Abstract
Pharmacologic inactivation or genetic deletion of adenosine A2A receptors protects ischemic neurons in adult animals, but studies in neonatal hypoxia-ischemia (H-I) are inconclusive. The present study in neonatal piglets examined the hypothesis that A2A receptor signaling after reoxygenation from global H-I contributes to injury in highly vulnerable striatal neurons where A2A receptors are enriched. A2A receptor immunoreactivity was detected in striatopallidal neurons. In nonischemic piglets, direct infusion of the selective A2A receptor agonist CGS 21680 through microdialysis probes into putamen increased phosphorylation of N-methyl-D-aspartic acid (NMDA) receptor NR1 subunit and Na+,K+-ATPase selectively at protein kinase A (PKA)-sensitive sites. In ischemic piglets, posttreatment with SCH 58261, a selective A2A receptor antagonist, improved early neurologic recovery and preferentially protected striatopallidal neurons. SCH 58261 selectively inhibited the ischemia-induced phosphorylation of NR1, Na+,K+-ATPase, and cAMP-regulated phosphoprotein 32 KDa (DARPP32) at PKA-sensitive sites at 3 hours of recovery and improved Na+,K+-ATPase activity. SCH 58261 also suppressed ischemia-induced protein nitration and oxidation. Thus, A2A receptor activation during reoxygenation contributes to the loss of a subpopulation of neonatal putamen neurons after H-I. Its toxic signaling may be related to DARPP32-dependent phosphorylation of PKA-sensitive sites on NR1 and Na+,K+-ATPase, thereby augmenting excitotoxicity-induced oxidative stress after reoxygenation.
Keywords: adenosine, dopamine, global ischemia, neonatal ischemia, receptors
Introduction
Adenosine, a potent neuromodulator in the brain, works through its G protein-coupled receptors to modulate numerous physiologic functions, including neuronal excitability and release of neurotransmitters such as glutamate. Cerebral ischemia is known to increase the brain's extracellular concentration of adenosine.1 Whereas adenosine A1 receptors are thought to play a protective role by limiting presynaptic glutamate release, postsynaptic Gs-coupled A2A receptors are thought to contribute to ischemic injury.2 In adult animals, pharmacologic inhibition or genetic deletion of A2A receptors reduces neuronal injury after global and focal cerebral ischemia.3, 4 In contrast to these studies on the adult brain, only a few studies have been reported on the role of A2A receptor in neonatal hypoxia-ischemia (H-I), and these have provided inconsistent results. One study showed that genetic deletion of A2A receptors in immature mice aggravated neuronal injury.5 Another study found that treatment with A2A receptor antagonist after the insult provided moderate protection in postnatal rats but that pretreatment was ineffective.6 Therefore, the role of A2A receptors in neonatal H-I remains unclear.
Postsynaptic A2A receptors are concentrated in striatal medium spiny neurons that project to globus pallidus.7 Their function is thought to be analogous to that of dopamine D1 receptors, which are localized in striatal medium spiny neurons that project to substantia nigra.8, 9 D1 receptors, which are also Gs protein-coupled receptors, control neuronal excitability and glutamatergic neurotransmission via protein kinase A (PKA)-dependent phosphorylation of various proteins, such as the dopamine- and cAMP-regulated phosphoprotein 32 kDa (DARPP32), N-methyl-D-aspartic acid (NMDA) receptor, and Na+,K+-ATPase.10 Treatment of newborn piglets with the selective dopamine D1 receptor antagonist SCH 23390 reduced the H-I-induced phosphorylation of DARPP32, NMDA receptor NR1 subunit, and Na+,K+-ATPase selectively at PKA-dependent sites; partially restored Na+,K+-ATPase activity; lessened protein nitration; and partially protected striatal neurons from H-I injury.11 These observations raise the possibility that activation of A2A receptors contributes to striatal neuronal injury by PKA-dependent phosphorylation of key proteins involved in excitotoxicity, analogous to the actions of D1 receptors. Whereas A2A receptor activation has been shown to increase DARPP32 phosphorylation at Thr34 in vitro,12 effects of A2A receptor signaling on NR1 and Na+,K+-ATPase have not been well studied.
In the current work, we produced asphyxic cardiac arrest in newborn piglets to induce neurodegeneration in the selectively vulnerable putamen,13 where H-I induces PKA- and PKC-dependent phosphorylation of the NMDA receptor NR1 subunit and Na+,K+-ATPase.11, 14 This model allows us to evaluate the role of A2A receptors in neonatal H-I and to determine whether the A2A receptor–cAMP–PKA pathway is involved in ischemic neuronal injury. Here, we tested the hypotheses that systemic administration of SCH 58261, a selective adenosine A2A receptor antagonist, (1) protects striatal neurons from H-I injury, (2) attenuates H-I-induced increase in phosphorylation selectively at PKA-sensitive sites on DARPP32, NMDA receptor NR1 subunit, and Na+,K+-ATPase, (3) improves recovery of Na+,K+-ATPase activity, and (4) reduces nitrosative and oxidative stress.
To evaluate whether protection by the A2A receptor antagonist was selective for striatopallidal neurons expressing A2A receptors compared with striatonigral neurons expressing D1 receptors, we used immunohistochemistry of Grp6- and Ebf1-positive cell bodies as a surrogate marker of neurons expressing A2A and D1 receptors, respectively, in the neuropil. Because A2A receptors are also expressed on vascular smooth muscle, we examined whether the antagonist affected the recovery of cerebral blood flow (CBF) after H-I. Finally, we determined whether the direct infusion of selective adenosine A2A receptor agonist CGS 21680 into nonischemic putamen via microdialysis could recapitulate the pattern of H-I-induced protein phosphorylation that was specifically sensitive to inhibition by the A2A receptor antagonist.
Materials and methods
All experimental protocols were approved by the Animal Care and Use Committee of the Johns Hopkins University and performed in accordance with National Institutes of Health guidelines. A total of 88 male piglets (2 to 2.5 kg, 4–7 days old) were used in this study.
Asphyxic Cardiac Arrest
Asphyxic cardiac arrest was produced as described previously.11 In brief, piglets were anesthetized with pentobarbital (50 mg/kg, intraperitoneal) and orally intubated. The femoral artery and vein were catheterized under aseptic conditions. Inspired O2 was decreased to 10.0±0.2% for 40 minutes (hypoxia). Then, piglets were ventilated with 21% O2 for 5 minutes (required for cardiac resuscitation) before their airway was occluded for 7 minutes (asphyxia, to produce cardiac arrest). Piglets were resuscitated by mechanical ventilation with 50% O2, manual chest compressions, and, if necessary, intravenous injection of epinephrine until return of spontaneous circulation. After resuscitation, inspired O2 was gradually reduced to 30% to maintain arterial O2 saturation greater than 95%. Sham-operated animals received only catheterization but no hypoxia or asphyxia. Arterial blood pressure, arterial blood gases, pH, glucose concentration, and rectal temperature were monitored until piglets regained consciousness. Those surviving for 3 hours for biochemical measurements and 6 hours for CBF measurements were kept sedated with a continuous intravenous infusion of fentanyl (10 μg/kg per hour) and pancuronium (0.2 mg/kg per hour).
