Abstract
The blood–brain barrier (BBB) plays critical roles in the maintenance of central nervous system (CNS) homeostasis. Dysfunction of the BBB occurs in a number of CNS diseases, including Alzheimer's disease (AD). A prevailing hypothesis in the AD field is the amyloid cascade hypothesis that states that amyloid-β (Aβ) deposition in the CNS initiates a cascade of molecular events that cause neurodegeneration, leading to AD onset and progression. In this review, the participation of the BBB in the amyloid cascade and in other mechanisms of AD neurodegeneration will be discussed. We will specifically focus on three aspects of BBB dysfunction: disruption, perturbation of transporters, and secretion of neurotoxic substances by the BBB. We will also discuss the interaction of the BBB with components of the neurovascular unit in relation to AD and the potential contribution of AD risk factors to aspects of BBB dysfunction. From the results discussed herein, we conclude that BBB dysfunction contributes to AD through a number of mechanisms that could be initiated in the presence or absence of Aβ pathology.
Keywords: Alzheimer's disease, blood–brain barrier, cerebrospinal fluid, diabetes, inflammation, neurovascular unit
Introduction
Alzheimer's disease (AD) is the most common type of dementia, and affects ∼36 million individuals worldwide (http://www.alz.co.uk/research/world-report). The majority of individuals afflicted with AD initially present with symptoms of memory loss and cognitive impairment late in life (≥65 years old). These symptoms increase in severity as AD progresses, often become so debilitating that institutionalization is necessary, and are eventually fatal. Therefore, AD is a disease with tremendous socioeconomic cost. Because of the growing aged population and lack of treatments that can prevent or slow disease progression, future AD prevalence is expected to increase to an extent that it may threaten the sustainability of healthcare systems worldwide. This necessitates a global effort to better understand AD, with the goal of developing treatments that can prevent or slow AD progression.
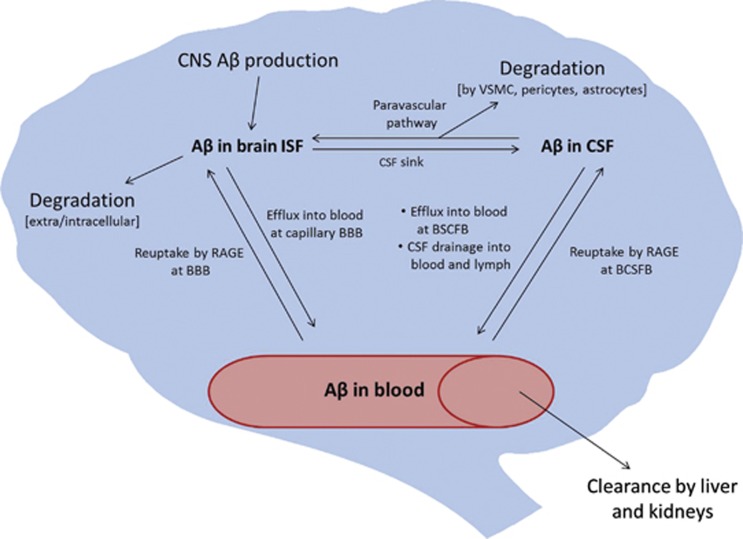
Much of the focus in AD research has been on amyloid-β peptide (Aβ) and tau, which are the protein constituents of the hallmark senile plaques and neurofibrillary tangles that pathologically define AD. The amyloid cascade hypothesis, initially proposed by Hardy and Higgins in 1992,1 updated by Hardy and Selkoe in 2002,2 and still a prevalent focus of AD research today, states that deposition of Aβ in the brain is the initiating event in AD. Although the hypothesis remains generally accepted, it is also increasingly evident that Aβ plays a complex, multifaceted role in AD progression. In rare, familial forms of AD, mutations in the Aβ precursor protein (APP) or in presenilin proteins, the enzymes that cleave Aβ from APP, cause increased Aβ deposition. In the remainder of AD cases, it is thought that Aβ deposition results from deficient clearance.2 Multiple routes of clearance have been elucidated for Aβ, including enzymatic degradation,3 bulk flow of cerebrospinal fluid (CSF),4 and transport across central nervous system (CNS)–blood barriers.5 These pathways are illustrated in Figure 1, and will be described in detail in later sections. In this review, we will discuss the participation of the blood–brain barrier (BBB) in AD with special focus on its potential roles both upstream and downstream of the amyloid cascade.
Figure 1.
Clearance pathways of amyloid-β (Aβ) from the brain. BBB, blood–brain barrier; CNS, central nervous system; BCSFB, blood–cerebrospinal fluid barrier; CSF, cerebrospinal fluid; ISF, interstitial fluid; RAGE, receptor for advanced glycation endproducts; VSMC, vascular smooth muscle cell.
Overview of the Blood–Brain Barrier
Endothelial cells of brain capillaries are the primary anatomic units of the BBB.6 These cells are highly specialized to support the essential functions of the BBB that include: (1) acting as a diffusion barrier, (2) transporting substances in and out of the brain, and (3) serving as an interface for communication between the CNS and periphery.7 Barrier properties of brain endothelial cells are conferred through expression of tight junctions at cell–cell contacts, absent fenestrae, degradative enzymes, reduced pinocytosis, and efflux transporters.6, 8 Tight junctions also restrict lateral diffusion of membrane proteins, and therefore support transporter function by maintaining their polarity.6 The BBB transporters play many roles in supporting proper CNS function by allowing selective passage of molecules in and out of the brain. Supportive cells such as astrocytes and pericytes are closely apposed to the capillary endothelium and play essential roles in BBB induction and maintenance.9, 10, 11, 12, 13 Neurons and microglia also communicate with BBB endothelial cells.6 Collectively, these cell types are referred to as the neurovascular unit (NVU). Because the BBB plays critical roles in maintaining CNS homeostasis, its dysfunction contributes to multiple diseases. The dysfunction of BBB includes (1) BBB disruption, which results in leakage of circulating substances into the CNS that can be neurotoxic; (2) transporter dysfunction, which has consequences such as inadequate nutrient supply, buildup of toxic substances in the CNS, and increased entry of compounds that are normally extruded; and (3) altered protein expression and secretions by endothelial cells and other cell types of the NVU that can result in inflammatory activation, oxidative stress, and neuronal damage. All three effects have been reported in AD, and are described in the remainder of this review.
Disruption of the Blood–Brain Barrier in Alzheimer's Disease
The possibility that the BBB is leaky in AD, that is, it does not prevent the uncontrolled entry into the brain of blood proteins and other molecules, has been investigated for ∼30 years. This is clearly an important question as disruption of even a transient or localized nature could have devastating consequences for brain function, inducing a cascade of events involving neurotoxicity, neuroinflammation, and oxidative stress that eventually could produce the AD phenotype. Indeed, some, but not all, animal models of AD exhibit BBB disruption. But to determine whether BBB disruption is actually a feature of AD, one can only consider clinical material.
Disruption of BBB can be readily shown in conditions such as stroke and multiple sclerosis, but showing a disrupted BBB in AD has been more controversial. Many papers have examined the question of BBB disruption in AD, some concluding that disruption occurs and some concluding that it does not. Here, we review selected papers on this topic to give a sense of the problems and surrounding controversies. In general, investigations fall into three categories: comparison of CSF/serum ratios of substances of peripheral origin, usually of albumin; histologic examination for evidence of blood proteins in the CNS; and imaging studies. These will each be considered below.
Albumin Cerebrospinal Fluid/Serum Ratios
The rationale as crystallized by Tibbling et al14 is very straightforward: albumin in the CNS is derived from peripheral sources; therefore, an increased amount of albumin in the CNS can be used as an index of BBB disruption. Tibbling et al14 established reference values for CSF/serum ratios for albumin that could be used as an indicator of BBB disruption and for the immunoglobulin G (IgG) index that could be used to indicate antibody synthesis within the CNS. Alafuzoff et al15 soon showed that CSF albumin levels were elevated in both patients with AD and even more so in patients with multi-infarct dementia; however, CSF/serum albumin ratios showed statistically significant elevations in multi-infarct versus AD patients and multi-infarct versus controls, but not between controls and AD. Elovaara et al16 showed that CSF/serum albumin ratios were elevated in both ambulatory and institutionalized AD patients in comparison with age-matched controls. Hampel et al17 compared ratios from patients with early-onset AD, late-onset AD, and major depression with age-matched controls. They found no statistical differences among the groups, but they did claim that 24% of major depression patients and 18% of AD patients exhibited a ‘pathologic albumin ratio.' Wada18 found that the CSF/serum ratio for albumin was elevated in both AD and vascular dementia patients. Skoog et al19 examined the CSF/serum albumin ratio in 85-year-old patients with AD, with vascular dementia, or who were free of dementia. The ratio was elevated in those with AD and vascular dementia and was predictive of those who developed dementia during the study, but was not correlative with dementia severity. The highest elevations were seen in the individuals who were Apolipoprotein E3 (ApoE3) negative, with the ratio for nondemented individuals not carrying the ApoE3 allele being statistically different from that of ApoE3 carriers. The ratio for demented non-ApoE3 carriers was arithmetically, but not statistically, elevated and there were no statistical differences between carriers versus noncarriers for ApoE2 or ApoE4. Using a reference value of <9.2 as normal, Algotsson and Winblad20 found that 42% of men and 13% of women with AD had elevated ratios; plasma creatinine was also a predictor of elevated ratios. Bowman et al21 found that ratios were elevated in ∼22% of AD patients and was predictive of changes in cognitive testing and ventricular volume. In a study by Blennow et al22 of 118 patients with AD and 50 healthy controls, CSF/serum ratios for albumin were found to be elevated only in those who had vascular disease accompanying their AD, but not in those patients with AD alone. Farrall and Wardlaw23 reviewed 21 studies that examined CSF/serum albumin ratios in AD. Seven of these studies included separate analysis of patients with vascular disease. We calculate from those seven studies that the mean ratio for the AD patients ranged from 86% to 138% of the controls (mean±s.d.: 122±20%, P<0.05) and for the vascular dementia patients from 105% to 184% (141±27%, P<0.01).
Substances other than albumin have also been used to assess BBB function in AD. Immunoglobulin G levels, either as the CSF/serum ratios or as the IgG index of Tibbling et al,14 have often been assessed but variably interpreted, with some authors using them as an index of immune function/inflammation and others using them as another indicator of BBB disruption.15, 16, 17, 21 Other substances whose CSF or CSF/serum ratios have been used to indicate BBB disruption in AD are secretory calcium-dependent phospholipase A224 and oligoclonal bands.25
Not all studies have found increases in CSF/serum albumin ratios. Mecocci et al26 found increased ratios in their multi-infarct dementia patients but not in their AD patients. Kay et al27 found no increase in the ratio in AD patients nor a correlation between albumin levels in CSF and brain atrophy. Frolich et al28 also failed to find any differences in the ratio between AD and controls. Each of these studies also assessed IgG and found no abnormalities for that substance as well.
