patients undergo a procedure. Researchers using DBS for movement disorders have suggested that a learning effect might be especially prominent as the first 6–10 patients undergo a procedure.35,36 Several factors are thought to contribute to gains in effectiveness and safety with experience in using DBS for movement disorders. These include improved diagnosis of potential candidates; selection of patients more likely to respond; advances in surgical technique and enhanced patient management, including device programming, during chronic treatment.37–43
As described above, targeting changed systematically during this work, whereas patient selection did not. The key difference appears to be have been the use of a progressively more posterior implantation site. Most of the change in targeting occurred between implantation of the first and second patient subgroups. Stimulation technique also changed. Relatively high intensities were used in the initial group. Energies (for example, charge densities) were lower in the subsequent two patient cohorts. This suggests that the posterior VC/VS target site may be closer to structures key to therapeutic effects of DBS for OCD, rather than that lower stimulation intensities were inherently more effective.
The modified VC/VS target lies at the junction of the anterior capsule, AC and posterior ventral striatum. During this work, the VC/VS target became somewhat more medial due to the trajectory of the capsule in this region. Regarding the dorsal–ventral dimension, VC/VS stimulation may be most effective when electrodes nearest to and including the intersection between the AC and the lower margin of the anterior capsule are activated.23 These are typically contacts 0 and/or 1 with the DBS lead used at the four centers. Interestingly, ongoing work in nonhuman primates suggests that pathways connecting medial and orbital frontal cortex to the thalamus, implicated in OCD,14 appear on preliminary analysis to travel in just this region (S Haber, personal communication). It should be noted, however, that the VC/VS target also impinges upon adjacent structures, including the bed nucleus of the stria terminalis, which might be involved in the therapeutic actions and patterns of adverse effects observed. Moreover, specific subregions within the ventral striatum portion of the target might be differentially involved.8
PET data acquired during VC/VS DBS are consistent with an effect on those pathways
O15-PET imaging in a subset of patients from this series found that acute high frequency DBS increased perfusion in orbitofrontal cortex, anterior cingulate cortex, striatum, pallidum and thalamus compared to control conditions.44 Future neuroimaging studies of neural networks affected by VC/VS stimulation should be informative. Moreover, fluorodeoxyglucose positron emission tomography (FDG-PET) imaging in a different subset of these patients found that metabolism in the subgenual cingulate cortex, measured before surgery, was directly correlated with the extent of OCD improvement during DBS,45 raising the possibility that neuroimaging might predict the probability of clinical benefit of DBS in individuals. Response prediction would be highly desirable in reducing exposure to the risks inherent in this procedure.
Although DBS was generally well tolerated, there were significant adverse effects. The relative reduction in stimulation-related adverse effects from group A to groups B and C may have reflected the use of lower stimulation energies in the latter two groups. The reduced need for stimulation energy as experience was gained was possibly due to more optimal targeting. Surgical SAEs included implantation-related hemorrhage, a single seizure and an infected surgical incision. Acute stimulation effects included increased anxiety and/or depression, mood elevation (including hypomania), impaired cognition or sensorimotor (usually orofacial) effects. These reversed rapidly with parameter changes. In one case, hypomania persisted over days. The other major effect was worsening of depression, nonspecific anxiety and in obsessions and compulsions when stimulation was interrupted. Mood worsening occurred earliest and was the most clinically prominent effect after DBS ceased. These observations, together with reported efficacy of identical lesion procedures for both OCD and depression, helped to prompt subsequently investigations of VC/VS stimulation for highly refractory depression.46
Even though depression worsening was usually the first symptomatic change noted clinically when stimulation was interrupted, no patient attempted suicide during chronic DBS. Patients have died by suicide during stimulation for movement disorders,47 or, in one case, DBS for OCD at a more anterior target similar to that for capsulotomy,5 Abelson et al. commented that the suicide in that case appeared most likely to be a consequence of profound demoralization and depression rather than a result of stimulation. Most patients referred for OCD neurosurgery suffer both profound demoralization after years of severe impairment and have comorbid depression as well. Several patients had suicidal thinking before enrollment at different centers. Suicides have occurred during evaluations for neurosurgery, or after acceptance but before the procedure (L Gabriels, personal communication; S Rasmussen, personal communication). Risk of suicide in this population must therefore be considered to be potentially high at all phases of evaluation and treatment. Psychiatric use of DBS requires dedicated interdisciplinary teams expert in patient selection, implantation, stimulation and, particularly in this regard, in long-term management of severely ill psychiatric patients.