A low (0.01 mg/kg), medial (0.1 mg/kg), or high (1 mg/kg) dose of the A2A antagonist SCH 58261 (Sigma-Aldrich, St Louis, MO, USA) was injected intravenously at 5 minutes of recovery or at an equivalent time in sham-operated piglets. The loading dose was followed by a corresponding continuous infusion of 0.0033 mg/kg per hour (low dose), 0.033 mg/kg per hour (medial dose), or 0.33 mg/kg per hour (high dose) for 6 hours. Vehicle-treated piglets received the equivalent volume of 0.5% DMSO in 0.9% saline. Neuronal cell death in putamen progresses between 6 and 24 hours after reoxygenation.13, 15 Neuronal damage was evaluated after 4 days of recovery to allow for a possible delay in the maturation of injury after SCH 58261 treatments. For biochemical and CBF studies, the medial dose was selected because it showed the greatest mean protection among the three doses. Biochemical measurements were made at 3 hours of recovery to avoid the effect of later neuronal loss on the measurements. In another experiment, piglets were pretreated with the medial dose of SCH 58261 15 minutes before the start of H-I.
Cerebral Blood Flow Measurement
In separate cohorts of piglets, cortical perfusion was monitored continuously by laser-Doppler flowmetry (LDF) (Moor Instruments, Axminster, UK) until 6 hours of recovery. The laser-Doppler probe was placed on the cortical surface (8 mm anterior and 4 mm lateral from the bregma) through a small craniotomy and an incision in the dura.
CGS 21680 Infusion
To examine the effects of A2A receptor activation on phosphorylation of key proteins in vivo, we inserted a microdialysis probe (CMA 12; CMA/Microdialysis, Solna, Sweden) into the putamen (8 mm anterior and 7.5 mm lateral from the bregma; 16.5 mm below the dura) of piglets that did not undergo H-I. Artificial cerebrospinal fluid was perfused at a rate of 2.5 μL/min through the probe without recirculation for 1 hour starting 1 hour after implantation. Then, the infusate was switched to 10 μmol/L CGS 21680 (Tocris, Bristol, UK) or vehicle (0.05% DMSO in artificial cerebrospinal fluid) for another hour, after which the piglets were perfused transcardially with ice-cold phosphate-buffered saline. The putamen was rapidly dissected bilaterally and processed for western blot analysis. On western blots, the optical density for the putamen ipsilateral to the vehicle or drug perfusion for each brain was normalized by the optical density for the contralateral putamen that had no infusion.
Western Blotting
Tissues were homogenized, and membrane-enriched fractions were collected, as described previously.11 Twenty microgram protein samples were separated by 4% to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were probed with the following primary antibodies: mouse anti-NR1 (1:1,000; BD Pharmingen, San Jose, CA, USA), rabbit anti-phospho-NR1 Ser896, anti-phospho-NR1 Ser897 (1:1,000; Millipore, Billerica, MA, USA), mouse anti-Na+,K+-ATPase α1 (1:10,000; Sigma-Aldrich) or Na+,K+-ATPase α3 (1:10,000; Affinity Bioreagents, Golden, CO, USA), rabbit anti-phospho Na+,K+-ATPase α Ser23 or Na+,K+-ATPase α Ser943 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-synaptophysin (1:20,000; Millipore), and mouse anti-nitrotyrosine (1:40,000; Millipore). Synaptophysin was used as a protein loading standard for membrane-enriched fractions.
Protein Oxidation Assay
Oxidative modification of proteins in putamen at 3 hours after H-I was determined with an OxyBlot protein oxidation detection kit (Millipore) for carbonyl groups, as described previously.16
Na+,K+-ATPase Biochemical Assay
The biochemical activity of Na+,K+-ATPase was measured in putamen samples obtained at 3 hours of recovery as described previously.11 In brief, cell membrane-enriched putamen samples were reacted with/without ouabain in the presence of ATP. The enzymatic hydrolysis of ATP was terminated by adding trichloroacetic acid. The ATPase activity was measured as a function of ouabain-sensitive liberation of inorganic phosphate by the colorimetric assay.
Neurobehavioral Assessment
In the 4-day survivors, a neurologic deficit score (0=best outcome, 154=worst outcome) was used to quantify overall neurologic function based on seven different components: (1) level of consciousness; (2) brain stem function; (3) sensory responses; (4) motor function; (5) behavioral activities; (6) spatial orientation; and (7) excitability.17
Histologic, Immunohistochemical, and Double Immunofluorescence Staining
Anesthetized piglets were perfused transcardially with ice-cold phosphate-buffered saline and 4% paraformaldehyde at 3 hours or 4 days of recovery. Brains were removed and bisected mid-sagittally. For those recovering 4 days from sham surgery or H-I, the left forebrain was cut into 1-cm slabs and embedded in paraffin for histology with hematoxylin and eosin staining of 10-μm sections and the assessment of viable neurons by profile counting. The right forebrain was cryoprotected, frozen, and cut into serial 60-μm coronal sections through the putamen for stereological analysis of viable neurons in putamen and for immunohistochemistry. Neuronal cell death in putamen is normally complete within 1 day of recovery.13, 15 To allow for the possibility that drug treatment might delay neuronal cell death, viable neurons were counted at 4 days of recovery.
Profile counting was performed on three level-matched sections in anterior, medial, and posterior putamen (bregma level: +15 mm, +9 mm, and +5 mm).18 For each section, an experimenter blinded to treatment counted the number of ischemic and nonischemic neuronal profiles in seven, randomly chosen, nonoverlapping microscopic fields at 1000 × power. The values were averaged from 21 fields to obtain a single value of viable neurons per square millimeter for each piglet to be used in the statistical analysis.
We used double immunofluorescence staining to assess whether A2A receptor and Ebf1 or Gpr6 are colocalized in striatal neurons. Sections were blocked in 10% normal horse serum and incubated with primary antibodies overnight at 4°C. Primary antibodies included mouse anti-A2A receptor and rabbit anti-Ebf1 (1:100; Santa Cruz) or rabbit anti-Gpr6 (1:100; MBL International, Woburn, MA, USA). Antibody binding was visualized by incubating sections with Alexa Fluor 488- or Alexa Fluor 555-conjugated secondary antibodies (1:500; Invitrogen, Eugene, OR, USA).
Immunohistochemical assessments were made on Ebf1 and Gpr6 in sham-operated and H-I piglet brain. Free-floating sections were blocked in 10% normal horse serum and incubated with anti-Ebf1 or anti-Gpr6 antibody, followed with biotinylated IgG (1:200; Vector Laboratories, Burlingame, CA, USA) and VECTASTAIN Elite ABC reagent (Vector). Immunostaining was developed with diaminobenzidine (Vector) as a chromogen. Negative controls were treated without primary antibodies and showed no positive signals. For quantification of immunoreactivity, seven 400 × optical fields were randomly selected in each anterior, medial, and posterior putamen section (bregma level: +15 mm, +9 mm, and +5 mm),18 and the number of immunopositive cells was quantified with ImageJ software (version 1.42q, NIH).