Both Frolich et al28 and Chen29 considered reasons why conflicting results have been found with regard to CSF/serum ratios for albumin and other substances. First, many studies, especially early ones, have been less rigorous in differentiating AD from vascular dementias. In many of the studies that did distinguish between AD and vascular dementias, no details are given regarding the criteria used to categorize patients, making comparisons among studies difficult. Even today, the criteria that should be used for diagnosing vascular dementias are debated.30 Nevertheless, vascular dementias as categorized in these studies would have an increased likelihood of having disruptions of the BBB. Inclusion of patients with such disruptions would tend to produce false elevations in CSF/serum ratios if they were included in the AD group. Second, perfect control groups are difficult to assemble; those with younger healthier controls might overestimate any elevation in ratios and those with sicker controls or with diseases that affect BBB integrity might underestimate any elevation. Third, it may be that only a subgroup of AD patients have BBB disruption that is measurable by ratios or that a subgroup of individuals with BBB disruption are protected by other factors from developing AD. In either scenario, a subgroup would be difficult to detect and could greatly sway results in an individual study, especially when that study has a limited number of patients that by the nature of circumstances have undefined preselection biases. The major problem, however, with using CSF/serum ratios as a proxy for BBB disruption is that factors other than disruption affect the level of albumin in the CSF. In particular, the rate of CSF turnover is greatly slowed in aging and even more so in AD.29, 31 The decreases in CSF production and reabsorption result in increases in CSF albumin levels and the levels of other substances not otherwise exported or catabolized by the CNS. These results make CSF/serum ratios unreliable indicators of BBB integrity. These studies do, however, indicate a dysfunction in other aspects of CSF dynamics in AD that could have profound effects on CNS function.
Histologic Studies
Most of these studies have relied on immunocytochemical techniques to detect the presence of plasma or serum proteins in the CNS. Assuming the proteins are not produced in the CNS, they can be used to indirectly indicate BBB disruption. Wisniewski and Kozlowski32 examined brains from 7 patients with AD aged 63 to 85 years and 5 nondemented controls aged 29 to 77 years for albumin and immunoglobulin staining. They found light staining for both albumin and immunoglobulins in controls and in the regions of brain with single or no amyloid plaques. In areas with heavy plaque burden, they found intensive staining of the plaques and the neuropile, with the heaviest staining being in the corona of the amyloid plaques. They also found perivascular staining in AD brains that was indicative of BBB disruption. In a later study, Wisniewski et al33 found albumin staining around microvessels that also had amyloid plaques or angiopathy and suggested that the staining resulted from an affinity of extravasated albumin for amyloid.
Substances other than albumin and immunoglobulins have been used to suggest BBB disruption. Peptides related or derived from hemoglobin are found in increased concentration in cerebellums from AD patients.34 These peptides may have arisen from an increased leakage of blood in AD as suggested by the work of De Reuck35 or by decreased clearance of these peptides by the AD brain. Prothrombin is not produced by the normal brain but shows increased levels in AD brains consistent with leakage across a disrupted BBB.36 Prothrombin levels in brain were elevated and it was found to be surrounding microvessels. Levels were highest in patients with the highest Braak stage and with at least one ApoE4 allele, but did not correlate with cerebral amyloid angiopathy. However, prothrombin mRNA is expressed by brain under some conditions.37, 38 If prothrombin is expressed by the AD brain, these results could be because of defective clearance mechanisms for prothrombin. Claudio39 found increased vesicularization of brain endothelial cells from AD patients, consistent with increased transcytotic disruption of the BBB.
Others have failed to find evidence for BBB disruption in AD. Alafuzoff et al40 stained for prealbumin, C1q, C3c, and fibrinogen, as well as albumin and immunoglobulins, concluding that the BBB is equally compromised in patients with AD as in the nondemented elderly. Rozemuller et al41 examined the effects of post-mortem delay in obtaining tissue, of formalin fixation, and of clinical course on immunohistochemical findings. They found that post-mortem delay and clinical course had no consistent effects but that formalin fixation, especially short-term formalin fixation, resulted in increased levels of staining for albumin, IgG, and complement fixation in comparison with fresh frozen cryostat sections. They found both AD cases without albumin staining of brain tissue and controls with staining, although staining was more common in AD cases (15 out of 28 AD cases versus 4 out of 18 normal controls and 7 out of 13 cases with non-AD neuropathological disorders). Although this could indicate that a subgroup of patients with AD do not have BBB disruption, they favored the conclusion that staining was a function of agonal and post-mortem changes in brain with those changes being more pronounced for damaged or degenerating cells. For these reasons, they concluded, ‘In our opinion, this approach, studying post-mortem brain tissue, cannot be used to (dis)prove dysfunction of the blood-brain barrier in the pathogenesis of Alzheimer's disease.'
Using fibrinogen and immunoglobulins as markers, Tomimoto et al42 found evidence for BBB disruption in patients with ischemic cerebrovascular disease but not with AD. Munoz et al43 compared AD patients with and without infarcts, age-matched controls with and without infarcts, and patients with multi-infarct dementia, and found no difference among groups for albumin, IgG, complement C3c component, or serum amyloid P staining.
Others have examined histologic material for cerebrovascular lesions in dementias, including AD. One clear finding of these studies is that patients with evidence for only a single cause of dementia such as AD, vascular dementia, or Lewy body disease are a minority. De Reuck35 examined the occurrence of small cerebral bleeds in various neurodegenerative diseases. He found that ∼50% of age-matched nondemented controls had microscopic bleeds. The percent of microscopic bleeds in AD patients was not reported, but in a severity scale of microscopic bleeds, those with AD had a worse score than age-matched controls and those with AD plus cerebral amyloid angiopathy still worse. Deramecourt et al44 devised a systematic semiquantitative scoring system for vascular pathology that included microinfarcts and gross infarcts as well as presence of arteriosclerosis, amyloid angiopathy, and perivascular hemosiderin leakage, applying it to 135 brains from patients with dementia. Approximately 50% of these patients had mixed lesions (a combination of vascular dementia, AD, or dementia with Lewy bodies), ∼20% with vascular dementia only, and ∼15% each with AD or dementia with Lewy bodies only. Of the many interesting findings from this study, one was that the most common lesion was arteriosclerosis, although a few patients from each category did not have this finding. In general, AD patients had much lower scores than vascular dementia patients, including the total absence of large and microinfarcts. Although the differences between the pure vascular dementia group and the pure neurodegenerative groups were striking, a control group was not analyzed and hence it is unclear how the vascular score for age-matched, nondemented controls would have compared with that for AD.
Overall, the clearest conclusions from histologic studies are that those with vascular dementia clearly have evidence of small vessel disease including that consistent with BBB disruption, that such evidence is more common in vascular dementia than in AD, that there are cases of AD with little or no evidence of BBB disruption, and that the patients with only a single disease, such as AD or vascular dementia, are in the minority of any study. The evidence also strongly suggests that vascular lesions occur to some extent in nondemented age-matched controls and that studies that rely on immunostaining as evidence of leakage must be particularly careful regarding issues such as time from death to fixation and fixation techniques. Those studies relying on immunostaining should also consider whether the increased detection of the index substance in the CNS is only dependent on its blood-to-brain leakage and not also affected by its induction of CNS production, alteration in clearance from the CNS, or increased CNS retention. Given these considerations, it is difficult to conclude that histologic studies have shown that AD patients without a cerebrovascular component have increased BBB disruption when compared with age-matched controls. However, as the histologic studies show that the majority of AD patients also have evidence of infarct (and the majority of vascular dementia patients have evidence of neurodegenerative disease), the studies suggest that the most relevant clinical question may not be whether the BBB is disrupted in AD, but whether or how neurodegenerative diseases and cerebrovascular disease interact to promote the onset and progression of dementia. Such interactions might not only induce BBB disruption, but contribute to the many other altered BBB functions found in AD.
Imaging
Several studies using various modalities of imaging have addressed the question of whether the BBB is disrupted in AD. Two computed tomography (CT) studies45, 46 and a positron emission tomography (PET) study with [68Ga]EDTA47 did not find increased BBB permeability in AD. A magnetic resonance imaging (MRI) study by Starr et al48 found evidence for leakage in AD patients, but this was not significantly different from the rate of leakage found in age-matched, nondemented controls. Bronge and Wahlund,49 using MRI to specifically examine white matter lesions in 10 demented patients (including 5 with elevated CSF/serum albumin ratios), found no evidence for BBB leakage. Wang et al50 found with MRI that the hippocampi of patients had a lower vascular space, but not a statistically significant difference in BBB leakage. Overall, imaging studies have not found evidence for BBB disruption in patients with AD.
Summary of Results for Blood–Brain Barrier Disruption
The CSF/serum ratios for serum proteins, immunohistochemical evaluations, and imaging modalities have all been useful in showing BBB disruption in a host of clinical conditions, including dementia resulting from infarcts. Application of these techniques to AD has led some to conclude that AD patients, or at least a subset of AD patients, have disruptions of the BBB. Others have not found such evidence, whereas some others have pointed out the difficulties in applying CSF/serum ratios and immunohistochemical approaches to AD. Several MRI, CT, and PET studies have failed to show convincing evidence of BBB disruption in AD. Any method will have a lower limit of detection and any disruption of the BBB, no matter how transient or limited in scope, could be relevant, especially in a susceptible population. To date, however, it is difficult to claim that BBB disruption is a major driver of pathology in AD patients who do not also have a vascular component to their dementia.
Defects in Blood–Brain Barrier Transporters That May Contribute to Alzheimer's Disease
Transport of Amyloid-β Efflux
The first observation of Aβ efflux from the brain was by Ghersi-Egea et al,51 who showed that Aβ in CSF was rapidly cleared into the bloodstream. Shibata et al52 subsequently showed that Aβ in the brain parenchyma was cleared primarily across the BBB via the low-density lipoprotein receptor-related protein-1 (LRP-1). In the same study, and later in others, LRP-1 expression was found to be decreased in the AD brain microvasculature.52, 53 As a result, the neurovascular hypothesis of AD was proposed that originally stated that defects in Aβ efflux across the BBB because of LRP-1 deficiency contribute to Aβ accumulation in the brain and hence promote AD.5 This hypothesis has since been validated and expanded on by multiple groups. The participation of LRP-1 in BBB efflux of Aβ is supported by the finding that knockdown of LRP-1 in the BBB using antisense oligonucleotides causes impaired Aβ efflux, accumulation of Aβ in the brains of young, wild-type mice, and cognitive impairments.54 The association of impaired Aβ efflux with AD was substantiated by observations of decreased Aβ efflux in rodent models of AD55, 56 as well as in aged squirrel monkeys57 and in a small sample set of humans with AD.58
More recently, it has become evident that other transporters in the BBB facilitating Aβ efflux also become impaired in AD. One example is the multidrug transporter P-glycoprotein (Pgp). Evidence supporting this role for Pgp includes: (1) observations of Pgp-dependent efflux of Aβ in vitro,59, 60 (2) an inverse correlation of Aβ deposition and microvascular Pgp expression in human brain tissue,61 (3) decreased Aβ efflux and enhanced Aβ deposition in mice that lack Pgp,62 (4) impaired microvascular Pgp function in a transgenic AD mouse model that is restored by pharmacologic intervention shows corresponding improvement in Aβ efflux and reduced Aβ deposition,62, 63 and (5) showing Pgp dysfunction in human AD using clinical PET imaging studies.64 Because of its luminal location,65 it has been proposed that Pgp facilitates the extrusion of Aβ from the endothelial cell into the bloodstream after Aβ has been internalized from brain interstitial fluid (ISF) by LRP-1.63 However, our group has found that under inflammatory conditions, antioxidant treatment that preserves LRP-1 but not Pgp function also preserves Aβ efflux.66 This suggests that LRP-1 can also function independently of Pgp. Furthermore, Pgp may function independently of LRP-1 by limiting the influx of blood-borne Aβ into the brain.67 The pathways governing influx will be described in the next section. The cellular prion protein68 and the multidrug transporters ABCG2 and 4 have also been shown to contribute to Aβ efflux across the BBB,69 although AD-relevant changes of these are presently unclear.