The data here reflect the effects of open chronic DBS, so a placebo effect is possible. However, persistence of improvement up to 3 or more years, in patients selected for chronic, highly refractory OCD (a group which generally resists placebo effects) argues against this. It is also difficult to see how a placebo effect could account for the improved outcomes in the second and third groups implanted compared to those in the first patients. For that to occur, those patients implanted more recently would have to be systematically more susceptible to placebo effects, which seems very unlikely. A learning effect, which has been well documented in other clinical applications of DBS, seems more plausible.
The data also argue against the possibility that a ‘microlesion’ effect after lead insertion contributed significantly to improvement. DBS interruption (essentially masked as neither patients nor investigators knew when it would occur) was consistently associated with symptomatic worsening. An insertion effect also appears unlikely, as OCD symptoms and functional status were essentially unchanged after implantation but before DBS started. In a subset of these patients, OCD severity was unchanged during sham DBS for 1–2 months after implantation (Goodman et al., personal communication). Moreover, symptomatic improvements were not maintained during masked stimulation withdrawal in one subgroup in this study,4 a finding that on preliminary analysis appears to be replicated in subsequent patients.
Although held constant before and for at least 3 months after implantation, medication regimens differed across patients, another potential source of variability. However, medications were modified most in patients who improved least. Those with stable medication regimens showed greater YBOCS severity reductions (−53.8%) than those whose medications changed (−34.0%). This, most likely, represents an attempt on the part of the psychiatrist to bring about improvement in those patients not obtaining sufficient benefit from DBS. Medication changes thus seem unlikely to account for the therapeutic gains noted.
A larger, sham-controlled study is also needed to address the possibility that outcomes may differ across patients with different predominant OCD symptom subtypes (for example, Leckman et al.29 and Pinto et al.48). Different neural circuits may underlie different subtypes49,50 and DBS targeting fronto-basal circuitry might improve some symptom subtypes better than others. It is of interest that in two of the three patients in whom DBS was discontinued due to inadequate benefit, the clinical picture was dominated by ‘incompleteness’, that is, the need to repeat actions until a sense of rightness is obtained.51 However, other patients with incompleteness symptoms (along with other types) did improve. The three patients in the overall series clinically judged to have primary incompleteness (BH2, BH4 and UF2) were distributed one each in the first, second and third patient cohorts, making it unlikely that the distribution of this OCD influenced the relatively better outcomes in the latter two groups. Although not formally excluded, no patients with primary hoarding were enrolled. Future studies are needed to determine the effect of DBS on that phenotype.
In conclusion, these combined data provide further support to prior reports indicating encouraging therapeutic effects after VC/VS DBS, a procedure that was well tolerated over long-term use. Overall, clinically significant symptom reductions and functional improvements were seen in about two-thirds of highly treatment-resistant patients. Results were generally better for patients implanted more recently. The main factor accounting for this ‘learning curve’ effect appeared to be the use of a more posterior implantation target in the later vs the earlier patients studied, suggesting that the neural networks relevant to therapeutic improvement might be modulated more effectively in this region. These data show that DBS can be successfully implemented as an OCD therapy by dedicated interdisciplinary teams.
Acknowledgments
We acknowledge the following individuals for their contributions to this multidisciplinary effort (listed by research team). Butler/Brown (BH): Richard Marsland, William Wong, M Evans, Norman Moore, Ronald Gaertner, Robert Bumgartner, Robert Gross, for management of study patients; Rouba Youssef, Katherine Rowinski, Erin Einbinder and Kathleen Barreiro for data management and administrative support. Leuven/Antwerp (LV): Kris van Kuyck, Marleen Welkenhuysen, Laura Luyten and John Das for their parallel animal research and technical support; Paul De Sutter, Bjorn Meyerson, Sergej, Andréewitch and Christian Rück, for contributing their neurosurgical and psychiatric experience; Cleveland Clinic (CC): Deborah Vegh RN, Natalie Sykuta RN for management of study patients; University of Florida (UF): Russell M Bauer, Gary Geffken, Candy L Hill, Chuck Jacobson, Nikki Ricciuti, Eric A Storch for clinical, technical and administrative support; Medtronic Inc. (MT): Keith Mullett, Frans Gielen, Roy Testerman for technical advice.