Stereological Measurements
We used unbiased stereology to gain a more detailed estimate of the total number of viable neurons in the entire putamen for the group that received the medial dose of SCH 58261. The low- and high-dose groups were not subjected to this analysis because profile counting indicated no additional benefit with these doses. Comparisons were made among the vehicle-treated and drug-treated sham and H-I groups. Stereological measurements were made by using a Nikon Eclipse 90i microscope (Nikon, Tokyo, Japan) attached to a Qimage Retiga-2000R camera, which was connected to a workstation with Stereo Investigator software (Version 10; MicroBrightField, Williston, VT, USA). A set of cresyl violet-stained sections of 60-μm thick were selected at random from every 16 evenly spaced sections of the entire rostrocaudal extent of the piglet putamen.
We used an optical fractionator method to estimate the number of neurons in the putamen under 40 × objective lens. The optical disector height (thickness) was 12 μm with a 2-μm top and bottom guard zone. A viable neuron could be distinguished from glia based on its size, presence of a visible rim of cytoplasm around the nucleus, and a prominent nucleolus. A neuron was counted only when its nucleus first came into focus within the optical disector counting frame. It was not counted when its nucleus came into focus within the guard zone or if the nucleus was touching the left or bottom side of the disector frame. The mean section thickness, measured at every counting frame site, was used for final calculation of the number of neurons in the putamen.
The putamen volume of each region was estimated with the Cavalieri method. This method was implemented by summing the number of intersecting points on a defined grid that fell within the target area. The calculation of the volume of putamen is based on the number of points of grid counted within the putamen, the grid spacing area (1.2 mm2), the section thickness (60 μm), and the distance between sections (960 μm).
Data and Statistical Analysis
All values are expressed as means±s.d. The neurologic deficit score was analyzed by two-way analysis of variance (ANOVA) for repeated measures. Other measurements were analyzed with Student's t-test for comparing two groups or with one-way ANOVA followed by the Student–Newman–Keuls multiple range test for comparing more than two groups. P<0.05 was considered as statistically different.
Results
Neuronal Damage in Striatum After Hypoxia-Ischemia
Successful resuscitation from H-I was achieved in 49 of 68 piglets, including 35 of 47 piglets intended for 4-day survival for neurobehavioral and histologic assessment. Within individual 4-day survival groups, successful cardiac resuscitation was achieved in 8 of 10 piglets in the vehicle group, 1 of 6 piglets in the SCH 58261 pretreatment group, 8 of 9 piglets in the low-dose SCH 58261 posttreatment group, 9 of 11 piglets in the medial-dose SCH 58261 posttreatment group, and 9 of 11 piglets in the high-dose SCH 58261 posttreatment group. Some piglets were euthanized early because of inability to extubate within 10 hours of recovery or severe generalized seizures (0/1 (no extubation/seizures), 1/0, 0/1, 1/0, and 1/1 in the vehicle, SCH 58261 pretreatment, and low-, medial-, and high-dose SCH 58261 posttreatment groups, respectively, resulting in 7, 0, 7, 8, and 7 survivors in these groups). Because piglets pretreated with SCH 58261 were difficult to resuscitate and did not survive, we did not carry out any additional pretreatment studies.
Piglets that received posttreatment had similar levels of arterial PO2 without arterial hypotension during the 40-minute period of hypoxia. They also exhibited comparable decreases in arterial pressure, PO2, and pH and increases in arterial PCO2 during the period of airway occlusion (Supplementary Table). After SCH 58261 treatment was begun at 5 minutes after resuscitation, arterial PO2, PCO2, pH, and mean arterial pressure remained in the normal physiologic range and showed no significant difference from values in the vehicle-treated group over the first 3 hours of recovery before the piglets regained consciousness.
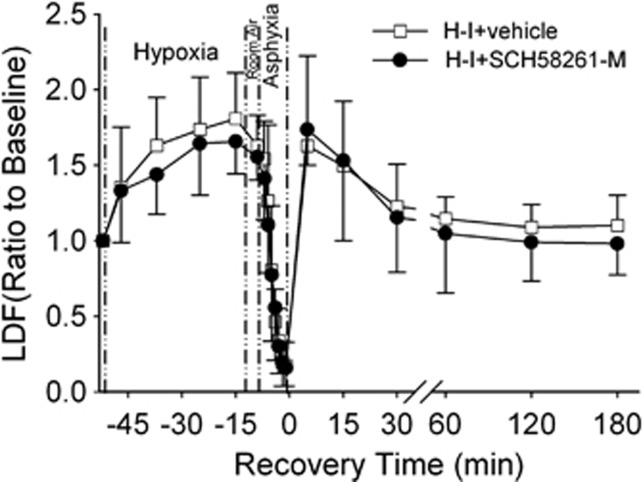
To determine if SCH 58261 affected recovery of CBF, we monitored cortical perfusion in a separate cohort of piglets treated with vehicle (n=4) or the medial dose of SCH 58261 (n=4). Hypoxia produced the expected increase in perfusion, and asphyxia produced the expected decrease in perfusion that accompanies hypotension (Figure 1). After resuscitation, the initial postischemic hyperemia was similar between groups, and no delayed hypoperfusion was detected in either group.
Figure 1.
Laser-Doppler flow (LDF) over somatosensory cortex during baseline, hypoxia, asphyxia, and the first 6 hours of recovery after hypoxia-ischemia (H-I) (means±s.d.; n=4 per group). Piglets were treated with vehicle or the medial dose of SCH 58261 (SCH58261-M; 0.1 mg/kg) after H-I.
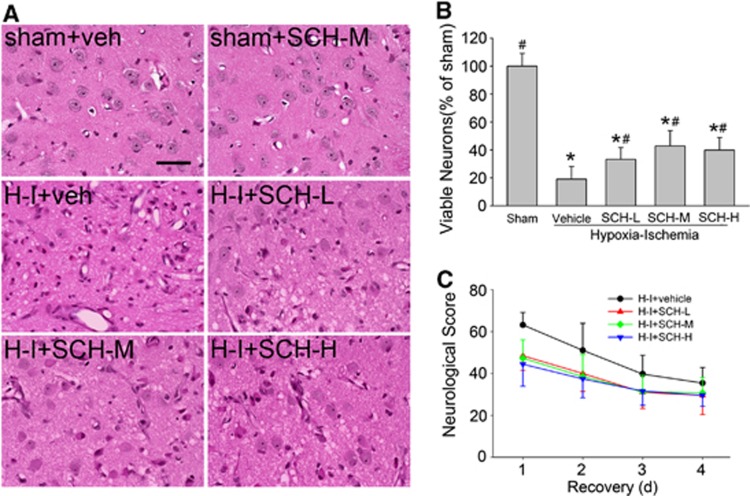
At 4 days of recovery, sham-operated piglets exhibited normal striatal cytoarchitecture and cellular morphology. Consistent with previous work,11, 13 H-I distorted the anatomic structure, with neurons exhibiting cytoplasmic microvacuolation, eosinophilia, and nuclear pyknosis in striatum (Figure 2A). Because the numbers of viable neurons in vehicle-treated and SCH 58261-treated sham piglets (n=4 each) were not significantly different, their values were combined into one sham group for statistical comparisons with values in the H-I groups. Profile counting indicated that the number of viable neurons in the putamen of the H-I vehicle group was 19±9% of that in the sham-operated piglets. This value was not different from that obtained in previous work with other vehicle treatments.11 With low-, medial-, and high-dose SCH 58261 treatment, more viable neurons were evident, although a considerable number of pyknotic cells were still present. The number of viable putamen neurons was significantly increased to 33±8%, 43±11%, and 40±9% after low, medial, and high doses, respectively. However, no significant difference was detected among these three doses of SCH 58261 (Figure 2B).
