Abstract
Gastric marginal zone B-cell lymphoma of MALT type (MALT lymphoma) arises in the context of chronic inflammation induced by the bacterial pathogen Helicobacter pylori. Although generally considered an indolent disease, MALT lymphoma may transform to gastric diffuse large B-cell lymphoma (gDLBCL) through mechanisms that remain poorly understood. By comparing microRNA expression profiles of gastric MALT lymphoma and gDLBCL, we have identified a signature of 27 deregulated microRNAs(miRNAs) that share the characteristic of being transcriptionally repressed by Myc. Myc overexpression was consequently detected in 80% of gDLBCL but only 20% of MALT lymphomas spotted on a tissue microarray. A highly similar signature of Myc-repressed miRNAs was further detected in nodal DLBCL. Small interfering RNA–mediated knock-down of Myc blocked proliferation of DLBCL cell lines. Of the Myc-repressed miRNAs down-regulated in malignant lymphoma, miR-34a showed the strongest antiproliferative properties when overexpressed in DLBCL cells. We could further attribute miR-34a's tumor-suppressive effects to deregulation of its target FoxP1. FoxP1 overexpression was detected in gDLBCL but not in gastric MALT lymphoma; FoxP1 knock-down efficiently blocked DLBCL proliferation. In conclusion, our results elucidate a novel Myc- and FoxP1-dependent pathway of malignant transformation and suggest miR-34a replacement therapy as a promising strategy in lymphoma treatment.
Introduction
Gastric marginal zone B-cell lymphoma of MALT type is a low-grade extranodal B-cell lymphoma that arises in the context of chronic gastric inflammation induced by persistent Helicobacter pylori infection.1 In its early stages, MALT lymphoma is an indolent and localized disease that can be treated by antibiotic eradication therapy targeting the underlying infection.2 In line with the concept that gastric MALT lymphomas are antigen-driven tumors, the surface immunoglobulins of MALT lymphoma B cells are clonal, somatically hypermutated, and have undergone positive selection.3 We have shown recently that MALT lymphoma tumor immunoglobulins (Igs) are polyreactive; that is, they bind with similar affinity to various unrelated self and foreign antigens and show a biased use of Ig VH gene segments previously linked to polyreactive and autoreactive antibodies.4 Early MALT lymphomas further require T-cell help in the form of soluble T-helper cell–derived signals, most probably B-cell mitogenic cytokines such as IL-4 and IL-5.5
Low-grade MALT lymphomas may progress to more aggressive disease, either through the acquisition of 1 of 3 characteristic chromosomal translocations resulting in the constitutive activation of the NF-κB signaling pathway,2 or through the histologically evident transformation to high-grade gastric diffuse large B-cell lymphoma (gDLBCL).6,7 High-grade transformation of Helicobacter-associated MALT lymphoma accounts for most of the gDLBCL cases, whereas primary gDLBCL is rare.6,7 gDLBCL is characterized by antigen-independent growth, resistance to Helicobacter eradication therapy, and a number of genetic alterations that may contribute to high-grade transformation.8 In particular, TP53 mutations,9 Bcl6 overexpression,10 and the aberrant DNA hypermethylation of tumor suppressor genes11 have been shown to be associated with high-grade transformation. However, the precise molecular mechanisms underlying the transition from low-grade MALT lymphoma to gDLBCL remain largely unclear.
