Abstract
Endothelial nitric oxide synthase (eNOS) dysfunction is related to secondary injury and lesion expansion after cerebral ischemia. To date, there are few reports about postischemic alterations in the eNOS regulatory system. The purpose of the present study was to clarify eNOS expression, Ser1177 phosphorylation, and monomer formation after cerebral ischemia. Male Wistar rats were subjected to transient focal cerebral ischemia. Endothelial nitric oxide synthase messenger RNA (mRNA) and protein expression increased ∼8-fold in the ischemic lesion. In the middle cerebral artery core, eNOS-Ser1177 phosphorylation increased 6 hours after ischemia; however, there was an approximately 90% decrease in eNOS-Ser1177 phosphorylation observed 24 hours after ischemia that continued until at least 7 days after ischemia. Endothelial nitric oxide synthase monomer formation also increased 24 and 48 hours after ischemia (P<0.05), and protein nitration progressed in parallel with monomerization. To assess the effect of a neuroprotective agent on eNOS dysfunction, we evaluated the effect of fasudil, a Rho-kinase inhibitor, on eNOS phosphorylation and dimerization. Postischemic treatment with fasudil suppressed lesion expansion and dephosphorylation and monomer formation of eNOS. In conclusion, functional deterioration of eNOS progressed after cerebral ischemia. Rho-kinase inhibitors can reduce ischemic lesion expansion as well as eNOS dysfunction in the ischemic brain.
Keywords: endothelium, focal ischemia, nitric oxide
Introduction
Endothelial dysfunction contributes to the expansion of ischemic lesions through microcirculatory disturbances after cerebral ischemia.1 Endothelium-derived nitric oxide (NO) is an important signal transduction molecule that preserves and maintains brain microcirculation. Nitric oxide increases cyclic guanosine monophosphate in target cells and regulates vascular contraction, platelet aggregation, and white blood cell adhesion to the endothelium. In endothelial cells, NO is synthesized by endothelial nitric oxide synthase (eNOS), a key molecule that maintains vascular homeostasis.
Endothelial nitric oxide synthase, which is constitutively expressed in the endothelial cells, produces NO under regulation systems, including calcium–calmodulin phosphorylation and dimerization. In particular, Ser1177 is an important phosphorylation residue that activates eNOS.2, 3 Phosphorylation of eNOS-Ser1177 influences cerebral blood flow (CBF) and infarct size after brain ischemia.4, 5, 6 Recently, we reported that eNOS function was impaired in adult spontaneous hypertensive rat brain microvessels.5 Endothelial nitric oxide synthase phosphorylation is reduced upon blood pressure elevation, and treatment with anti-hypertensive drugs preserves eNOS phosphorylation and ameliorates ischemic brain damage. This result indicates that eNOS phosphorylation is related to endothelial function and cerebral ischemia pathophysiology. Dimer formation is also required for eNOS to produce NO.7 Reactive oxygen species inhibit eNOS dimerization, which results in reduced NO production as well as superoxide production. Superoxide reacts with NO to produce peroxynitrite, which is harmful for the ischemic brain. This eNOS condition, called eNOS uncoupling, may relate to the lesion expansion after cerebral ischemia.
The pre-ischemic eNOS condition is closely related to cerebral ischemia severity. In the ischemic brain, loss of eNOS activity results in reduced CBF and ischemic lesion expansion.8, 9 Increased eNOS activity before ischemia ameliorates CBF reduction and lesion expansion.5, 6, 10, 11 Nonetheless, postischemic alterations in eNOS are not fully understood. There are reports about postischemic eNOS that demonstrate increased eNOS protein expression after cerebral ischemia.12, 13 However, there are few reports that show profiles of the eNOS regulatory system, such as phosphorylation and dimerization, after cerebral ischemia. Because ischemic lesions can expand gradually over 24 hours because of the microcirculatory disturbances via endothelial dysfunction,14, 15 investigations of the postischemic eNOS condition are necessary to understand cerebral ischemia pathology and assess the therapeutic approach. The purpose of this study was to elucidate the temporal and spatial profiles of eNOS expression, phosphorylation, and dimerization after focal cerebral ischemia. Additionally, we assessed the effect of neuroprotective agents on eNOS dysfunction. The Rho-kinase inhibitor fasudil is known to ameliorate ischemic brain injury.11, 15, 16 In this study, we evaluated the effect of fasudil on eNOS phosphorylation and dimerization after cerebral ischemia.
Materials and Methods
Animal Model
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Osaka University Graduate School of Medicine and all experiments were performed in accordance with the Regulations on Animal Experiments at Osaka University. Adult male Wistar rats weighing 300 to 340 g were used. The middle cerebral artery (MCA) was occluded for 80 minutes by using an intraluminal filament technique as previously described.5, 6 Animals were anesthetized via facemask with 1.5% halothane. The right common carotid artery was exposed, and the internal carotid artery was isolated and carefully separated. The tip of a 4-0 nylon monofilament was rounded by heating. It was introduced from the internal carotid artery bifurcation. Eighty minutes after ischemia induction, the filament was withdrawn to allow reperfusion. Neurologic symptoms were assessed after reperfusion. Rats were used for experiments if they failed to fully extend the left forepaw or circled to the left. Rats with symptoms that were too severe, such as coma, seizure, or respiratory disturbance, were excluded. Rectal temperature was monitored and maintained at 37.0±0.5°C during the procedure.