Figure 2.
Effects of SCH 58261 on neuronal damage and neurologic deficits in piglets subjected to hypoxia-ischemia (H-I). Piglets exposed to H-I or sham operation were administered vehicle or low-, medial-, or high-dose SCH 58261 (SCH-L, SCH-M, or SCH-H) after H-I. (A) Representative photographs of hematoxylin and eosin (H&E)-stained sections at 4 days of recovery. Scale bar=40 μm. (B) Quantitative results for viable putamen neurons (expressed as a percentage of the mean value of the sham group). Data are means±s.d. (n=7 to 8 per group). *P<0.05 versus sham-operated group; #P<0.05 versus H-I vehicle group; ANOVA followed by the Student–Newman–Keuls test. (C) Neurologic scores during the 4-day recovery. Two-way analysis of variance (ANOVA) with repeated measures indicated a significant overall effect of treatment (P<0.001) and recovery time (P<0.001).
The neuronal injury produced by H-I was less severe and more variable in the caudate nucleus than in the putamen. The number of viable neurons, expressed as a percentage of the mean value of the sham groups, was 44±35%, 63±26%, 56±24%, and 66±26% in the groups treated with vehicle and low, medial, and high doses of SCH 58261, respectively. These values were not significantly different.
No neurobehavioral abnormalities were observed in sham-operated piglets treated with vehicle or SCH 58261. In contrast, neurobehavioral deficits were evident after H-I, as published previously.11 Most vehicle-treated piglets showed impaired consciousness, no light and/or no auditory reflexes, and low muscle tone; some failed to exhibit a response to pain stimulation on the first day of recovery. These deficits diminished on subsequent days. Two-way repeated measures ANOVA indicated an overall protective effect of the three doses of SCH 58261 treatment (P<0.001) and time (P<0.001) (Figure 2C). No significant difference was found among groups treated with different doses of SCH 58261.
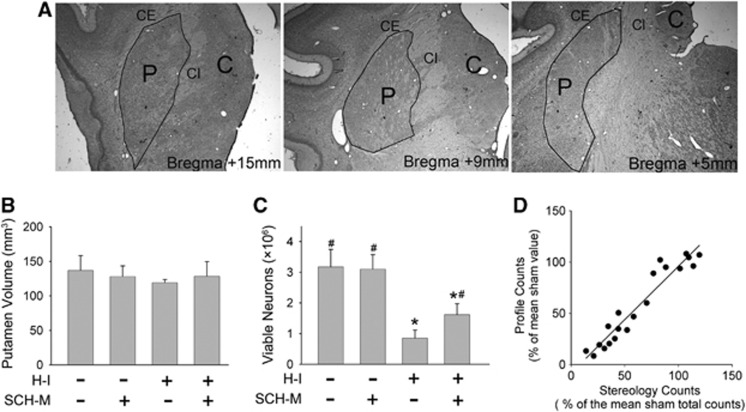
We used unbiased stereolgy to obtain an estimate of the total number of surviving neurons in the putamen and the volume of putamen after medial-dose SCH 58261 treatment. This method reduces possible bias from brain shrinkage caused by cell loss or tissue processing and from possible variance in the anteroposterior viable cell distribution. Examples of putamen boundaries at different coronal levels are shown in Figure 3A. SCH 58261 and H-I had no significant effect on the volume of the putamen (Figure 3B). The antagonist did not change the number of putamen neurons after sham surgery. However, SCH 58261 significantly increased the total number of viable neurons in putamen at 4 days of recovery from H-I (Figure 3C). Linear regression analysis on viable neurons showed a close relationship between stereological counts and profile counting (r=0.96; Figure 3D).
Figure 3.
Effect of hypoxia-ischemia (H-I) on putamen. (A) Putamen (P) is clearly distinguished from caudate nucleus (C) by capsula interna (CI) and from cerebral cortex and claustrum by capsula externa (CE) under 2 × objective lens. Its boundaries are outlined on the outer rim of CI and inner rim of CE. No clear anatomic definition separates the rostral putamen from globus pallidus or caudal putamen from globus pallidus and nucleus basalis. Therefore, the outline was drawn artificially between the lowest points of CE and CI. Stereological analysis of putamen volume (B), number of viable putamen neurons (C), and the relationship between stereology counts and profile counts (D) in piglets posttreated with the medial dose of SCH 58261 (n=4 to 6 per group). *P<0.05 versus sham vehicle group; #P<0.05 versus H-I vehicle group; analysis of variance (ANOVA) followed by the Student–Newman–Keuls test. Linear correlation analysis was used to assess the relationship between the number of viable neurons as determined by stereology and the number determined by profile counting (r=0.96).
