Abstract
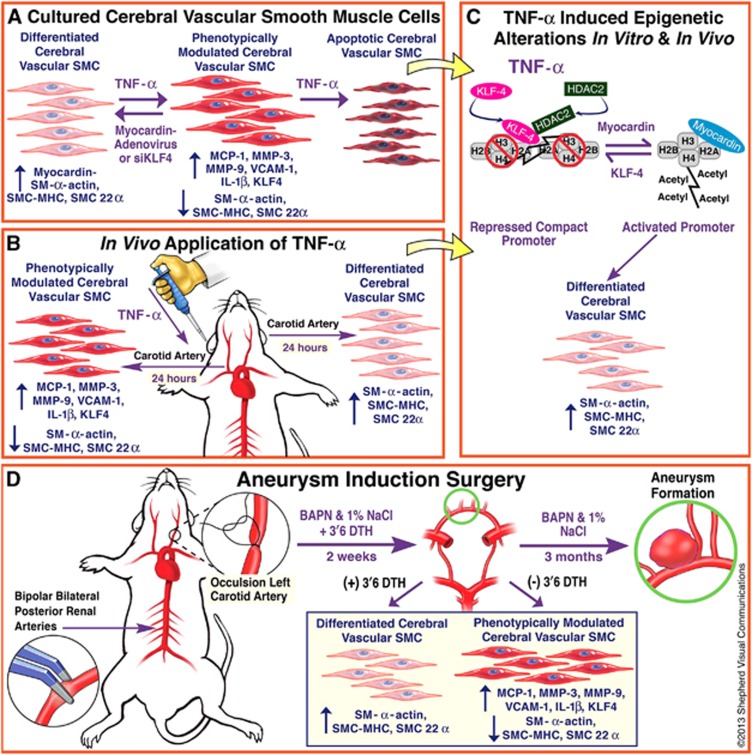
Little is known about vascular smooth muscle cell (SMC) phenotypic modulation in the cerebral circulation or pathogenesis of intracranial aneurysms. Tumor necrosis factor-alpha (TNF-α) has been associated with aneurysms, but potential mechanisms are unclear. Cultured rat cerebral SMCs overexpressing myocardin induced expression of key SMC contractile genes (SM-α-actin, SM-22α, smooth muscle myosin heavy chain), while dominant-negative cells suppressed expression. Tumor necrosis factor-alpha treatment inhibited this contractile phenotype and induced pro-inflammatory/matrix-remodeling genes (monocyte chemoattractant protein-1, matrix metalloproteinase-3, matrix metalloproteinase-9, vascular cell adhesion molecule-1, interleukin-1 beta). Tumor necrosis factor-alpha increased expression of KLF4, a known regulator of SMC differentiation. Kruppel-like transcription factor 4 (KLF4) small interfering RNA abrogated TNF-α activation of inflammatory genes and suppression of contractile genes. These mechanisms were confirmed in vivo after exposure of rat carotid arteries to TNF-α and early on in a model of cerebral aneurysm formation. Treatment with the synthesized TNF-α inhibitor 3,6-dithiothalidomide reversed pathologic vessel wall alterations after induced hypertension and hemodynamic stress. Chromatin immunoprecipitation assays in vivo and in vitro demonstrated that TNF-α promotes epigenetic changes through KLF4-dependent alterations in promoter regions of myocardin, SMCs, and inflammatory genes. In conclusion, TNF-α induces phenotypic modulation of cerebral SMCs through myocardin and KLF4-regulated pathways. These results demonstrate a novel role for TNF-α in promoting a pro-inflammatory/matrix-remodeling phenotype, which has important implications for the mechanisms behind intracranial aneurysm formation.
Keywords: Cerebral aneurysm, Cerebral vascular smooth muscle cells, Smooth muscle cell phenotypic modulation, TNF-alpha
Introduction
Vascular smooth muscle cells (SMCs), unlike the terminally differentiated cardiac or skeletal muscle cells, retain remarkable plasticity. In response to environmental stimuli or cues, SMCs can undergo profound changes in phenotype, switching from cells principally concerned with contraction to cells that possess a pro-inflammatory, pro-matrix remodeling phenotype.1, 2 Prior studies have noted significant alterations in cerebral blood vessels in response to injury and ischemia, resulting in an inflammatory response.3 Phenotypic modulation is characterized by a decreased expression of contractile proteins (such as smooth muscle myosin heavy chain (SM-MHC), SM-α-actin, and SM-22α with increased expression of matrix metalloproteinases (MMPs) and other inflammatory mediators. Phenotypic modulation of vascular SMCs is known to be important in the pathogenesis of atherosclerosis and other vascular diseases.1, 2
There are well-established regional differences in SMC phenotype at the molecular level among different vascular beds,4 and little is known about SMC differentiation and phenotypic switching in the cerebral circulation. Accumulating data suggest that SMC phenotypic modulation is involved in the pathogenesis of intracranial aneurysms (IA),5, 6 and progression of atherosclerosis within the aneurysmal sac has correlated with aneurysmal growth and rupture.7 Furthermore, inflammation and inflammatory cytokines have been directly implicated in the pathogenesis of IA.8 More specifically, a potentially critical role for TNF-α in the pathology of IA has been suggested from recent data,9, 10 although a possible mechanism has not been established.
Tumor necrosis factor-alpha is a pro-inflammatory cytokine that is a constituent of the innate immune system's response to various forms of stress (infectious, chemical, and mechanical).11 It has been demonstrated to be proatherogenic through multiple biologic effects including influences on the endothelium (producing endothelial dysfunction), SMC apoptosis, and atherosclerotic plaque destabilization through extracellular matrix remodeling.12, 13 Although TNF-α and pro-inflammatory mediators have been found to be upregulated after cerebrovascular injury and ischemia,3, 14 a potential direct role for TNF-α in SMC phenotypic modulation has not been investigated.
The aims of the present study were:1 to evaluate a potential direct role of TNF-α in producing phenotypic modulation of cultured cerebral SMCs including repression of SMC marker genes and induction of pro-inflammatory, matrix-remodeling genes that may have a critical role in the pathogenesis of cerebral aneurysms;2 to determine whether TNF-α produces similar phenotypic modulation of SMCs in vivo, and3 to test the hypothesis that TNF-α-induced phenotypic modulation of SMCs is mediated by Kruppel-like transcription factor 4 (KLF4)—a potent repressor of SMC differentiation marker genes,1, 2 and pluripotency factor involved in reprogramming of somatic cells.15
Materials and Methods
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and in accordance with the ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Thomas Jefferson University (Permit Number: 833). All surgery was performed under Isofluorane anesthesia. All efforts were made to minimize suffering. An outline of experimental time line is demonstrated in Figure 1. Cerebral blood vessels (circle of Willis) from rats were harvested for cerebral vascular SMC culture and treated with TNF-α (Millipore, Billerica, MA, USA) for quantitative polymerase chain reaction (PCR), western blot, chromatin immunoprecipitation (CHIP), evaluation of apoptosis, and assessment after adenovirus promoter transfection (See Supplementary Materials and Materials and Methods).
Figure 1.
Time-line of experiments. (A) Cerebral blood vessels (circle of Willis) from rats were harvested for cerebral vascular smooth muscle cell (SMC) culture and treated with tumor necrosis factor-alpha (TNF-α) for quantitative polymerase chain reaction, western blot, evaluation of apoptosis, and assessment after adenovirus promoter transfection. (B) After experiments in vitro, experiments were carried out after application of pluronic gel containing TNF-α to the adventitial surface of rat carotid arteries to directly evaluate phenotypic modulation in vivo. (C) Additionally, chromatin immunoprecipitation assays were carried out to determine epigenetic alterations in vitro and in vivo. (D) Subsequently, the role of TNF-α and the TNF-α inhibitor 3,6′-dithiothalidomide was assessed early on in an established rodent cerebral aneurysm model induced by hypertension and hemodynamic stress. BAPN, β-aminopropionitrile; SMC-MHC, smooth muscle cell myosine heavy chain.