MicroRNAs (miRNAs) are an abundant class of small noncoding RNAs that modulate the expression of their target genes at the posttranscriptional level. Aberrant expression of specific miRNAs has been associated with both solid-organ and hematopoietic malignancies,12 including chronic lymphocytic leukemia,13 lung cancer,14,15 and ovarian cancer.16,17 Most human miRNAs are located at fragile sites or cancer-associated genomic regions.17 For example, the frequent down-regulation of the fragile region encoding miR-15a and miR-16-1 promotes chronic lymphocytic leukemia through deregulation of the Bcl2 oncogene.13 The widespread deregulation of the miRNA transcriptome appears to be a hallmark of cancer and has been attributed to deletions, amplifications, or mutations of miRNA loci,16,17 epigenetic silencing,18 or the aberrant transcriptional regulation of miRNA genes.16 Several studies have shown the potential of miRNA expression profiles as diagnostic and prognostic markers of cancers,15 which may be more useful than expression analysis of protein-coding genes for the classification and stratification of cancer subtypes.12
Here, we have used a microarray approach to identify miRNAs that are differentially regulated in gastric low-grade MALT lymphoma and its transformed high-grade disease counterpart. Interestingly, we found that a characteristic set of Myc-repressed miRNAs was down-regulated in the high-grade but not the low-grade cases studied. Aberrant Myc expression indeed correlated with high-grade transformation as analyzed immunohistochemically with a gastric lymphoma tissue microarray. Bioinformatic target prediction combined with functional analyses showed that one of the miRNAs found to be down-regulated in high-grade gDLBLC, miR-34a, represents a bona fide tumor suppressor miRNA in DLBCL. miR-34a acts through posttranscriptional control of its direct target FoxP1, a hematopoietic oncoprotein overexpressed in gDLBCL. In conclusion, our findings identify a new mechanism that links the aberrant expression of Myc and the resulting repression of the tumor suppressor miRNA miR-34a to FoxP1 deregulation in high-grade transformation of gastric B-cell lymphoma.
Methods
Patient material and lymphoma cell lines
For miRNA expression analysis of archived patient material, consecutive cases of H pylori–positive gastritis, H pylori–positive gastric low-grade MALT lymphoma, and gDLBCL were drawn from the surgical pathology files of the Institute of Pathology at the Cantonal Hospital St Gallen and from the files of the Institute for Surgical Pathology, University Hospital Zurich. Diagnosis was performed according to the classification system of the World Health Organization (WHO) on formalin-fixed, paraffin-embedded (FFPE) tissue. All data were blinded to guarantee patients' protection. All procedures were in agreement with the guidelines for use of human material in research issued by the Ethics Committees of the Cantonal Hospital St Gallen and the Canton of Zurich. The DLBCL and marginal zone lymphoma cell lines used were SUDHL4 and SUDHL6 (both DLBCL of germinal center B-cell or GCB type), U2932 (DLBCL, activated B-cell or ABC type), SUDHL7 (DLBCL, unclassified), and SSK41 (marginal zone lymphoma). All cell lines were maintained at 37°C in RPMI 1640 medium supplemented with 20% heat-inactivated FBS and antibiotics.
RNA extraction, microRNA expression profiling by microarray analysis, and locked nucleic acid real-time PCR for microRNA quantification
Total RNA was extracted from three 20-μm slices per sample of FFPE material with the use of the RecoverAll total RNA Isolation kit (Ambion). Total RNA concentrations were measured with an ND-1000 spectrophotometer (NanoDrop Technologies). RNA integrity was confirmed on an Agilent 2100 Bioanalyzer (Agilent Technologies). All microarray experiments were performed with the Agilent Human miRNA Microarray Kit Version 10.0. Total RNA (100 ng) was hybridized per sample and processed according to the manufacturer's instructions. The arrays were scanned with an Agilent Technology G2565B scanner. The scanned images were gridded and analyzed with Agilent Feature Extraction Software Version 9.5. The 795 human mature miRNAs included on the array were represented by 2421 probes; the average intensity of all probes corresponding to one miRNA was calculated. Normalization was performed with quantile normalization implemented in the Bioconductor package PreprocessCore. The false discovery rate was computed with the Benjamini-Hochberg algorithm. All raw data of the microarray experiments are publicly accessible under the accession number GSE24485 (http://www.ncbi.nlm.nih.gov/geo/). The expression of mature miRNAs was analyzed with the miRCURY locked nucleic acid (LNA) microRNA PCR system following the manufacturer's instructions (Exiqon). Briefly, 10 ng of total RNA was subjected to cDNA synthesis with either miRNA- or U6 small nuclear RNA (snRNA)–specific primers. The cDNA template was diluted 1:10, and real-time PCR reactions were performed following the manufactures' recommendations (LightCycler; Roche). Calculations of miRNA expression levels were performed with the comparative ΔΔCt method and normalized against U6 snRNA levels.
