Abstract
Polymicrogyria (PMG) is one of a large group of human cortical malformations that collectively account for a significant percentage of patients with epilepsy, congenital neurological deficits, and intellectual disability. PMG is characterized by an excess of small gyri and abnormal cortical lamination. The most common distribution is bilateral, symmetrical, and maximal, in the region surrounding the sylvian fissures, and is known as “bilateral perisylvian polymicrogyria” (BPP). Most cases are sporadic, although several families have been observed with multiple affected members, usually following an X-linked inheritance pattern. Here we report the first genetic locus for BPP mapped by linkage analysis in five families. Linkage places the critical region for BPP at Xq28 (LOD score 3.08 in Xq28, distal to DXS8103 by multipoint analysis). We suggest that this region contains a gene that is necessary for correct neuronal organization and that the identification of this gene will both enhance our understanding of normal cortical development and accelerate the identification of other genes responsible for PMG.
Polymicrogyria (PMG) is a malformation of cortical development in which the brain surface is irregular and the normal gyral pattern is replaced by multiple small, partly fused gyri separated by shallow sulci. Microscopic examination shows a simplified four-layered or unlayered cortex (Crome 1952; Ferrer 1984). Several patterns of PMG—including bilateral frontal, bilateral perisylvian, and bilateral mesial occipital PMG—have been described on the basis of their topographic distribution (Kuzniecky et al. 1993; Guerrini et al. 1997, 2000; Barkovich et al. 1999). All but the perisylvian form appear to be rare. Bilateral perisylvian PMG (BPP [MIM 260980]) often results in a typical clinical syndrome that is manifested by mild mental retardation, epilepsy, and pseudobulbar palsy, which causes difficulties with expressive speech and feeding (Kuzniecky et al. 1993).
Recognition of the various malformations of cortical development has increased significantly since the use of magnetic resonance imaging (MRI) has become widespread. Collectively, these malformations account for a significant proportion of epilepsy, mental retardation, and congenital neurological deficit, with the incidence of PMG and related cortical malformations likely to be ∼1/2,500 live births. This estimate comes from several epidemiological studies, which have shown a prevalence of 0.5% for epilepsy. Among patients with epilepsy, ∼20% have intractable seizures, and ∼40% of children (and 25% of adults) with intractable seizures have cortical dysplasia, including PMG and focal cortical dysplasia (Vinters et al. 1992; Kuzniecky and Jackson 1995, p. 345).
The pathogenesis of PMG remains poorly understood, but there is evidence for extrinsic causes, including intrauterine cytomegalovirus infection (Barkovich and Lindan 1994) and placental perfusion failure often related to twinning (Baker 1996). In addition, a severe and unlayered form of PMG is a prominent feature of Zellweger syndrome, which is associated with mutations in several genes that are involved in peroxisome biogenesis (Gould and Valle 2000).
Recent reports have described the familial recurrence of cases of PMG, the majority of which show a pattern that is consistent with X-linked inheritance (Borgatti et al. 1999; Guerreiro et al. 2000). In some, only males have PMG, whereas, in others, individuals of both sexes are affected but with more-severe expression in males. Further support for an X-linked locus for PMG comes from our study group of 220 patients with PMG, in which the majority (60%) of patients were males and in which males were generally more severely affected than females (R. J. Leventer and W. B. Dobyns, unpublished data).