After experiments in vitro, experiments were carried out after application of pluronic gel (Sigma-Aldrich, St Louis, MO, USA) containing TNF-α to the adventitial surface of rat carotid arteries to directly evaluate phenotypic modulation in vivo. Additionally, CHIP assays were carried out to determine epigenetic alterations in vivo (Figure 1). Subsequently, the role of TNF-α was assessed early on in an established rodent cerebral aneurysm model induced by hypertension and hemodynamic stress.16 The TNF-α inhibitor 3,6′-dithiothalidomide was synthesized as previously described17, 18, 19 and activity was assessed in an early rodent cerebral aneurysm model (Figure 1). Further details regarding the Materials and Methods can be found in the Supplementary Information available at the Journal of Cerebral Blood Flow & Metabolism website—www.nature.com/jcbfm.
Results
Tumor Necrosis Factor-Alpha Potently Repressed Smooth Muscle Cell Marker Gene Promoter Activity and Messenger RNA levels in Cultured Cerebral Smooth Muscle Cells
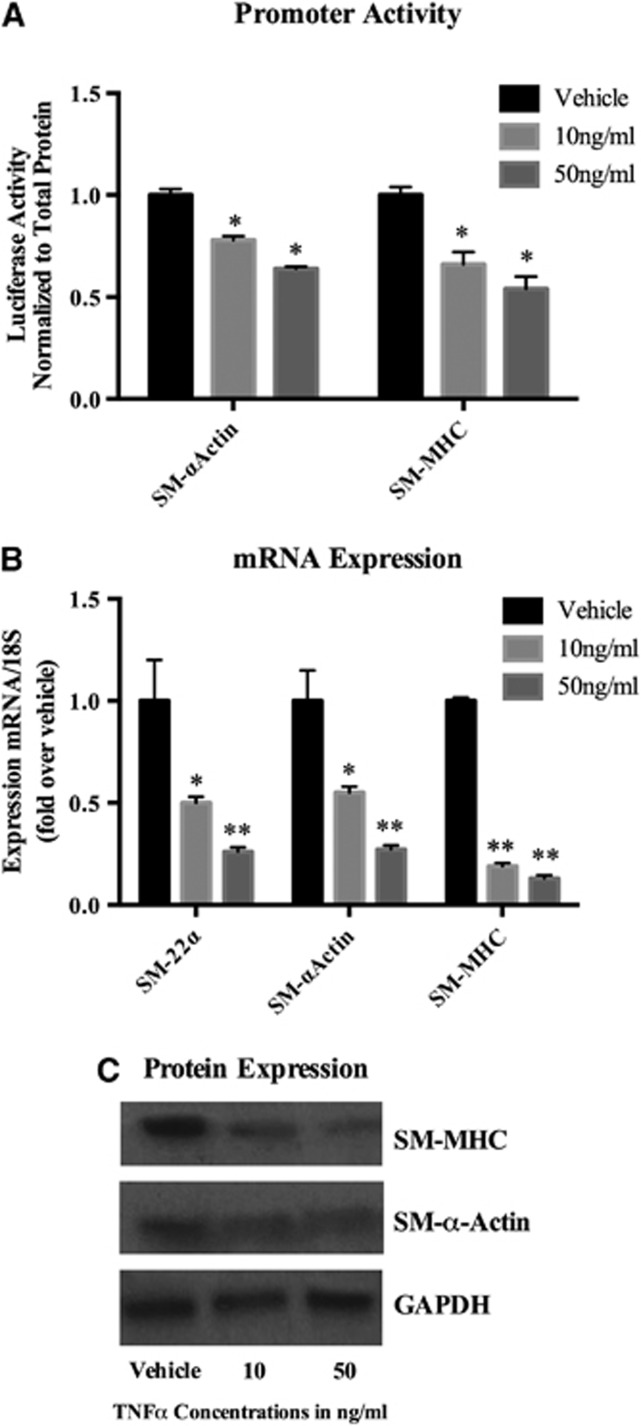
To examine a potential direct effect of TNF-α on inducing phenotypic modulation in cultured cerebral SMCs, cells were first transfected with the various SMC promoter–reporter constructs and treated for 24 hours with TNF-α (Figure 1). Tumor necrosis factor-alpha potently repressed SM-MHC and SM-α-actin promoter activity in a dose-dependent fashion (Figure 2A). Quantitative real-time (RT)–PCR analysis demonstrated that cerebral SMCs treated with TNF-α for 24 hours exhibit a profound suppression of SM-α-actin, SM-MHC, and SM-22-α in a dose-dependent fashion (Figure 2B). Western blot analysis confirmed a reduction in protein expression of SM-α-actin and SM-MHC in response to TNF-α stimulation (Figure 2C). Further, TNF-α induces apoptosis in vascular SMCs in a dose-dependent fashion (Supplementary Figure 1).
Figure 2.
Tumor necrosis factor-alpha (TNF-α) suppressed promoter activity, messenger RNA (mRNA) levels, and protein expression of smooth muscle cell (SMC) marker genes. (A) Smooth muscle myosine heavy chain (SM-MHC)-luc and SM α-actin-luc promoter-luciferase constructs were transiently transfected into cerebral vascular SMCs for 48 hours and were treated with TNF-α for 24 hours. Luciferase activity was measured, normalized to total protein content, and then expressed as fold increase over vehicle. Values represent mean±s.e.m. *P<0.001 vs vehicle. (B) Cultured cerebral SMCs were treated for 24 hours with the indicated concentration of TNF-α or vehicle. Real-time polymerase chain reaction (RT–PCR) was performed, normalized to 18S ribosomal RNA (rRNA), and expressed as fold increase over vehicle. Values represent mean±s.e.m. *P<0.05; **P<0.005; ***P<0.0001 vs vehicle. (C) Cultured SMCs were starved for 72 hours and further treated with TNF-α with the indicated range of concentration for another 72 hours. Total protein lysate of SMCs (0.2 μg) were subjected to western blot analysis of SM-MHC and SM-α-actin protein expression. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Tumor Necrosis Factor-Alpha-Activated Expression of a Cohort of Pro-inflammatory Genes
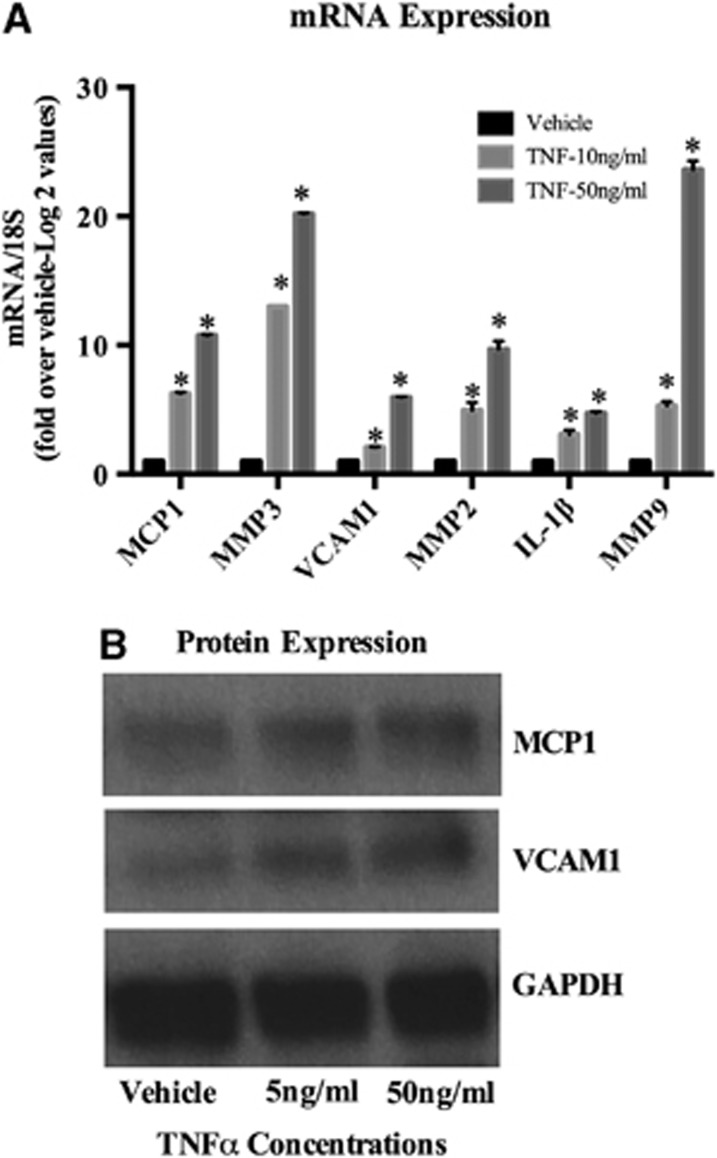
Phenotypic modulation of vascular SMCs can also be characterized by activation of a repertoire of pro-inflammatory genes.1, 2 Based in part upon established work in the field of cerebral aneurysms,8 we selected a number of inflammatory genes that may have a role in cerebral aneurysm pathogenesis that also contain KLF4 consensus binding sites that are conserved across species in their promoter region. Quantitative real-time RT–PCR and western blot analysis revealed that TNF-α treatment for 24 hours markedly increased expression of MCP-1, MMP-3, MMP-9, VCAM-1, and IL-1β messenger RNA (mRNA), respectively, in a dose-dependent manner (Figure 3). Similarly, protein expression of inflammatory genes was increased in a dose-dependent manner (Figure 3).
Figure 3.