Based on present results, it is conceivable that deficient BBB efflux of Aβ could both initiate and be initiated by the amyloid cascade. Pathologic states such as inflammation, obesity, diabetes, stroke, and others are known to alter the function of many transporters in the BBB. Interestingly, many of these conditions are also considered risk factors for AD. There is evidence that such risk factors alter the function of Aβ transporters in the BBB, and details of this will be described in a later section. More severe defects in Aβ transport are also likely to occur as a result of Aβ accumulation in the CNS. Amyloid-β has a much higher propensity to transition to β-sheet conformation and aggregate as its concentration increases.70 Optimal BBB efflux occurs for Aβ in its monomeric state, and transporter affinity decreases with increased aggregation/β-sheet content.71, 72 Therefore, increased Aβ accumulation in the CNS would preclude its efflux. Amyloid-β also decreases microvascular expression of its transporters. This is evident for LRP-1 and Pgp in transgenic mouse models of AD,63, 71 and in nontransgenic mice treated with Aβ.73 Impaired Aβ efflux is also observed in the SAMP8 mouse model of AD, which derives from a spontaneous mutation found to cause accelerated senescence.56 These mice show a modest increase in APP and Aβ compared with transgenic models, yet have marked age-associated cognitive impairment.74, 75 Evidence supports that the Aβ efflux deficit occurs as a result of Aβ accumulation in this model as well, as antisense oligonucleotide that reduces APP expression and Aβ accumulation also restores Aβ efflux to normal levels.76 Together, these results suggest that deficits in efflux of Aβ across the BBB feedforward as AD progresses, and unique therapeutic interventions may be necessary at each stage of disease.
Transport of Amyloid-β Influx and Systemic Clearance
The amyloid precursor protein is expressed in a variety of tissues other than brain, and low levels of Aβ are detectable in the circulation.77 Before the realization of an efflux system for Aβ, it was tested whether circulating Aβ could contribute to Aβ deposition in the brain. Studies in rodents showed that a 28-amino-acid fragment, as well as the 40- and 42-amino-acid forms of Aβ, were taken up by capillaries and could completely cross the BBB from the circulation.78, 79, 80, 81, 82 Later, the transporter that mediates luminal-to-abluminal transcytosis of Aβ was identified as the receptor for advanced glycation endproducts (RAGE).83 In transgenic rodent AD models, influx was shown to be a substantial contributor to Aβ deposition; blocking the Aβ/RAGE interaction significantly reduced the appearance of plaques in these models.83, 84 Furthermore, RAGE upregulation is observed in the CNS microvasculature of humans with AD, as analyzed by histochemical methods in post-mortem tissue.85, 86
Systemic clearance of Aβ is facilitated by the liver and, to a lesser extent, the kidneys and spleen.55, 87 Lipoprotein receptor-related protein-1 was shown to be the predominant transporter that mediates clearance by the liver. The short half-life of circulating Aβ (on the scale of minutes),55, 87 and the capacity of the liver to clear Aβ at levels far exceeding its physiologic concentration in blood suggest that there is a biologic necessity to protect against elevations in circulating Aβ.87 This also suggests that rapid systemic clearance of Aβ prevents reuptake by RAGE after efflux. Liver ligation was shown to be an effective method to maintain high levels of Aβ in the circulation up to 1 hour after intravenous injection. In the same study, liver-ligated rats with sustained increased levels of Aβ in blood showed markedly reduced efflux of Aβ from the brain.88 Despite the results of these studies in rodents, data on plasma Aβ changes in human AD have been contradictory. Increased,89, 90 decreased,91, 92 or unchanged93, 94 circulating levels of Aβ have been reported in clinical studies. Furthermore, it remains unclear whether changes that are detected have any predictive value for cognitive decline.91, 92, 94
Lack of human evidence showing changes in circulating levels of Aβ in AD suggests that an alternative mechanism is relevant for influx. An early study showed that Aβ entering the brain from the circulation is unbound.82 Amyloid-β in the circulation is bound to a number of proteins naturally occurring in blood such as albumin,95 ApoE and ApoJ,96, 97 and a soluble form of LRP-1 (sLRP-1).98 The latter was shown to bind the majority of circulating Aβ, and Aβ bound to sLRP-1 prevents RAGE-dependent influx but enhances systemic clearance.98 This evidence supports a peripheral sink hypothesis,99 where binding proteins in serum facilitate systemic clearance of Aβ and prevent its uptake by RAGE. Peripheral sink mechanisms may therefore at least in part explain how therapeutics such as antibodies against Aβ100 and other binding proteins such as gelsolin101 and sLRP-198 lower Aβ levels in the CNS. Furthermore, individuals with AD have slightly lower circulating levels of sLRP-1, as well as increased oxidative damage to sLRP-1, which markedly lowers its binding affinity for Aβ.98 This would increase the pool of free circulating Aβ that is available for RAGE-dependent influx. Future studies are necessary to investigate the utility of sLRP-1, both as a plasma biomarker of AD and as an Aβ-lowering therapeutic.
GLUT-1 Transporter, Alzheimer's Disease, and the Blood–Brain Barrier
Glucose use by brain is decreased in AD, with low glucose metabolism predating clinical symptoms, predicting subsequent decline in cognitive function, and with further decreases in glucose use correlating with those cognitive declines.102 Glucose uptake from blood to brain is dependent on GLUT-1 expression by the BBB, with GLUT-1 increasing the transport rate of D-glucose across the BBB by ∼30- to 100-fold.103 With the capillary bed comprising ∼0.1% of brain weight and the brain using ∼20% of the body's glucose, it is not surprising that GLUT-1 is highly expressed by the BBB or that a reduction in GLUT-1 expression is associated with seizures and mental retardation.104 The brain microvessels in AD, including those from the hippocampus, have a reduction in the expression of GLUT-1 protein,105, 106, 107 but not a decrease in GLUT-1 mRNA expression.108
Decreased expression of GLUT-1 in the BBB raises the question of whether this is because of decreased demand by a dysfunctional brain or whether the brain is dysfunctional in AD because the BBB is not delivering adequate amounts of glucose, essentially starving the brain. The answer to this question is vital: if GLUT-1 activity is a primary defect in AD, then providing more energy to the brain would be the major step to preventing and treating AD. Harik109 postulated that the decrease in GLUT-1 in the BBB was secondary because no corresponding decrease in GLUT-1 was found in the erythrocytes of the AD patients, with erythrocytes being a readily accessible tissue that contains GLUT-1. This contrasts with De Vivo's disease, an inherited condition in which GLUT-1 is underexpressed in both the BBB and erythrocytes. The brain capillaries of the aged APP/PS1 transgenic mouse have both an absolute decrease in GLUT-1 expression and a decreased density of GLUT-1 that seems to occur after a critical level of Aβ peptide accumulates in brain.110 In imaging studies of humans, however, glucose use by brain correlates with ApoE genotype but not with fibrillated Aβ peptide load in brain as assessed in vivo by two different compounds.111, 112
In conclusion, GLUT-1 expression and activity is decreased in the BBB of AD patients and in transgenic animal models. Although the reason for this decrease is not known, current thinking seems to lean toward it being in response to the decreased metabolic demand by brain.
Cerebrospinal Fluid/Interstitial Fluid Bulk Flow, Brain Barriers, and Alzheimer's Disease
The Role of Cerebrospinal Fluid/Interstitial Fluid Bulk Flow in Alzheimer's Disease, and Participation of the Blood–Brain Barrier
The CSF has many critical functions in the CNS, including physical protection, regulation of intracranial pressure, waste removal, and providing a supportive milieu (for detailed review, see Johanson et al4). The CSF is produced by specialized ependymal cells in the choroid plexus (CP) that form the blood–CSF barrier (BCSFB). These cells, like the BBB, have tight junctions that restrict paracellular diffusion of solutes and express specialized transporters that carefully regulate the molecular composition of CSF.113 In humans, the entire CSF volume turns over ∼4 to 6 times per day. In AD,114 this rate is markedly decreased because of a combination of decreased CSF production and increased CSF volume caused by brain atrophy and ventricular enlargement.4 Decreased CSF turnover in aging is associated with decreased clearance of solutes ectopically administered in CSF,115 including Aβ.116
The ability of CSF to supply nutrients to and clear waste from the brain ISF depends on its ability to mix with ISF. No barrier exists between ISF and CSF, and therefore solute transfer can occur between both fluid compartments. It has been proposed that a predominant mechanism driving solute transfer is bulk flow of ISF into CSF compartments. In this model, CSF acts as a ‘sink' for solutes in ISF.117, 118 A distinct paravascular anatomic pathway for CSF volume transfer has also been shown,119, 120 and a recent study has elucidated a role for aquaporin 4 (AQP4), a water channel that is localized to astrocytic endfeet of the microvasculature, in this pathway.121 Iliff et al121 found that Aβ follows this paravascular pathway, implicating its importance in AD. These findings show that Aβ in CSF also has access to vascular clearance, either through uptake and degradation by vascular mural cells122, 123 or by efflux across the BBB after entry into ISF.52 Conversely, sluggish bulk flow of CSF/ISF may cause Aβ accumulation in the perivascular spaces,124 and contribute to vascular toxicity in AD. This may also be important to cerebral amyloid angiopathy (CAA) that is observed in a majority of pathologically diagnosed AD cases.125 Finally, the data of Iliff et al121 suggest that AQP4 may play an important role in AD. Both increases and decreases in AQP4 expression have been reported in human AD brains.126, 127 In a recent study, it was shown that astrocytes upregulate AQP4 expression in response to lower concentrations of Aβ42, whereas exposure to higher concentrations results in downregulation of AQP4.128 Knockout of AQP4 (ref. 121) and reduction of AQP4 by endothelial-specific agrin knockout129 result in Aβ accumulation in the brain with no apparent signs of BBB disruption.121, 129 However, AQP4 knockout in astrocytes protects against Aβ-induced activation and toxicity.128 Therefore, variable changes in AQP4 may reflect a varying necessity for promoting Aβ clearance versus protecting against gliosis as Aβ concentrations rise to pathologic levels. In contrast to AQP4, aquaporin 1 (AQP1) localization in the CNS is primarily at CP epithelial cells under physiologic conditions and plays an important role in CSF dynamics.130 In pathologic conditions, AQP1 is detected in astrocytes.131 In AD, astrocytic upregulation of AQP1 and localization to senile plaques has been reported,132, 133, 134 and therefore AQP1 may also have important functional implications for CSF/ISF dynamics and AD progression.
Blood–Cerebrospinal Fluid Barrier Transport in Aging and Alzheimer's Disease
The BCSFB, like the BBB, is an active transport interface for a number of peptides between the blood and CSF compartments. Impaired BCSFB function could therefore cause CNS disturbances that would promote AD. This is evident with respect to reduced CSF production in AD,31 whose consequences on CSF turnover were discussed in the previous section. Evidence also supports, however, that CP epithelial cells play a direct role in regulating levels of Aβ in CSF. Choroid plexus epithelial cells show saturable uptake and complete transcytosis of Aβ that favors efflux in the CSF-to-blood direction.135 The BCSFB, like the BBB, expresses transporters for Aβ that include LRP-1,136 LRP-2/megalin,137 Pgp,138 and RAGE.139 The efflux transporters LRP-1 and Pgp in the CP increase with aging,139 which is opposite to expression patterns observed with aging in the BBB. Similarly, the bidirectional transporter LRP-2 and influx transporter RAGE were decreased and unchanged with age, respectively.139 These changes were associated with decreased concentrations of Aβ42 in the CP, suggesting that the BCSFB compensates for age-dependent transporter dysfunctions in the BBB.139 In humans with AD, increased Aβ deposition in the CP is observed.140 Furthermore, Aβ deposition in CP epithelial cells causes oxidative damage, disruption of the BCSFB, and cell death.140 This supports that transporter dysfunction and Aβ deposition in the BCSFB is an important pathologic distinction from normal aging and AD, and likely contributes mechanistically to AD pathogenesis.