Ebf1 and Gpr6 Immunoreactivity at 4 Days of Recovery in Hypoxia-Ischemia Piglets
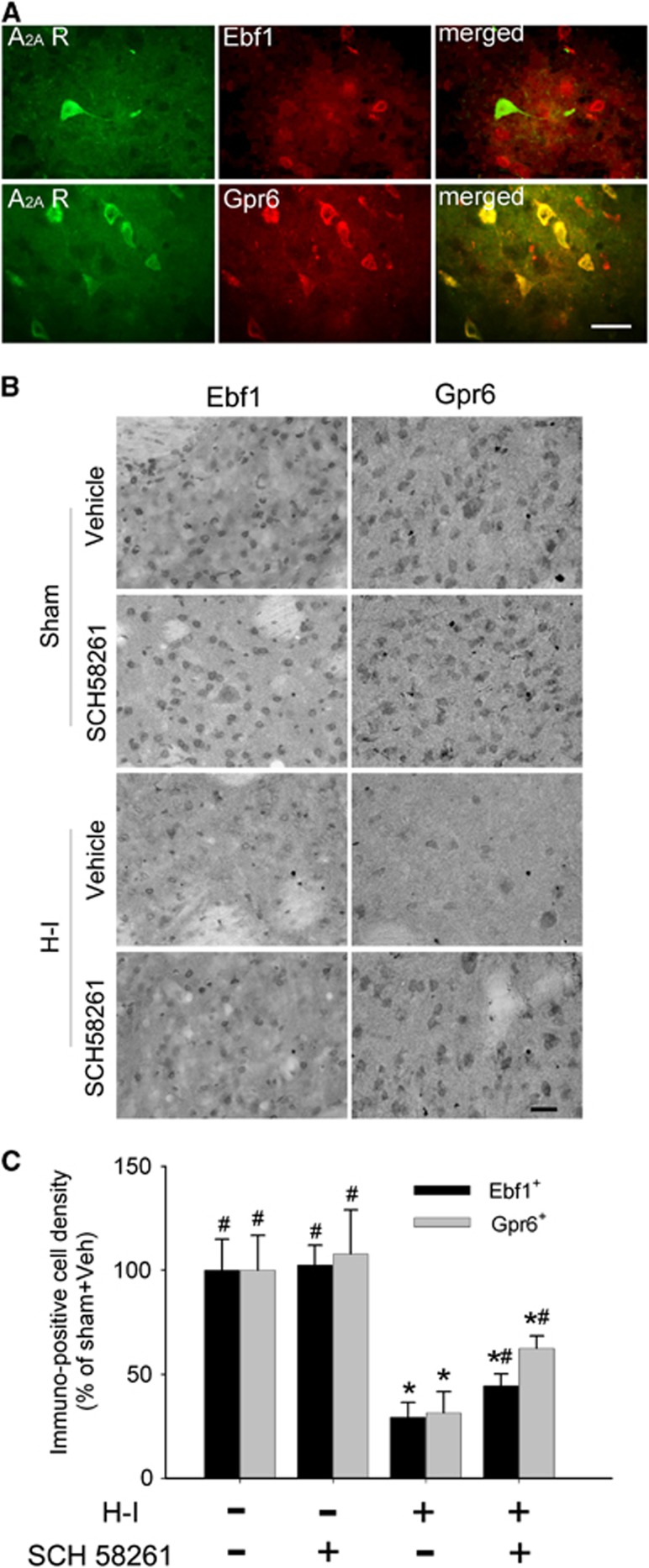
Double immunofluorescence results in putamen showed that A2A receptor signals colocalized in most cell bodies that expressed Gpr6 rather than in those that expressed Ebf1 (Figure 4A). Immunohistochemical analysis further indicated widespread Ebf1- and Gpr6-positive signals throughout putamen of sham-operated piglets (Figure 4B). SCH 58261 did not change the distribution pattern or the level of Ebf1 and Gpr6 immunoreactivity in putamen of sham-operated animals. Hypoxia-ischemia significantly decreased the number of Ebf1- and Gpr6-positive cells in putamen at 4 days of recovery. Ebf1-positive cells decreased to 29±7% and Gpr6-positive cells decreased to 31±10% of the number in the sham-operated, vehicle-treated group (n=6 each). SCH 58261 treatment significantly attenuated the reduction in Ebf1-positive cells to 44±6% (n=6) and in Gpr6-positive cells to 62±6% (n=6) of the corresponding sham values (Figure 4C). The proportion of Grp6-positive cells was significantly greater than the proportion of Ebf1-positive cells (P<0.001).
Figure 4.
SCH 58261 treatment attenuates the loss of Ebf1- and Gpr6-positive cells in putamen at 4 days of recovery after hypoxia-ischemia (H-I). (A) Double immunofluorescence of Ebf1 or Gpr6 and adenosine A2A receptor (A2A R) in piglet putamen at 3 hours of recovery after H-I. Scale bar=20 μm. (B) Representative immunohistochemical staining shows Ebf1 or Gpr6 signals in putamen of piglets at 4 days of recovery after sham surgery or H-I. Scale bar=20 μm. (C) Quantitative results indicate that H-I induced a loss of Ebf1- and Gpr6-positive cells in putamen at 4 days of recovery; both were attenuated by SCH 58261 treatment. All data are shown as means±s.d. *P<0.05 versus sham vehicle group; #P<0.05 versus H-I vehicle group; one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test.
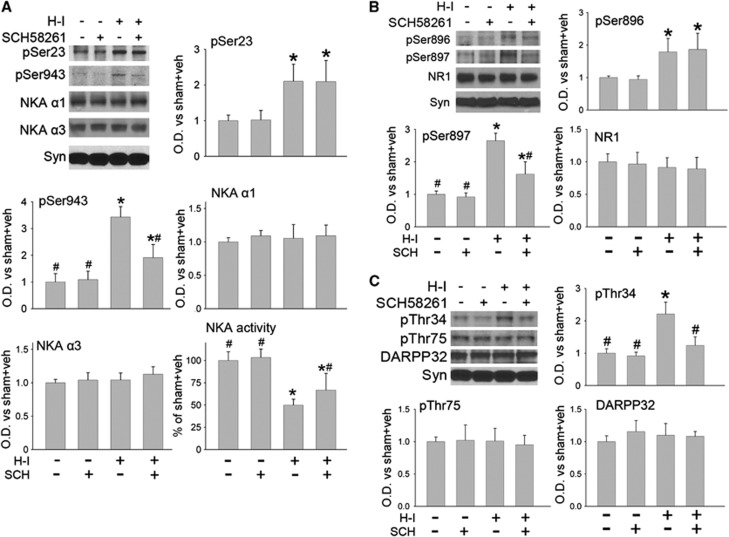
Effect on Na+,K+-ATPase Phosphorylation and Activity and on DARPP32 and NR1 Phosphorylation
Treatment of sham-operated piglets with SCH 58261 (n=4) did not significantly change the basal phosphorylation state of Ser23 or Ser943 on Na+,K+-ATPase α subunits or ATPase activity (Figure 5A) compared with that in vehicle-treated sham piglets (n=4). As expected based on previous work,11 H-I significantly increased the level of Ser23 and Ser943 phosphorylation (n=6) and substantially decreased the Na+,K+-ATPase activity (n=7) in the putamen at 3 hours of recovery. Treatment with SCH 58261 after H-I (n=6) markedly blunted the increase in PKA-dependent phosphorylation at Ser943 but not the PKC-dependent phosphorylation at Ser23. SCH 58261 posttreatment also significantly increased Na+,K+-ATPase activity after H-I compared with vehicle treatment (n=7), although the value remained significantly less than that in the sham groups. SCH 58261 and H-I had no significant effect on the expression levels of the neuronal-specific Na+,K+-ATPase α3 subunit or the more widespread α1 isoform at 3 hours of recovery.
Figure 5.
Effect of medial-dose SCH 58261 treatment after hypoxia-ischemia (H-I) on (A) phospho- and total Na+,K+-ATPase (NKA) expression and activity, (B) phospho- and total N-methyl-D-aspartic acid (NMDA) receptor NR1 subunit expression, and (C) phospho- and total DARPP32 expression in a membrane-enriched fraction of putamen at 3 hours of recovery (n=4 to 7 per group). Synaptophysin (Syn) was used as a loading control. Optical density (O.D.) data (means±s.d.) were normalized to the sham vehicle value. *P<0.05 versus sham vehicle group; #P<0.05 versus H-I vehicle group; one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test.
Protein kinase A and PKC can also phosphorylate NR1 at Ser897 and Ser896, respectively.19 Hypoxia-ischemia increased the level of phosphorylation at both sites in putamen at 3 hours of recovery (n=6). SCH 58261 treatment (n=6) selectively reduced this increase in phospho-NR1 at Ser897 but not at Ser896. However, SCH 58261 did not change the basal level of phospho-NR1 Ser896 or phospho-NR1 Ser897 in sham-operated piglets (n=4 each). Moreover, H-I and SCH 58261 treatment did not affect the levels of total NR1 protein at 3 hours of recovery (Figure 5B).