Tumor necrosis factor-alpha (TNF-α)-induced messenger (mRNA) and protein expression of pro-inflammatory genes. (A) Cultured cerebral smooth muscle cells (SMCs) were treated with the indicated concentration of TNF-α for 24 hours. Real-time polymerase chain reaction was performed, normalized to 18S ribosomal RNA (rRNA), and expressed as fold increase over vehicle. Values represent mean±s.e.m. *P<0.001 vs vehicle. *P<0.0001 vs vehicle. (B) Cultured SMCs were starved for 72 hours and further treated with TNF-α with the indicated concentration for another 72 hours. Total protein lysate of SMCs (0.2 μg) were subjected to western blot analysis of monocyte chemoattractant protein-1 (MCP-1) and vascular cell adhesion molecule-1 (VCAM-1) protein expression. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control.
Tumor Necrosis Factor-Alpha Induced Expression of the Transcription Factor, Kruppel-like transcription factor 4, A Potent Regulator of Smooth Muscle Cell Phenotypic Modulation
The transcription factor KLF4 is known to be a potent regulator of SMC differentiation.1, 2 To determine if TNF-α induced suppression of SMC differentiation marker genes and activation of pro-inflammatory genes were accompanied by concomitant changes in expression of KLF4, cultured cerebral SMCs were treated with TNF-α for various periods of time. Using quantitative real-time RT–PCR, TNF-α markedly increased expression of KLF4 (over six-fold) beginning as early as 2 hours after TNF-α treatment (Supplementary Figure 2A). The maximal response appeared to occur at a TNF-α concentration of 10 ng/mL. Western blot also confirmed increased protein expression of KLF4 compared with glyceraldehyde 3-phosphate dehydrogenase control (Supplementary Figure 2B).
Small Interfering RNA-Induced Suppression of Kruppel-like transcription factor 4 Inhibited Tumor Necrosis Factor-alpha-induced Repression of Smooth Muscle Cell Marker Genes and Activation of Pro-Inflammatory Genes
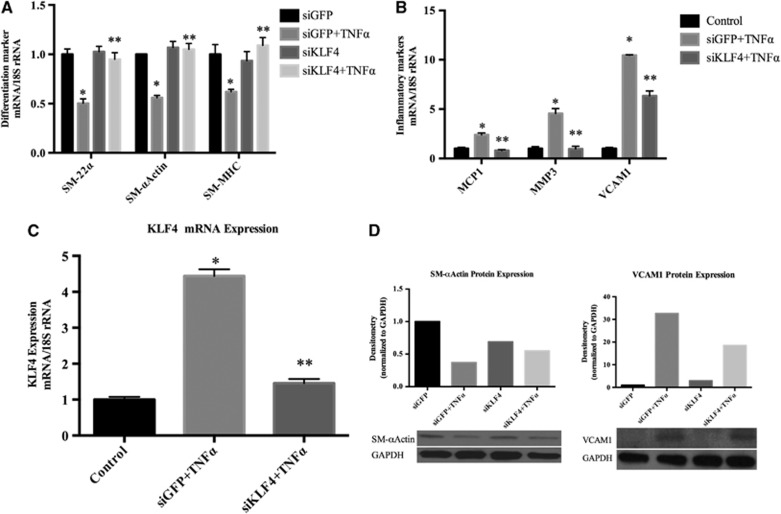
To evaluate whether KLF4 is required for TNF-α-induced suppression of SMC marker genes and activation of expression of pro-inflammatory genes, cultured cerebral SMCs were treated with TNF-α +/− an small interfering RNA (siRNA) oligonucleotide specific for KLF4 or with a control siRNA to green fluorescent protein (GFP). KLF4 siRNA abrogated TNF-α-induced suppression of SM-α-actin, SM-MHC, and SM-22-α while the control GFP siRNA had no effect (Figure 4A). Moreover, KLF4 siRNA oligonucleotide essentially completely prevented the activation of expression of MCP-1, VCAM-1, and MMP-3 while control GFP siRNA did not (Figure 4B). Control studies showed that the KLF4 siRNA completely abolished the increase in expression of KLF4 mRNA in response to TNF-α stimulation while the GFP siRNA did not (Figure 4C).
Figure 4.
Tumor necrosis factor-alpha (TNF-α) induced suppression of smooth muscle cell (SMC) marker genes and induction of pro-inflammatory genes was mediated by Kruppel-like transcription factor 4 (KLF4). (A-C) Cultured cerebral SMCs were transfected with KLF4 small interfering (siRNA), oligonucleotides (short interfering RNA specific to KLF4 (siKLF4)), or nonspecific control oligonucleotides (siGFP) with TNF-α (50 ng/mL) or vehicle treatment. Total RNA samples were isolated and differentiation marker genes (SM-22α, SM-α-actin and smooth muscle myosine heavy chain (SM-MHC)), inflammatory marker genes (MCP-1, VCAM-1, and MMP-3), and KLF4 messenger RNA (mRNA) expression were analyzed by real-time polymerase chain reaction. Values were normalized to 18S ribosomal RNA (rRNA) and represent mean±s.e.m. The experiment was repeated four times and the representative data are shown. (A) *P<0.05 vs vehicle; **P<0.005 vs siGFP. (B) *P<0.01 vs vehicle; **P<0.0001 vs siGFP. (C) *P<0.0001 vs vehicle; **P<0.0002 vs siGFP. (D) Three micrograms of total protein lysate of SMCs transfected with KLF4 small interfering RNA (siRNA) or green fluorescent protein (GFP) siRNA were subjected to western blot analysis using anti-SM-α- actin, anti-VCAM-1 (vascular cell adhesion molecule-1), and anti-GAPDH (glyceraldehyde 3-phosphate dehydrogenase) antibodies. The band intensity was quantified by densitometry. Relative band intensity was normalized to the band intensity of GAPDH and presented as fold increase over vehicle.