Tissue Sampling
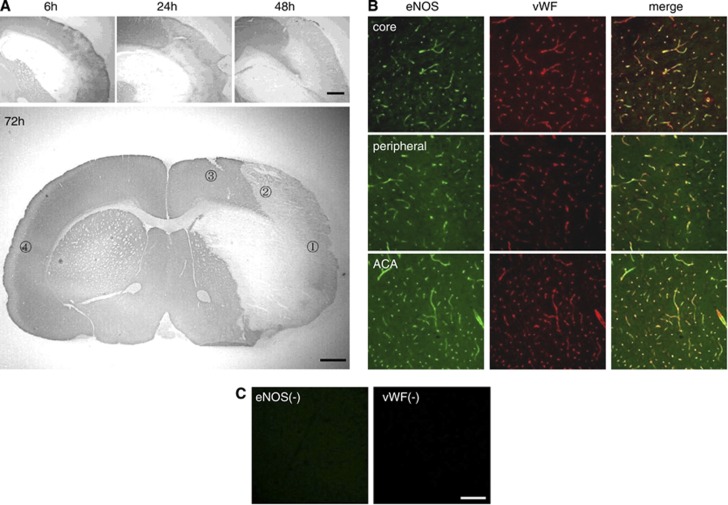
The brains were divided coronally into the rostral and caudal parts. The rostral parts were subjected to microtubule-associated protein 2 immunohistochemistry to confirm ischemic lesions. The brain tissue samples were taken from the caudal parts to be subjected to real-time polymerase chain reaction and western blot. Tissue samples were obtained from each brain region: the MCA core, MCA peripheral area, and anterior cerebral artery (ACA) area in the cerebral cortex (Figure 1A).
Figure 1.
Ischemic lesions and endothelial nitric oxide synthase (eNOS) expression after transient focal brain ischemia. (A) Microtubule-associated protein immunostaining in the ischemic brain. The ischemic lesion in the brain cortex gradually expanded from the middle cerebral artery (MCA) core to the MCA peripheral area. The bottom panel shows the ischemic lesion 72 hours after ischemia and the regions of interest for assessment; (1) Middle cerebral artery core, (2) MCA peripheral area, (3) anterior cerebral artery (ACA) area, (4) contralateral side. (B) Double immunostaining of eNOS (green) and von Willebrand factor (vWF) (red) in the MCA core, MCA peripheral area, and ACA area. The eNOS signals were mainly localized in the brain microvessels. (C) Immunohistochemistry without primary antibodies showed specificity of eNOS and vWF signals. Scale bar, 1 mm in panel A, 100 μm in panels B and C.
Immunohistochemistry
The brains were immediately frozen in powdered dry ice and cut into 14-μm-thick coronal sections. The sections were fixed in 4% paraformaldehyde for 15 minutes at room temperature. Nonspecific staining was blocked using 10% normal serum (Vector Laboratories, Burlingame, CA, USA; Jackson Immunoresearch, West Grove, PA, USA). Sections were incubated with the primary antibodies, mouse anti-microtubule-associated protein 2 antibody (Sigma, St Louis, MO, USA), rabbit anti-von Willebrand factor antibody (vWF; Abcam, Cambridge, MA, USA), and mouse anti-eNOS antibody (BD Transduction Laboratories, San Diego, CA, USA) diluted in Tris-buffered saline with 0.1% Triton X-100 at 4°C overnight. After washing in Tris-buffered saline, the sections were incubated with biotinylated secondary antibodies for 60 minutes at room temperature. The ABC Elite kit (Vector Laboratories) was used and staining was detected using a diaminobenzidine–peroxidase reaction. Fluorescence-conjugated secondary antibodies (Jackson Immunoresearch) were used for immunofluorescence after incubation with primary antibodies. Fluorescent images were observed using laser scanning confocal microscopy (FV1000-D; Olympus, Tokyo, Japan).
Real-Time Polymerase Chain Reaction
Total RNA was isolated using Trizol (Invitrogen, Carlsbad, CA, USA) from brain tissue. RNA was reverse transcribed using random hexamers and the High-Capacity cDNA Reverse Transcription Kit according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Real-time polymerase chain reaction was performed using the TaqMan Universal Master Mix and TaqMan Gene Expression Assays (Applied Biosystems). Relative gene expression data were obtained using the delta–delta CT method. Endothelial nitric oxide synthase mRNA expression was normalized to β-actin as an internal control.
Western Blot
Brain homogenate supernatants were mixed with Laemmli sample buffer containing 2-mercaptoethanol and were boiled for 5 minutes. The protein samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and separated proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA, USA). Membranes were incubated with the following primary antibodies: mouse anti-eNOS antibody (BD Transduction Laboratory), rabbit anti-phospho-eNOS antibody (Cell Signal Technology, Beverly, MA), and mouse anti-β-actin antibody (Sigma). Proteins were detected using the ECL detection system (GE Healthcare, Buckinghamshire, UK) after incubation with secondary antibodies (GE Healthcare). Signals were quantified using the ImageJ program and the results are expressed as the ratio of phospho-eNOS to eNOS or normalized to β-actin as an internal control.
