Abstract
Poststroke hyperglycemia is associated with a poor outcome yet clinical management is inadequately informed. We sought to determine whether clinically relevant levels of hyperglycemia exert detrimental effects on the early evolution of focal ischemic brain damage, as determined by magnetic resonance imaging, in normal rats and in those modeling the ‘metabolic syndrome'. Wistar Kyoto (WKY) or fructose-fed spontaneously hypertensive stroke-prone (ffSHRSP) rats were randomly allocated to groups for glucose or vehicle administration before permanent middle cerebral artery occlusion. Diffusion-weighted imaging was carried out over the first 4 hours after middle cerebral artery occlusion and lesion volume calculated from apparent diffusion coefficient maps. Infarct volume and immunostaining for markers of oxidative stress were measured in the fixed brain sections at 24 hours. Hyperglycemia rapidly exacerbated early ischemic damage in both WKY and ffSHRSP rats but increased infarct volume only in WKY rats. There was only limited evidence of oxidative stress in hyperglycemic animals. Acute hyperglycemia, at clinically relevant levels, exacerbates early ischemic damage in both normal and metabolic syndrome rats. Management of hyperglycemia may have greatest benefit when performed in the acute phase after stroke in the absence or presence of comorbidities.
Keywords: animal models, diffusion-weighted MRI, focal ischemia, glucose, hyperglycemia
Introduction
Poststroke hyperglycemia predicts poor outcome independent of age, stroke type, or severity and occurs in over 60% of patients without a diagnosis of diabetes and in more than 90% of diabetic patients.1, 2 Around 56% of hyperglycemic acute stroke patients are subsequently diagnosed with insulin resistance, manifesting as impaired glucose tolerance, impaired fasting glucose, or the ‘metabolic syndrome.' This phenotype is a significant risk factor for cardiovascular disease and comprises combinations of insulin resistance, hypertension, hypertriglyceridemia, and obesity.3, 4 Current guidelines for stroke recommend routine blood glucose monitoring, and intervention with insulin for hyperglycemia5, 6 although clinical evidence of benefit from treatment is lacking. Moreover, recent findings raise concerns about the safety of insulin use in predominantly non-diabetic acute populations with stroke7, 8 or other medical conditions.9, 10 In light of the potential risk of hypoglycemia with insulin regimes in stroke, it is important to question whether the harmful effects of acute hyperglycemia pertain to all stroke patients, or whether they differ in those with and without pre-existing co-morbidities. Such evidence would aid the design of future glucose-lowering clinical trials.
In animal models of focal cerebral ischemia, hyperglycemia is widely reported to increase infarct size.11, 12, 13, 14, 15, 16, 17, 18 However, a recent systematic review highlighted the uncertain relevance of many animal studies to the typical clinical picture of poststroke hyperglycemia.19 Streptozotocin, which destroys the insulin producing beta cells of the pancreas, has been used to induce hyperglycemia in rats11, 15, 17, 18 although this more closely models type 1 diabetes rather than what is usually observed in the clinic.7, 20, 21, 22 Glucose infusion models have also been used to emulate poststroke hyperglycemia; however, extremely high levels of blood glucose have been used in many studies, and such levels are rarely recorded clinically.12, 13, 14 In acute stroke, 24% of the patients had blood glucose >8.6 mmol/L within the first 6 hours after onset, with a further 13% developing blood glucose >8.6 mmol/L in the first 48 hours.22 Very high blood glucose is uncommon, however, in a combined data set of acute stroke trials from the VISTA archive, blood glucose on hospital admission, a median of 5 hours after stroke onset was 10 to 15 mmol/L in 9.6% of patients and was >15 mmol/L in only 5.0%.1 It is currently not clear whether clinically relevant elevations in blood glucose exacerbate ischemic brain damage in animal models other than those of type 1 diabetes, and if a detrimental effect occurs in animals with and without co-morbidities.
Given the labor-intensive nature of glucose-lowering strategies and the potential for patient harm from hypoglycemia, future clinical trials need to optimally identify the target population. A key issue still to be resolved is the time window within which hyperglycemia exerts its detrimental effects. Final infarct size has been the focus in the majority of animal studies, which have examined the effects of hyperglycemia. However, the progression of tissue at risk to infarction has been reported to be exacerbated by elevated blood glucose levels in stroke patients studied within the first hours after onset.23, 24, 25 Moreover, hyperglycemia exacerbates risk of intracerebral hemorrhage in patients treated with intravenous thrombolysis within 4.5 hours. Other studies indicate that elevated glucose over extended periods of up to 48 hours is also a strong predictor of adverse outcome1, 22 but guidelines offer no proposed time when an intervention should be considered. Single-slice magnetic resonance imaging studies indicate that the detrimental effects of high blood glucose occur early during lesion evolution after transient or permanent focal ischemia in the rat streptozotocin model of type 1 diabetes.15, 17 Subsequent developments in multislice diffusion-weighted imaging (DWI) mean that it is now possible to gain a comprehensive assessment of lesion expansion in the acute phase. In the present study, we have used this approach to investigate the effects of clinically relevant levels of hyperglycemia in rats with and without comorbidities associated with stroke, namely features of the ‘metabolic syndrome'. In humans, this comprises the constellation of central obesity, dyslipidemia, hypertension, and hyperglycemia that increase liability to type 2 diabetes and atherosclerotic vascular disease, with insulin resistance the unifying physiologic abnormality.26 More than 40% of stroke patients meet criteria for metabolic syndrome,27 and the presence of the syndrome signifies increased risk for incident stroke.28 Features of the metabolic syndrome can be modeled using spontaneously hypertensive stroke-prone (SHRSP) rats fed a fructose-rich diet for 14 days (ffSHRSP). In addition to hypertension, these rats develop hyperlipidemia and glucose intolerance.29 In the current study, we examined the effects of hyperglycemia in both ffSHRSP rats and the normotensive reference strain, Wistar Kyoto, which do not exhibit the comorbidities listed above. We hypothesized that hyperglycemia exerts its detrimental effects on lesion growth after induction of ischemia and that this would occur in both rats with and without features of metabolic syndrome. Final infarct size was assessed by quantitative histology and tissue markers of oxidative stress were examined in fixed brain based on the proposed involvement of excess superoxide production in the chronic effects of hyperglycemia in diabetic complications.30
Materials and Methods
Animals
Experiments were performed under license granted by the Home Office, UK, according to the UK Animals (Scientific Procedures) Act, 1986. Male adult rats (n=10 per group; 10 weeks old) were obtained from breeding colonies within the Institute of Cardiovascular and Medical Sciences, University of Glasgow. Wistar Kyoto rats were randomly allocated to normoglycemic (WKY) or hyperglycemic (WKY+G) groups and maintained on normal rat chow for 2 weeks. Spontaneously hypertensive stroke-prone (SHRSP) rats were maintained on chow containing 60% fructose (Hope Farms, Woerden, The Netherlands) for 2 weeks29 and randomly allocated to normoglycemic (ffSHRSP) or hyperglycemic (ffSHRSP+G) groups. Separate cohorts of WKY (n=5) and ffSHRSP (n=5) rats to those used for ischemia studies were examined for features of metabolic syndrome according to previously described methods.29 Adiposity was calculated as the sum of epididymal and retroperitoneal fat pad weights expressed as a percentage of body weight. For measurements of cholesterol, triglycerides, and insulin in blood, a terminal sample was collected after overnight fasting. These technical requirements required that separate groups of animals from those being subjected to focal cerebral ischemia be used. In rats used for ischemic studies, systolic blood pressure was measured by tail cuff plethysmography in the conscious state in all rats 3 to 5 days before stroke surgery to confirm the hypertensive status of ffSHRSP compared with WKY. Rats were fasted overnight before surgery and water was available ad libitum.