These findings were corroborated by examining the changes at the protein level. Cerebral SMCs were transfected with either GFP siRNA or KLF4 siRNA. Total protein extracts were subjected to western blot analysis. Tumor necrosis factor-alpha stimulation suppressed expression of SM-α-actin in cells transfected with GFP siRNA while this suppression was eliminated by KLF4 siRNA (Figure 4D). However, TNF-α stimulated the expression of VCAM-1 in cells transected with siGFP while this was inhibited by short interfering RNA specific to KLF4 (siKLF4) (Figure 4D). Expression of β-actin was not altered with either GFP siRNA or KLF4 siRNA transfection (Supplementary Figure 3A).
Tumor Necrosis Factor-Alpha Decreased Expression of Smooth Muscle Cell Marker Genes and Increased Expression of Pro-Inflammatory Genes In Vivo in Rat Carotid Arteries
To delineate whether TNF-α also suppressed expression of SMC marker genes and activated expression of pro-inflammatory/matrix-remodeling genes in vivo, the F-127 pluronic gel system20, 21 was used to apply TNF-α or vehicle to the adventitial surface of rat carotid arteries in vivo (Figure 1). Quantitative real-time RT–PCR analysis demonstrated that TNF-α induced significant suppression of SM-α-actin, SM-MHC, and SM-22-α after 24 hours as well as a significant increase in KLF4 mRNA levels after 6 hours of treatment, vs vehicle-treated vessels (Supplementary Figure 4A). Additionally, TNF-α produced a marked increase in pro-inflammatory genes (Supplementary Figure 4B). The effects of TNF-α were localized and expression of these genes was not altered in the aorta or liver. Additionally, control β-actin mRNA expression was unchanged in TNF-α-treated vessels (Supplementary Figure 3B). Collectively, these data demonstrate that TNF-α decreases the expression of SMC marker genes, increases expression of KLF4, and increases expression of pro-inflammatory/matrix-remodeling genes in vivo.
Aneurysm Induction Surgery is Associated with a Suppression of Smooth Muscle Cell-α-Actin and an Increase in Expression of Tumor Necrosis Factor-Alpha, Kruppel-Like Transcription Factor 4 and Pro-inflammatory/Matrix-Remodeling Genes
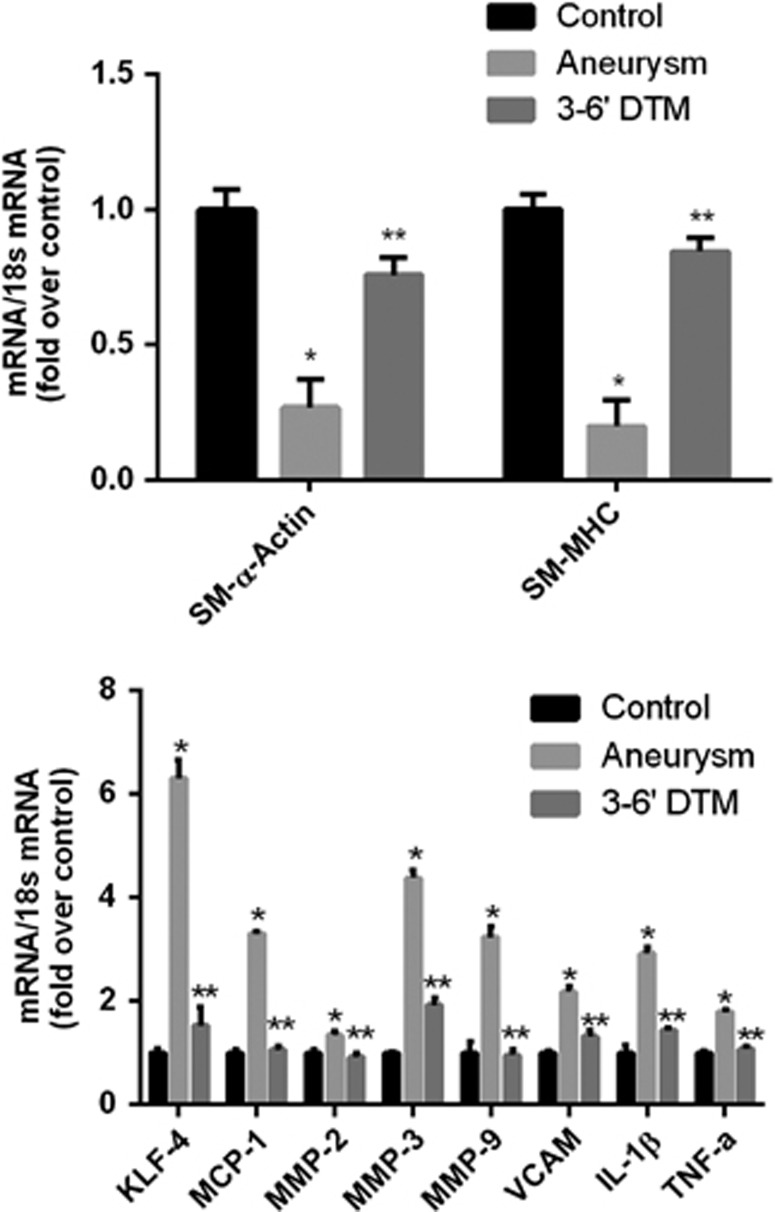
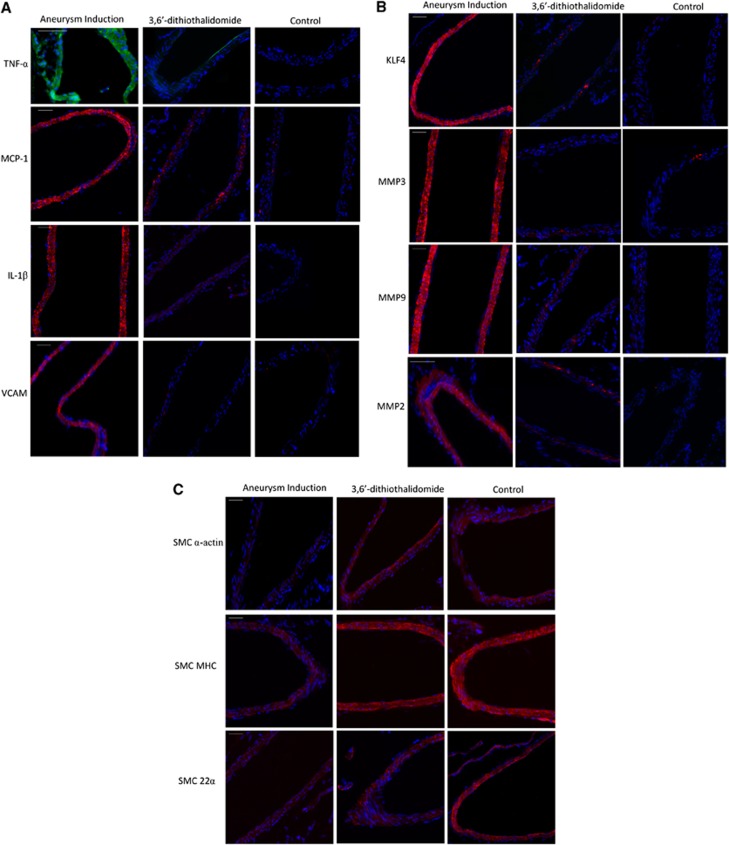
To evaluate if changes in SMC marker genes, KLF4, TNF-α, and pro-inflammatory/matrix-remodeling genes occur at an early time point after aneurysm induction surgery (before frank aneurysm formation), we employed an established rodent model of cerebral aneurysm formation that involves induced hypertension and hemodynamic stress through unilateral carotid artery ligation (Figure 1).16 Animals undergoing treatment developed hypertension as compared with untreated controls (Supplementary Figure 5). Two weeks after aneurysm induction surgery, quantitative real-time RT–PCR (Figure 5) and immunofluorescence staining (Figure 6) were performed on vessels of the Circle of Willis. Aneurysm induction surgery resulted in a significant reduction in SM-MHC and SM-α-actin with an increase in expression of TNF-α, KLF4, MMPs (including MMP-2, -3, and -9), MCP-1, and VCAM-1 compared with Circle of Willis vessels from age-matched controls. Daily intraperitoneal injections with the synthesized TNF-α inhibitor 3,6′-dithiothalidomide reversed these alterations. Expression of SM-22α was also decreased after aneurysm induction, but this was not significantly altered with 3,6′-dithiothalidomide treatment. This model typically induces aneurysm formation between 3 and 6 months (Supplementary Figure 6). Taken together, these data demonstrate that TNF-α is increased early after aneurysm induction surgery and concomitant changes in expression of SMC marker gene SM-α-actin, KLF4, and pro-inflammatory/matrix-remodeling genes occur in a similar fashion as demonstrated with TNF-α treatment of SMCs in vitro and rat carotid arteries in vivo.