Footnotes
Disclosures
Financial support and other disclosures (listed by center): Butler Hospital/Brown (BH): Medtronic Inc. (investigator-initiated research) and by a NARSAD Independent Investigator Award (BDG). Cleveland Clinic (CC): Medtronic Inc. (investigator-initiated research). Leuven/Antwerp (LV): Research Fund KU Leuven (projects OT-98-31, VIS-02-007 and OT-03-57), the Institute for the Promotion of Innovation by Science and Technology in Flanders (SBO50151); the Fonds voor Wetenschappelijk Onderzoek - Vlaanderen (G.0598.06 and G.0273.97.N) (BN); the Verkennende Internationale Samenwerking (VIS ZKB1159) and the Research Mandate of the University of Antwerp (LAG). Stimulation devices provided by Medtronic Inc. (QUEST program, L1170). University of Florida (UF): Funded by NIMH 5R21MH064161-03 (WKG, PI) and by General Clinical Research Center MO1-RR00082. UF has a contract with Medtronic for DBS fellowship training and programming training for physicians focusing on DBS in Movement Disorders. Individual Authors: BDG, SAR and DAM were unpaid consultants to Medtronic Inc.; DAM and LAG became paid MDT consultants after these data were collected. Medtronic personnel roles: PHS and MTR focused on DBS technology, targeting and data analysis; JEG performed statistical analysis of the data. BJN has received research and teaching grants from Medtronic, and is co-holder of a patent for the use of DBS for OCD.
References
- 1.Greenberg BD, Price LH, Rauch SL, Jenike MA, Malone D, Friehs G, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin North Am. 2003;14:199–212. doi: 10.1016/s1042-3680(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive- compulsive disorder. Am J Psychiatry. 2002;159:269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- 3.Nuttin BJ, Gabriels LA, Cosyns PR, Meyerson BA, Andreewitch S, Sunaert SG, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52:1263–1272. doi: 10.1227/01.neu.0000064565.49299.9a. discussion 1272–1264. [DOI] [PubMed] [Google Scholar]
- 4.Gabriels L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003;107:275–282. [PubMed] [Google Scholar]
- 5.Abelson JL, Curtis GC, Sagher O, Albucher RC, Harrigan M, Taylor SF, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Anderson D, Ahmed A. Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation. Case report. J Neurosurg. 2003;98:1104–1108. doi: 10.3171/jns.2003.98.5.1104. [DOI] [PubMed] [Google Scholar]
- 7.Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- 8.Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26:293–299. doi: 10.1016/j.jchemneu.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Aouizerate B, Martin-Guehl C, Cuny E, Guehl D, Amieva H, Benazzouz A, et al. Deep brain stimulation for OCD and major depression. Am J Psychiatry. 2005;162:2192. doi: 10.1176/appi.ajp.162.11.2192. [DOI] [PubMed] [Google Scholar]
- 10.Bingley T, Leksell L, Meyerson BA, Rylander G. Long term results of stereotactic capsulotomy in chronic obsessive-compulsive neurosis. In: Sweet WH, Obrador Alcalde S, Martín-Rodríguez JG, editors. Neurosurgical Treatment in Psychiatry, Pain, and Epilepsy; Proceedings of the Fourth World Congress of Psychiatric Surgery; 7–10 September, 1975; Madrid, Spain. Baltimore: University Park Press; 1977. pp. 287–299. [Google Scholar]
- 11.Noren G, Lindquist C, Rasmussen SA, Greenberg BD, Friehs G, Chougule PB, et al. Gamma knife capsulotomy for obsessive-compulsive disorder (abstract) J Neurosurg. 2002;96:414–415. [Google Scholar]
- 12.Lippitz BE, Mindus P, Meyerson BA, Kihlstrom L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery. 1999;44:452–458. doi: 10.1097/00006123-199903000-00005. discussion 458–460. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg BD, Norén G, Rauch SL, Malloy P, Marsland R, Jenike MA, et al. Lesion characteristics and clinical response after gamma capsulotomy for intractable OCD. Proceedings of the 40th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, PR. 2001. p. 116. [Google Scholar]