Thr34 and Thr75 of DARPP32 are phosphorylated by PKA and cyclin-dependent kinase 5, respectively.10 SCH 58261 treatment alleviated the H-I induction of phospho-DARPP32 Thr34 (n=6), but it had no effect on the basal level of phospho-DARPP32 Thr34 in sham-operated piglets (n=4 each). Hypoxia-ischemia and SCH 58261 treatment did not affect the levels of phospho-DARPP32 Thr75 or total DARPP32 protein (Figure 5C).
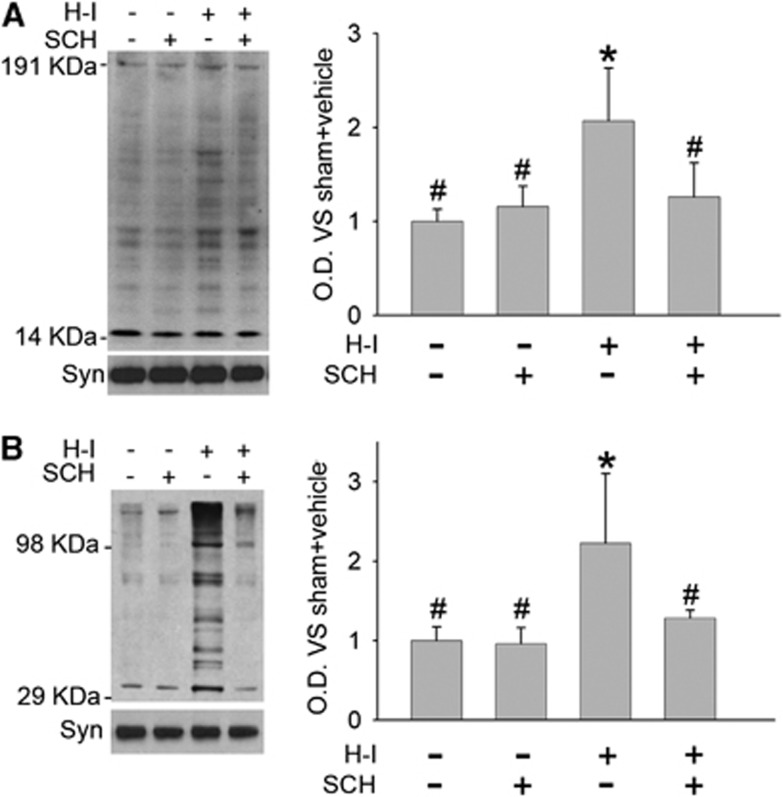
Nitrosative and Oxidative Stress After Hypoxia-Ischemia
3-Nitrotyrosine immunoreactivity and protein carbonyl formation were used as markers of nitrosative and oxidative stress. Hypoxia-ischemia increased 3-nitrotyrosine immunoreactivity in membrane-enriched fractions of putamen tissues at 3 hours of recovery (Figure 6A). Treatment with SCH 58261 significantly reduced the immunoreactivity to levels similar to that in the sham-vehicle group. In addition, SCH 58261 had no effect on 3-nitrotyrosine immunoreactivity in the sham group.
Figure 6.
Effect of medial-dose SCH 58261 treatment on hypoxia-ischemia (H-I)-induced nitrative and oxidative stress in putamen at 3 hours of recovery. Western blot analysis showed that SCH 58261 decreased H-I-induced 3-nitrotyrosine (A) and carbonyl (B) immunoreactivity on multiple protein bands in a membrane-enriched fraction of putamen at 3 hours of recovery (n=4 to 6 per group). Optical density (O.D.; means±s.d.) was integrated over multiple protein bands for nitrotyrosine (14 to 191 kDa) and carbonyls (29 to 98 kDa). *P<0.05 versus sham vehicle group; #P<0.05 versus H-I vehicle group; one-way ANOVA followed by the Student–Newman–Keuls test.
Oxyblot analysis of carbonyl groups indicated similar results. Carbonyl formation was significantly increased in the proteins of the membrane-enriched fraction at 3 hours of recovery from H-I, and this increase was attenuated by SCH 58261 treatment (Figure 6B).
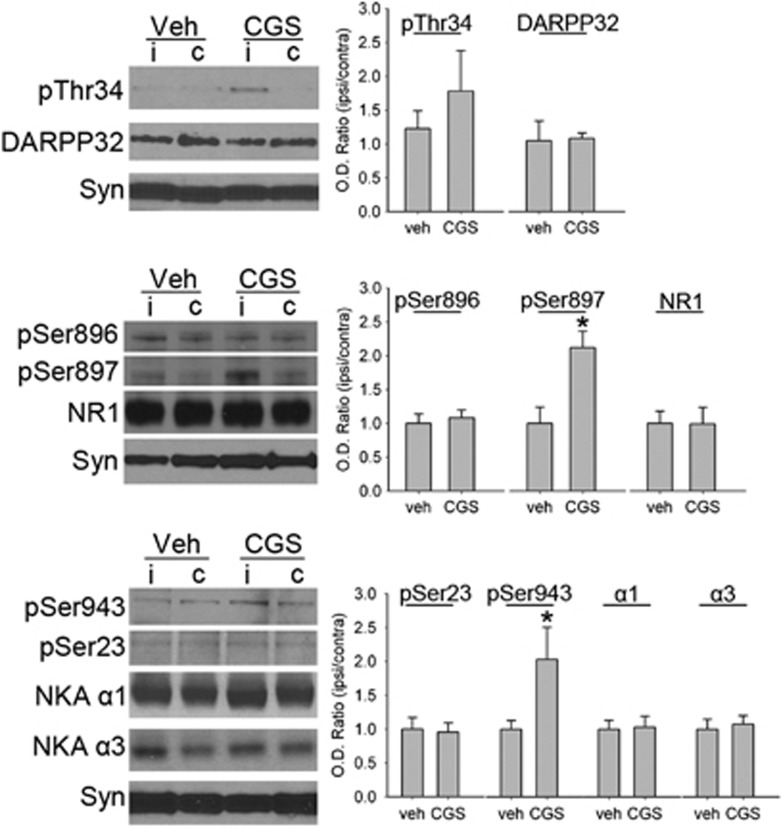
CGS 21680 Infusion Changes Phosphorylation of DARPP-32, NR1, and Na+,K+-ATPase
We infused CGS 21680 (10 μmol/L) through microdialysis probes into normal piglet putamen (n=4) to determine if A2A receptor activation could directly increase PKA-dependent phosphorylation on DARPP-32, NR1, and Na+,K+-ATPase. CGS 21680 increased phosphorylation selectively at the PKA-dependent sites Ser897 in NR1 and Ser943 in Na+,K+-ATPase compared with phosphorylation levels in the contralateral putamen (Figure 7). The change in Thr34 phosphorylation did not attain statistical significance (P<0.14). No significant change was observed at the PKC-dependent sites in NR1 or Na+,K+-ATPase. Vehicle infusion did not alter the phosphorylation level at PKC- or PKA-sensitive sites (n=4). The levels of total NR1, Na+,K+-ATPase α1 and α3, and DARPP32 protein were unaffected by CGS 21680 or vehicle infusion.
Figure 7.