Middle Cerebral Artery Occlusion
Anesthesia was induced (5% isoflurane) and maintained by artificial ventilation with 2% to 3% isoflurane in nitrous oxide–oxygen (70:30). One femoral artery was cannulated for continuous monitoring of mean arterial blood pressure and heart rate (Biopac MP150, Biopac Systems, Goleta, CA, USA). PaO2, PaCO2, and pH were measured (blood gas analyzer; Rapidlab 248, Bayer, Germany) from arterial blood samples obtained once during surgery then hourly after middle cerebral artery occlusion (MCAO) for 4 hours. Blood gases were maintained within the physiologic range by adjusting ventilator settings. Rectal temperature was continually monitored and maintained at 37±0.5°C during surgery. Ten minutes before MCAO rats either received a single intraperitoneal injection (10 mL/kg) of 15% glucose in distilled water (WKY+G and ffSHRSP+G) or vehicle (WKY and ffSHRSP). Permanent focal cerebral ischemia was induced by distal occlusion of the middle cerebral artery (MCA) using diathermy with modification.31 After exposure via a craniectomy, the point at which the MCA crossed the inferior cerebral vein was identified and the MCA was electrocoagulated using diathermy forceps 2 mm distally from this point. After this, the MCA was cut with microscissors to confirm a complete occlusion. Arterial blood glucose was measured immediately before glucose/vehicle administration and at each subsequent hour for 4 hours using a commercially available glucometer (Accu-Chek, Roche, Germany).
Magnetic Resonance Imaging
Magnetic resonance imaging data were acquired on a Bruker Biospec 7T/30 cm system equipped with an inserted gradient coil (121 mm ID, 400 mT/m) and a 72 mm birdcage resonator. Immediately after MCAO, anesthetized animals were placed prone in a rat cradle and the head restrained. A 4-channel rat brain phased-array surface coil was placed above the head. Electrocardiography (ECG) leads and a pressure sensor were attached for cardiac and respiratory monitoring, respectively. A water jacket was used to maintain core temperature at 37±0.5°C.
Diffusion-weighted imaging was performed each hour post MCAO for 4 hours to map the evolution of the ischemic lesion (Spin Echo-Echo planar Imaging (SE-EPI) TE: 43 milliseconds; TR: 4,000.3 milliseconds; in plane resolution of 260 μm; 3 directions: x, y, z; B values: 0, 1,000 second/mm2, eight slices of 1.5 mm thickness). Quantitative apparent diffusion coefficient (ADC) maps (mm2/second) were calculated from the DWI images using Paravision 5 software (Bruker, Germany) and subsequently analyzed using ImageJ software (http://rsb.info.nih.gov/ij/). Diffusion thresholds of abnormality may vary between different strains of rodent because of potential differences in the evolution of ischemic damage and the quality of collateral blood supply during the acute phase poststroke. The diffusion threshold will also change depending on the time point after stroke onset. Therefore, we have previously established strain-specific thresholds for both diffusion- and perfusion-weighted imaging in both WKY and SHRSP rats at 4 hours after MCAO.32 These diffusion thresholds (WKY: 0.61 mm2/second, SHRSP 0.59 mm2/second) were applied in the present study.
Infarct Measurement
Animals were recovered from anesthesia after magnetic resonance scanning and re-anesthetized for perfusion fixation with 4% paraformaldehyde 24 hours after MCAO. Infarct volumes were determined by quantitative histology using eight coronal sections stained with hematoxylin and eosin sampled from throughout MCA territory as described previously.33
4-Hydroxynonenal and Nitrotyrosine Immunohistochemistry
Sections adjacent to those used for infarct measurements were processed for immunohistochemistry with an antibody raised against the lipid peroxidation by-product 4-hydroxynonenal (4-HNE, Clone 198960, R&D Systems, Abingdon, UK) as described previously.34 Light microscopy was used to transcribe the location of increased 4-HNE immunostaining (compared with contralateral tissue) onto a separate set of eight coronal stereotaxic line diagrams, to those used for infarct measurements, and ImageJ was used to determine the volume of 4-HNE staining for each animal. This analysis was performed masked to the infarct volume data. Spare sections available for four coronal planes were stained with an antibody raised against nitrotyrosine (AB5411, Millipore, Watford, UK), a marker of nitric oxide-induced nitrative stress, and areas of increased immunostaining calculated at each of the four planes examined.
Sample Size Calculation, Randomization, Blinding, and Statistical Analysis
Standard deviations of ADC lesion measurements obtained from a previous study using single-slice imaging of SHRSP rats were used to inform the sample size calculation.35 Animals were randomly allocated upon arrival from the breeding colony to normoglycemic or hyperglycemic groups by the toss of a coin. The investigator was masked to animal identity during the analysis of ADC lesion, infarct size, and immunohistochemistry. Blood glucose levels, ADC lesion volumes, and nitrotyrosine immunopositive areas were assessed using a 2-way analysis of variance repeated measures test with Bonferroni post hoc test. Infarct and 4-HNE immunopositive volumes and physiologic variables were assessed using a 1-way analysis of variance with Bonferroni post hoc test. Data are presented as mean±standard deviation (s.d.) or a scatter plot with means indicated.