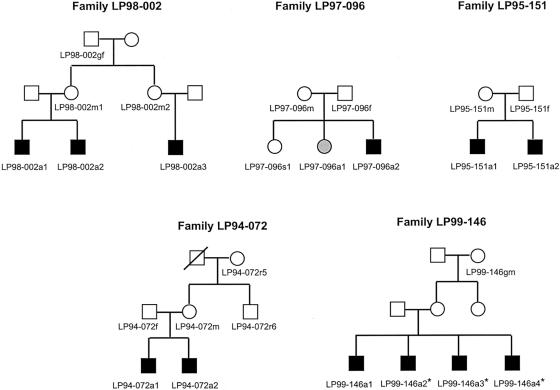
From 1991 to 2001, we ascertained MRI from >250 patients with PMG who were referred to our Brain Malformation Research Project. All MRI studies were reviewed by two investigators (W.B.D. and R.J.L.) who were familiar with the radiological features of PMG. We identified 10 multiplex families with PMG, of which 8 had BPP, defined as bilateral PMG with maximal severity in the perisylvian regions; of these 8 families with BPP, 7 had a pattern of transmission compatible with X-linked inheritance, and 5 of these agreed to participate in the research study (fig. 1). Informed consent was obtained with appropriate institutional approval, and blood samples were obtained from all affected children, most of their parents, and other relatives when possible. Clinical data and MRI scans were available for the majority of affected family members (table 1 and fig. 2). All affected individuals had BPP, although the severity of the malformation differed among them. Accordingly, we developed a grading system to account for the variations in severity that were seen among patients with BPP (see Appendix A and table 1). Affected boys had a more severe grade of BPP compared to that of the one affected girl. MRI studies were obtained for all affected patients with the exception of patients LP99-146a2, LP99-146a3, and LP99-146a4. These individuals were diagnosed as having clinically probable BPP, since they had clinical symptoms that were remarkably similar to those of their brother (LP99-146a1), who had definite BPP on MRI. The mother of these boys has epilepsy and mental retardation and thus may be a manifesting carrier, but requests for DNA and imaging studies were refused. The mother of patients LP94-072a1 and LP94-072a2 has microcephaly (2.8 SD below mean) but does not have PMG, epilepsy, or cognitive impairment. MRI was not available for other unaffected family members.
Figure 1.
Pedigrees of families included in analysis. All affected children (black symbols) have definite or probable BPP. In family LP97-096, individual LP97-096a1 (gray symbol) has a milder form of BPP as compared to her affected brother (i.e., individual LP97-096a2). MRI scans were not performed on the patients denoted by an asterisk (*).
Table 1.
Clinical and Imaging Characteristics in Affected Children with BPP
| Subject (Sex) | BPP Gradea | ClinicalCharacteristicsb |
| LP94-072a1 (M) | 2 | MIC/DD/SD/E/FW |
| LP94-072a2 (M) | 2 | MIC/DD/SD/FW |
| LP95-051a1 (M) | 1 | MIC/DD |
| LP95-051a2 (M) | 1 | MIC/DD |
| LP97-096a1 (M) | 2 | DD/SD |
| LP97-096a2 (F) | 4 | DD/SD |
| LP98-002a1 (M) | 2 | SD/E |
| LP98-002a2 (M) | 2 | SD/E |
| LP98-002a3 (M) | 2 | SD/E |
| LP99-146a1 (M) | 2 | DD/SD/FW/S |
| LP99-146a2 (M) | NA | DD/SD/FW |
| LP99-146a3 (M) | NA | DD/SD/FW |
| LP99-146a4 (M) | NA | DD/SD/FW |
See Appendix A. NA = not available.
MIC = congenital microcephaly; DD = global developmental delay; SD = prominent speech delay; E = epilepsy; FW = facial weakness.
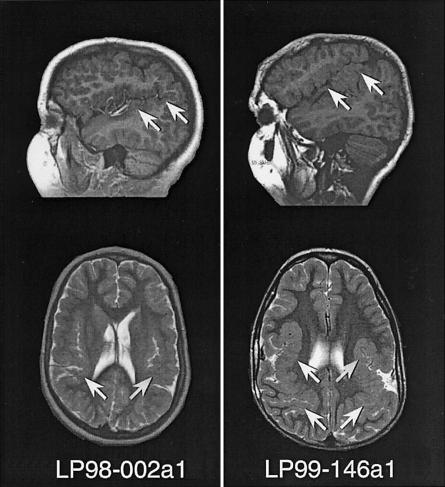
Figure 2.