Alzheimer's Disease and the Neurovascular Unit
Many cell types in addition to brain endothelial cells contribute to the essential function of the BBB, including pericytes, microglia, astrocytes, and neurons. These cell types and the extracellular matrix are collectively referred to as the NVU.141 This section will discuss how pathologic changes in the NVU in AD could cause BBB dysfunction, and potentially lead to subsequent neurodegeneration.
Pericytes
Among all cell types of the NVU, pericytes have the closest anatomic association with the capillary endothelium. They are located beneath a layer of the basement membrane, and direct communication between pericytes and endothelial cells is possible through gap and peg-and-socket junctions. The ratio of pericytes to endothelial cells in the brain ranges from 1:5 in rats to 1:3 in humans in the CNS, which is much higher than in peripheral tissues.142 Pericytes are important for induction and maintenance of BBB functions as supported by (1) findings of higher transendothelial electrical resistance and reduced permeability to fluorescein when pericytes are cocultured with brain endothelial cells in vitro,143 and (2) a leaky BBB in pericyte-deficient mice.9, 10, 12 The CNS pericytes are also able to differentiate into multiple cell types, and therefore are a reservoir of multipotent stem cells in the brain.144 In AD, it was found that pericyte coverage of capillaries in the cortex and hippocampus was markedly reduced compared with nondemented controls, and this correlated with reduced BBB integrity.145 Because Aβ is toxic to pericytes in vitro,146 it is possible that pericyte degeneration observed in AD is a consequence of Aβ accumulation. However, other risk factors for AD such as stroke,147 traumatic brain injury,148 and diabetes149 also cause microvascular pericyte degeneration in the CNS. Therefore, pericyte loss in AD could have multiple causes both up- and downstream of Aβ.
Although anatomically distinct, pericytes have many similarities to vascular smooth muscle cells (VSMCs). For example, like VSMCs, pericytes have receptors for vasoactive substances and can regulate capillary diameter and local blood flow.150 Vascular smooth muscle cells play an important role in Aβ uptake and clearance by degradation, and their ability to clear Aβ is impaired in AD.122 This function in VSMCs is dependent on Aβ endocytosis by LRP-1 or scavenger receptors,122, 123, 151 followed by lysosomal degradation.123, 151 Whether pericytes have a similar Aβ clearing function is presently unknown, but they do express LRP-1.152, 153
Astrocytes
Astrocytes are the most abundant cell type in the brain, and have important functions in the BBB. Their endfeet surround the endothelium of capillaries, arterioles, and venules.154 At capillaries, the astrocytic endfeet are closely apposed to the endothelium and surrounding basement membrane such that interstitial space is absent.155 This proximity allows for cross-talk between astrocytes and capillary endothelial cells that promotes the phenotypic specialization of both cell types. In vitro, coculture of brain endothelial cell monolayers with astrocytes or astrocyte supernatant strengthens barrier properties of the monolayer and increases the integrity of tight junctions.156, 157 Astrocytes also enhance expression of BBB transporters such as Pgp in brain endothelial cells.158 Induction of the BBB phenotype is strongest when astrocyte processes are allowed to contact the endothelial monolayer,13 suggesting an importance of physical contact in addition to secreted factors. Brain endothelial cells exert reciprocal effects on astrocytes in coculture, such as influencing the localization of AQP4 on astrocyte plasma membranes.13 Therefore, communication between endothelial cells and astrocytes is important for a number of BBB-centric processes discussed in previous sections of this review (i.e., disruption, transporter function, CSF bulk flow) that become altered in AD.
Astrocytes can internalize and degrade Aβ,159 resulting in reduced Aβ accumulation in the CNS.160 In AD, astrocytes are found in proximity to Aβ plaques,161 and perivascular astrocytes accumulate Aβ.154 Furthermore, in response to Aβ–ApoE complexes, astrocytes from nondemented human brain tissue upregulate their expression of scavenger receptor B1 and neprilysin, which participate in the internalization and degradation of Aβ, respectively.162 This function is lost in astrocytes derived from AD brains,162 suggesting that the Aβ clearance function of astrocytes is impaired in AD. Exposure of astrocytes to Aβ also results in astrogliosis, oxidative stress, and impaired glutamate uptake.163 This has clear negative consequences for neuronal survival,164 but the contribution of Aβ-induced astrocytic changes to BBB function is less clear. It has been shown that astrocyte changes precede widespread amyloid pathology and BBB disruption in a mouse model that develops plaques and CAA.165 This suggests that Aβ-induced astrocyte changes in AD may contribute to BBB dysfunction, but the mechanisms by which this occurs have yet to be determined.
Microglia/Perivascular Macrophages
The resident immune cells of the brain are microglia that derive from monocyte populations during embryonic development, but may also be replenished by circulating monocytes that enter the brain during adulthood.166 Microglia constantly survey the CNS,167 and transition to an activated phenotype on contact with an immune stimulus. Activated microglia interact with the BBB through secretions of cytokines and vasoactive substances, and physically shield blood vessels after injury.167 They are therefore an important constituent of the neurovascular unit. In AD, activated microglia are found surrounding amyloid plaques,168 and microglia activation correlates with cognitive impairment in living AD patients.169 Microglia activation has somewhat conflicting roles in AD. Proinflammatory molecules secreted from activated microglia could result in direct or indirect neurotoxicity. The BBB may contribute to this indirect neurotoxicity through disruption, secretions, and/or aberrant transport in response to microglial activation. The details of AD-related BBB changes in response to inflammation will be discussed in a later section. Conversely, activated microglia can promote Aβ clearance through phagocytosis.168, 170 Phagocytosis of Aβ was shown to be a property of a specialized subset of brain macrophages that are recruited from the circulation, migrate to parenchymal plaques, and inhibit plaque deposition.170, 171 Perivascular macrophages also phagocytose Aβ, and protect against vascular deposition of Aβ in a mouse model of cerebral amyloid angiopathy.172 Furthermore, peripheral blood mononuclear cells from individuals with AD show inefficient phagocytosis of Aβ compared with healthy controls.173 Together, these data support that deficient phagocytosis of Aβ by brain macrophages could contribute to Aβ deposition in AD. Although participation of the BBB in recruitment of these cells into the brain is implicit, the mechanistic details of this process are presently not well understood.
Neurons
The brain is an organ with an exceptionally high metabolic demand, and is highly vascularized to meet such a demand. This allows for neurons to regulate their own blood supply according to their metabolic needs through communication with the endothelium. Dysregulation of this communication (neurovascular uncoupling) is thought to promote AD, and this topic has been extensively reviewed in a previous issue of this journal.174 Therefore, this section will discuss neurovascular cross-talk in the context of how soluble factors produced in the BBB in AD influence neurodegeneration. A substantial amount of information on factors whose expression and/or secretion is altered in the BBB in AD has been elucidated by Grammas et al175 (for review, see ref. 175). Of these secreted products, some were found to be neurotoxic (e.g., thrombin) and others neuroprotective (endothelin-1, and CCL5/RANTES).176, 177, 178 However, an overall neurotoxic effect was observed after neuronal coculture with AD microvessels or conditioned medium.179 Recent work by the same group has suggested that vascular endothelial growth factor (VEGF) may contribute to AD neurodegeneration.180 Vascular endothelial growth factor is an angiogenic factor that is produced by endothelial cells in response to insults such as oxidative stress and ischemia.181 Vascular endothelial growth factor can be neuroprotective in ischemic stroke182 and glutamate excitotoxicity,183 and therefore may have therapeutic applications in such conditions. However, Sanchez et al180 found that VEGF secretions by microvessels isolated from AD brains resulted in exceptionally high concentrations ex vivo compared with controls. Neurotoxicity was observed when comparable concentrations of VEGF were applied to neurons in culture, and previously observed protective effects apparent at low concentrations were absent.180 Together, these results suggest that despite upregulation of some neuroprotective factors, the net contribution of the microvasculature in AD is a neurotoxic milieu.
Alzheimer's disease Risk Factors and the Blood–Brain Barrier
Despite the continued focus on Aβ and tau in AD, it is increasingly evident that other pathologic events and conditions such as inflammation, oxidative stress, ApoE4 genotype, diabetes/metabolic syndrome, head injury, and vascular pathologies are associated with AD. This section will consider these changes in the context of BBB participation, and their potential contribution to AD neurodegeneration.
Inflammation/Oxidative Stress
Evidence supports that both inflammation and oxidative stress are early events in AD184, 185 and activation of inflammatory and oxidative stress pathways in the BBB recapitulate many of the AD-associated deficiencies described in previous sections. The BBB endothelial cells respond to inflammatory stimuli such as cytokines,186 LPS,187, 188 Aβ,189, 190, 191 and a truncated tau fragment192 by activating signaling pathways that result in the upregulation of proinflammatory second messengers and reactive oxygen/nitrogen species, ultimately causing BBB disruption and paracrine activation of surrounding cells that are responsive to such stimuli, such as astrocytes193 and pericytes153, 194 of the NVU. Inflammation impairs systemic clearance of Aβ by the liver and kidneys, as well as bulk flow of CSF.195 Furthermore, BBB transporter changes occur in response to inflammation and oxidative stress that can promote the accumulation of Aβ in the brain. Increased oxidative damage to LRP-1 in the CNS and circulation has been observed in human AD.98, 196 In a mouse lacking the antioxidant vitamin E, LRP-1-dependent brain efflux and systemic clearance of Aβ are impaired.197 Both LRP-1 and Pgp are functionally downregulated in the BBB in mice with systemic inflammation induced by LPS,66, 198 and this corresponds with impaired Aβ efflux.66, 198 In contrast to AD, decreased function of these transporters after LPS was not attributed to reduced protein levels or oxidative damage.66 Despite this, the antioxidant N-acetylcysteine (Nac) was found to protect against LPS-induced Aβ efflux impairment and LRP-1 dysfunction, but not Pgp dysfunction in the BBB.66 Furthermore, Nac treatment in the same study was found to exert the majority of its antioxidant and anti-inflammatory effects in the blood, despite its ability to cross the BBB. This suggests that pathological influences of inflammation and oxidative stress in AD may not be limited to the CNS compartment, but may also arise from interactions of circulating factors with the BBB. Although presently a relatively unexplored topic, these interactions are likely to be highly relevant in a variety of conditions that may predispose an individual to AD, including those discussed below.
Apolipoprotein E Genotype
Apolipoprotein E is an apolipoprotein that facilitates lipid transport. Humans have a polymorphic APOE allele that results in three isoforms: APOE2, APOE3, and APOE4. The predominant APOE allele in human populations is APOE3, with mean frequency at 77.9%, whereas APOE4 and APOE2 are substantially less abundant, at 13.7% and 8.4%, respectively.199 The APOE4 allele is the strongest genetic risk factor for nonfamilial AD.200 The influences of ApoE4 on CNS function and AD pathogenesis have been described in a recent review,201 and this section will primarily consider functional differences of ApoE isoforms in the context of their influence on the BBB that are relevant to AD. The ApoE production occurs in both the CNS and peripheral compartments, where it is made primarily by astrocytes and the liver, respectively.201 An early pharmacokinetic study in guinea pigs showed that circulating ApoE does not cross the BBB,202 and all three ApoE isoforms limit the influx of circulating Aβ.202, 203 Unlike Aβ in complex with ApoE2 and 3, circulating ApoE4–Aβ complexes can cross the BBB.202 All isoforms of ApoE show significant efflux across the BBB, although at slower rates than both Aβ40 and Aβ42.204 Furthermore, the efflux rate of ApoE4 across the BBB is slower than that for ApoE2 and 3.204 Efflux of ApoE2 and 3 is dependent on LRP-1 and very-low-density-lipoprotein receptor (VLDLR), whereas ApoE4 efflux is primarily through VLDLR and is LRP-1 independent.204 When in complex with Aβ, all three isoforms of ApoE reduce the efflux rate of Aβ.72, 204 This reduction is most pronounced for ApoE4–Aβ complexes, which was attributed to the slower endocytosis rate of VLDLR versus LRP-1.204 Despite these findings for ApoE complexed to Aβ ex vivo, efflux rates of Aβ in ApoE knockout mice or transgenic mice expressing human ApoE3 or 4 are not significantly different than wild type.205 Efflux of Aβ is also unchanged when ApoE2–4 is preadministered to the brain.72 Therefore, endogenous factor(s) in the brain likely prevent ApoE–Aβ interactions in the brain that would slow its efflux.