Results
Physiologic Variables
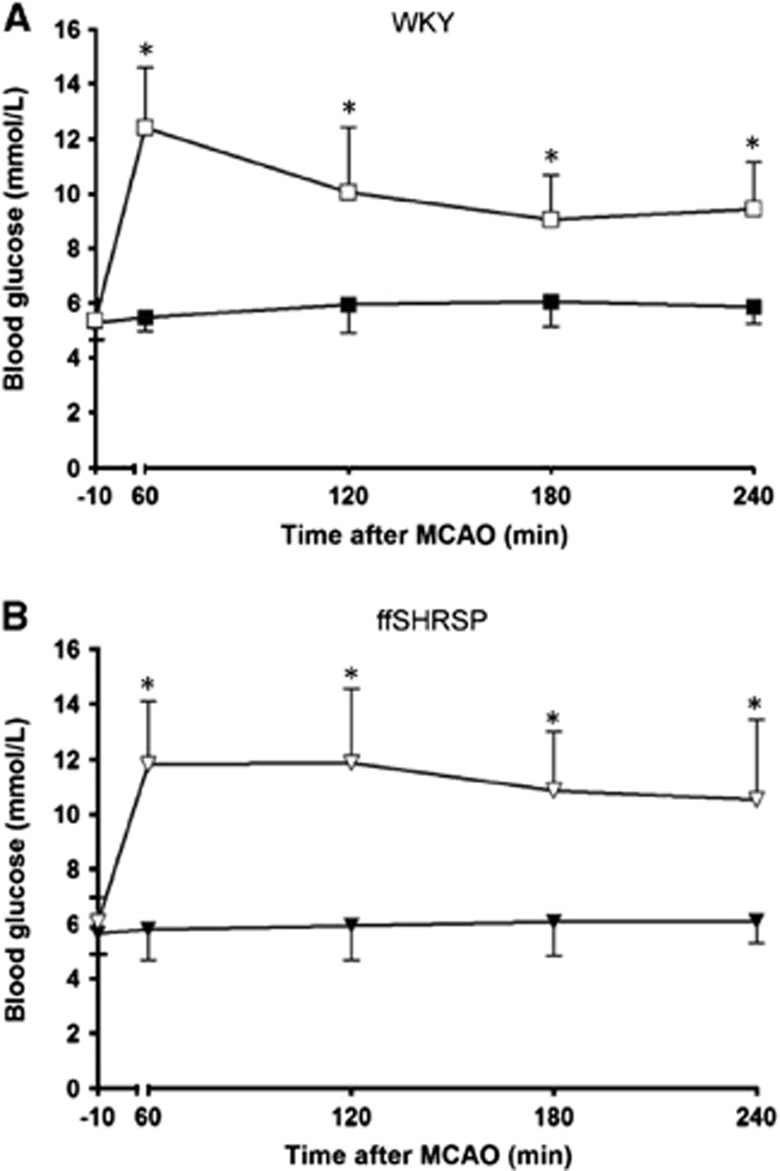
In the separate cohorts of rats not used in ischemia studies, ffSHRSP rats displayed impaired glucose tolerance, hypertriglyceridemia, hyperinsulinemia, and increased adiposity compared with WKY (Table 1). For those rats used in ischemia studies, ffSHRSP rats exhibited hypertension and weighed significantly less compared with WKY, consistent with previously reported differences between these two strains (Table 2). During anesthesia, ffSHRSP rats had significantly higher mean arterial blood pressure than WKY rats. There was no difference in temperature, blood gas, or pH measurements between any of the four groups (Table 2). After intraperitoneal glucose administration, peak blood glucose levels in both WKY and ffSHRSP rats were similar (Figure 1). At all time points examined during the MR scanning period, blood glucose levels in rats injected with glucose were significantly greater than in vehicle-treated controls (Figure 1). There was no mortality in any of the four groups after MCAO surgery.
Table 1. Features of metabolic syndrome in ffSHRSP rats.
| WKY | ffSHRSP | |
|---|---|---|
| Adiposity (% fat pad weight) | 1.40±0.29 | 2.40±0.09** |
| Plasma triglycerides (mmol/L) | 0.28±0.04 | 0.65±0.11*** |
| Total cholesterol (mmol/L) | 2.53±0.09 | 1.74±0.11*** |
| HDL cholesterol (mmol/L) | 2.212±0.07 | 1.58±0.12*** |
| Insulin (μg/l) | 0.30±0.07 | 0.46±0.14* |
Abbreviations: ffSHRSP, fructose-fed spontaneously hypertensive stroke-prone; WKY, Wistar-Kyoto. Rats (n=5 per group) were from the same breeding colonies as those used for ischemia studies. Data are expressed as mean±s.d. Groups compared by two-tailed Student t-test: *P>0.05; **P>0.001; ***P>0.0001 compared with WKY.
Table 2. Physiologic variables.
|
Before anesthesia |
During anesthesia |
||||||
|---|---|---|---|---|---|---|---|
| Group | Weight (g) | Systolic BP (mmHg) | PaO2(mm Hg) | PaCO2 (mm Hg) | pH | MABP (mm Hg) | Temperature (°C) |
| WKY | 271±16 | 123.6±6.9 | 139.1±17.4 | 38.2±4.0 | 7.39±0.05 | 93.4±12.1 | 37.1±0.5 |
| WKY+G | 274±15 | 122.7±6.5 | 140.8±17.3 | 39.9±5.3 | 7.40±0.06 | 96.1±8.9 | 37.3±0.5 |
| ffSHRSP | 219±13** | 177.9±5.9** | 136.5±14.2 | 36.5±2.4 | 7.40±0.07 | 116.7±13.5** | 37.2±0.4 |
| ffSHRSP+G | 225±13## | 183.3±8.4## | 134.2±13.9 | 37.0±2.1 | 7.41±0.06 | 114.6±10.2## | 37.3±0.6 |
Abbreviations: BP, blood pressure; ffSHRSP, fructose-fed spontaneously hypertensive stroke-prone; MABP, mean arterial blood pressure; WKY, Wistar-Kyoto. Body weight measured on the day of MCAO; systolic BP measured 3 to 5 days before MCAO; PaO2, PaCO2, pH measured at time of MCAO; MABP, temperature (rectal) expressed as mean for anesthesia period. **P<0.001 versus WKY; ##P<0.001 versus WKY+glucose. Data presented as mean±s.d.