Western blot analysis shows effects of microdialysis infusion of 10 μmol/L CGS 21680 (CGS) into piglet putamen on phospho- and total protein of DARPP32, N-methyl-D-aspartic acid (NMDA) receptor NR1 subunit, and Na+,K+-ATPase (NKA) in a membrane-enriched fraction of putamen from piglets that did not undergo hypoxia-ischemia (H-I) (n=4 per group). Synaptophysin (Syn) was used as a loading control. Optical density (O.D.) data (means±s.d.) were normalized to the value of the contralateral putamen that had no microdialysis probe insertion. *P<0.05 versus vehicle group, Student's t-test. c: putamen contralateral to infusion; i: putamen ipsilateral to infusion; veh: vehicle treatment.
Discussion
Our results showed that posttreatment of piglets with the selective adenosine A2A receptor antagonist SCH 58261 after resuscitation from asphyxic cardiac arrest (1) reduces early neurologic deficits and protects striatal neurons, especially the striatopallidal neurons; (2) reduces H-I-induced phosphorylation at PKA-dependent sites on DARPP-32, NR1, and Na+,K+-ATPase; (3) mitigates H-I-induced suppression of Na+,K+-ATPase activity; and (4) reduces nitrosative and oxidative damage to proteins. In addition, activation of A2A receptors with the selective agonist CGS 21680 in nonischemic brain produced phosphorylation at the same PKA-dependent sites on NR1 and Na+,K+-ATPase as those inhibited by the A2A antagonist after H-I.
A2A Receptor Antagonist Protects Neurons After Neonatal Hypoxia-Ischemia
Extracellular adenosine can markedly increase during cerebral ischemia and have a critical role in limiting or augmenting damage.1 Activation of presynaptic A1 receptors is thought to provide protection by inhibiting release of excitatory neurotransmitters.20 In contrast, activation of postsynaptic A2A receptors may promote excitotoxicity. Therefore, the postsynaptic receptors represent a therapeutic target for treating brain ischemia,2, 21 as supported by work in adult models of global and focal brain ischemia.3, 4 In addition, A2A receptors in astrocytes have been reported to contribute to β-amyloid-induced downregulation of astrocyte glutamate transporter expression and conceivably may have similar effects in downregulating glutamate uptake after H-I.22 However, in immature brain, the role of A2A receptors is less clear. Genetic deletion of A2A receptors worsened brain injury and impaired behavioral performance after cerebral H-I in 7-day-old mice.5 In contrast, SCH 58261 posttreatment after cerebral H-I showed protection in postnatal rats, but pretreatment was ineffective.6 Divergent outcomes in genetic and drug studies may be related to deleterious adaptive changes in A2A receptor null mice. These mice showed impaired LDF response to hypoxia.23 Although these null mice have been reported to have a normal ratio of ipsilateral-to-contralateral LDF during an H-I insult,5 it is possible that the absolute level of CBF was reduced in both hemispheres during H-I and recovery because of arterial hypotension and impaired cerebral vasodilation to hypotension and hypoxia with loss of cerebrovascular A2A receptors.24 Interestingly, we found that cardiac resuscitation was difficult in piglets treated with SCH 58261 before the induction of H-I. The antagonist likely impaired coronary vasodilation to H-I and thereby could have augmented the ischemic insult to the heart. With SCH 58261 treatment after reoxygenation, we found no effect on the recovery of LDF in cerebral cortex. Although it is possible that SCH 58261 could have reduced blood flow selectively in striatum, it is unlikely that such a reduction during reperfusion would have been responsible for the observed reduction in neuronal injury.
PKA-Dependent Phosphorylation Is Involved in Neuronal Death After Hypoxia-Ischemia
Protein kinase A-dependent phosphorylation is involved in regulating the functions of many proteins, such as Na+,K+-ATPase and NMDA receptors. Na+,K+-ATPase has critical roles in maintaining physiologic ion gradients, osmotic balance, resting membrane potential, and neuronal excitability. Although the precise mechanism is still unclear, PKA can suppress Na+,K+-ATPase activity in the striatum.25 In addition, PKA decreases Na+,K+-ATPase activity in a cooperative manner with PKC.26 Contrary to the suppressive effect on Na+,K+-ATPase, PKA phosphorylation of NMDA receptors27 enhances NMDA currents28 and Ca2+ influx29 and promotes forward trafficking of the receptor from the endoplasmic reticulum to the synapse.30 However, effects of A2A receptors on phosphorylation of Na+,K+-ATPase and NMDA receptors are not well described.
In the current study, H-I induced the PKA- and PKC-dependent phosphorylation of Na+,K+-ATPase and reduced its activity at 3 hours of recovery. Hypoxia-ischemia had a similar effect on the NMDA receptor NR1 subunit. In addition, H-I increased PKA-dependent phosphorylation of Thr34 on DARPP-32, which is known to inhibit protein phosphatase 1 and permit sustained phosphorylation at PKA-dependent serine sites. These findings are consistent with those of previous reports.11, 14, 17 To date, studies in immature brain have not examined the mechanisms of action of A2A receptors specifically in striatum. SCH 58261 treatment achieved reductions in phosphorylation similar to those achieved with a D1 receptor antagonist in H-I piglets.11 It attenuated the H-I-induced, PKA-dependent phosphorylation of DARPP-32 Thr34, NR1 Ser897, and Na+,K+-ATPase Ser943 and improved striatal Na+,K+-ATPase activity after H-I. Moreover, infusion of the A2A agonist CGS 21680 increased the levels of PKA-dependent phosphorylation at NR1 Ser897 and Na+,K+-ATPase Ser943. Although it is not precisely known how phosphorylation of Ser897 on NR1 modulates NMDA receptor function, the A2A receptor–cAMP–PKA cascade does enhance NMDA-mediated calcium signaling.31 Therefore, activation of the A2A receptor–cAMP–PKA cascade does lead to phosphorylation of NMDA receptors and Na+,K+-ATPase in vivo, and this phosphorylation may contribute to amplifying postischemic excitatory injury in striatum.
It should be noted that Gs protein-coupled adenosine A2A receptor and Gi protein-coupled dopamine D2 receptor colocalize in striatopallidal medium spiny neurons and interact antagonistically to regulate cAMP level and PKA activity. The tonic effects of dopamine on D2 receptors suppress the A2A receptor–cAMP–PKA cascade under physiologic conditions.32 The positive effect of an A2A antagonist in piglets after reoxygenation suggests that A2A receptor stimulation of PKA is not completely suppressed by D2 receptors during the early hours of recovery from H-I.