Apolipoprotein E also plays a role in maintenance of BBB integrity. This was first shown in vitro using primary mouse brain endothelial cells cocultured with astrocytes from wild-type, ApoE knockout, or ApoE3 or 4 transgenic mice.206 Barrier properties of the endothelial cell monolayer when cultured with ApoE3 astrocytes were comparable to wild type. However, co-culture with ApoE-null or ApoE4 astrocytes significantly lowered the integrity of the monolayer. Wild-type ApoE promotes tight junction assembly through activation of protein kinase Cη and occludin phosphorylation at threonine residues. The ApoE4 and ApoE-null astrocytes are less able or unable to activate this pathway, respectively. Activation of this pathway by wild-type or ApoE3 astrocytes depended on interaction with LRP-1, but not LDLR or VLDLR.206 Bell et al207 recently expanded on these findings by showing that ApoE promotes BBB integrity in vivo through its interaction with LRP-1. Neurovascular leakage was observed in targeted-replacement ApoE4 and ApoE-null mice, which was caused by upregulation of the proinflammatory cytokine cyclophilin A (CypA) in the pericytes. Cyclophilin A caused BBB disruption by activating MMP9 (matrix metallopeptidase 9),207 a gelatinase that degrades the extracellular matrix and causes disruption of tight junctions.208 Together, these findings show that ApoE3 interacts with LRP-1 in the BBB to suppress inflammation and promote tight junction integrity. The inability of ApoE4 to exert these protective effects in experimental models suggests that human APOE4 carriers may have BBB abnormalities and/or increased risk for BBB disruption.
Diabetes/Metabolic Syndrome
Diabetes mellitus is hyperglycemia that results from insufficient insulin action, the insufficiency caused by either decreased insulin production by pancreatic β-cells (type 1 diabetes) or by insulin receptor resistance (type 2 diabetes). Both types of diabetes are associated with cognitive decline and an increased risk of developing AD.209, 210 One probable factor linking these diseases is insulin resistance in the CNS.211 Under physiologic conditions, CNS insulin signaling regulates feeding and cognition.212 Therefore, some have proposed that AD should be considered ‘type 3 diabetes.'213
Because of a lack of detectable insulin production in the CNS with the exception of a small group of cells in the olfactory mucosa,214 CNS insulin is derived primarily from the circulation through BBB transport.215 Pharmacokinetic studies of insulin uptake by the brain confirm that insulin crosses the BBB by a saturable transport system.216, 217, 218 Insulin transport across the BBB increases in a rodent model of diabetes219 and associated pathologic factors such as inflammation220 and triglycerides.221 However, insulin transport is decreased in obese dogs and rodents.221, 222, 223 Together, these results indicate that multiple conditions of the metabolic syndrome influence insulin transport across the BBB. The BBB therefore plays a likely role in mediating CNS insulin resistance, but the precise mechanisms of this are presently unclear.
It is also evident that altered BBB functions in the diabetic state recapitulate many of those observed in AD. Insulin transport in the BBB is increased in a transgenic mouse model of AD. In a rodent model of diabetes, LRP-1-dependent BBB efflux of Aβ is impaired,224 whereas RAGE-dependent influx of Aβ increases.225 However, Pgp function is unaltered.226 Disruption of BBB occurs in a rodent model of diabetes,227 and has also been reported in diabetic humans.228 One factor contributing to BBB disruption is pericyte loss229 that occurs from hyperglycemia-induced mitochondrial oxidative stress in a rodent model of diabetes.149 Pericyte loss in this model is blocked by the carbonic anhydrase inhibitor topiramate that shifts glucose metabolism to aerobic glycolysis and prevents generation of superoxide.149 Together, these results suggest that diabetes causes BBB dysfunction that could contribute to AD.
Traumatic Brain Injury
Traumatic brain injury (TBI) increases the risk for developing AD by 2- to 4.5-fold, depending on severity of the injury.230 Individuals who die from TBI have elevated Aβ plaques231 and tau pathology is evident in those who have experienced repeated head injuries such as boxers, football players, and combat veterans.230 Disruption of BBB occurs as an early response to TBI, but resolves by 1 week after injury.232 Endothelial cell activation, tight junction disruption, and migration of pericytes away from brain capillaries occur within this time period.148, 232 Recently, Pop et al233 investigated TBI-induced changes in the rat BBB at 60 days after injury. Microvascular Pgp was downregulated at this time point, whereas no significant changes were observed in microvascular expression of LRP-1, RAGE, or claudin 5. Claudin 5 was upregulated in larger vessels, and no BBB disruption was evident. These changes were associated with Aβ accumulation in proximity to blood vessels and microglia, and impaired spatial memory.233 Together, these results show that BBB changes promoting Aβ accumulation and cognitive impairment in TBI persist long after BBB disruption has resolved. However, early pathologies caused by transient opening of the BBB also influence secondary injury in the brain.234 Therefore, BBB disruption may contribute to long-term transporter changes even after its resolution after TBI.
Cardiovascular Disease
Cardiovascular disease is a risk factor for AD, and is associated with other AD risk factors such as diabetes and obesity.230 Furthermore, cerebrovascular disease is often evident in AD.235 Ischemia caused by cerebrovascular disease can cause Aβ accumulation, inflammation, and oxidative stress in the CNS that trigger BBB dysfunction.236 In a rodent model of brain ischemia induced by microsphere embolism, microvascular accumulation of Aβ occurs and coincides with parenchymal Aβ deposition and tau hyperphosphorylation.237 This raises the question of whether vascular pathologies themselves contribute to AD.238
Conclusion
From the information presented in this review (summarized in Table 1), it is evident that Aβ accumulation in the CNS can be both a cause and consequence of BBB dysfunction in AD. However, other Aβ-independent pathologies can phenotypically mimic BBB dysfunction observed in AD, and many changes in the BBB cause neurotoxicity independently of Aβ. This highlights the complexity of AD, and the likelihood that AD has diverse etiologies that converge on Aβ and tau. A lack of clearcut evidence of BBB disruption in AD necessitates the consideration of other critical functions of the BBB that may become impaired in AD, such as altered transport and communication within the neurovascular unit. We conclude that the BBB plays a multifaceted role in AD both upstream and downstream of the amyloid cascade, and is therefore important to consider in future efforts towards understanding this devastating and widespread disease.
Table 1. Potential causes and consequences of proposed BBB dysfunction in AD.
| BBB dysfunction | Upstream causes | Downstream consequences | ||
|---|---|---|---|---|
| Disruption | Aβ oligomers, truncated tau, inflammation, oxidative stress, diabetes, ApoE4 allele, TBI, vascular disease | Leakage of serum components into CNS, neurotoxicity, inflammatory activation | ||
| Altered transport | Of Aβ | ↓LRP-1 | Aβ oligomers, inflammation, oxidative stress, diabetes | Aβ accumulation in the CNS |
| ↓Pgp | Aβ oligomers, inflammation, TBI | Aβ accumulation in the CNS, altered xenobiotic transport | ||
| ↑RAGE | Aβ oligomers, diabetes, inflammation, oxidative stress | Aβ accumulation in the CNS, vascular inflammation | ||
| Of glucose | ↓GLUT-1 | Aβ oligomers, ApoE4 allele | Energy deprivation, neuronal dysfunction | |
| Altered secretions | Of thrombin | Oxidative stress, ischemia | Neurotoxicity, BBB disruption | |
| Of VEGF | Oxidative stress, ischemia | Neurotoxicity, BBB disruption, angiogenesis | ||
Aβ, amyloid-β; AD, Alzheimer's disease; ApoE, Apolipoprotein E; BBB, blood–brain barrier; CNS, central nervous system; LRP-1, lipoprotein receptor-related protein-1; Pgp, P-glycoprotein; RAGE, receptor for advanced glycation endproducts; TBI, traumatic brain injury; VEGF, vascular endothelial growth factor.
Acknowledgments
We thank Ms Emily Wing for assistance with figure preparation.
The authors declare no conflict of interest.