Figure 1.
Blood glucose levels before and after middle cerebral artery occlusion (MCAO) in Wistar Kyoto (WKY) (A) and fructose-fed spontaneously hypertensive stroke-prone (ffSHRSP) (B) rats. Glucose or vehicle was administered 10 minutes before MCAO (−10). Peak blood glucose levels in WKY and ffSHRSP rats after glucose administration were similar. In rats administered glucose, blood glucose levels remained elevated above the vehicle-treated group for at least 4 hours after MCAO. *P<0.001 glucose versus vehicle. Data presented as mean±s.d. Filled symbols, vehicle; open symbols, glucose.
Apparent Diffusion Coefficient Lesion Volume
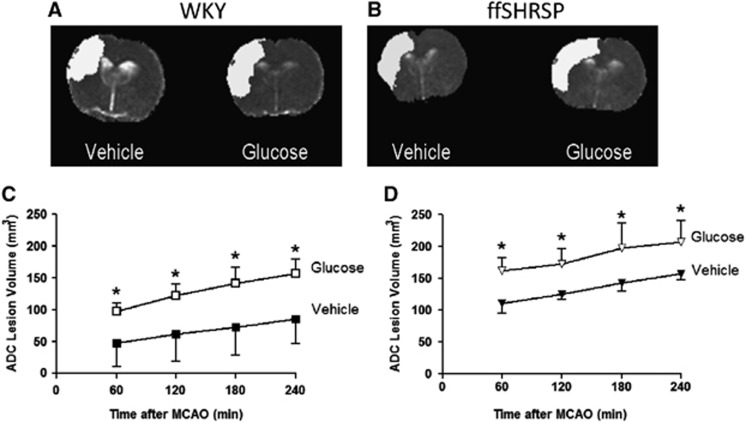
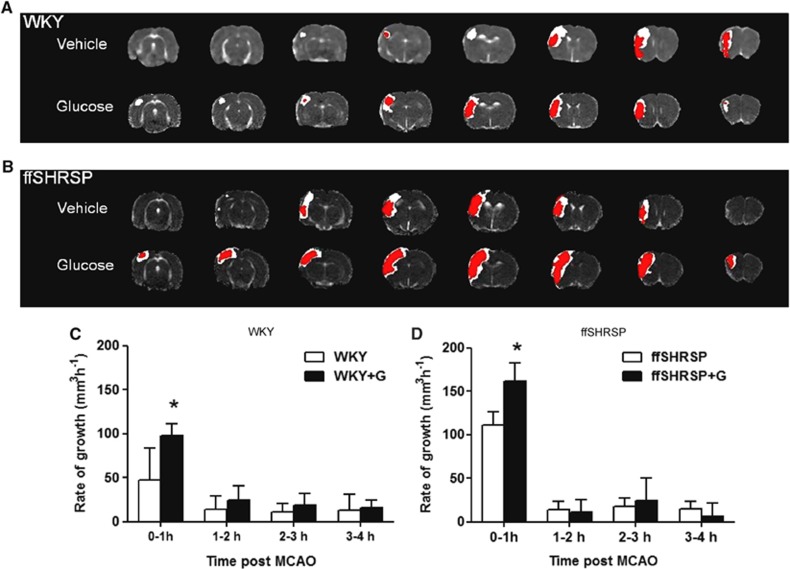
At the earliest time point examined, 1 hour after MCAO, the mean ADC lesion was significantly larger in hyperglycemic than in normoglycemic WKY rats and the effect of hyperglycemia was maintained over the subsequent 3 hours (Figure 2). The mean ADC lesion in hyperglycemic ffSHRSP rats was larger than in normoglycemic ffSHRSP at 1 hour after MCAO and the effect of hyperglycemia on ADC lesion volume was maintained over subsequent time points in this strain also (Figure 2). Thus, hyperglycemia exacerbated early ischemic brain damage in rats with or without features of metabolic syndrome. The comparative spatial profiles of lesions at 1 and 4 hours are illustrated for median animals in each of the four groups in Figure 3. Lesions are anatomically more widespread in hyperglycemic animals in both WKY and ffSHRSP groups and the apparent difference between lesions at 1 and 4 hours is greater in hyperglycemic animals in the majority of slices. The hourly rate of lesion growth was calculated by subtracting the lesion volume at a given time point from the subsequent measurement (Figure 3). There was a significant effect of hyperglycemia on lesion growth between 0 to 1 hour in both WKY and ffSHRSP groups. After this, hourly lesion growth was similar in normo- and hyperglycemic groups in both WKY and ffSHRSP rats. Thus, the detrimental impact of hyperglycemia was rapid, occurring predominantly in the first hour after MCAO in the presence or absence of features of metabolic syndrome.
Figure 2.
Apparent diffusion coefficient (ADC) lesion evolution. Representative ADC maps from a single slice illustrating ischemic damage (white) at 1 hour in Wistar-Kyoto (WKY) (A) and fructose-fed spontaneously hypertensive stroke-prone (ffSHRSP) (B) rats. Volumetric analysis of ADC lesions over eight coronal slices 1 to 4 hours after middle cerebral artery occlusion (MCAO) in WKY rats (C) and ffSHRSP rats (D) injected with either vehicle or glucose solution prior to MCAO. Hyperglycemia exacerbated damage at all time points examined in both WKY and ffSHRSP rats. *P<0.001 compared with normoglycemic, vehicle-treated controls. Data presented as mean±s.d.
Figure 3.
Apparent diffusion coefficient lesions at 1 and 4 hours after middle cerebral artery occlusion (MCAO). (A,B): the eight coronal slices collected from the median animal in each group with the areas of ischemic injury at 1 hour (red area) and 4 hours (white area) superimposed. C,D: the rate of ADC lesion growth over each hour was calculated by subtraction. Hyperglycemic groups indicated by +G. Data are mean±s.d. *P<0.001 compared with normoglycemic controls at that time period.