Figure 5.
Aneurysm induction surgery is associated with a suppression of smooth muscle cell (SMC)-α-actin, smooth muscle cell myosine heavy chain (SM-MHC), and an increase in expression of TNF-α, Kruppel-like transcription factor 4 (KLF4), and pro-inflammatory/matrix-remodeling genes in vivo, which is reversed with treatment with the TNF-α synthesis inhibitor 3,6′-dithiothalidomide. Messenger RNA (mRNA) expression of anterior cerebral circulation 2 weeks after aneurysm induction surgery. Aneurysm induction surgeries were performed as detailed in Materials and Methods. Circle of Willis from animals undergoing aneurysm induction surgery and daily intraperitoneal injections of 3,6′-dithiothalidomide (N=6), aneurysm induction surgery and vehicle (N=6), or controls treated only with vehicle (N=6) were harvested after 14 days. RNA was extracted, real-time polymerase chain reaction was performed, and expression levels of SM-α-actin, SM-MHC, KLF4, TNF-α, and pro-inflammatory marker genes were normalized to 18S ribosomal RNA (rRNA) and represent fold increase over control. Messenger RNA (mRNA) expression of SM-α-actin, SM-MHC was decreased and expression of KLF4, TNF-α, and pro-inflammatory marker genes was increased after aneurysm induction surgery, as compared with controls. Treatment with daily intraperitoneal injections of 3,6′-dithiothalidomide significantly reversed the decreased expression of SM-α-actin and SM-MHC and the increase in expression of KLF4, TNF-α, and pro-inflammatory marker genes. Values represent mean±s.e.m. *P<0.001 vs control; **P<0.001 aneurysm induction versus 3,6′-dithiothalidomide. 3,6′DTM represents 3,6′-dithiothalidomide.
Figure 6.
After aneurysm induction surgery, immunohistochemistry staining demonstrates decreased expression of SMC-α-actin and smooth muscle myosine heavy chain (SM-MHC) and increased expression of tumor necrosis factor-alpha (TNF-α,) Kruppel-like transcription factor 4 (KLF4), and pro-inflammatory/matrix-remodeling genes in vivo, which is reversed with treatment with the TNF-α synthesis inhibitor 3,6′-dithiothalidomide. Immunofluorescence staining of the Circle of Willis 2 weeks after aneurysm induction surgery (N=6) demonstrated increased expression of (A) TNF-α (green ), monocyte chemoattractant protein-1 (MCP-1) (red), interleukin-1 beta (IL-1β) (red ), vascular cell adhesion molecule-1 (VCAM) (red ), (B) KLF4 (red), matrix metalloproteinase (MMP-2) (red), MMP-3 (red ), MMP-9 (red ), and decreased expression of (C) SM-α-actin (red), SM-MHC (red) as compared with controls. Alterations in animals undergoing aneurysm induction surgery were reversed with daily intraperitoneal injections of 3,6′-dithiothalidomide (N=6). Expression of SM-22α (red) was decreased after aneurysm induction surgery, but changes in expression were not reversed with 3,6′-dithiothalidomide treatment. Nuclei (blue) are counter stained with 4',6-Diamidino-2-phenylindole (DAPI). 3,6′DTM represents 3,6′-dithiothalidomide. Scale bar represents 100 μm.
Myocardin Increased Smooth Muscle Cell Marker Gene Promoter Activity and Induced Expression of Smooth Muscle Cell Marker Genes in Cultured Cerebral Smooth Muscle Cells
Because of regional heterogeneity in factors regulating vascular SMC differentiation according to the vascular bed, we set out to determine if the highly potent SRF coactivator, myocardin, was able to increase SMC marker gene promoter activity and mRNA level in cerebral SMCs. Cultured rat cerebral SMCs were transfected with various SMC promoter–reporter constructs and co-transfected with the myocardin or dominant-negative myocardin transfection vectors. Transfection with the myocardin vector significantly increased SM-α-actin and SM-MHC promoter activity (Supplementary Figure 7A) while transfection with the dominant-negative myocardin vector repressed SM-α-actin and SM-MHC promoter activity (Supplementary Figure 7B). Examination of endogenous gene expression using real-time RT–PCR demonstrated that myocardin transfection potently increased expression of SM-α-actin, SM-22α, and SM-MHC mRNA (Supplementary Figure 7C). Conversely, transfection with a dominant-negative myocardin construct suppressed expression of SM-α-actin, SM-22α, and SM-MHC mRNA (Supplementary Figure 7D). When cultured cerebral vascular SMCs were infected with dominant-negative myocardin, there was a significant reduction in expression of myocardin mRNA, as compared with vehicle (Supplementary Figure 8A). Additionally, transfection with myocardin adenovirus significantly increased myocardin expression, as compared with vehicle (Supplementary Figure 8B).
Tumor Necrosis Factor-Alpha Repressed Expression of Myocardin in a Kruppel-Like Transcription Factor 4-Dependent Manner
To evaluate potential mechanisms by which TNF-α represses expression of SMC marker genes, we examined its effects on expression of myocardin. Tumor necrosis factor-alpha significantly attenuated expression of myocardin mRNA (Supplementary Figure 9A) while siKLF4 prevented this decrease (Supplementary Figure 9B). Tumor necrosis factor-alpha also significantly reduced expression of myocardin mRNA in vivo (while simultaneously increasing expression of KLF4) when applied with pluronic gel to rat carotid artery (Supplementary Figure 9C). These data suggest that KLF4 represses expression of vascular SMC marker genes, at least in part, via KLF4-dependent repression of myocardin.