Non-Reducing Low-Temperature SDS-PAGE
To detect the eNOS dimer, non-reducing low-temperature SDS-PAGE was performed as previously reported, with some modification.17 Briefly, the brain homogenate supernatants were mixed with Laemmli sample buffer (without 2-mercaptoethanol or boiling) and subjected to low-temperature SDS-PAGE. The electrophoresis tank was placed in an ice bath during electrophoresis to keep the low temperature. The procedure after the transfer process was performed as above. The degree of eNOS monomer formation is expressed as monomer/(monomer+dimer).
3-Nitrotyrosine Immunohistochemistry
Perfusion-fixed brain tissue was used for 3-nitrotyrosine (3-NT) immunohistochemistry. Rats were transcardially perfused with Zamboni's solution (2% paraformaldehyde and 0.2% picric acid), and the brains were postfixed overnight at 4°C in the same fixative. Brains were cut into frozen 14-μm-thick coronal sections. The subsequent procedure was performed same as other immunohistochemistry. A rabbit anti-3-NT antibody (Abcam) was used as the primary antibody.
Evaluation of Fasudil on Brain Lesions and eNOS Dysfunction
Fasudil (10 mg/kg body weight), a Rho-kinase inhibitor, was injected intraperitoneally. To evaluate fasudil's effect on eNOS dephosphorylation, monomerization, and brain injury after ischemia, fasudil was injected immediately and 24 hours after ischemia. Brain samples were obtained 30 hours after ischemia for western blot. Ischemic brain lesions were evaluated 48 hours after ischemia using 2,3,5-triphenyltetrazolium chloride staining of 2-mm-thick coronal brain sections. Lesion volume was determined by integrating the appropriate area and section thickness.
Statistical Analyses
All values are expressed as the mean±s.e. Kruskal–Wallis tests were used for multiple comparisons. Comparisons between the two groups were performed using Mann–Whitney U test. P<0.05 was considered statistically significant.
Results
Ischemic Lesion Expansion and eNOS Localization in the Brain After Focal Ischemia
Among all operated rats, 16.3% were excluded because of death, subarachnoid hemorrhage, no neurologic deficit, seizure, respiratory disturbance, and coma. No rats died during the operation and postoperative mortality was 4.1%. The incidence of subarachnoid hemorrhage was 4.9%. Immunoreactivity of microtubule-associated protein 2, a neuronal marker, was lost in parts of the striatum and cerebral cortex 6 hours after ischemia (Figure 1A). Neuronal loss gradually expanded after ischemia to the peripheral area of the MCA perfusion territory in the cortex. Twenty-four hours after ischemia, expression of vWF, an endothelial marker, was preserved in the MCA core, where massive neuronal loss had occurred (Figure 1B). Endothelial nitric oxide synthase immunoreactivity mainly colocalized with vWF in both ischemic lesions and the intact area. Immunohistochemistry performed without the primary antibodies showed the specificity of the eNOS and vWF signals (Figure 1C).
eNOS mRNA Expression After Focal Brain Ischemia
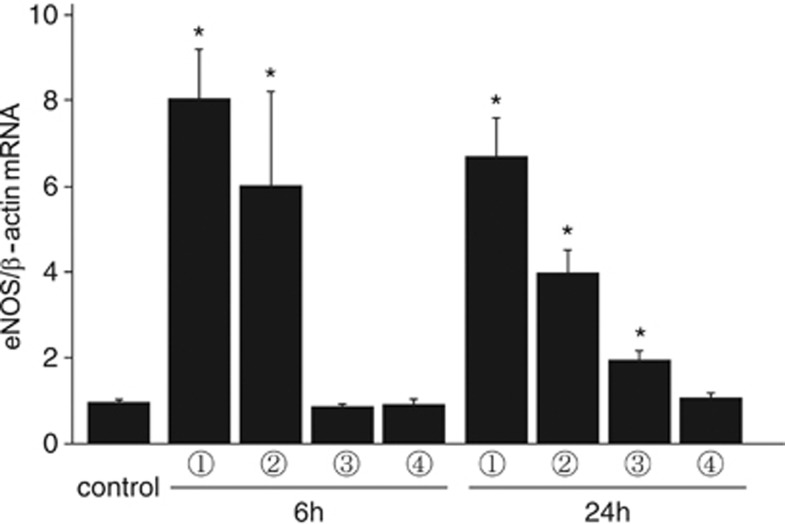
The temporal profile of eNOS mRNA was investigated using real-time polymerase chain reaction. In the MCA core and peripheral area of the cerebral cortex, the relative amount of eNOS mRNA expression increased 8.1±1.2-fold and 6.0±2.2-fold 6 hours after ischemia, respectively, and 6.7±0.8-fold and 4.0±0.5-fold 24 hours after ischemia, respectively (Figure 2). Interestingly, eNOS mRNA expression also increased significantly in the ACA area 24 hours after ischemia. In the contralateral side, eNOS mRNA expression was not different between the control and ischemic brains.