MRI scans of patients LP98-002a1 and LP99-146a1, showing sagittal T1 (top) and axial T2 (bottom) images for both patients. The images show bilateral extended sylvian fissures lined by polymicrogyric cortex, showing characteristic stippling of the gray-white junction and an irregular, overfolded cortical ribbon (arrows).
For linkage analysis, microsatellite markers were selected on the basis of the Généthon human genetic linkage map (Dib et al. 1996) or were identified after manual inspection of the Xq28 genomic sequence distal to DXS8103 (for markers KN01 and KN02, details are available on request). Genotypes were determined using PCR amplification and resolution on a polyacrylamide gel for allele numbering on a LiCor automated sequencer. To compute the LOD scores (Z), we used the LINKAGE package (Lathrop et al. 1984) for two-point analysis and GENEHUNTER (Kruglyak et al. 1996) for multipoint analysis and haplotype reconstruction. The frequency of the disease allele was chosen arbitrarily as 0.0001. Genetic distances were used as described by Dib et al. (1996).
To genotype the selected families, we used a set of 40 polymorphic markers that were evenly distributed along the human X chromosome. The mean genetic distance between the markers is 5 cM, and the mean physical distance is 4 Mb. In family LP98-002, access to the grandpaternal DNA allowed us to perform haplotype reconstruction in order to determine the phase of each X chromosome of the two mothers of the affected patients and, thus, to unambiguously localize the meiotic recombination breakpoints in their offspring. This analysis shows that the only region of the X chromosome that is shared by the affected boys in this family lies distal to DXS8103 (fig. 3) in a region that has a genetic size of 5.6 cM. Next, we genotyped the other four families. In the latter families, the grandpaternal DNA could not be obtained, and, hence, the exclusion mapping could only be done on the basis of discordant allele sharing in the affected children. In family LP97-096, the Xpter–DXS7105 region is excluded. In family LP95-151, the DXS56–DXS8064 region is the only region excluded. In family LP94-072, the Xpter–DXS7104 and DXS1003–DXS8064 regions are excluded. In family LP99-146, all of the X chromosome except for the DXS1059–DXS1001 and DXS8103–Xqter regions is excluded (data not shown).
Figure 3.
X-chromosome haplotypes in family LP98-002. Only informative individuals are indicated. The crossovers in the affected children are labeled by an “X.” The critical region (DXS8103–Xqter) is the only region on the X chromosome that the three affected boys share.
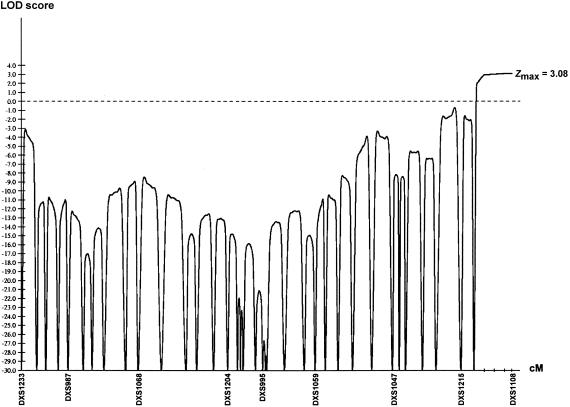
We used the genotyping results that were obtained for the five families to run linkage-analysis programs. The pooling of these five families for a two-point linkage analysis yielded a positive LOD score only in the Xq28 region, since the rest of the X chromosome was excluded, with a LOD score of −∞ at every position along the X chromosome. A significant LOD score was obtained for three of the four Xq28 markers that were used in this study (table 2). The maximal LOD score (Zmax) was obtained for DXS1073 and DXS1108 (Z=2.58 at θ=0). Multipoint linkage analysis slightly increased the LOD score in the same region (Z=3.08 in Xq28, distal to DXS8103) (fig. 4).
Table 2.