Small RNA cloning, sequence annotation, and differential expression detection
Small RNA cloning was performed by Vertis Biotechnology as described.19 The small RNA fraction from 4 cases each of nodal DLBCL and follicular lymphoma was isolated with the miRVana-microRNA isolation kit (Ambion), run on 12.5% polyacrylamide gel, and stained with SYBR greenII. The 15- to 40-nt fraction was eluted and poly-adenylated with poly(A) polymerase, and an adaptor was ligated to the 5′-phosphate of the miRNAs (5′-end adaptor, 43 nucleotides: 5′-GCCTCCCTCGCGCCATC AGCTNNNNGACCTTGGCTGTCACTCA-3′; NNNN represents a barcode sequence). First-strand cDNA synthesis was performed with an oligo(dT)-linker primer and M-MLV-RNaseH reverse transcriptase (3′-end oligo dT linker primer: 5′-GCCTTGCCAGCC CGCTCAGACGAGACATCGCCCCG C(T)25-3′). The resulting cDNAs were amplified in 22 cycles with the use of the high-fidelity Phusion polymerase (Finnzymes). The resulting 120- to 135-bp amplification products were electroeluted from 6% phosphatase antialkaline gels. After isolation with Nucleospin Extract II (Macherey and Nagel), the cDNA was dissolved in 5mM Tris/HCl (pH 8.5) at a concentration of 10 ng/mL and used in single-molecule sequencing. Massively parallel sequencing was performed by 454 Life Sciences with the use of the Genome Sequencer 20 system. Sequence analysis and small RNA–derived library annotation was done semimanually as described.19 Relative miRNA abundance in indolent nodal lymphoma and nodal DLBCL was compared on the basis of the miRNA reads normalized to the total number of annotated miRNAs.
Immunohistochemical staining, Western blotting, and FISH
The gastric lymphoma tissue microarray used in this study was previously described20 and included a total of 76 specimens, comprising 39 cases of gastric low-grade MALT lymphomas and 37 cases of gDLBCL. The following primary antibodies were used: anti-Myc (N-262; Santa Cruz Biotechnology), anti-FoxP1 (ICI2; Abcam), anti-p53 (M7001; Dako), and anti-p21/WAF (NCL-WAF-1; Novocastra). Myc and FoxP1 levels were assessed by counting the number of positively staining tumor cells and graded with the following expression scale: a negligible level of staining of 0%-10% was recorded as negative, whereas low expression was between 10% and 60%, and high expression was recorded when 60%-100% tumor cells stained positive. Pictures were taken at room temperature with a Leica Leitz DM RB microscope (20×/0.5 NA) equipped with a Leica DFC 420C camera. Images were acquired using the Leica Application Suite 3.3.0 software. Protein extracts from cell lines were made in 2× Laemmli sample buffer (4% SDS, 20% glycerol, 120mM Tris [pH 6.8]). Proteins were separated by SDS/PAGE and transferred onto nitrocellulose membranes. Membranes were probed with antibodies against FoxP1 (JC12; Abcam) or against α-tubulin (Santa Cruz Biotechnology) to control for equivalent gel loading. The MYC gene status was assessed by FISH with the use of dual-color, break-apart probes (Vysis/Abott), whereas the FOXP1 gene status was determined by bacterial artificial chromosome clones RP11-118O11, RP11-1031N18, RP11-430J3 and RP11-154H23, RP11-321A23, RP11-266022 flanking the FOXP1 locus, both as described.21,22 Slides were counterstained with 125 ng/mL 4′,6-diamino-2-phenylindole in antifade solution. FISH signals were scored with a Zeiss fluorescence microscope equipped with double-band pass filters for simultaneous visualization of Spectrum Green and Spectrum Orange signals. Cases on the tissue microarray (TMA) were considered evaluable for FISH if ≥ 200 tumor cell nuclei per core displayed positive signals. Splits were recorded as the percentage of cells bearing an abnormality of all analyzed cells. The cutoff score to consider a case rearranged was the mean plus 3 standard deviations of split nuclei in reactive lymph nodes and tonsils (ie, 4% for MYC and 3% for FOXP1). The minimal distance of split signals was defined as ≥ 3 single signal widths.