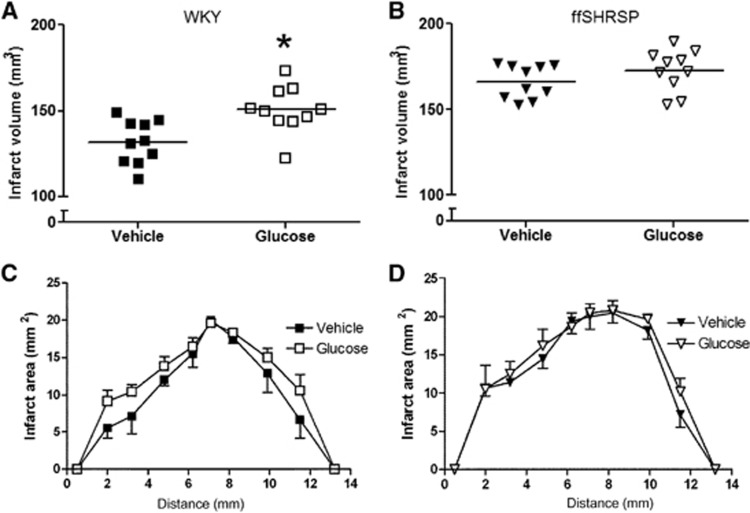
Infarct Volume
Infarct volume was significantly larger in hyperglycemic than in normoglycemic WKY rats (Figure 4). The most pronounced differences were observed in sections at the rostral and caudal poles of the infarct (Figure 4). Infarct volume in hyperglycemic ffSHRSP rats was not increased compared with normoglycemic controls (Figure 4), the rostro–caudal distribution of the lesion being similar in both groups (Figure 4). Thus, hyperglycemia in the early period after MCAO exacerbated infarct size at 24 hours only in rats lacking features of metabolic syndrome.
Figure 4.
Infarct volume 24 hours after middle cerebral artery occlusion (MCAO) in Wistar-Kyoto (WKY) (A) and fructose-fed spontaneously hypertensive stroke-prone (ffSHRSP) rats (B). Data points indicate individual rats and the horizontal bar represents the mean. Hyperglycemia increased the volume of infarction in WKY (*P<0.001 glucose compared with vehicle administration) but not ffSHRSP rats. The rostro–caudal profile of the infarct is shown for WKY (C) and ffSHRSP (D). Infarct area was measured at eight coronal planes; the first and last points on the x-axis are extrapolated for calculation of volume. Differences in the amount of ischemic damage between glucose and vehicle-treated WKY rats are most evident at the rostral and caudal poles of the lesion (C). Data presented as mean±s.d.
Immunostaining for Markers of Oxidative stress
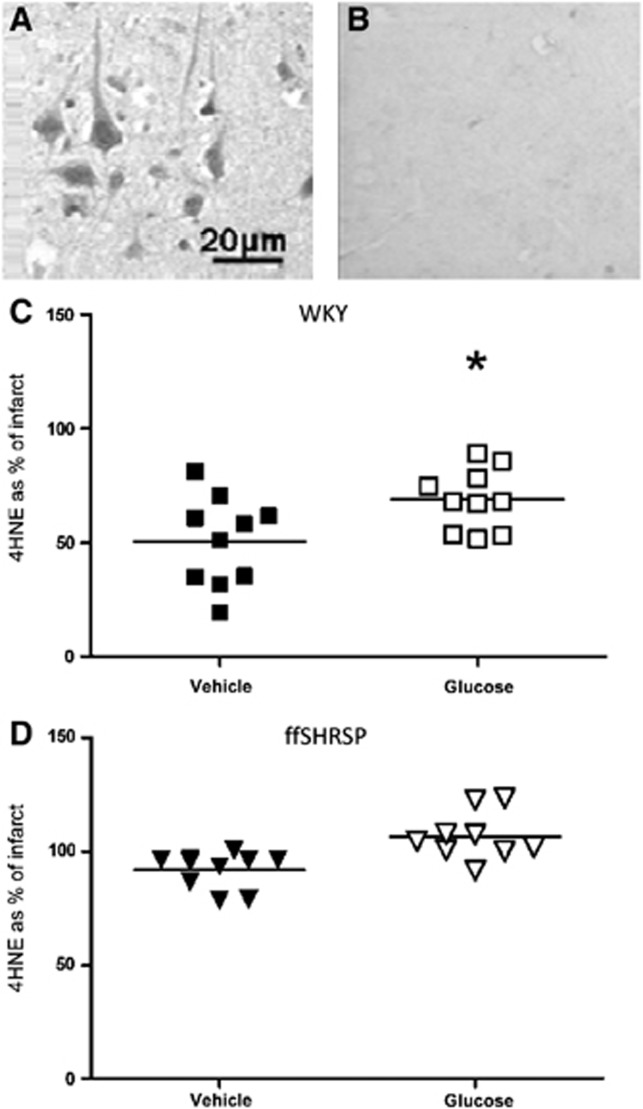
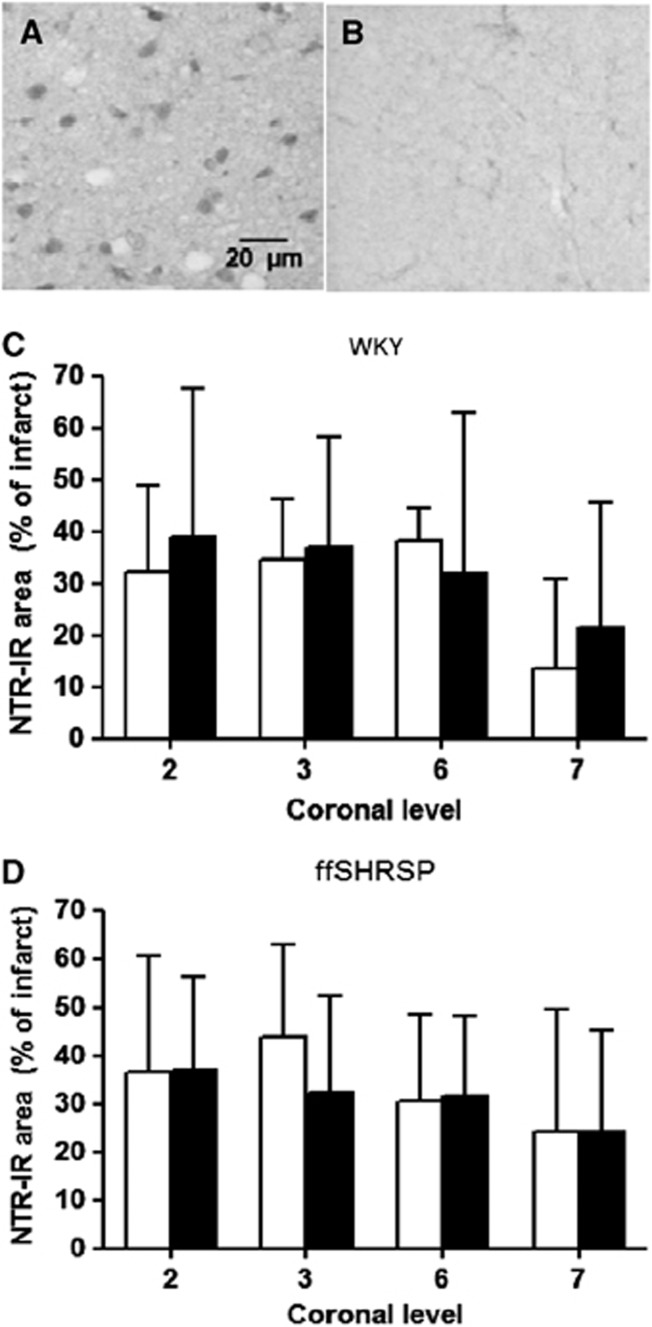
Compared with the contralateral hemisphere, 4-HNE immunoreactivity was higher in ischemically damaged tissue being present in neurons and axons in both gray and white matter (Figure 5). Volumetric analysis of the tissue containing increased 4-HNE immunopositivity mirrored the group differences observed for infarct volume and therefore for each animal, the data were expressed as a % of infarct volume (Figure 5). In hyperglycemic WKY rats, 4-HNE staining occupied 18% more of the infarct than in the normoglycemic animals, a modest but statistically significant increase (Figure 5). In ffSHRSP rats, ischemic tissue had more intense 4-HNE staining than the contralateral hemisphere. Although there was a trend towards the same direction of change as in the WKY rats, there was no statistical difference between normo- and hyperglycemic groups in the proportion of infarcted tissue occupied by this lipid peroxidation marker in ffSHRSP rats. This part of the study may have been underpowered. Nitrotyrosine immunoreactivity was more intense in ischemically damaged tissue compared with contralateral tissue, but this was less striking than the hemispheric difference observed with 4-HNE staining. The proportion of infarcted tissue occupied by nitrotyrosine staining was similar in both normo- and hyperglycemic groups in both WKY and ffSHRSP rats (Figure 6). Taken together with the 4-HNE data, the data indicate only limited evidence for increased oxidative/nitrative stress associated with acute hyperglycemia.
Figure 5.
4-HNE immunoreactivity at 24 hours after middle cerebral artery occlusion. The intensity of 4-HNE immunopositivity was greater in the ischemic (A) compared with the non-ischemic (B) hemisphere in all rats examined. Images show 4-HNE immunoreactivity within the infarct (A) and in the equivalent area of the contralateral hemisphere (B). The volume of tissue containing 4-HNE immunoreactivity was determined using the method used to calculate infarct volume over eight matching coronal planes. Group differences in the volume of 4-HNE immunostaining simply mirrored those observed for infarct volume and therefore data were expressed as % of infarct volume in Wistar-Kyoto (WKY) (C) and fructose-fed spontaneously hypertensive stroke-prone (ffSHRSP) (D) rats administered vehicle or glucose. *P<0.001 glucose compared with vehicle. Data points represent individual rats and the horizontal bar represents the mean.