Tumor Necrosis Factor-Alpha Induced Binding of the Transcription Factor SP1 to the Promoter Region of Kruppel-Like Transcription Factor 4
To further assess the mechanisms behind KLF4 activation, CHIP assays were used to assess the role of transcription factory SP1 and histone modifications at the KLF4 promoter region. Tumor necrosis factor-alpha treatment in cerebral vascular SMCs resulted in increased binding of SP1 at the promoter region of KLF4 along with increased histone acetylation, both of which are characteristic of promotion of KLF4 gene expression (Supplementary Figure 10).2
Tumor Necrosis Factor-Alpha Induced Binding of Kruppel-Like Transcription Factor 4 to the Promoter Regions of Smooth Muscle Cell Marker Genes and Myocardin
To examine potential direct interactions of KLF4 with the promoter regions of SMC marker genes and myocardin, we performed CHIP assays (Figure 1). Tumor necrosis factor-alpha resulted in increased binding of KLF4 to the promoter regions of SM-α-actin, SM-MHC, and myocardin at 6 hours after treatment in cultured cerebral vascular SMCs (Supplementary Figure 11A). Moreover, when TNF-α was applied to rat carotid arteries in vivo, CHIP assays demonstrated a similar increased binding of KLF4 to the promoter regions of SM-α-actin, SM-MHC, and myocardin (Supplementary Figure 11B).
Tumor Necrosis Factor-Alpha Induced Binding of Kruppel-Like Transcription Factor 4 to the Promoter Regions of Monocyte Chemoattractant Protein-1
To determine the interactions between KLF4 and pro-inflammatory gene expression, CHIP assays were used to assess alterations at the MCP-1 promoter region both in vivo (Supplementary Figure 11C) and in vitro (Supplementary Figure 11D). Tumor necrosis factor-alpha induces binding of KLF4 to the promoter region of MCP-1 and increases histone acetylation. Increased histone acetylation promotes transcription of the downstream gene. These studies demonstrate that both direct binding of KLF4 to the promoter region and histone acetylation may have an important role in MCP-1 regulation.
Tumor Necrosis Factor-Alpha-Induced Suppression of Smooth Muscle Cell Marker Genes was Accompanied by Recruitment of Histone Deacetylase 2, Promoter Hypoacetylation, and Changes in Promoter Methylation
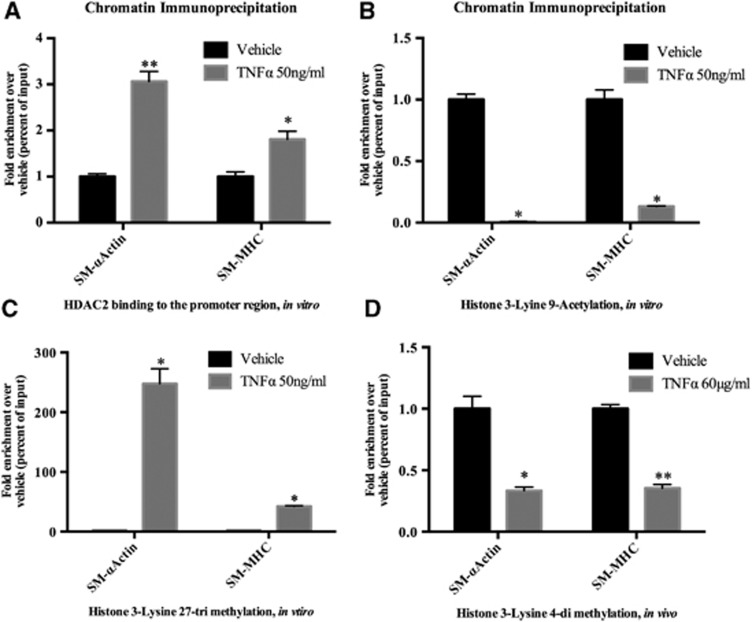
To determine if epigenetic mechanisms may potentially mediate TNF-α-induced suppression of SMC marker genes, we examined whether TNF-α recruited histone deactylases (HDACs) and resulted in changes in acetylation at the promoter regions of the respective genes. Tumor necrosis factor-alpha recruited histone deacetylase 2 to the promoter regions of SM-α-actin and SM-MHC at 6 hours after treatment in cultured cerebral SMCs (Figure 7A). Additionally, TNF-α produced hypoacetylation of the histones at the promoter regions of these genes (Figure 7B). On examining changes in hypoacetylation of the histones at the promoter regions of SM-α-actin and SM-MHC when TNF-α was applied to rat carotid arteries in vivo, there was a nonsignificant trend toward similar changes (data not shown). To evaluate potential changes in promoter methylation that may induce gene suppression, further CHIP assays were performed. Tumor necrosis factor-alpha produced H3K27 trimethylation in the histones at the promoter regions of SM-α-actin and SM-MHC at 6 hours after treatment in cultured cerebral vascular SMCs, characteristic of transcriptional suppression22 (Figure 7C). Application of TNF-α to rat carotid arteries appeared to produce decreases in H3K4 dimethylation that has also been associated with transcriptional repression22 (Figure 7D). In summary, TNF-α appears to recruit histone deacetylase 2 to the promoter region of SMC marker genes producing hypoacetylation and also appears to result in changes in promoter methylation that lead to gene transcriptional repression.
Figure 7.
Tumor necrosis factor-alpha (TNF-α)-induced suppression of smooth muscle cell (SMC) marker genes was accompanied by recruitment of histone deacetylase 2 (HDAC2), promoter hypoacetylation, and changes in promoter methylation. (A) Cultured cerebral SMCs were treated with TNF-α (50 ng/mL) for 6 hours. Association of HDAC2 to the CArG-containing promoter region of SMC marker genes (SM-α-actin and smooth muscle myosine heavy chain (SM-MHC)) was determined with chromatin immunoprecipitation (ChIP) assay using anti-HDAC2 antibody. Values represent fold increase over vehicle and are expressed as mean±s.e.m. *P<0.01; **P<0.0007 vs vehicle. (B & C). Similar to the above, ChIP assays were performed with the following antibodies: anti-H3K9Ac and anti-H3K27triMe. Values represent fold increase over vehicle and represent mean±s.e.m. *P<0.0001 vs vehicle. (D): Pluronic gel containing TNF-α (60 μg/mL) was applied to the rat carotid arteries (n=12). Vessels were harvested and ChIP assay performed using anti-H3K4diMe. Values represent fold increase over vehicle and mean±s.e.m. *P<0.0001; **P<0.0005 vs vehicle. CArG, Cytosine Cytosine [Adenosine/Thymine]6 Guanine Guanine.
Discussion
Significant progress has been made in understanding vascular SMC biology, although mechanisms underlying control of SMC phenotypic modulation in disease states remain poorly defined. Well-established regional differences in SMC phenotype at the molecular level have been documented and previously little was known about SMC differentiation in the cerebral circulation. Although prior studies have found that cerebral vascular injury and ischemia may result in an inflammatory response3, 14 and accumulating evidence suggests SMC phenotypic modulation and inflammation may have a role in the pathogenesis of cerebral aneurysms,8 potential mechanisms remain incompletely understood. The current study presents novel data demonstrating that TNF-α produces profound phenotypic modulation of cerebral SMCs characterized by decreased expression of SMC contractile genes and increased expression of pro-inflammatory, pro-matrix-remodeling genes in vitro and in vivo. Additionally, TNF-α is increased and similar SMC phenotypic modulation appears to occur within the cerebral circulation early in a rodent model of cerebral aneurysm formation, and this is reversed after daily treatment of the TNF-α synthesis inhibitor 3,6′-dithiothalidomide.