Figure 2.
Endothelial nitric oxide synthase (eNOS) messenger RNA (mRNA) expression after focal brain ischemia. Expression of eNOS mRNA was evaluated using real time reverse transcription polymerase chain reaction. In the middle cerebral artery core and peripheral area of the ischemic side, eNOS mRNA increased significantly 6 and 24 hours after ischemia. In the anterior cerebral artery area, eNOS mRNA increased 24 hours after ischemia. *P<0.05 versus control, n=5 in each group.
eNOS Expression and Phosphorylation in the Brain Cortex After Transient Focal Ischemia
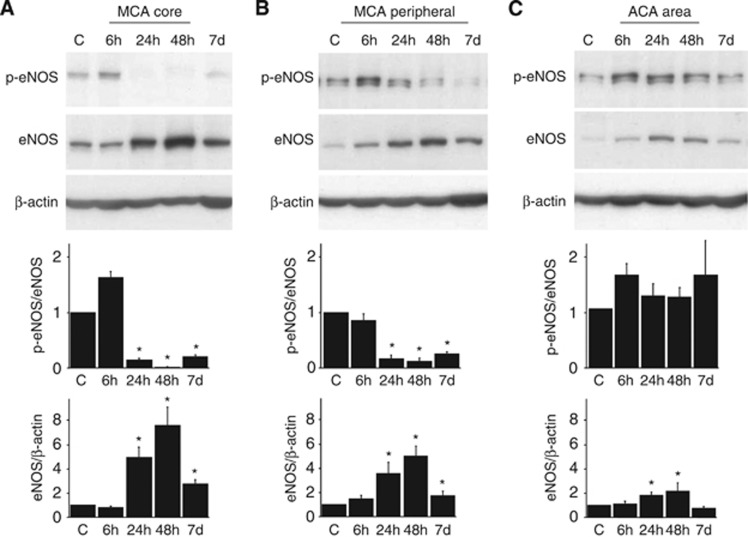
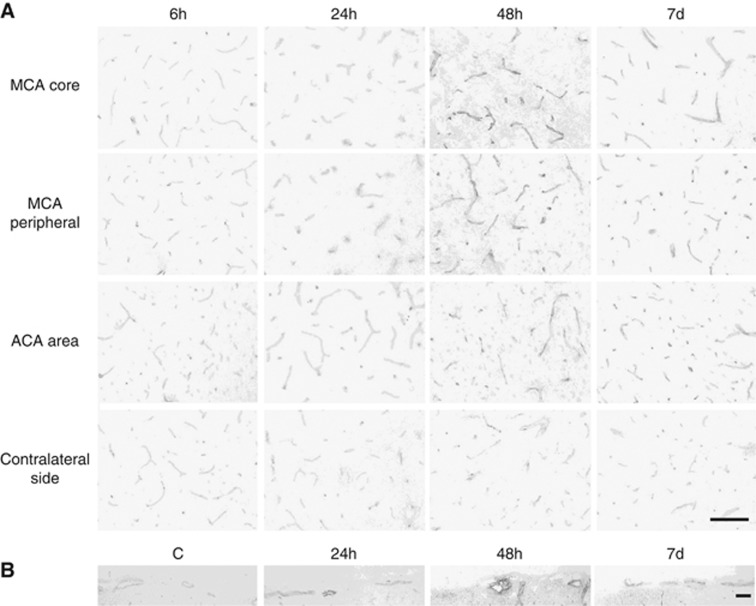
Endothelial nitric oxide synthase protein expression was markedly increased in the MCA area 24 hours after ischemia, 5.1±1.5-fold in the MCA core and 3.6±0.8-fold in the MCA peripheral area (Figures 3A and 3B). Increased eNOS protein expression lasted until 7 days after ischemia. Although eNOS phosphorylation tended to increase (1.5±0.2-fold; P=0.12) in the MCA core 6 hours after ischemia, it dramatically decreased 24 hours after ischemia, 89% decrease in the MCA core and 81% decrease in the MCA peripheral area (Figure 3A). In the ACA area, the adjacent region of the ischemic lesion, eNOS expression increased 24 and 48 hours after ischemia and eNOS phosphorylation was preserved (Figure 3C). Immunohistochemical analyses showed increased eNOS signals in the brain parenchymal small vessels (Figure 4A) and the larger vessels on the brain surface (Figure 4B).
Figure 3.
Endothelial nitric oxide synthase (eNOS) expression and phosphorylation in the brain cortex after transient focal ischemia. Endothelial nitric oxide synthase phosphorylation tended to increase 6 hours after ischemia in the middle cerebral artery (MCA) core (A). However, it markedly decreased in the MCA core (A) and MCA peripheral area (B) 24 hours after ischemia, although increased eNOS expression was observed in both areas. In the anterior cerebral artery (ACA) area, eNOS expression increased without decreased eNOS phosphorylation (C). *P<0.05 versus control, n=5 in each group.