Cell lines, transfections, and luciferase reporter assays
On-target plus smartpool small interfering RNAs (siRNAs) for MYC, FOXP1, and a scrambled negative control was purchased from Dharmacon (Thermo Scientific). Additionally for FOXP1 RUA the following sense sequence was used: AAGAACCACAGGCAACAAUC Precursor microRNA oligonucleotides (pre–miR-let-7a, pre–miR-34a, pre–miR-23a, pre–miR-26a, pre–miR-150, and pre–miR-15) and scrambled negative control oligonucleotides were purchased from Ambion. For the purpose of siRNA or miRNA introduction into DLBCL cells, 1 × 106 cells were nucleoporated with an Amaxa Nucleoporator with the specified amount of pre-miR miRNA precursor or siRNA. After 48 hours, cells were harvested for RNA and protein analysis. After 72 hours, tumor cell proliferation was quantified by [3H]-thymidine incorporation assay as previously described.5 The pmirGLO Dual-Luciferase miRNA Target Expression Vector was purchased from Promega. HEK293T cells were seeded into 21-well plates at 1 × 105 cells/well 24 hours before transfection. Reporter plasmid (1 μg) containing the FOXP1 3′-UTR (untranslated region) or its mutants and 30nM mir-34a precursor molecules were cotransfected into each well with the use of the Fugene 6 transfection reagent (Roche) in triplicates. Luciferase assays were performed 24 hours after transfection with the use of the Dual-Luciferase Reporter Assay System (Promega) with a Spectramax M5 reader (Molecular Devices).
DNA methylation analysis of the miR-34a promoter
Specific oligonucleotides for methylation-specific PCR of the miR-34a CpG island were described previously.23 Genomic DNA was isolated from FFPE tissue and fresh material with the use of the RecoverAll total RNA Isolation kit (Ambion) and the DNeasy Blood and Tissue Kit (QIAGEN), respectively. gDNA (2 μg) was converted with sodium bisulfite as previously reported.24 After amplification of the bisulfite-converted DNA, the methylation status was assessed by PCR as described previously.23 PCR products were separated by electrophoresis on polyacrylamide gels and visualized under ultraviolet light after staining with ethidium bromide.