- 14.Rauch SL. Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders. Neurosurg Clin N Am. 2003;14:213–223. vii–viii. doi: 10.1016/s1042-3680(02)00114-6. [DOI] [PubMed] [Google Scholar]
- 15.Kopell BH, Greenberg B, Rezai AR. Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol. 2004;21:51–67. doi: 10.1097/00004691-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Velasco M, Velasco F, Jimenez F, Carrillo-Ruiz JD, Velasco AL, Salin-Pascual R. Electrocortical and behavioral responses elicited by acute electrical stimulation of inferior thalamic peduncle and nucleus reticularis thalami in a patient with major depression disorder. Clin Neurophysiol. 2006;117:320–327. doi: 10.1016/j.clinph.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. 1999;354:1526. doi: 10.1016/S0140-6736(99)02376-4. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31:2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- 19.Okun MS, Bowers D, Springer U, Shapira NA, Malone D, Rezai AR, et al. What’s in a ‘smile? Intra-operative observations of contralateral smiles induced by deep brain stimulation. Neurocase. 2004;10:271–279. doi: 10.1080/13554790490507632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapira NA, Okun MS, Wint D, Foote KD, Byars JA, Bowers D, et al. Panic and fear induced by deep brain stimulation. J Neurol Neurosurg Psychiatry. 2006;77:410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuttin B, Gybels J, Cosyns P, Gabriels L, Meyerson B, Andreewitch S, et al. Deep brain stimulation for psychiatric disorders. Neurosurg Clin N Am. 2003;14:xv–xvi. doi: 10.1016/s1042-3680(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics. New York: 2001. The Structured Clinical Interview for DSM-IV. [Google Scholar]
- 23.Machado AG, Malone DA, Kopell BH, Greenberg BD, Rezai AR. Deep Brain Stimulation (DBS) for the Treatment of Refractory Obsessive Compulsive Disorder (OCD) Assessment of Optimal Therapeutic Cathode Location. American Association of Neurological Surgery; San Francisco: 2006. [Google Scholar]
- 24.Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, et al. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatry. 2007;78:310–314. doi: 10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Kuyck K, Demeulemeester H, Feys H, De Weerdt W, Dewil M, Tousseyn T, et al. Effects of electrical stimulation or lesion in nucleus accumbens on the behaviour of rats in a T-maze after administration of 8-OH-DPAT or vehicle. Behav Brain Res. 2003;140:165–173. doi: 10.1016/s0166-4328(02)00295-4. [DOI] [PubMed] [Google Scholar]
- 26.Nuttin BJ, Gabriels L, van Kuyck K, Cosyns P. Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am. 2003;14:267–274. doi: 10.1016/s1042-3680(02)00117-1. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36:267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- 29.Leckman JF, Grice DE, Boardman J, Zhang H, Vitale A, Bondi C, et al. Symptoms of obsessive-compulsive disorder. Am J Psychiatry. 1997;154:911–917. doi: 10.1176/ajp.154.7.911. [DOI] [PubMed] [Google Scholar]
- 30.Nierenberg AA, Wright EC. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(Suppl 22):7–11. [PubMed] [Google Scholar]
- 31.Ballenger JC. Clinical guidelines for establishing remission in patients with depression and anxiety. J Clin Psychiatry. 1999;60(Suppl 22):29–34. [PubMed] [Google Scholar]
- 32.Keck PE, Jr, Lipinski JF, Jr, White K. An inverse relationship between mania and obsessive-compulsive disorder: a case report. J Clin Psychopharmacol. 1986;6:123–124. [PubMed] [Google Scholar]
- 33.Kubu C, Malone DA, Rezai AR, Machado A, Rasmussen SA, Chelune G, et al. Improvements in memory and visuospatial skills after deep brain stimulation of the anterior limb of the internal capsule results in patients with treatment-resistant obsessive compulsive disorder. Personal communication. May, 2008.