Figure 4.
Endothelial nitric oxide synthase (eNOS) immunohistochemistry in the brain cortex after transient focal ischemia. Endothelial nitric oxide synthase expression in the parenchymal small vessels increased in the middle cerebral artery (MCA) core, MCA peripheral area, and anterior cerebral artery (ACA) area, compared with the contralateral side (A). Signals of eNOS also increased in the larger vessels on the brain surface (B). Scale bar, 100 μm in panels A and B.
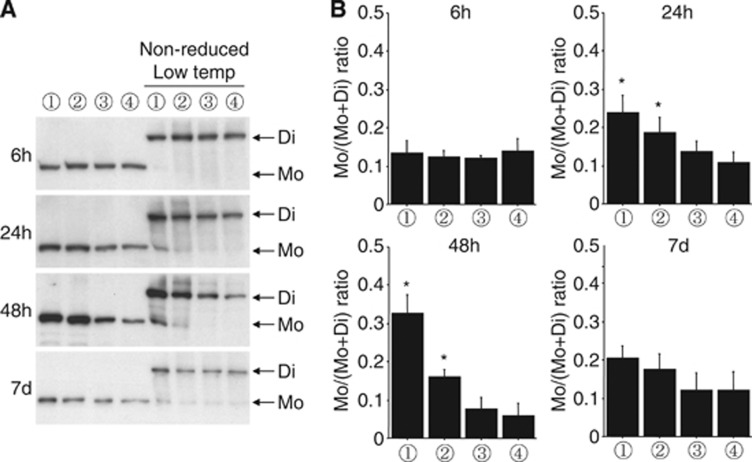
Increased eNOS Monomers After Transient Focal Ischemia
In normal endothelial cells, eNOS works as a homodimer to produce NO. However, eNOS monomers can be the source of superoxide anions in pathologic states. We evaluated eNOS monomerization by using non-reducing low-temperature SDS-PAGE (Figure 5). Monomer levels were not increased 6 hours after ischemia. In parallel with the progression of neuronal loss, eNOS monomers were increased 24 and 48 hours after ischemia in the MCA core. Lower levels of eNOS monomer were observed in the MCA peripheral region compared with the MCA core. This result suggests that the progression of eNOS monomerization is related to the degree of ischemia-induced tissue damage. Increases in eNOS monomers were not significant 7 days after ischemia. In the ACA region, eNOS monomerization was not altered.
Figure 5.
Increased endothelial nitric oxide synthase (eNOS) monomerization after transient focal ischemia. Western blot (A) and quantitative analyses (B). Non-reducing low-temperature SDS-PAGE was performed to assess eNOS dimer and monomer levels. Increased eNOS monomers were observed in the middle cerebral artery (MCA) core and peripheral area 24 and 48 hours after ischemia. Lane numbers indicate the brain area; (1) MCA core, (2) MCA peripheral area, (3) anterior cerebral artery area, (4) contralateral side, as shown in Figure 1A. *P<0.05 versus contralateral side, n=5 in each group.
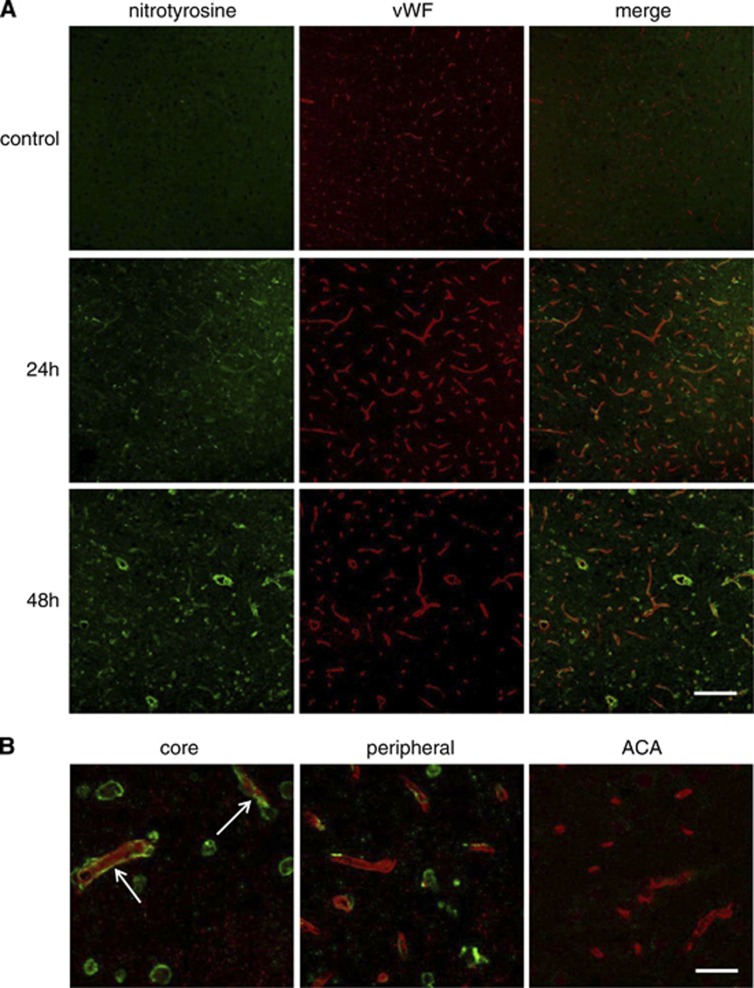
Increased 3-nitrotyrosine Expression in Parallel with eNOS Monomerization
In the MCA core, 3-NT signals increased 24 and 48 hours after ischemia (Figure 6A). Some 3-NT signals colocalized with vWF signals, an endothelial cell marker. Forty-eight hours after ischemia, 3-NT signals in the endothelial cells were detected more obviously in the MCA core than in the MCA peripheral area (Figure 6B). Increased 3-NT signals in the endothelial cells may be temporally and spatially related to eNOS monomerization. Few 3-NT signals could be detected in the ACA area.