Two-Point LOD Scores between the X-Chromosome Markers and the Locus for BPP in the Families Studied
|
Two-Point LOD Score at θ = |
|||||
| Marker | .0 | .1 | .2 | .3 | .4 |
| DXS1233 | −∞ | .06 | .16 | .14 | .07 |
| DXS1060 | −∞ | −1.52 | −.57 | −.16 | .01 |
| DXS7103 | −∞ | −1.08 | −.38 | −.08 | .03 |
| DXS7104 | −∞ | −2.96 | −1.44 | −.68 | −.25 |
| DXS987 | −∞ | −.19 | .05 | .06 | .02 |
| DXS7163 | −∞ | −2.55 | −1.31 | −.69 | −.29 |
| DXS7105 | −∞ | −1.60 | −.71 | −.32 | −.12 |
| DXS8010 | −∞ | −2.77 | −1.45 | −.75 | −.31 |
| DXS997 | −∞ | −1.41 | −.72 | −.39 | −.18 |
| DXS1068 | −∞ | −.30 | −.01 | .04 | .02 |
| DXS993 | −∞ | −2.45 | −1.24 | −.65 | −.28 |
| DXS8035 | −∞ | −3.11 | −1.57 | −.79 | −.32 |
| DXS1003 | −∞ | −2.51 | −1.24 | −.61 | −.23 |
| DXS1039 | −∞ | −1.76 | −.85 | −.43 | −.18 |
| DXS1204 | −∞ | −1.76 | −.85 | −.43 | −.18 |
| DXS1194 | −∞ | −1.37 | −.65 | −.31 | −.12 |
| AR | −∞ | −2.20 | −1.04 | −.50 | −.20 |
| DXS1275 | −∞ | −2.90 | −1.44 | −.73 | −.30 |
| DXS56 | −∞ | −3.56 | −1.76 | −.87 | −.33 |
| DXS995 | −∞ | −3.43 | −1.78 | −.89 | −.34 |
| DXS1196 | −∞ | −3.11 | −1.57 | −.79 | −.32 |
| DXS990 | −∞ | −2.26 | −1.04 | −.46 | −.15 |
| DXS1231 | −∞ | −2.92 | −1.37 | −.60 | −.19 |
| DXS1120 | −∞ | −2.47 | −1.17 | −.52 | −.17 |
| DXS1059 | −∞ | −2.47 | −1.17 | −.52 | −.17 |
| DXS8081 | −∞ | .38 | .37 | .24 | .10 |
| DXS8064 | −∞ | −.83 | −.23 | −.01 | .04 |
| DXS1001 | −∞ | −2.26 | −1.04 | −.46 | −.15 |
| DXS8044 | −∞ | .36 | .31 | .18 | .05 |
| DXS1047 | −∞ | −.33 | .09 | .15 | .09 |
| DXS8074 | −∞ | −1.27 | −.58 | −.27 | −.10 |
| DXS984 | −∞ | −.33 | .09 | .15 | .09 |
| DXS1227 | −∞ | −.86 | −.24 | −.02 | .04 |
| DXS8084 | −∞ | −.33 | .09 | .15 | .09 |
| DXS1215 | −∞ | .40 | .39 | .26 | .11 |
| DXS8103 | −∞ | −.42 | −.05 | .06 | .06 |
| KN01 | 2.18 | 1.69 | 1.20 | .72 | .31 |
| KN02 | 1.18 | .93 | .69 | .44 | .20 |
| DXS1073 | 2.58 | 2.02 | 1.44 | .88 | .39 |
| DXS1108 | 2.58 | 2.02 | 1.44 | .88 | .39 |
Figure 4.
Result of multipoint linkage analysis in set of five families with BPP. Real genetic distances between the markers were used to perform the calculations. For clarity purposes, only one of every five markers is indicated on the genetic distance axis. The maximum LOD score is 3.08 in the region distal to DXS8103.
Since mutation in a gene on the X chromosome may cause skewed X inactivation in the lymphocytes of female carriers, we assessed X-inactivation patterns in each woman in these families. We used the polymorphic-repeat sequence of the androgen-receptor gene—AR, which lies on Xq12—to perform these experiments with lymphoblast DNA prepared from fresh blood samples as described elsewhere (Villard et al. 2001). X inactivation was found to be random for each female, including the one affected girl with mild BPP, in the families that we studied (data not shown).