SCH 58261 Preferentially Rescues Striatopallidal Neurons After Hypoxia-Ischemia
Adenosine A2A receptors and dopamine D2 receptors mainly reside postsynaptically in striatopallidal medium spiny neurons; most dopamine D1 receptors are located postsynaptically in striatonigral neurons.2, 9 Thus, we anticipated that SCH 58261 would selectively rescue striatopallidal neurons with D2 and A2A receptors. However, quantification of the precise number of D1-, D2-, and A2A-positive cells is difficult because these receptors are diffusely distributed throughout the neuropil with less localization in striatal cell bodies.11, 33 Ebf1 and Gpr6 can be used as alternative cell markers for D1- and D2-receptor-positive cells because they selectively locate in striatal neurons that project to nigra and globus pallidus, respectively.34, 35 As expected from the colocalization of A2A receptors with Gpr6-positive neurons, SCH 58261 treatment protected a greater proportion of Gpr6-positive cells than of Ebf1-positive cells after H-I injury. However, the A2A antagonist also protected a small portion of Ebf1-positive neurons. One explanation is that crosstalk in the signaling between the two subpopulations of medium spiny neurons results in partial protection of one population when the excitotoxic stress in the other population is reduced. Another explanation could be related to the presence of striatal A2A receptors presynaptically in the terminals of glutamatergic cortical-striatal pathway to striatal neurons that preferentially project to substantia nigra.36 These presynaptic A2A receptors form a heteromeric complex with A1 receptors and facilitate glutamatergic neurotransmission by blocking the normal A1 receptor-mediated inhibition of glutamate release.37 Consequently, high levels of extracellular adenosine after H-I could activate presynaptic, low-affinity A2A receptors, and augment glutamate release. Pharmacologic and genetic deletion of A2A receptors supports this possibility in ischemic adult brain.38, 39 Therefore, presynaptic and postsynaptic actions of A2A receptors could be involved in the observed neuroprotection of both populations of medium spiny neurons in the selectively vulnerable putamen.
Neonatal H-I encephalopathy is a major cause of brain injury in newborns and can result in long-term devastating consequences. Interestingly, prolonged caffeine treatment for bronchopulmonary dysplasia in preterm human neonates also decreased the incidence of mild cerebral palsy and improved mental development at 18 months of age.40 Although caffeine has multiple targets, its antagonist effects on A2A receptors in striatum may have contributed to the improved neurologic outcome. Our study shows that treatment with the selective adenosine A2A receptor antagonist SCH 58261 after H-I blunts phosphorylation of target proteins that are known to amplify neuronal excitability, alleviates nitrosative and oxidative protein alterations, accelerates neurologic recovery, and reduces ischemic injury to striatal neurons, particularly those known to project to globus pallidus. These findings in a large-animal model of neonatal H-I encephalopathy provide strong support that blocking A2A receptor activation during early recovery from H-I is neuroprotective in selectively vulnerable striatum and suggest that this pharmacologic approach deserves further preclinical investigation for potential neonatal H-I therapy.
Acknowledgments
The authors acknowledge the editorial assistance of Claire F Levine, MS.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This research was supported by National Institutes of Health grant NS060703 (RCK), an American Heart Association-Phillips Resuscitation Research Fellow Award (Z-JY), and an American Heart Association Research Fellow Award (BW).
Supplementary Material
References
- Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann NY Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and "fine tuning" modulation. Prog Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, et al. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aden U, Halldner L, Lagercrantz H, Dalmau I, Ledent C, Fredholm BB. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34:739–744. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Gilland E, Fredholm BB, Hagberg H. Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology. 1997;36:1327–1338. doi: 10.1016/s0028-3908(97)00139-1. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr., et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol. 2007;83:277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, et al. Dopamine receptor modulation of hypoxic-ischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metab. 2007;27:1339–1351. doi: 10.1038/sj.jcbfm.9600440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, et al. Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci USA. 2000;97:1856–1860. doi: 10.1073/pnas.97.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Guerguerian AM, Brambrink AM, Traystman RJ, Huganir RL, Martin LJ. Altered expression and phosphorylation of N-methyl-D-aspartate receptors in piglet striatum after hypoxia-ischemia. Brain Res Mol Brain Res. 2002;104:66–80. doi: 10.1016/s0169-328x(02)00285-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, et al. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Mueller-Burke D, Koehler RC, Martin LJ. Rapid NMDA receptor phosphorylation and oxidative stress precede striatal neurodegeneration after hypoxic ischemia in newborn piglets and are attenuated with hypothermia. Int J Dev Neurosci. 2008;26:67–76. doi: 10.1016/j.ijdevneu.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew DM, Koehler RC, Guerguerian AM, Shaffner DH, Traystman RJ, Martin LJ, et al. Hypothermia for 24 hours after asphyxic cardiac arrest in piglets provides striatal neuroprotection that is sustained 10 days after rewarming. Pediatr Res. 2003;54:253–262. doi: 10.1203/01.PDR.0000072783.22373.FF. [DOI] [PubMed] [Google Scholar]
- Salinas-Zeballos M, Zeballos GA, Gootman PM. A Sterotaxic Atlas of the Developing Swine (Sus scrofa) Forebrain. Vol. 2. Plenum Press: New York; 1986. [Google Scholar]
- Cheung HH, Teves L, Wallace MC, Gurd JW. Increased phosphorylation of the NR1 subunit of the NMDA receptor following cerebral ischemia. J Neurochem. 2001;78:1179–1182. doi: 10.1046/j.1471-4159.2001.0780051179.x. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Fillenz M. Adenosine agonists can both inhibit and enhance in vivo striatal dopamine release. Eur J Pharmacol. 1990;180:137–143. doi: 10.1016/0014-2999(90)90601-2. [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M, Augusto E, Machado NJ, dos Santos-Rodrigues A, Cunha RA, Agostinho P. Astrocytic adenosine A2A receptors control the amyloid-beta peptide-induced decrease of glutamate uptake. J Alzheimers Dis. 2012;31:555–567. doi: 10.3233/JAD-2012-120469. [DOI] [PubMed] [Google Scholar]
- Miekisiak G, Kulik T, Kusano Y, Kung D, Chen JF, Winn HR. Cerebral blood flow response in adenosine 2a receptor knockout mice during transient hypoxic hypoxia. J Cereb Blood Flow Metab. 2008;28:1656–1664. doi: 10.1038/jcbfm.2008.57. [DOI] [PubMed] [Google Scholar]
- Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, et al. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010;30:808–815. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto Ferreira M, DeLucia R, Luiz Aizenstein M, Glezer I, Scavone C. Fencamfamine modulates sodium, potassium-ATPase through cyclic AMP and cyclic AMP-dependent protein kinase in rat striatum. J Neural Transm. 1998;105:549–560. doi: 10.1007/s007020050078. [DOI] [PubMed] [Google Scholar]
- Cheng XJ, Hoog JO, Nairn AC, Greengard P, Aperia A. Regulation of rat Na(+)-K(+)-ATPase activity by PKC is modulated by state of phosphorylation of Ser-943 by PKA. Am J Physiol. 1997;273:C1981–C1986. doi: 10.1152/ajpcell.1997.273.6.C1981. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, et al. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnati LF, Ferre S, Lluis C, Franco R, Fuxe K. Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev. 2003;55:509–550. doi: 10.1124/pr.55.3.2. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, et al. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Cui Y, Ostlund SB, Balleine BW, Yang XW. Genetic control of instrumental conditioning by striatopallidal neuron-specific S1P receptor Gpr6. Nat Neurosci. 2007;10:1395–1397. doi: 10.1038/nn1987. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Lujan R, Uchigashima M, Simoes AP, Lerner TN, Borycz J, et al. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26:2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L, Duan W, Tian H, Li C, Zhu J, Chen JF, et al. Adenosine A 2A receptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res. 2009;1297:185–193. doi: 10.1016/j.brainres.2009.08.050. [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, et al. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–250. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357:1893–1902. doi: 10.1056/NEJMoa073679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.