Figure 6.
Increased 3-NT signals in the ischemic brain. 3-NT immunohistochemistry revealed that 3-NT signals increased 24 hours after ischemia, but substantially increased 48 hours after ischemia (A). Forty-eight hours after ischemia, there were increased 3-NT signals in the endothelial cells (arrow) in the middle cerebral artery (MCA) core compared with the MCA peripheral and anterior cerebral artery (ACA) area (B). Scale bar, 100 μm in panel A and 25 μm in panel B. vWF, von Willebrand factor.
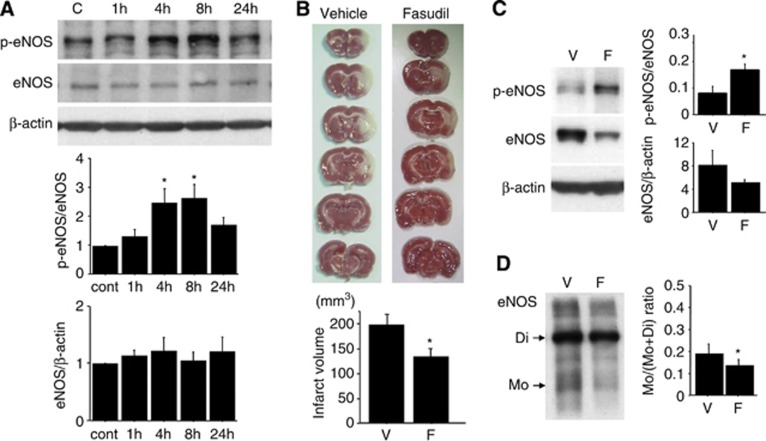
Effect of the Rho-kinase Inhibitor Fasudil on Brain Lesions and eNOS Dysfunction After Ischemia
A single injection of fasudil increased eNOS phosphorylation levels in the normal brain 4 to 8 hours after treatment (Figure 7A). This result suggests that the Rho-kinase inhibitor enhances eNOS activity in the brain in vivo. Next, we investigated the effect of fasudil on tissue injury and eNOS dysfunction after ischemia. Brain lesion size was evaluated using 2,3,5-triphenyltetrazolium chloride staining and was significantly smaller in the fasudil-treated group (Figure 7B). Endothelial nitric oxide synthase phosphorylation in the MCA core was preserved in fasudil-treated brains, compared with vehicle-treated brains (Figure 7C). Additionally, eNOS monomerization was partially prevented by fasudil treatment (Figure 7D). These results suggest that fasudil reduces brain injury as well as eNOS dysfunction after ischemia.
Figure 7.
The effect of fasudil on ischemic lesion expansion and endothelial nitric oxide synthase (eNOS) dysfunction. (A) Systemic treatment of fasudil (10 mg/kg body weight) enhances eNOS phosphorylation in the normal brain cortex, which lasts up to 8 hours after injection. *P<0.05 versus control, n=6 in each group. (B) Fasudil injection immediately and 24 hours after ischemia ameliorates ischemic brain injury 48 hours after ischemia. *P<0.05 versus vehicle-treated group, n=9 in each group. (C, D) According to the reduction in brain tissue injury, eNOS phosphorylation and dimerization were significantly preserved upon fasudil treatment. Assays were performed 30 hours after ischemia. *P<0.05 versus vehicle-treated group, n=5 in each group.
Discussion
This report is the first to describe the temporal profiles of eNOS phosphorylation and monomerization after focal brain ischemia. Phosphorylation of eNOS-Ser1177 is increased in the ischemic core region 6 hours after ischemia, but is markedly decreased in the ischemic lesion 24 hours after ischemia. Endothelial nitric oxide synthase monomerization significantly increased 24 and 48 hours after ischemia. These results indicate that eNOS function deteriorates despite increased eNOS expression in the injured brain after ischemia.