Figure 6.
Nitrotyrosine immunoreactivity at 24 hours after middle cerebral artery occlusion. The intensity of nitrotyrosine immunoreactivity was greater in the ischemic (A) compared with the non-ischemic (B) hemisphere in all rats examined. Nitrotyrosine immunoreactivity was present in cell bodies in gray and white matter within the infarct. Because of limited tissue availability, compared with that available for histology and 4-HNE immunostaining, nitrotyrosine immunohistochemistry was performed at four of the eight coronal levels used to measure infarct and 4-HNE immunostaining and is therefore presented as area measurements at each of the four planes. These correspond to the two most rostral and caudal levels indicated in Figures 4C,B. Areas of nitrotyrosine immunostaining were expressed as a % of infarct area at the corresponding coronal plane in Wistar-Kyoto (WKY) (C) and fructose-fed spontaneously hypertensive stroke-prone (ffSHRSP) (D) rats administered vehicle (white bars) or glucose (black bars). Data presented as mean±s.d.
Discussion
The data reported here demonstrate that hyperglycemia at the time of MCA occlusion significantly increases lesion growth in the immediate postocclusion phase. The levels of blood glucose observed were consistent with those previously reported in stroke patients.1, 22 The clinical significance of our observations is that the earlier glucose-lowering therapies are initiated in hyperglycemic stroke patients the greater the benefit is likely to be. Conversely, such therapies may be futile in patients who do not have an evolving lesion and this may have contributed to the lack of clinical benefit reported in trials of insulin treatment.7, 8 Previous studies of hyperglycemia in animal models of stroke, while useful in establishing the principle that elevated blood glucose exacerbates brain damage, did not use models that reproduce features of typical human poststroke hyperglycemia; the majority used models of type 1 diabetes and/or very high concentrations of blood glucose (16 to 30 mmol/L).19 The current study examined levels of hyperglycemia, which are typically encountered clinically in acute stroke patients and also assessed its impact in the context of stroke comorbidities. The detrimental effect of hyperglycemia was pronounced as early as 1 hour after MCAO and persisted over the subsequent 3 hours, which is consistent with data from a previous diffusion-weighted magnetic resonance imaging study of permanent MCAO in rats, although in the presence of substantially higher levels of blood glucose (∼25mmol/L) than those modeled in the present study (∼12 mmol/L).17 Our data further demonstrate that clinically relevant levels of hyperglycemia accelerate early lesion growth in both the absence and presence of features associated with metabolic syndrome. Apparent diffusion coefficient lesions in ffSHRSP rats were larger than those in WKY groups regardless of glycemic status and this is likely attributable to the known differences between SHRSP and WKY strains.35 We have previously reported larger ADC lesions in SHRSP rats (not fructose fed) compared with WKY over the first 4 hours after intraluminal filament occlusion of the middle cerebral artery.32 A potential explanation for a lesser effect of hyperglycemia in ffSHRSP rats is that hypertension and poor collateral flow36 have prevailing roles in exacerbating the evolution of damage. In ffSHRSP, there may have been less penumbral tissue than in WKY rats and therefore the amount of target tissue where hyperglycemia could exert its detrimental effect was smaller. However, the primary aim of the study was to determine if hyperglycemia had a detrimental effect on lesion evolution in the absence and presence of comorbid features associated with the metabolic syndrome and the data demonstrate that this was the case.