Our results show that a locus for BPP maps to the distal long arm of the X chromosome (Xq28), in a 5.6-cM region flanked by DXS8103 and the telomere. This is the first report of the localization of a gene that is responsible for perisylvian PMG in the human genome. This result was obtained with five families with multiple male occurrences of BPP and one very mildly affected female.
Two of the families that were included in this study (families LP95-051 and LP94-072) each had two affected brothers as the only affected individuals in the family. This could be owing to either autosomal recessive or X-linked inheritance. However, both the exclusion-mapping results obtained in family LP98-002, which show a clear X-linked inheritance pattern, and the multipoint linkage analysis results for the five families (Z=3.08 in distal Xq28) indicate that a locus for BPP does indeed exist in this region of the human X chromosome.
We found it interesting that obligate carriers did not show a skewed X-inactivation pattern, as has been observed in many other X-linked diseases. However, this result is not completely unexpected for a brain-specific disease, such as BPP. It is possible that the gene responsible for this particular phenotype will not be expressed in lymphocytes and thus will not promote clonal selection against the mutated X in these cells. Alternatively, the pattern of X inactivation in the brain may be different than that in lymphocytes.
Another interesting finding observed in family LP98-002 was that the grandpaternal X-chromosome haplotype was found to be associated with the disease in the affected children. The maternal grandfather in this family has no history of learning disability, suprabulbar palsy, or epilepsy and thus is unlikely to be affected. However, the vast majority of patients with PMG have the sporadic form, which suggests that genetic forms of PMG often arise as new mutations. This observation also supports decreased penetrance and causal heterogeneity. Thus, we do not find it surprising to detect possible germinal mosaicism in the maternal grandfather in this family.
Further studies now need to be undertaken to identify the molecular basis of BPP—specifically, mutation screening of candidate genes in the critical region needs to be done. The Xq28 region has been extensively mapped both physically and transcriptionally. It is currently completely cloned in the form of YAC/PAC contigs, and a very detailed transcriptional map is available. There are currently 66 genes described in this interval. Among these, 14 have already been shown to be the cause of genetic diseases, when mutated.
To detect a potential recombination distal to DXS8103, we plan to genotype additional markers in our families. In addition, we will include additional suitable families in the linkage analysis as they are identified. However, even if additional recombinants are not identified, this region of the human X chromosome is sufficiently well characterized for us to undertake mutation screening in candidate genes. In this region, candidate genes that are appropriate for screening include FLNA, which encodes actin-binding protein filamin–1 and is implicated in the cortical malformation periventricular nodular heterotopia; PCNK, which encodes a calcium/calmodulin protein kinase and is highly expressed in the brain; and the GABA-A receptor–ε subunit gene, which encodes a full-length protein only in the brain.
The study of individuals with cortical malformations has provided us with an opportunity to identify genes and new molecular mechanisms for normal cortical development. We anticipate that the cloning of the first gene for perisylvian PMG will offer important biological clues to understand the molecular basis of this relatively common cortical malformation. In addition to assisting with clinical issues, such as genetic counseling and prenatal testing, the identification of the genetic basis of BPP will contribute to the understanding of the molecular cues that are required to orchestrate the normal organization of the cerebral cortex and, specifically, the formation of one of the most primary and functionally important regions of the brain—that is, the sylvian fissures and the surrounding perisylvian cortex.
Acknowledgments
We thank the affected families for participating in this study, Patti Mills for patient liaison, and Sheila Pressman, Kristin Petras, Jessica Roseberry and Alyssa Gross for technical assistance. We thank Prof. David Ledbetter and Prof. Michel Fontès for their advice and support in the establishment of this collaborative project.
Appendix A: Grading System for BPP
The following grades refer to bilateral, symmetrical PMG with maximal severity in the perisylvian regions:
-
1.