References
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2:a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer's neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Banks WA. Drug delivery to the brain in Alzheimer's disease: consideration of the blood-brain barrier. Adv Drug Deliv Rev. 2012;64:629–639. doi: 10.1016/j.addr.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbling G, Link H, Thman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Adolfsson R, Bucht G, Winblad B. Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci. 1983;60:465–472. doi: 10.1016/0022-510x(83)90157-0. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Icén A, Palo J, Erkinjuntti T. CSF in Alzheimer's disease. Studies on blood-brain barrier function and intrathecal protein synthesis. J Neurol Sci. 1985;70:73–80. doi: 10.1016/0022-510x(85)90189-3. [DOI] [PubMed] [Google Scholar]
- Hampel H, Kötter HU, Möller HJ. Blood-cerebrospinal fluid barrier dysfunction for high molecular weight proteins in Alzheimer disease and major depression: indication for disease subsets. Alzheimer Dis Assoc Disord. 1997;11:78–87. doi: 10.1097/00002093-199706000-00004. [DOI] [PubMed] [Google Scholar]
- Wada H. Blood-brain barrier permeability of the demented elderly as studied by cerebrospinal fluid-serum albumin ratio. Intern Med. 1998;37:509–513. doi: 10.2169/internalmedicine.37.509. [DOI] [PubMed] [Google Scholar]
- Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–971. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- Algotsson A, Winblad B. The integrety of the blood-brain barrier in Alzheimer's Disease. Acta Neurol Scand. 2007;115:403–408. doi: 10.1111/j.1600-0404.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Kaye JA, Moore M, Waichunas D, Carlson NE, Quinn JF. Blood-brain barrier impairment in Alzheimer's diease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Fredman P, Karlsson I, Gottfries CG, Svennerholm L. Blood-brain barrier disturbance in patients with Alzheimer's disease is related to vascular factors. Acta Neurol Scand. 1990;81:323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease - systematic review and metaanalysis. Neurobiol Aging. 2007;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Chalbot S, Zetterberg H, Blennow K, Fladby T, Andreasen N, Grundke-Iqbal I, et al. Blood-cerebrospinal fluid barrier permeability in Alzheimer's disease. J Alzheimers Dis. 2011;25:505–515. doi: 10.3233/JAD-2011-101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Kötter HU, Padberg F, Körschenhausen DA, Möller HJ. Oligoclonal bands and blood—cerebrospinal-fluid barrier dysfunction in a subset of patients with Alzheimer disease: comparison with vascular dementia, major depression, and multiple sclerosis. Alzheimer Dis Assoc Disord. 1999;13:9–19. doi: 10.1097/00002093-199903000-00002. [DOI] [PubMed] [Google Scholar]
- Mecocci P, Parnetti L, Reboldi GP, Santucci C, Gaiti A, Ferri C, et al. Blood-brain-barrier in a geriatric population: barrier function in degenerative and vascular dementias. Acta Neurol Scand. 1991;84:210–213. doi: 10.1111/j.1600-0404.1991.tb04940.x. [DOI] [PubMed] [Google Scholar]
- Kay AD, May C, Papadopoulos NM, Costello R, Atack JR, Luxenberg JS, et al. CSF and serum concentrations of albumin and IgG in Alzheimer's disease. Neurobiol Aging. 1987;8:21–25. doi: 10.1016/0197-4580(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Frolich L, Kornhuber J, Ihl R, Fritze J, Maurer K, Riederer P. Integrity of the blood-CSF barrier in dementia of Alzheimer type: CSF/serum ratios of albumin and IgG. Eur Arch Psychiatry Clin Neurosci. 1991;240:363–366. doi: 10.1007/BF02279767. [DOI] [PubMed] [Google Scholar]
- Chen R. Is it appropriate to use albumin CSF/plasma ratio to assess blood brain barrier permeability. Neurobiol Aging. 2011;32:1338–1339. doi: 10.1016/j.neurobiolaging.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Iemolo F, Duro G, Rizzo C, Castiglia L, Hachinski V, Caruso C. Pathophysiology of vascular dementia. Immun Ageing. 2009;6:13. doi: 10.1186/1742-4933-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer's type. Neurology. 2001;57:1763–1766. doi: 10.1212/wnl.57.10.1763. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Kozlowski PB. Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT) Ann NY Acad Sci. 1982;396:119–129. doi: 10.1111/j.1749-6632.1982.tb26848.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Vorbrodt AW, Wegeil J. Amyloid angiopathy and blood-brain barrier changes in Alzheimer's disease. Ann NY Acad Sci. 1997;826:161–172. doi: 10.1111/j.1749-6632.1997.tb48468.x. [DOI] [PubMed] [Google Scholar]
- Siemmon JR, Hughes CM, Campbell GA, Flood DG. Increased levels of hemoglobin-derived and other peptides in Alzheimer's disease cerebellum. J Neurosci. 1994;14:2225–2235. doi: 10.1523/JNEUROSCI.14-04-02225.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reuck JL. The significance of small cerebral bleeds in neurodegenerative dementia syndromes. Aging Dis. 2012;3:307–312. [PMC free article] [PubMed] [Google Scholar]
- Zipser BD, Johanson CD, Gonzalez L, Berzin TM, Tavares R, Hulette CN, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in Rat brain: expression by mesencephalic dopaminergenic neurons and codistribution with prothrombin mRNA. J Neurosci. 1995;15:2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamahata H, Takeshima H, Kuratsu JI, Sarker KP, Tanioka K, Wakimaru N, et al. The role of thrombin in the neo-bascularization of malignant gliomas: an intrinsic modulator for the up-regulation of vascular endothelial growth factor. Int J Oncol. 2002;20:921–928. [PubMed] [Google Scholar]
- Claudio L. Ultrastructural features of the blood-brain barrier in biopsy tissue from Alzheimer's disease patients. Acta Neuropathol. 1996;91:6–14. doi: 10.1007/s004010050386. [DOI] [PubMed] [Google Scholar]
- Alafuzoff I, Adolfsson R, Grundke-Iqbal I, Winblad B. Blood-brain barrier in Alzheimer dementia and in non-demented elderly. Acta Neuropathol (Berlin) 1987;73:160–166. doi: 10.1007/BF00693782. [DOI] [PubMed] [Google Scholar]
- Rozemuller JM, Eikelenboom P, Kamphorst W, Stam FC. Lack of evedence for dysfunction of the blood-brain barrier in Alzheimer's disease: an immunohistochemical study. Neurobiol Aging. 1988;9:383–391. doi: 10.1016/s0197-4580(88)80085-x. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Akiguchi I, Suenaga T, Nishimura M, Wakita H, Nakamura S, et al. Alterations of the blood-brain barrier and glial cells in white-matter lesions in cerebrovascular and Alzheimer's disease patients. Stroke. 1996;27:2069–2074. doi: 10.1161/01.str.27.11.2069. [DOI] [PubMed] [Google Scholar]
- Munoz DG, Erkinjuntti T, Gaytan-Garcia S, Hachinski V. Serum protein leakage in Alzheimer's disease revisited. Ann NY Acad Sci. 1997;826:173–189. doi: 10.1111/j.1749-6632.1997.tb48469.x. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage C-A, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1040. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, Caccioppo D, Lapin GD, Ragin A, Groothuis DR. Blood-brain barrier integrity in Alzheimer's disease patients and elderly control subjects. J Neuropsychiatry Clin Neurosci. 1998;10:78–84. doi: 10.1176/jnp.10.1.78. [DOI] [PubMed] [Google Scholar]
- Dysken MW, Nelson MJ, Hoover KM, Kuskowski M, McGeachie R. Rapid dynamic CT scanning in primary degenerative dementia and age-matched controls. Biol Psychiatry. 1990;28:425–434. doi: 10.1016/0006-3223(90)90410-4. [DOI] [PubMed] [Google Scholar]
- Schlageter NL, Carson RE, Rapoport SI. Examination of blood-brain barrier permeability in dementia of the Alzheimer type with [68Ga]EDTA and positron emission tomography. J Cereb Blood Flow Metab. 1987;7:1–8. doi: 10.1038/jcbfm.1987.1. [DOI] [PubMed] [Google Scholar]
- Starr JM, Farrall AJ, Armitage P, McGurn B, Wardlaw J. Blood-brain barrier permeability in Alzheimer's disease: a case-control MRI study. Psychiatry Res. 2009;171:232–241. doi: 10.1016/j.pscychresns.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Bronge L, Wahlund LO. White matter lesions in dementia: an MRI study on blood-brain barrier dysfunction. Dement Geriatr Cogn Disord. 2000;11:263–267. doi: 10.1159/000017248. [DOI] [PubMed] [Google Scholar]
- Wang H, Golob EJ, Su MY. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging. 2006;24:695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Gorevic PD, Ghiso J, Frangione B, Patlak CS, Fenstermacher JD. Fate of cerebrospinal fluid-borne amyloid beta-peptide: rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem. 1996;67:880–883. doi: 10.1046/j.1471-4159.1996.67020880.x. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue JE, Flaherty SL, Johanson CE, Duncan JA, 3rd, Silverberg GD, Miller MC, et al. RAGE, LRP-1, and amyloid-beta protein in Alzheimer's disease. Acta Neuropathol. 2006;112:405–415. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Hwang MC, Farr SA, Murphy MP, Fleegal-DeMotta MA, et al. Testing the neurovascular hypothesis of Alzheimer's disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla KK, Curran GL, Holasek SS, Gilles EJ, Wengenack TM, Poduslo JF. Pharmacokinetic analysis of the blood-brain barrier transport of 125I-amyloid beta protein 40 in wild-type and Alzheimer's disease transgenic mice (APP,PS1) and its implications for amyloid plaque formation. J Pharmacol Exp Ther. 2005;313:1370–1378. doi: 10.1124/jpet.104.081901. [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Verma S, Morley JE. Efflux of human and mouse amyloid beta proteins 1-40 and 1-42 from brain: impairment in a mouse model of Alzheimer's disease. Neuroscience. 2003;121:487–492. doi: 10.1016/s0306-4522(03)00474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, et al. Brain clearance of Alzheimer's amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002;10:359–368. doi: 10.1080/10611860290031831. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer's disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam FC, Liu R, Lu P, Shapiro AB, Renoir JM, Sharom FJ, et al. beta-Amyloid efflux mediated by p-glycoprotein. J Neurochem. 2001;76:1121–1128. doi: 10.1046/j.1471-4159.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- Kuhnke D, Jedlitschky G, Grube M, Krohn M, Jucker M, Mosyagin I, et al. MDR1-P-Glycoprotein (ABCB1) mediates transport of Alzheimer's amyloid-beta peptides—implications for the mechanisms of Abeta clearance at the blood-brain barrier. Brain Pathol. 2007;17:347–353. doi: 10.1111/j.1750-3639.2007.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, et al. Deposition of Alzheimer's beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12:535–541. doi: 10.1097/00008571-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz AM, Miller DS, Bauer B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer's disease. Mol Pharmacol. 2010;77:715–723. doi: 10.1124/mol.109.061754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Assema DM, Lubberink M, Bauer M, van der Flier WM, Schuit RC, Windhorst AD, et al. Blood-brain barrier P-glycoprotein function in Alzheimer's disease. Brain. 2012;135:181–189. doi: 10.1093/brain/awr298. [DOI] [PubMed] [Google Scholar]
- Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326 (Pt 2:539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Hansen K, Banks WA. Inflammation-induced dysfunction of the low-density lipoprotein receptor-related protein-1 at the blood-brain barrier: protection by the antioxidant N-acetylcysteine. Brain Behav Immun. 2012;26:1085–1094. doi: 10.1016/j.bbi.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela P, Gosselet F, Saint-Pol J, Sevin E, Boucau MC, Boulanger E, et al. Apical-to-basolateral transport of amyloid-beta peptides through blood-brain barrier cells is mediated by the receptor for advanced glycation end-products and is restricted by P-glycoprotein. J Alzheimers Dis. 2010;22:849–859. doi: 10.3233/JAD-2010-100462. [DOI] [PubMed] [Google Scholar]
- Pflanzner T, Petsch B, Andre-Dohmen B, Muller-Schiffmann A, Tschickardt S, Weggen S, et al. Cellular prion protein participates in amyloid-beta transcytosis across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:628–632. doi: 10.1038/jcbfm.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do TM, Noel-Hudson MS, Ribes S, Besengez C, Smirnova M, Cisternino S, et al. ABCG2- and ABCG4-mediated efflux of amyloid-beta peptide 1-40 at the mouse blood-brain barrier. J Alzheimers Dis. 2012;30:155–166. doi: 10.3233/JAD-2012-112189. [DOI] [PubMed] [Google Scholar]
- Hortschansky P, Schroeckh V, Christopeit T, Zandomeneghi G, Fandrich M. The aggregation kinetics of Alzheimer's beta-amyloid peptide is controlled by stochastic nucleation. Protein Sci. 2005;14:1753–1759. doi: 10.1110/ps.041266605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Ito S, Ohtsuki S, Kamiie J, Nezu Y, Terasaki T. Cerebral clearance of human amyloid-beta peptide (1-40) across the blood-brain barrier is reduced by self-aggregation and formation of low-density lipoprotein receptor-related protein-1 ligand complexes. J Neurochem. 2007;103:2482–2490. doi: 10.1111/j.1471-4159.2007.04938.x. [DOI] [PubMed] [Google Scholar]