Infarct volume 24 hours post MCAO was increased by hyperglycemia only in animals lacking features of metabolic syndrome. This is consistent with a systematic review of patient data, which reported that for stress hyperglycemia, relative risks of mortality and poor functional outcome were increased after stroke in non-diabetic patients, but not in those with a diagnosis of diabetes.37 This clinical observation may therefore reflect the pathophysiological effects of hyperglycemia we have demonstrated in ffSHRSP rats. An issue to be considered when assessing factors that have detrimental effects on infarct size in rodent models of stroke is the potential for a ‘ceiling effect.' If damage is near maximal in the baseline state, it will be difficult to detect an increase induced by additional pathophysiology. To allow for an adverse effect of hyperglycemia on infarct size to be detected, we purposefully chose to use a distal MCAO model, which produces smaller lesions than those created by intraluminal filament placement. Even so, infarcts in normoglycemic ffSHRSP rats were anatomically extensive; hence the ceiling effect is a possible reason why hyperglycemia had minimal impact on infarct volume. This should be taken into account when interpreting the finding that hyperglycemia did not increase infarct size in ffSHRSP rats. It will be important in future preclinical studies to determine if glucose-lowering therapies administered in the acute phase are beneficial both in terms of infarct evolution and functional outcome in rats with and without co-morbidities such as hypertension and insulin resistance. Moreover, it will be important to examine the therapeutic window in such studies. The current study measured blood glucose only for the first 4 hours after MCAO but poststroke hyperglycemia in patients has been reported up to 48 hours22 after onset so the persistently elevated levels over the subsequent period may contribute to the resolution of the final infarct size.
The primary endpoints in this study were ADC lesion growth and infarct size; however, the method used for the latter also presented a limited opportunity to examine sections by immunohistochemistry for markers of oxidative and nitrative stress. The rationale for this was based on evidence from studies of chronic, rather than acute, hyperglycemia implicating excessive release of superoxide from mitochondria, in complications, including neuropathy, associated with diabetes.30, 38 In addition, oxidative stress due to activation of nicotinamide adenine dinucleotide phosphate-oxidase has been implicated in the exacerbation of ischemia/reperfusion injury.39 Our assumption was that the presence of markers indicating free radical-mediated damage in the tissue at 24 hours after MCAO would reflect events that had previously taken place within the evolving lesion while tissue was at risk. However, since the tissue examined was already infarcted, we cannot exclude the possibility that any such processes could have occurred secondary to irreversible damage. In hyperglycemic WKY rats, 4-HNE staining occupied 18% more of the infarcted tissue volume than in normoglycemic animals, a modest but statistically significant difference. However, this was not accompanied by an increase in the amount of damaged tissue containing nitrotyrosine immunoreactivity. Moreover, the extent of both 4-HNE and nitrotyrosine staining within the infarct was not different between normo- and hyperglycemic ffSHRSP rats. These data do not provide convincing evidence in support of a role for oxidative stress in the detrimental effects of hyperglycemia during the evolution of focal ischemic damage, though as our approach was limited more detailed and sensitive analyses at earlier time points after MCAO are necessary to elucidate this potential mechanism further. One further consideration is the existing level of oxidative stress in SHRSP rat brain,40 which may have precluded detection of additional damage consequent to ischemia and hyperglycemia. Additional studies are required to ascertain the mechanisms by which hyperglycemia impacts on ischemic lesion growth.
In summary, our data demonstrate that hyperglycemia has a significant detrimental effect on the evolution of ischemic brain damage in the immediate postocclusion period both in the absence and presence of comorbidities associated with metabolic syndrome. In the GIST-UK trial, which failed to show a clinical benefit of insulin treatment in hyperglycemic stroke patients, the median time for treatment was 13 hours and of the 933 patients recruited, only 8 received insulin treatment within 3 hours of onset.7 Inclusion of patients who did not have penumbral tissue on which hyperglycemia could exert its effects may therefore have reduced the ability to detect its impact on outcome, in addition to exposing many patients to the potential risk of hypoglycemia when they were unlikely to benefit. Future clinical trials should begin glycemic control regimes as early as possible in patients with hyperglycemia and ensure balance of patients with and without underlying metabolic syndrome between treatment arms.
Acknowledgments
The authors thank Jim Mullin, Lindsay Gallagher, and staff at the Wellcome Surgical Institute for technical assistance.
The authors declare no conflict of interest.
Footnotes
Financial support: Chief Scientist Office (Scotland); Medical Research Council, UK; Scottish Imaging Network: A Platform for Scientific Excellence. Funded by the Chief Scientist Office (Scotland); Medical Research Council PhD studentship (DT) and the Scottish Imaging Network: A Platform for Scientific Excellence (KWM).