PMG most severe in perisylvian region, extending beyond perisylvian region to involve one or both poles.
-
2.
PMG most severe in perisylvian region, extending beyond perisylvian region but not to either pole.
-
3.
PMG of entire perisylvian region only.
-
4.
PMG of posterior perisylvian region only.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for BPP [MIM 260980])
References
- Baker EM, Khorasgani MG, Gardner-Medwin D, Gholkar A, Griffiths PD (1996) Arthrogryposis multiplex congenita and bilateral parietal polymicrogyria in association with the intrauterine death of a twin. Neuropediatrics 27:54–56 [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Hevner R, Guerrini R (1999) Syndromes of bilateral symmetrical polymicrogyria. Am J Neuroradiol 20:1814–1821 [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Lindan CE (1994) Congenital cytomegalovirus infection of the brain: imaging analysis and embryologic considerations. Am J Neuroradiol 15:703–715 [PMC free article] [PubMed] [Google Scholar]
- Borgatti R, Triulzi F, Zucca C, Piccinelli P, Balottin U, Carrozzo R, Guerrini R (1999) Bilateral perisylvian polymicrogyria in three generations. Neurology 52:1910–1913 [DOI] [PubMed] [Google Scholar]
- Crome L (1952) Microgyria. J Pathol Bacteriol 64:479–495 [DOI] [PubMed] [Google Scholar]
- Dib C, Fauré S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Selboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Ferrer I (1984) A Golgi analysis of unlayered polymicrogyria. Acta Neuropathol 65:69–76 [DOI] [PubMed] [Google Scholar]
- Gould SJ, Valle D (2000) Peroxisome biogenesis disorders: genetics and cell biology. Trends Genet 16:340–345 [DOI] [PubMed] [Google Scholar]
- Guerreiro MM, Andermann E, Guerrini R, Dobyns WB, Kuzniecky R, Silver K, Van Bogaert P, Gillain C, David P, Ambrosetto G, Rosati A, Bartolomei F, Parmeggiani A, Paetau R, Salonen O, Ignatius J, Borgatti R, Zucca C, Bastos AC, Palmini A, Fernandes W, Montenegro MA, Cendes F, Andermann F (2000) Familial perisylvian polymicrogyria: a new familial syndrome of cortical development. Ann Neurol 48:39–48 [PubMed] [Google Scholar]
- Guerrini R, Barkovich AJ, Sztriha L, Dobyns WB (2000) Bilateral frontal polymicrogyria: a newly recognized brain malformation syndrome. Neurology 54:909–913 [DOI] [PubMed] [Google Scholar]
- Guerrini R, Dubeau F, Dulac O, Barkovich AJ, Kuzniecky R, Fett C, Jones-Gotman M, Canapicchi R, Cross H, Fish D, Bonanni P, Jambaque I, Andermann F (1997) Bilateral parasagittal parietooccipital polymicrogyria and epilepsy. Ann Neurol 41:65–73 [DOI] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Kuzniecky RI, Andermann F, Guerrini R (1993) The congenital bilateral perisylvian syndrome: study of 31 patients. The CPBS Multicenter Collaborative Study. Lancet 341:608–612 [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Jackson G (1995) Magnetic resonance in epilepsy. New York, Raven Press [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard L, Lévy N, Xiang F, Kpebe A, Labelle V, Chevillard C, Zhang Z, Schwartz CE, Tardieu M, Chelly J, Anvret M, Fontès M (2001) Segregation of a totally skewed pattern of X chromosome inactivation in four familial cases of Rett syndrome without MeCP2 mutation: implications for the disease. J Med Genet 38:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinters HV, Fisher RS, Cornford ME, Mah V, Lenard Secor D, DeRosa MJ, Comair YG, Peacock WJ, Shields WD (1992) Morphological substrates of infantile spasms: studies based on surgically resected cerebral tissue. Childs Nerv Syst 8:8–17 [DOI] [PubMed] [Google Scholar]