- Brenn A, Grube M, Peters M, Fischer A, Jedlitschky G, Kroemer HK, et al. Beta-amyloid downregulates MDR1-P-glycoprotein (Abcb1) expression at the blood-brain barrier in mice. Int J Alzheimers Dis. 2011;2011:690121. doi: 10.4061/2011/690121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, et al. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21:1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Bernardo AE, Farr SA, Uezu K, Tumosa N, et al. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kumar VB, Farr SA, Nakaoke R, Robinson SM, Morley JE. Impairments in brain-to-blood transport of amyloid-beta and reabsorption of cerebrospinal fluid in an animal model of Alzheimer's disease are reversed by antisense directed against amyloid-beta protein precursor. J Alzheimers Dis. 2011;23:599–605. doi: 10.3233/JAD-2010-100021. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, et al. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Barrera CM, Maness LM. Lack of saturable transport across the blood-brain barrier in either direction for beta-amyloid1-28 (Alzheimer's disease protein) Brain Res Bull. 1991;27:819–823. doi: 10.1016/0361-9230(91)90215-6. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Ghiso J, Mackic JB, McComb JG, Weiss MH, Frangione B. Blood-brain barrier transport of circulating Alzheimer's amyloid beta. Biochem Biophys Res Commun. 1993;197:1034–1040. doi: 10.1006/bbrc.1993.2582. [DOI] [PubMed] [Google Scholar]
- Maness LM, Banks WA, Podlisny MB, Selkoe DJ, Kastin AJ. Passage of human amyloid beta-protein 1-40 across the murine blood-brain barrier. Life Sci. 1994;55:1643–1650. doi: 10.1016/0024-3205(94)00331-9. [DOI] [PubMed] [Google Scholar]
- Martel CL, Mackic JB, McComb JG, Ghiso J, Zlokovic BV. Blood-brain barrier uptake of the 40 and 42 amino acid sequences of circulating Alzheimer's amyloid beta in guinea pigs. Neurosci Lett. 1996;206:157–160. doi: 10.1016/s0304-3940(96)12462-9. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Haggard JJ, Biere AL, Selkoe DJ. Permeability and residual plasma volume of human, Dutch variant, and rat amyloid beta-protein 1-40 at the blood-brain barrier. Neurobiol Dis. 1997;4:27–34. doi: 10.1006/nbdi.1997.0132. [DOI] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes B, Provias J. Evidence for altered LRP/RAGE expression in Alzheimer lesion pathogenesis. Curr Alzheimer Res. 2008;5:432–437. doi: 10.2174/156720508785908937. [DOI] [PubMed] [Google Scholar]
- Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, et al. Systemic catabolism of Alzheimer's Abeta40 and Abeta42. J Biol Chem. 2004;279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- Marques MA, Kulstad JJ, Savard CE, Green PS, Lee SP, Craft S, et al. Peripheral amyloid-beta levels regulate amyloid-beta clearance from the central nervous system. J Alzheimers Dis. 2009;16:325–329. doi: 10.3233/JAD-2009-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- Pesaresi M, Lovati C, Bertora P, Mailland E, Galimberti D, Scarpini E, et al. Plasma levels of beta-amyloid (1-42) in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:904–905. doi: 10.1016/j.neurobiolaging.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Tamaoka A, Fukushima T, Sawamura N, Ishikawa K, Oguni E, Komatsuzaki Y, et al. Amyloid beta protein in plasma from patients with sporadic Alzheimer's disease. J Neurol Sci. 1996;141:65–68. doi: 10.1016/0022-510x(96)00143-8. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, Londos E, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiso J, Matsubara E, Koudinov A, Choi-Miura NH, Tomita M, Wisniewski T, et al. The cerebrospinal-fluid soluble form of Alzheimer's amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem J. 1993;293 (Pt 1:27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Sagare A, Zlokovic BV. The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer's disease. Curr Pharm Des. 2008;14:1601–1605. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Solomon B, Maness LM, Kastin AJ. Antibodies to beta-amyloid decrease the blood-to-brain transfer of beta-amyloid peptide. Exp Biol Med (Maywood) 2002;227:609–615. doi: 10.1177/153537020222700808. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Saito M, LaFrancois J, Gaynor K, Olm V, Wang L, et al. Novel therapeutic approach for the treatment of Alzheimer's disease by peripheral administration of agents with an affinity to beta-amyloid. J Neurosci. 2003;23:29–33. doi: 10.1523/JNEUROSCI.23-01-00029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measrues of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1278. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radio-labelled amino acids, amines and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991;325:703–709. doi: 10.1056/NEJM199109053251006. [DOI] [PubMed] [Google Scholar]
- Harik SI. Changes in the glucose transporter of brain capillaries. Can J Physiol Pharmacol. 1992;70 S1:S113–S117. doi: 10.1139/y92-252. [DOI] [PubMed] [Google Scholar]
- Horwood N, Davies DC. Immunolabelling of hippocampal microvessel glucose transporter protein is reduced in Alzheimer's disease. Virchows Arch. 1994;425:69–72. doi: 10.1007/BF00193951. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Harik SI. Abnormalities of the glucose transporter at the blood-brain barrier and in brain in Alzheimer's disease. Prog Clin Biol Res. 1989;317:415–421. [PubMed] [Google Scholar]
- Mooradian AD, Chung HC, Shah GN. GLUT-1 expression in the cerebra of patients with Alzheimer's disease. Neurobiol Aging. 1997;18:469–474. doi: 10.1016/s0197-4580(97)00111-5. [DOI] [PubMed] [Google Scholar]
- Harik SI, Kalaria RN. Blood-brain barrier abnormalities in Alzheimer's disease. Ann NY Acad Sci. 1991;640:47–52. doi: 10.1111/j.1749-6632.1991.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Graven C, Dederen PJ, Tanila H, van Groen T, Kiliaan AJ. Amyloid beta deposition is related to decreased glucose transporter-1 levels and hippocampal atrophy in brains of aged APP/PS1 mice. Brain Res. 2007;1181:93–103. doi: 10.1016/j.brainres.2007.08.063. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Ghosh PM, Madison C, Laforce RJ, Corbetta-Rastelli C, WEiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain. 2013;136 (Pt:844–858. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Initiative AsDN Apolipoprotein E, not fibrillar beta-amyloid, reduces cerebral glucose metabolism in normal aging. J Neurosci. 2012;32:18227–18233. doi: 10.1523/JNEUROSCI.3266-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Stopa EG, McMillan PN. The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol. 2011;686:101–131. doi: 10.1007/978-1-60761-938-3_4. [DOI] [PubMed] [Google Scholar]
- Silverberg GD, Mayo M, Saul T, Rubenstein E, McGuire D. Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2003;2:506–511. doi: 10.1016/s1474-4422(03)00487-3. [DOI] [PubMed] [Google Scholar]
- Henriksson L, Voigt K. Age-dependent differences of distribution and clearance patterns in normal RIHSA cisternograms. Neuroradiology. 1976;12:103–107. doi: 10.1007/BF00333125. [DOI] [PubMed] [Google Scholar]
- Preston JE. Ageing choroid plexus-cerebrospinal fluid system. Microsc Res Tech. 2001;52:31–37. doi: 10.1002/1097-0029(20010101)52:1<31::AID-JEMT5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH, Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967;17:196–205. doi: 10.1001/archneur.1967.00470260086010. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431–439. [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Deane R, Chow N, Long X, Sagare A, Singh I, et al. SRF and myocardin regulate LRP-mediated amyloid-beta clearance in brain vascular cells. Nat Cell Biol. 2009;11:143–153. doi: 10.1038/ncb1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer's amyloid-beta. J Neurosci. 2012;32:16458–16465. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Massey A, Kuo YM, Roher AE. Cerebral amyloid angiopathy: accumulation of A beta in interstitial fluid drainage pathways in Alzheimer's disease. Ann NY Acad Sci. 2000;903:110–117. doi: 10.1111/j.1749-6632.2000.tb06356.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- Moftakhar P, Lynch MD, Pomakian JL, Vinters HV. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2010;69:1201–1209. doi: 10.1097/NEN.0b013e3181fd252c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159:1055–1069. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Wu Q, Yuan C, Gao J, Xiao M, Gu M, et al. Aquaporin-4 mediates astrocyte response to beta-amyloid. Mol Cell Neurosci. 2012;49:406–414. doi: 10.1016/j.mcn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Rauch SM, Huen K, Miller MC, Chaudry H, Lau M, Sanes JR, et al. Changes in brain beta-amyloid deposition and aquaporin 4 levels in response to altered agrin expression in mice. J Neuropathol Exp Neurol. 2011;70:1124–1137. doi: 10.1097/NEN.0b013e31823b0b12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- McCoy E, Sontheimer H. MAPK induces AQP1 expression in astrocytes following injury. Glia. 2010;58:209–217. doi: 10.1002/glia.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi A, Yamamoto T, Shimizu K, Ugawa Y, Nishizawa M, Takahashi H, et al. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:750–759. doi: 10.1097/NEN.0b013e3182632566. [DOI] [PubMed] [Google Scholar]
- Perez E, Barrachina M, Rodriguez A, Torrejon-Escribano B, Boada M, Hernandez I, et al. Aquaporin expression in the cerebral cortex is increased at early stages of Alzheimer disease. Brain Res. 2007;1128:164–174. doi: 10.1016/j.brainres.2006.09.109. [DOI] [PubMed] [Google Scholar]
- Misawa T, Arima K, Mizusawa H, Satoh J. Close association of water channel AQP1 with amyloid-beta deposition in Alzheimer disease brains. Acta Neuropathol. 2008;116:247–260. doi: 10.1007/s00401-008-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp Biol Med (Maywood) 2005;230:771–776. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyoshi M, Tachikawa M, Ohtsuki S, Ito S, Uchida Y, Akanuma S, et al. Amyloid-beta peptide(1-40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J Neurochem. 2011;118:407–415. doi: 10.1111/j.1471-4159.2011.07311.x. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, Martel CL, Matsubara E, McComb JG, Zheng G, McCluskey RT, et al. Glycoprotein 330/megalin: probable role in receptor-mediated transport of apolipoprotein J alone and in a complex with Alzheimer disease amyloid beta at the blood-brain and blood-cerebrospinal fluid barriers. Proc Natl Acad Sci USA. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96:3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, et al. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. doi: 10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas T, Ugalde C, Spuch C, Antequera D, Moran MJ, Martin MA, et al. Abeta accumulation in choroid plexus is associated with mitochondrial-induced apoptosis. Neurobiol Aging. 2010;31:1569–1581. doi: 10.1016/j.neurobiolaging.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol. 2012;45:327–347. doi: 10.1007/s12035-012-8244-2. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, et al. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Sengillo JD, Winkler EA, Walker CT, Sullivan JS, Johnson M, Zlokovic BV. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer's disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem. 1997;68:1135–1141. doi: 10.1046/j.1471-4159.1997.68031135.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J, et al. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J Cereb Blood Flow Metab. 2012;33:428–439. doi: 10.1038/jcbfm.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- Price TO, Eranki V, Banks WA, Ercal N, Shah GN. Topiramate treatment protects blood-brain barrier pericytes from hyperglycemia-induced oxidative damage in diabetic mice. Endocrinology. 2012;153:362–372. doi: 10.1210/en.2011-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior R, Wihl G, Urmoneit B. Apolipoprotein E, smooth muscle cells and the pathogenesis of cerebral amyloid angiopathy: the potential role of impaired cerebrovascular A beta clearance. Ann NY Acad Sci. 2000;903:180–186. doi: 10.1111/j.1749-6632.2000.tb06367.x. [DOI] [PubMed] [Google Scholar]
- Tooyama I, Kawamata T, Akiyama H, Kimura H, Moestrup SK, Gliemann J, et al. Subcellular localization of the low density lipoprotein receptor-related protein (alpha 2-macroglobulin receptor) in human brain. Brain Res. 1995;691:235–238. doi: 10.1016/0006-8993(95)00735-9. [DOI] [PubMed] [Google Scholar]
- Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DR. The role of astrocytes in amyloid beta-protein toxicity and clearance. Exp Neurol. 2012;236:1–5. doi: 10.1016/j.expneurol.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Thal DR. The pre-capillary segment of the blood-brain barrier and its relation to perivascular drainage in Alzheimer's disease and small vessel disease. ScientificWorldJournal. 2009;9:557–563. doi: 10.1100/tsw.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, et al. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107 (Pt 5:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- El Hafny B, Chappey O, Piciotti M, Debray M, Boval B, Roux F. Modulation of P-glycoprotein activity by glial factors and retinoic acid in an immortalized rat brain microvessel endothelial cell line. Neurosci Lett. 1997;236:107–111. doi: 10.1016/s0304-3940(97)00679-4. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Pihlaja R, Koistinaho J, Kauppinen R, Sandholm J, Tanila H, Koistinaho M. Multiple cellular and molecular mechanisms are involved in human Abeta clearance by transplanted adult astrocytes. Glia. 2011;59:1643–1657. doi: 10.1002/glia.21212. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Cotman CW. Early association of reactive astrocytes with senile plaques in Alzheimer's disease. Exp Neurol. 1995;132:172–179. doi: 10.1016/0014-4886(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Mulder SD, Veerhuis R, Blankenstein MA, Nielsen HM. The effect of amyloid associated proteins on the expression of genes involved in amyloid-beta clearance by adult human astrocytes. Exp Neurol. 2012;233:373–379. doi: 10.1016/j.expneurol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Oliveira CR, Agostinho P. Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience. 2008;156:898–910. doi: 10.1016/j.neuroscience.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer's disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- Merlini M, Meyer EP, Ulmann-Schuler A, Nitsch RM. Vascular beta-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAbeta mice. Acta Neuropathol. 2011;122:293–311. doi: 10.1007/s00401-011-0834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Jo Leukoc Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Rogers J, Lue LF. Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer's disease. Neurochem Int. 2001;39:333–340. doi: 10.1016/s0197-0186(01)00040-7. [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Gerhard A, Hinz R, Pavese N, Turkheimer FE, et al. Microglia, amyloid, and cognition in Alzheimer's disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Wisniewski HM, Barcikowska M, Kida E. Phagocytosis of beta/A4 amyloid fibrils of the neuritic neocortical plaques. Acta Neuropathol. 1991;81:588–590. doi: 10.1007/BF00310142. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Selective targeting of perivascular macrophages for clearance of beta-amyloid in cerebral amyloid angiopathy. Proc Natl Acad Sci USA. 2009;106:1261–1266. doi: 10.1073/pnas.0805453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avagyan H, Goldenson B, Tse E, Masoumi A, Porter V, Wiedau-Pazos M, et al. Immune blood biomarkers of Alzheimer disease patients. J Neuroimmunol. 2009;210:67–72. doi: 10.1016/j.jneuroim.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Nicolakakis N, Hamel E. Neurovascular function in Alzheimer's disease patients and experimental models. J Cereb Blood Flow Metab. 2011;31:1354–1370. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Sanchez A, Tripathy D, Luo E, Martinez J. Vascular signaling abnormalities in Alzheimer disease. Cleve Clin J Med. 2011;78 (Suppl 1:S50–S53. doi: 10.3949/ccjm.78.s1.09. [DOI] [PubMed] [Google Scholar]
- Yin X, Wright J, Wall T, Grammas P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer's disease. Am J Pathol. 2010;176:1600–1606. doi: 10.2353/ajpath.2010.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Grammas P. Endothelin-1 is elevated in Alzheimer's disease brain microvessels and is neuroprotective. J Alzheimers Dis. 2010;21:887–896. doi: 10.3233/JAD-2010-091486. [DOI] [PubMed] [Google Scholar]
- Tripathy D, Thirumangalakudi L, Grammas P. RANTES upregulation in the Alzheimer's disease brain: a possible neuroprotective role. Neurobiol Aging. 2010;31:8–16. doi: 10.1016/j.neurobiolaging.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammas P, Moore P, Weigel PH. Microvessels from Alzheimer's disease brains kill neurons in vitro. Am J Pathol. 1999;154:337–342. doi: 10.1016/S0002-9440(10)65280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, Tripathy D, Luo J, Yin X, Martinez J, Grammas P. Neurovascular unit and the effects of dosage in VEGF toxicity: role for oxidative stress and thrombin. J Alzheimers Dis. 2013;34:281–291. doi: 10.3233/JAD-121636. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. J Neurosci. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa L, Mir M, Asensio VJ, Olmos G, Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008;105:1080–1090. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- Perry G, Cash AD, Smith MA. Alzheimer disease and oxidative stress. J Biomed Biotechnol. 2002;2:120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, van Exel E, Veerhuis R, Rozemuller AJ, van Gool WA, Hoozemans JJ. Innate immunity and the etiology of late-onset Alzheimer's disease. Neurodegener Dis. 2012;10:271–273. doi: 10.1159/000334287. [DOI] [PubMed] [Google Scholar]
- Pan W, Stone KP, Hsuchou H, Manda VK, Zhang Y, Kastin AJ. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des. 2011;17:3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DD, Goldblum SE. Direct effects of endotoxin on the endothelium: barrier function and injury. Lab Invest. 1999;79:1181–1199. [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Velasquez FJ, Kotarek JA, Moss MA. Soluble aggregates of the amyloid-beta protein selectively stimulate permeability in human brain microvascular endothelial monolayers. J Neurochem. 2008;107:466–477. doi: 10.1111/j.1471-4159.2008.05618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deli MA, Veszelka S, Csiszar B, Toth A, Kittel A, Csete M, et al. Protection of the blood-brain barrier by pentosan against amyloid-beta-induced toxicity. J Alzheimers Dis. 2010;22:777–794. doi: 10.3233/JAD-2010-100759. [DOI] [PubMed] [Google Scholar]
- Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, de Vries HE. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15:1167–1178. doi: 10.1089/ars.2011.3895. [DOI] [PubMed] [Google Scholar]
- Kovac A, Zilkova M, Deli MA, Zilka N, Novak M. Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J Alzheimers Dis. 2009;18:897–906. doi: 10.3233/JAD-2009-1197. [DOI] [PubMed] [Google Scholar]
- Tada M, Diserens AC, Desbaillets I, de Tribolet N. Analysis of cytokine receptor messenger RNA expression in human glioblastoma cells and normal astrocytes by reverse-transcription polymerase chain reaction. J Neurosurg. 1994;80:1063–1073. doi: 10.3171/jns.1994.80.6.1063. [DOI] [PubMed] [Google Scholar]
- Takata F, Dohgu S, Matsumoto J, Takahashi H, Machida T, Wakigawa T, et al. Brain pericytes among cells constituting the blood-brain barrier are highly sensitive to tumor necrosis factor-alpha, releasing matrix metalloproteinase-9 and migrating in vitro. J Neuroinflammation. 2011;8:106. doi: 10.1186/1742-2094-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MA, Hartvigson PE, Morofuji Y, Owen JB, Butterfield DA, Banks WA. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J Neuroinflammation. 2012;9:150. doi: 10.1186/1742-2094-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, et al. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic Biol Med. 2010;49:1798–1803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y, Ito S, Ohtsuki S, Yamamoto N, Takahashi T, Iwata N, et al. Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem. 2009;284:33400–33408. doi: 10.1074/jbc.M109.054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer's disease. Brain Behav Immun. 2009;23:507–517. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel CL, Mackic JB, Matsubara E, Governale S, Miguel C, Miao W, et al. Isoform-specific effects of apolipoproteins E2, E3, and E4 on cerebral capillary sequestration and blood-brain barrier transport of circulating Alzheimer's amyloid beta. J Neurochem. 1997;69:1995–2004. doi: 10.1046/j.1471-4159.1997.69051995.x. [DOI] [PubMed] [Google Scholar]
- Bachmeier C, Paris D, Beaulieu-Abdelahad D, Mouzon B, Mullan M, Crawford F. A multifaceted role for apoE in the clearance of beta-amyloid across the blood-brain barrier. Neurodegener Dis. 2013;11:13–21. doi: 10.1159/000337231. [DOI] [PubMed] [Google Scholar]
- Deane R, Sagare A, Hamm K, Parisi M, Lane S, Finn MB, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Permanne B, Sigurdsson EM, Holtzman DM, Wisniewski T. Amyloid beta40/42 clearance across the blood-brain barrier following intra-ventricular injections in wild-type, apoE knock-out and human apoE3 or E4 expressing transgenic mice. J Alzheimers Dis. 2001;3:23–30. doi: 10.3233/jad-2001-3105. [DOI] [PubMed] [Google Scholar]
- Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Biol Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey C, Hamilton N, Schwartz AV, Simonsick EM, Satterfield S, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol. 2012;69:1170–1175. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix MC, Badonnel K, Meunier N, Tan F, Schlegel-Le Poupon C, Durieux D, et al. Expression of insulin system in the olfactory epithelium: first approaches to its role and regulation. J Neuroendocrinol. 2008;20:1176–1190. doi: 10.1111/j.1365-2826.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Jr., Fisher LD, Sipols AJ, et al. Evidence for entry of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. Quantitative aspects and implications for transport. J Clin Invest. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baura GD, Foster DM, Porte D, Jr., Kahn SE, Bergman RN, Cobelli C, et al. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo. A mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Huang W, Kastin AJ. Transport of insulin across the blood-brain barrier: saturability at euglycemic doses of insulin. Peptides. 1997;18:1423–1429. doi: 10.1016/s0196-9781(97)00231-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18:1577–1584. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- Urayama A, Banks WA. Starvation and triglycerides reverse the obesity-induced impairment of insulin transport at the blood-brain barrier. Endocrinology. 2008;149:3592–3597. doi: 10.1210/en.2008-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- Baskin DG, Stein LJ, Ikeda H, Woods SC, Figlewicz DP, Porte D, Jr., et al. Genetically obese Zucker rats have abnormally low brain insulin content. Life Sci. 1985;36:627–633. doi: 10.1016/0024-3205(85)90166-3. [DOI] [PubMed] [Google Scholar]
- Hong H, Liu LP, Liao JM, Wang TS, Ye FY, Wu J, et al. Downregulation of LRP1 [correction of LPR1] at the blood-brain barrier in streptozotocin-induced diabetic mice. Neuropharmacology. 2009;56:1054–1059. doi: 10.1016/j.neuropharm.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Liu LP, Hong H, Liao JM, Wang TS, Wu J, Chen SS, et al. Upregulation of RAGE at the blood-brain barrier in streptozotocin-induced diabetic mice. Synapse. 2009;63:636–642. doi: 10.1002/syn.20644. [DOI] [PubMed] [Google Scholar]
- Reichel V, Burghard S, John I, Huber O. P-glycoprotein and breast cancer resistance protein expression and function at the blood-brain barrier and blood-cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res. 2011;1370:238–245. doi: 10.1016/j.brainres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Huber JD, VanGilder RL, Houser KA. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660–H2668. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino LJ. Current hypotheses for the biochemical basis of diabetic retinopathy. Diabetes Care. 1992;15:1892–1901. doi: 10.2337/diacare.15.12.1892. [DOI] [PubMed] [Google Scholar]
- Alzheimer's Association 2012 Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease. Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, Badaut JA. Neurovascular perspective for long-term changes after brain trauma. Transl Stroke Res. 2011;2:533–545. doi: 10.1007/s12975-011-0126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop V, Sorensen DW, Kamper JE, Ajao DO, Paul Murphy M, Head E, et al. Early brain injury alters the blood-brain barrier phenotype in parallel with beta-amyloid and cognitive changes in adulthood. J Cereb Blood Flow Metab. 2013;33:205–214. doi: 10.1038/jcbfm.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick AS, Hwang BY, Appelboom G, Hwang RY, Piazza MA, Connolly ES., Jr. Serum biomarkers of spontaneous intracerebral hemorrhage induced secondary brain injury. J Neurol Sci. 2012;321:1–10. doi: 10.1016/j.jns.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Deramecourt V, Slade JY, Oakley AE, Perry RH, Ince PG, Maurage CA, et al. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78:1043–1050. doi: 10.1212/WNL.0b013e31824e8e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Black SE, Verhoeff NP. Alzheimer's disease, cerebrovascular disease, and the beta-amyloid cascade. Can J Neurol Sci. 2012;39:712–728. doi: 10.1017/s0317167100015547. [DOI] [PubMed] [Google Scholar]
- Han F, Fukunaga K. Beta-amyloid accumulation in neurovascular units following brain embolism. J Pharmacol Sci. 2009;111:101–109. doi: 10.1254/jphs.09r02cp. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes. J Neurol Sci. 2012;322:141–147. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]