Previous studies in cultured aortic SMCs have demonstrated that in response to environmental stimuli, SMCs can differentiate into cells primarily concerned with contraction or regress to a pro-inflammatory, pro-matrix remodeling phenotype.1 Myocardin has a key role in promotion of differentiated SMCs with upregulation of contractile proteins, including SM-MHC, SM-α-actin, and SM-22α.1 Studies have found that in disease states such as vascular injury,23 experimental atherosclerosis,24 and hemodynamic stress,25 phenotypic modulation results in repression of the SMC contractile phenotype that is, at least in part, regulated by KLF4.1 Observations in the present study provide evidence that SMC differentiation and phenotypic modulation occurs, at least in part, via similar mechanisms within the cerebral circulation. Both in vitro and in vivo experiments in cerebral SMCs demonstrated increased expression of SMC contractile proteins under the direct control of myocardin and KLF4.
During aneurysm formation, there is thinning and degeneration within the media as spindle-like vascular SMCs dissociate from each other, migrate to the intima, and proliferate.8 This phenotypic alteration is believed to be characterized by a decreased expression of contractile proteins and an increased expression of inflammatory mediators. Prior studies have found that in response to injury and ischemia, TNF-α and other pro-inflammatory mediators are upregulated in cerebral blood vessels.3, 14 Both in in vitro and in vivo experiments performed herein, TNF-α induced downregulation of myocardin, SM-α-actin, SM-MHC, and SM-22-α and upregulation of MMP-3, MMP-9, MCP-1, VCAM-1, and IL-1β in a dose-dependent manner. Upregulation of these remodeling proteins has been linked to inflammation and IA. Specifically, MMPs upregulate proteinases and angiogenic factors linked to degradation of the extracelluar matrix.26 Overexpression of MMPs has been demonstrated both in humans,27 where increased expression was observed in ruptured aneurysms, and in animal experiments, where inhibition of MMPs28 blocked aneurysm progression. Monocyte chemoattractant protein-1 is a key inflammatory mediator in atherosclerosis and is overexpressed in IA and subarachnoid hemorrhage after aneurysm rupture.29 In experimental models, MCP-1 was upregulated in aneurysm walls, and MCP-1 knockout mice demonstrated a decreased expression of MMPs and inhibition of aneurysmal progression.30 In both SMCs and endothelial cells, VCAM-1 is a potent mediator of inflammatory cell adhesion and found to be upregulated in both experimental aneurysm models31 as well as in humans after aneurysm rupture.32 Vascular cell adhesion molecule-1 helps bind key inflammatory cells that lead to further release of matrix-remodeling proteins and vessel wall breakdown in aneurysm models. Similarly IL-1β has been found in the vascular media of cerebral aneurysms and IL-1β knockout mice have been associated with decreased incidence of advanced aneurysmal changes and apoptosis through caspase regulation.33 Thus, TNF-α may have multiple roles in aneurysm progression including SMC phenotypic modulation, upregulation of vessel wall remodeling and inflammatory genes, increased expression of pro-inflammatory proteins, and localization of inflammatory cells to areas of vascular injury.
Although a number of studies have found upregulation of key inflammatory genes in IA, the specific mechanisms have not been completely elucidated. It is possible that a number of other inflammatory genes are involved,8 but attention has focused on MMP-3, MMP-9, MCP-1, VCAM-1, and IL-1β as most of these genes have conserved KLF4-binding domains. In experiments of vascular injury23 and atherosclerosis24 in aortic SMCs, studies have demonstrated that phenotypic modulation was dependent on KLF4. This is mediated by downregulation of the SMC marker genes and the myocardin promoter. In the present study, phenotypic modulation occurred through a similar mechanism. Tumor necrosis factor-alpha potently increased KLF4 expression while resulting in a corresponding decrease in myocardin. Chromatin immunoprecipitation assays demonstrated direct binding of KLF4 to the promoter regions of myocardin and SMC genes after treatment with TNF-α. Inhibition of KLF4 with siRNA reversed these alterations. Additionally, similar SMC phenotypic modulation appears to occur early within the cerebral circulation in a rodent model of cerebral aneurysm formation through hemodynamic stress and induced hypertension with an analogous increase in TNF-α, KLF4, pro-inflammatory genes and a significant reduction in SM-α-actin and SM-MHC. Epigenetic changes characteristic of induced transcriptional activation of MCP-1 and suppression of myocardin and SMC differentiation genes,22, 34, 35 as demonstrated in CHIP assays in vitro and in vivo, provides further evidence that KLF4 regulates SMC phenotypic modulation through activation of pro-inflammatory genes and inhibition of myocardin-mediated activation of SMC genes.23, 36
Although KLF4 is necessary, it may not be sufficient to fully account for TNF-α induced suppression of SMC genes. Chromatin immunoprecipitation assays demonstrated that transcription factor SP1 was found to bind and activate the KLF4 promoter. In addition to KLF4, a number of other repressor pathways have been implicated in SMC phenotypic modulation,1 and TNF-α activates a variety of alternative pathways implicated in cerebral aneurysm formation.9, 10, 37 Similarly, studies are necessary to address the activation mechanisms of TNF-α in aneurysm formation. In this study, we have found that hemodynamic stress and induced hypertension lead to upregulation of TNF-α. Tumor necrosis factor-alpha is a potent inflammatory cytokine released by numerous inflammatory cells and its induction has been linked to many risk factors for aneurysm formation, including hemodynamic stress, hypertension, aging, gender, alcohol, and smoking.1, 8, 37 Although there have been limited studies, TNF-α has been found to be overexpressed in human aneurysms.9 The most likely link is a combination of genetic, environmental, and hemodynamic factors that result in vascular injury and inflammation leading to TNF-α upregulation, vascular SMC phenotypic modulation and, ultimately, vessel wall degradation.8
Prior studies have found upregulation of TNF-α in human excised aneurysm domes, but no studies have assessed the incidence of aneurysm formation or progression in human trials of patients receiving TNF-α inhibitors. Further studies will need to directly test the role of TNF-α-mediated vascular SMC phenotypic modulation in aneurysm growth, and its relevance to clinical events such as rupture. In addition to its potent pro-inflammatory effects, TNF-α is involved in signal initiation of apoptosis6, 37 and increased expression of its proapoptotic downstream target Fas has been found in IA.9 After TNF-α initiates vascular SMC phenotypic modulation and alteration from the contractile phenotype to an inflammatory/matrix-remodeling phenotype, it may eventually trigger apoptosis with subsequent loss of both phenotypes and aneurysmal rupture.8 Above experiments with terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling demonstrated increased apoptosis after high-dose TNF-α exposure (Supplementary Figure 1) and may be the ultimate end product of TNF-α-induced modulation of vascular SMCs. As SMCs are responsible for production of elastic fibers38 and extracellular matrix, their death may cause a decline in contractile element synthesis and vessel wall structural integrity that ultimately lead to aneurysm formation or progression.39 The underlying mechanism may occur through TNF-α upregulation of KLF4, as KLF4 has been shown to activate macrophages,40 which not only induce expression MMPs in aneurysms, but also have a key role in apoptosis and phagocytosis of SMCs leading to aneurysm progression.28, 30 The number of such apoptotic cells has been shown to correlate with the probability of rupture of cerebral aneurysms.41, 42 In addition to repression of SMC differentiation marker genes, KLF4 is a pluripotency factor involved in the reprogramming of somatic cells.15 This may, in part, explain how inflammatory cells and cytokines promote vascular wall remodeling and aneurysm formation.28, 30
In summary, results of the present studies provide novel in vitro and in vivo evidence showing that TNF-α profoundly suppresses expression of SMC differentiation genes and myocardin while concomitantly activating expression of KLF4 and matrix remodeling/inflammatory genes. Although phenotypic modulation cannot be directly tested in cerebral aneurysms as carried out in cultured SMCs and rat carotid arteries—a model of intracranial atherosclerosis20, 21—TNF-α was increased early in an animal model of aneurysm formation in parallel with changes in expression of the SMC marker gene SM-α-actin, SM-MHC, KLF4, and pro-inflammatory/matrix-remodeling genes in a similar fashion. This process was inhibited by treatment with the TNF-α synthesis inhibitor 3,6′-dithiothalidomide. This process appears, at least in part, to be regulated by TNF-α induction of KLF4 with inhibition of myocardin. In vivo and in vitro experiments show that TNF-α results in epigenetic changes with markedly enhanced binding of KLF4 to the promoter regions of SMC marker genes and recruitment of histone deacetylase, resulting in histone modifications and altered gene expression. Taken together, these results demonstrate a direct, novel role of TNF-α in phenotypic modulation of SMCs with important implications for the mechanisms by which TNF-α contributes to intracranial aneurysm formation. These processes, importantly, appear to be reversible, as assessed by TNF-α synthesis inhibition with 3,6′-dithiothalidomide. Although prior studies have found upregulation of TNF-α in human excised aneurysm domes,9, 10 no studies have assessed the incidence of aneurysm formation or progression in human trials of patients receiving TNF-α inhibitors. Additionally, inhibitors of key inflammatory proteins have been shown to preclude aneurysm development and progression in animal models, and further studies may lead to a beneficial alternative medical therapy in humans.