- 34.Eisen JL, Mancebo MA, Pinto A, Coles ME, Pagano ME, Stout R, et al. Impact of obsessive-compulsive disorder on quality of life. Compr Psychiatry. 2006;47:270–275. doi: 10.1016/j.comppsych.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simuni T, Jaggi JL, Mulholland H, Hurtig HI, Colcher A, Siderowf AD, et al. Bilateral stimulation of the subthalamic nucleus in patients with Parkinson disease: a study of efficacy and safety. J Neurosurg. 2002;96:666–672. doi: 10.3171/jns.2002.96.4.0666. [DOI] [PubMed] [Google Scholar]
- 36.Starr PA, Christine CW, Theodosopoulos PV, Lindsey N, Byrd D, Mosley A, et al. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002;97:370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 37.Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, et al. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol. 2005;62:1250–1255. doi: 10.1001/archneur.62.8.noc40425. [DOI] [PubMed] [Google Scholar]
- 38.Welter ML, Houeto JL, Tezenas duMontcel S, Mesnage V, Bonnet AM, Pillon B, et al. Clinical predictive factors of subthalamic stimulation in Parkinson’s disease. Brain. 2002;125(Part 3):575–583. doi: 10.1093/brain/awf050. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, et al. Bilateral deep brain stimulation in Parkinson’s disease: a multicentre study with 4 years follow-up. Brain. 2005;128(Part 10):2240–2249. doi: 10.1093/brain/awh571. [DOI] [PubMed] [Google Scholar]
- 40.DBS for Parkinson’s Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson’s disease. N Engl J Med. 2001;345:956–963. doi: 10.1056/NEJMoa000827. [DOI] [PubMed] [Google Scholar]
- 41.Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001;56:548–551. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- 42.Umemura A, Jaggi JL, Hurtig HI, Siderowf AD, Colcher A, Stern MB, et al. Deep brain stimulation for movement disorders: morbidity and mortality in 109 patients. J Neurosurg. 2003;98:779–784. doi: 10.3171/jns.2003.98.4.0779. [DOI] [PubMed] [Google Scholar]
- 43.Su PC, Tseng HM, Liu HM. Unsuccessful deep brain stimulation in the subthalamic nucleus for advanced Parkinson’s disease. Mov Disord. 2003;18:350–351. doi: 10.1002/mds.10318. [DOI] [PubMed] [Google Scholar]
- 44.Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, et al. A functional neuroimaging investigation of deep brain stimulation in patients with obsessive-compulsive disorder. J Neurosurg. 2006;104:558–565. doi: 10.3171/jns.2006.104.4.558. [DOI] [PubMed] [Google Scholar]
- 45.Van Laere K, Nuttin B, Gabriels L, Dupont P, Rasmussen SA, Greenberg BD, et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47:740–747. [PubMed] [Google Scholar]
- 46.Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Long-term outcome of deep brain stimulation for treatment resistant depression. Personal communication. May, 2008.
- 47.Burkhard PR, Vingerhoets FJ, Berney A, Bogousslavsky J, Villemure JG, Ghika J. Suicide after successful deep brain stimulation for movement disorders. Neurology. 2004;63:2170–2172. doi: 10.1212/01.wnl.0000145603.48221.b5. [DOI] [PubMed] [Google Scholar]
- 48.Pinto A, Eisen JL, Mancebo MC, Greenberg BD, Stout RL, Rasmussen SA. Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive-compulsive disorder symptoms. Psychiatry Res. 2007;151:255–258. doi: 10.1016/j.psychres.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML. Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:564–576. doi: 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- 50.Saxena S, Brody AL, Maidment KM, Smith EC, Zohrabi N, Katz E, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry. 2004;161:1038–1048. doi: 10.1176/appi.ajp.161.6.1038. [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen SA, Eisen JL. The epidemiology and clinical features of obsessive compulsive disorder. Psychiatr Clin North Am. 1992;15:743–758. [PubMed] [Google Scholar]