Focal cerebral ischemia is known to increase eNOS protein expression in cerebral blood vessels.12, 13 In contrast, the present study reveals that eNOS-Ser1177 phosphorylation is markedly decreased in the injured area, suggesting that eNOS-mediated NO production is decreased after cerebral ischemia. These findings are consistent with previous reports.18, 19 Loss of eNOS decreases postischemic CBF, reduces the penumbral region, and increases the infarct area.8, 9 These findings indicate that eNOS activity is important in preventing lesion expansion after cerebral ischemia. Among the several regulatory systems of eNOS function, eNOS-Ser1177 phosphorylation is a major regulator of eNOS activity.2, 3 In the ischemic brain, loss of eNOS phosphorylation increases lesion size due to eNOS dysfunction.4 There are reports that eNOS phosphorylation is decreased in the brain under some pathologic conditions. Decreased eNOS phosphorylation is observed in the brain parenchymal small vessels under atherogenic conditions, such as hypertension.5 Microcirculatory disturbances due to eNOS dysfunction are one mechanism of lesion expansion in spontaneous hypertensive rat after cerebral ischemia. Adiponectin, an anti-atherogenic factor, can increase eNOS phosphorylation in the brain. Loss of adiponectin results in larger brain lesions in ischemic mice compared with wild-type mice.20 These results indicate that decreased eNOS phosphorylation is closely related to brain ischemia severity in various pathologic conditions. Because eNOS is necessary for angiogenesis and neurogenesis,21, 22, 23 eNOS may be involved in tissue repair and remodeling. In the present study, we demonstrate persistent decreases in eNOS phosphorylation for at least 7 days after ischemia. Therefore, it may be one factor that inhibits functional recovery in the chronic phase.
Under physiologic conditions, eNOS works as a homodimer to produce NO. In contrast, l-arginine or tetrahydrobiopterin insufficiency causes eNOS monomerization, called eNOS uncoupling.7 Uncoupled eNOS fails to produce NO, generates superoxide, and enhances tissue injury in vascular disease. Endothelial nitric oxide synthase uncoupling is observed in vivo in some pathologic conditions, such as hyperglycemia.24 It is also observed in subarachnoid hemorrhage and may contribute to enhanced brain injury.25 In the present study, we demonstrate that eNOS monomers increase in brain lesions after focal ischemia. Superoxide may be produced by eNOS monomers in the injured area. The reaction between superoxide and NO produces peroxynitrite and worsens brain tissue injury because of strong oxidative and nitrosative stress.26 In our study, eNOS monomerization increased 24 and 48 hours after ischemia in the ischemic lesion. Additionally tyrosine nitrosylation increased in the endothelial cells in parallel with eNOS monomerization. These findings indicate that eNOS dysfunction may be related to secondary injury and lesion expansion after cerebral ischemia.
To evaluate the profile of eNOS mRNA after cerebral ischemia, β-actin was used as an internal control because it had been used previously.27 Endothelial nitric oxide synthase mRNA expression increased significantly 6 and 24 hours after ischemia in the MCA core and peripheral area, suggesting that eNOS protein increases because of transcriptional activation. Interestingly, it was revealed that eNOS mRNA expression increased in the ACA area, which is adjacent to ischemic region. This may be related to changes of CBF, such as collateral flow from the ACA area to the MCA area. Only a few reports have documented eNOS mRNA expression after ischemia. One report used reverse transcription polymerase chain reaction to show that eNOS mRNA increases after focal cerebral ischemia.27 The finding of this report is consistent with our current findings, and our data adds quantitative information.
In the current study, we examined the effect of a neuroprotective agent on postischemic eNOS dysfunction in brain lesions. The Rho-kinase inhibitor fasudil ameliorates brain tissue injury via rapid augmentation of eNOS phosphorylation, which results in the suppression of eNOS dephosphorylation and monomerization in the injured area. Fasudil is known to exert a brain protective effect via eNOS activation against focal cerebral ischemia.11, 16 Rho-kinase is abnormally activated in endothelial cells after focal cerebral ischemia.15 Endothelial Rho-kinase activation decreases the half life of eNOS mRNA and thus suppresses eNOS expression and activity.28 Repeated fasudil treatment before ischemia increases eNOS expression and decreases ischemic brain lesions.11 Furthermore, acute treatment after ischemia also ameliorates ischemic brain injury.15, 16 Previous studies have shown that Rho-kinase inhibition increases eNOS-Ser1177 phosphorylation via the PI3K/Akt pathway in vitro and in the heart.29, 30 The present study demonstrates that eNOS-Ser1177 phosphorylation rapidly increases in the brain upon fasudil treatment. These results may explain the acute protective effect against cerebral ischemia. Six hours after ischemia, eNOS phosphorylation is increased in the MCA core region; this is thought to be an endogenous protective reaction against ischemic insult. Fasudil may enhance eNOS phosphorylation in the acute phase to suppress expansion of ischemic lesions. Additionally, we found that fasudil prevents eNOS dysfunction in ischemic lesions. Prevention of eNOS dephosphorylation and monomerization may contribute to the suppression of secondary injury in the ischemic brain and tissue remodeling in later phases.