The authors declare no conflict of interest. The following disclosures are all modest. AS Dumont: Consultant for ev3, Stryker. P Jabbour: Consultant for ev3, Codman, Mizhuo. S Tjoumakaris: Consultant for Stryker. LF Gonzales: Consultant for ev3. R Rosenwasser: Consultant for Boston Scientific.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by NIH grants K08NS067072 to ASD, NIH grants R01 HL57353 (GKO), R01 HL098538 (GKO), and R01 HL087867 to GKO, and NIH P01 HL091799 and P01 HL07544 Project 2 to WJK. NHG is supported by the Intramural Research Program of NIH.
Supplementary Material
References
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- Edvinsson LI, Povlsen GK. Vascular plasticity in cerebrovascular disorders. J Cereb Blood Flow Metab. 2011;31:1554–1571. doi: 10.1038/jcbfm.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Owens GK. Molecular determinants of vascular smooth muscle cell diversity. Circ Res. 2005;96:280–291. doi: 10.1161/01.RES.0000155951.62152.2e. [DOI] [PubMed] [Google Scholar]
- Kilic T, Sohrabifar M, Kurtkaya O, Yildirim O, Elmaci I, Günel M, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls Neurosurgery 200557997–1007.discussion 1997-1007. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Nagahiro S, Sano T, Satomi J, Satoh K. Phenotypic modulation of smooth muscle cells in human cerebral aneurysmal walls. Acta Neuropathol. 2000;100:475–480. doi: 10.1007/s004010000220. [DOI] [PubMed] [Google Scholar]
- Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994;53:399–406. doi: 10.1097/00005072-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32:1659–1676. doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman T, Berenstein V, Li X, Mayer J, Silane M, Shin YS, et al. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms Neurosurgery 200557558–564.discussion 558-564. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Paget A, Shin YS, Li X, Mayer J, Chaudhry H, et al. TNF-alpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc Health Risk Manag. 2008;4:805–817. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- Maddahi A, Kruse LS, Chen QW, Edvinsson L. The role of tumor necrosis factor-alpha and TNF-alpha receptors in cerebral arteries following cerebral ischemia in rat. J Neuroinflammation. 2011;8:107. doi: 10.1186/1742-2094-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Nagata I, Handa H, Hashimoto N, Hazama F. Experimentally induced cerebral aneurysms in rats: Part VI. Hypertension. Surg Neurol. 1980;14:477–479. [PubMed] [Google Scholar]
- Luo WM, Yu QS, Tweedie D, et al. Synthesis of aromatic substituted 6'-thaiothalidomides. Synthesis. 2008. pp. 3415–3422.
- Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, et al. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweedie D, Luo W, Short RG, Brossi A, Holloway HW, Li Y, et al. A cellular model of inflammation for identifying TNF-alpha synthesis inhibitors. J Neurosci Methods. 2009;183:182–187. doi: 10.1016/j.jneumeth.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, et al. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–638. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, et al. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007;100:1428–1441. doi: 10.1161/01.RES.0000266448.30370.a0. [DOI] [PubMed] [Google Scholar]
- Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res. 2004;95:981–988. doi: 10.1161/01.RES.0000147961.09840.fb. [DOI] [PubMed] [Google Scholar]
- Shi ZD, Abraham G, Tarbell JM. Shear stress modulation of smooth muscle cell marker genes in 2-D and 3-D depends on mechanotransduction by heparan sulfate proteoglycans and ERK1/2. PLoS One. 2010;5:e12196. doi: 10.1371/journal.pone.0012196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, et al. Matrix metalloproteinase-9 in cerebral aneurysms Neurosurgery 199741642–666.discussion 646-647. [DOI] [PubMed] [Google Scholar]
- Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38:162–169. doi: 10.1161/01.STR.0000252129.18605.c8. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, et al. Bone marrow-derived monocyte chemoattractant protein-1 receptor CCR2 is critical in angiotensin II-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:e174–e178. doi: 10.1161/01.ATV.0000143384.69170.2d. [DOI] [PubMed] [Google Scholar]
- Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Li X, Zhong L, Hao-Tong, Di J, Liu F, et al. MCP-1, ICAM-1 and VCAM-1 are present in early aneurysmal dilatation in experimental rats. Folia Histochem Cytobiol. 2010;48:455–461. doi: 10.2478/v10042-010-0042-y. [DOI] [PubMed] [Google Scholar]
- Rothoerl RD, Schebesch KM, Kubitza M, Woertgen C, Brawanski A, Pina AL. ICAM-1 and VCAM-1 expression following aneurysmal subarachnoid hemorrhage and their possible role in the pathophysiology of subsequent ischemic deficits. Cerebrovasc Dis. 2006;22:143–149. doi: 10.1159/000093243. [DOI] [PubMed] [Google Scholar]
- Moriwaki T, Takagi Y, Sadamasa N, Aoki T, Nozaki K, Hashimoto N. Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice. Stroke. 2006;37:900–905. doi: 10.1161/01.STR.0000204028.39783.d9. [DOI] [PubMed] [Google Scholar]
- McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116:36–48. doi: 10.1172/JCI26505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Reinberg D. Histone lysine demethylases and their impact on epigenetics. Cell. 2006;125:213–217. doi: 10.1016/j.cell.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Inci S, Spetzler RF.Intracranial aneurysms and arterial hypertension: a review and hypothesis Surg Neurol 200053530–540.discussion 540-532. [DOI] [PubMed] [Google Scholar]
- Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- Kondo S, Hashimoto N, Kikuchi H, Hazama F, Nagata I, Kataoka H.Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats Stroke 199829181–188.discussion 189. [DOI] [PubMed] [Google Scholar]
- Hara A, Yoshimi N, Mori H. Evidence for apoptosis in human intracranial aneurysms. Neurol Res. 1998;20:127–130. doi: 10.1080/01616412.1998.11740494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.