References
- Muir KW, McCormick M, Baird T, Ali M. Prevalence, predictors and prognosis of post-stroke hyperglycaemia in acute stroke trials: individual patient data pooled analysis from the Virtual International Stroke Trials Archive (VISTA) Cerebrovasc Dis Extra. 2011;1:17–27. doi: 10.1159/000324319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir CJ, Murray GD, Dyker AG, Lees KR. Is hyperglycaemia an independent predictor of poor outcome after acute stroke? Results of a long-term follow up study. BMJ. 1997;314:1303–1306. doi: 10.1136/bmj.314.7090.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CS, Scott JF, French JM, Alberti KG, O'Connell JE. Prevalence and prediction of unrecognised diabetes mellitus and impaired glucose tolerance following acute stroke. Age Ageing. 2004;33:71–77. doi: 10.1093/ageing/afh026. [DOI] [PubMed] [Google Scholar]
- McCormick MT, Muir KW. Prevalence of impaired glucose metabolism and metabolic syndrome in non-diabetic patients with acute post-stroke hyperglycaemia. Cerebrovasc Dis. 2006;21:62. [Google Scholar]
- Adams HP, Jr., del ZG, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation. 2007;115:e478–e534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- The European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010;67:570–578. doi: 10.1002/ana.21983. [DOI] [PubMed] [Google Scholar]
- Malmberg K, Ryden L, Wedel H, Birkeland K, Bootsma A, Dickstein K, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650–661. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Diemer NH. Focal ischemia of the rat brain, with special reference to the influence of plasma glucose concentration. Acta Neuropathol. 1987;73:131–137. doi: 10.1007/BF00693778. [DOI] [PubMed] [Google Scholar]
- Berger L, Hakim AM. Nimodipine prevents hyperglycemia-induced cerebral acidosis in middle cerebral artery occluded rats. J Cereb Blood Flow Metab. 1989;9:58–64. doi: 10.1038/jcbfm.1989.8. [DOI] [PubMed] [Google Scholar]
- Bomont L, MacKenzie ET. Neuroprotection after focal cerebral ischaemia in hyperglycaemic and diabetic rats. Neurosci Lett. 1995;197:53–56. doi: 10.1016/0304-3940(95)11899-8. [DOI] [PubMed] [Google Scholar]
- Slivka AP. Hypertension and hyperglycemia in experimental stroke. Brain Res. 1991;562:66–70. doi: 10.1016/0006-8993(91)91187-6. [DOI] [PubMed] [Google Scholar]
- Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM, et al. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab. 1997;17:553–559. doi: 10.1097/00004647-199705000-00009. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Transient focal ischemia in hyperglycemic rats is associated with increased cerebral infarction. Brain Res. 1987;408:79–85. doi: 10.1016/0006-8993(87)90360-x. [DOI] [PubMed] [Google Scholar]
- Huang NC, Wei J, Quast MJ. A comparison of the early development of ischemic brain damage in normoglycemic and hyperglycemic rats using magnetic resonance imaging. Exp Brain Res. 1996;109:33–42. doi: 10.1007/BF00228624. [DOI] [PubMed] [Google Scholar]
- Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Macdougall NJ, Muir KW. Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31:807–818. doi: 10.1038/jcbfm.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–1933. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- Fuentes B, Castillo J, San JB, Leira R, Serena J, Vivancos J, et al. The prognostic value of capillary glucose levels in acute stroke: the GLycemia in Acute Stroke (GLIAS) study. Stroke. 2009;40:562–568. doi: 10.1161/STROKEAHA.108.519926. [DOI] [PubMed] [Google Scholar]
- Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- Rosso C, Attal Y, Deltour S, Hevia-Montiel N, Lehericy S, Crozier S, et al. Hyperglycemia and the fate of apparent diffusion coefficient-defined ischemic penumbra. AJNR Am J Neuroradiol. 2011;32:852–856. doi: 10.3174/ajnr.A2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenillas JF, Moro MA, Davalos A. The metabolic syndrome and stroke: potential treatment approaches. Stroke. 2007;38:2196–2203. doi: 10.1161/STROKEAHA.106.480004. [DOI] [PubMed] [Google Scholar]
- Ninomiya JK, L'Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C. [DOI] [PubMed] [Google Scholar]
- Milionis HJ, Rizos E, Goudevenos J, Seferiadis K, Mikhailidis DP, Elisaf MS. Components of the metabolic syndrome and risk for first-ever acute ischemic nonembolic stroke in elderly subjects. Stroke. 2005;36:1372–1376. doi: 10.1161/01.STR.0000169935.35394.38. [DOI] [PubMed] [Google Scholar]
- Strahorn P, Graham D, Charchar FJ, Sattar N, McBride MW, Dominiczak AF. Genetic determinants of metabolic syndrome components in the stroke-prone spontaneously hypertensive rat. J Hypertens. 2005;23:2179–2186. doi: 10.1097/01.hjh.0000191904.26853.b8. [DOI] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Reid E, Graham D, Lopez-Gonzalez MR, Holmes WM, Macrae IM, McCabe C. Penumbra detection using PWI/DWI mismatch MRI in a rat stroke model with and without comorbidity: comparison of methods. J Cereb Blood Flow Metab. 2012;32:1765–1777. doi: 10.1038/jcbfm.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai H, Harland J, McCulloch J, Graham DI, Brown SM, Macrae IM. Specific expression of the cell cycle regulation proteins, GADD34 and PCNA, in the peri-infarct zone after focal cerebral ischaemia in the rat. Eur J Neurosci. 2002;15:1929–1936. doi: 10.1046/j.1460-9568.2002.02025.x. [DOI] [PubMed] [Google Scholar]
- McCracken E, Valeriani V, Simpson C, Jover T, McCulloch J, Dewar D. The lipid peroxidation by-product 4-hydroxynonenal is toxic to axons and oligodendrocytes. J Cereb Blood Flow Metab. 2000;20:1529–1536. doi: 10.1097/00004647-200011000-00002. [DOI] [PubMed] [Google Scholar]
- McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. 2009;40:3864–3868. doi: 10.1161/STROKEAHA.109.559021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle P, Heistad DD. Blood flow through cerebral collateral vessels one month after middle cerebral artery occlusion. Stroke. 1987;18:407–411. doi: 10.1161/01.str.18.2.407. [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Tomlinson DR, Gardiner NJ. Diabetic neuropathies: components of etiology. J Peripher Nerv Syst. 2008;13:112–121. doi: 10.1111/j.1529-8027.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- Suh SW, Shin BS, Ma H, Van HM, Brennan AM, Yenari MA, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michihara A, Shimatani M, Anraku M, Tomida H, Akasaki K. High levels of oxidative stress exist in the brain than serum or kidneys in stroke-prone spontaneously hypertensive rats at ten weeks of age. Biol Pharm Bull. 2010;33:518–521. doi: 10.1248/bpb.33.518. [DOI] [PubMed] [Google Scholar]