Some information is gained regarding the mechanism of fasudil against eNOS dephosphorylation and monomerization. One is the direct effect on eNOS. Increased eNOS phosphorylation by fasudil in the acute phase prevents eNOS dysfunction in the later phase. Another is the indirect effect. Because dephosphorylation and monomerization parallel neuronal loss and brain tissue injury, they may cause eNOS dysfunction. In such cases, reduction of neuronal loss by fasudil may contribute to the suppression of eNOS dysfunction. It is difficult to assess these hypotheses in the current in vivo study. Further study is necessary to clarify the exact mechanisms of action of fasudil.
In the present study, we used a partial reperfusion model.5, 6, 15 Carotid artery occlusion may influence CBF after reperfusion and result in mild hypoperfusion around the ischemic lesion and broader penumbra. Although the present results cannot be directly compared with the data obtained using a complete reperfusion model, this model can also be useful to investigate temporal and spatial changes in the penumbra.
In conclusion, eNOS phosphorylation is increased in the acute phase, but is markedly decreased in the later phases of ischemia. Uncoupling of eNOS is significantly increased in this phase. Because eNOS dysfunction may contribute to secondary injury and inhibit tissue repair, endothelial dysfunction in the later phases may be an important therapeutic target of cerebral ischemia. To correctly understand the contribution of eNOS to cerebral ischemia pathology, evaluating eNOS expression, phosphorylation, and monomerization is necessary.
Acknowledgments
Fasudil was kindly provided by Asahi Kasei Parma Corporation, Tokyo, Japan. The authors thank K Nishiyama for technical assistance and C Kurano for secretarial assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant Number 24591260.
References
- Terpolilli NA, Moskowitz MA, Plesnila N. Nitric oxide: considerations for the treatment of ischemic stroke. J Cereb Blood Flow Metab. 2012;32:1332–1346. doi: 10.1038/jcbfm.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama N, Yagita Y, Sasaki T, Omura-Matsuoka E, Terasaki Y, Sugiyama Y, et al. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J Neurosci Res. 2010;88:2889–2898. doi: 10.1002/jnr.22441. [DOI] [PubMed] [Google Scholar]
- Oyama N, Yagita Y, Kawamura M, Sugiyama Y, Terasaki Y, Omura-Matsuoka E, et al. Cilostazol, not aspirin, reduces ischemic brain injury via endothelial protection in spontaneously hypertensive rats. Stroke. 2011;42:2571–2577. doi: 10.1161/STROKEAHA.110.609834. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Lo EH, Hara H, Rogowska J, Trocha M, Pierce AR, Huang PL, et al. Temporal correlation mapping analysis of the hemodynamic penumbra in mutant mice deficient in endothelial nitric oxide synthase gene expression. Stroke. 1996;27:1381–1385. doi: 10.1161/01.str.27.8.1381. [DOI] [PubMed] [Google Scholar]
- Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36:2251–2257. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M, Zaloga C, Pollock JS, Forstermann U. Cerebral endothelial nitric oxide synthase expression after focal cerebral ischemia in rats. Stroke. 1993;24:2016–2022. doi: 10.1161/01.str.24.12.2016. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002;33:2704–2710. doi: 10.1161/01.str.0000033132.85123.6a. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Ohtsuki T, Kuwabara K, Yagita Y, Yanagihara T, et al. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31:1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Sasaki T, Terasaki Y, Todo K, Omura-Matsuoka E, et al. Rho-kinase activation in endothelial cells contributes to expansion of infarction after focal cerebral ischemia. J Neurosci Res. 2007;85:2460–2469. doi: 10.1002/jnr.21375. [DOI] [PubMed] [Google Scholar]
- Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, et al. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27:998–1009. doi: 10.1038/sj.jcbfm.9600406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema RC, Ju H, Zou R, Ryan JW, Venema VJ. Subunit interactions of endothelial nitric-oxide synthase. Comparisons to the neuronal and inducible nitric-oxide synthase isoforms. J Biol Chem. 1997;272:1276–1282. doi: 10.1074/jbc.272.2.1276. [DOI] [PubMed] [Google Scholar]
- Malinski T, Bailey F, Zhang ZG, Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1993;13:355–358. doi: 10.1038/jcbfm.1993.48. [DOI] [PubMed] [Google Scholar]
- Sugimura T, Sako K, Tohyama Y, Yonemasu Y. Consecutive in vivo measurement of nitric oxide in transient forebrain ischemic rat under normothermia and hypothermia. Brain Res. 1998;808:313–316. doi: 10.1016/s0006-8993(98)00822-1. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Chourbaji S, Bartsch C, Urani A, et al. Differential effect of endothelial nitric oxide synthase (NOS-III) on the regulation of adult neurogenesis and behaviour. Eur J Neurosci. 2004;20:885–895. doi: 10.1111/j.1460-9568.2004.03559.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Knight B, Tariq A, Jeon H, Shang X, et al. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:190–199. doi: 10.1038/jcbfm.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama N, Takizawa S, Ishida H, Hoshiai K, Shinohara Y, Nakazawa H. Peroxynitrite formation in focal cerebral ischemia-reperfusion in rats occurs predominantly in the peri-infarct region. J Cereb Blood Flow Metab. 1998;18:123–129. doi: 10.1097/00004647-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, et al. Central administration of angiotensin-(1-7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42:593–600. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, et al. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]