Results
Myc-repressed miRNAs are specifically down-regulated in high-grade–transformed gastric lymphoma
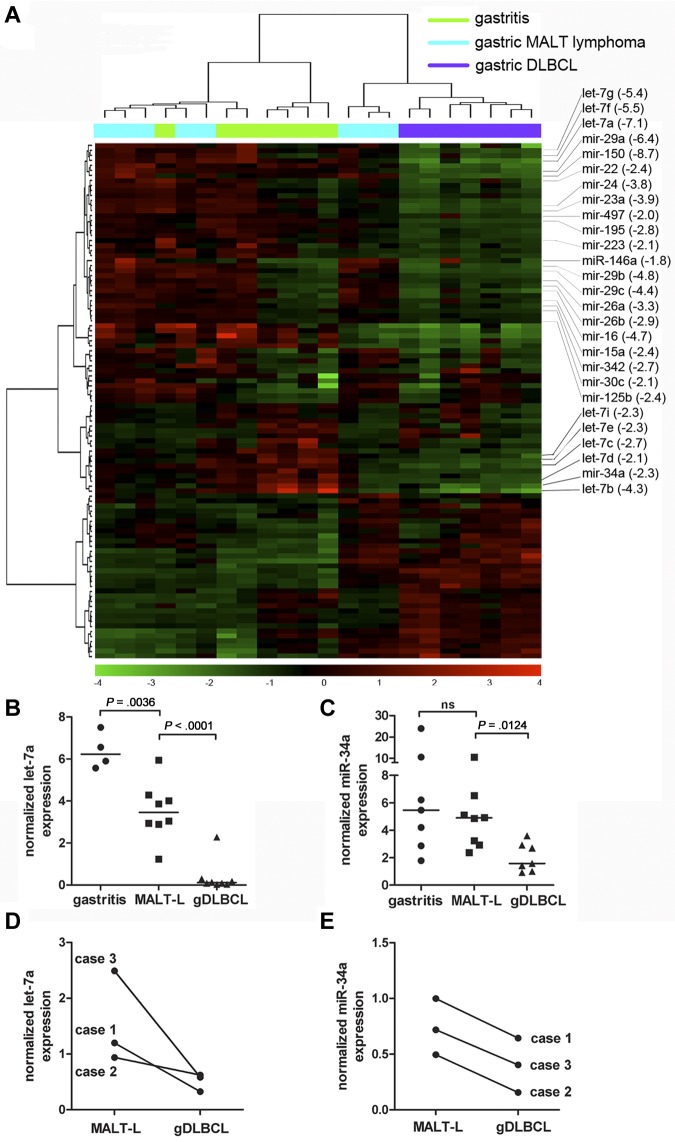
To obtain global miRNA expression signatures of gDLBCL and its precursor lesions, total RNA isolated from 7 to 8 cases each of Helicobacter-associated gastritis, low-grade MALT lymphoma, and gDLBCL was hybridized to Agilent miRNA microarrays, representing 795 human mature miRNAs. Unsupervised hierarchical clustering analysis showed a clear segregation of the gDLBCL cases from the low-grade MALT lymphomas and the gastritis samples (Figure 1A). The segregation of the latter 2 disease entities was incomplete (Figure 1A), reflecting the relative biologic similarity of gastritis and low-grade lymphomas. Statistical analysis of the dataset showed only 25 differentially expressed miRNAs between these 2 groups (P < .05; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), whereas 88 miRNAs exhibited significant differences in expression between low- and high-grade lymphomas (supplemental Table 2).
Figure 1.
A Myc-repressed miRNA signature distinguishes gastric DLBCL from low-grade MALT lymphoma. (A) The expression of 795 human mature miRNAsincluded in Version 10.0 of the miRBase database was analyzed for 7 cases of gastritis (green), 8 cases of low-grade MALT lymphoma (MALT-L; blue), and 7 gDLBCL cases (purple) with the use of microarray technology. The result of unsupervised hierarchical clustering of all miRNAs that varied across the 22 arrays with a standard deviation of log2 expression > 0.05 is shown in the heat diagram. All known Myc-repressed miRNAs with lower expression in gDLBCL compared with low-grade MALT-L are annotated along with their fold change in expression (averaged across groups) between gDLBCL and MALT-L (in brackets). (B-C) Quantification of let-7a (B) and miR-34a (C) expression in the samples shown in panel A by LNA real-time RT-PCR; absolute expression was normalized to U6 snRNA. P values were calculated by unpaired t test. Horizontal lines represent median values. (D-E) Quantification of let-7a (D) and miR-34a (E) expression in matched MALT-L and gDLBCL samples from 3 patients with gastric lymphoma.
To identify miRNAs with a putative tumor-suppressive role in high-grade transformation, we focused on the subset of 57 miRNAs that were down-regulated in high-grade but not in low-grade tumors (supplemental Table 2). Interestingly, a large fraction of the down-regulated miRNAs had previously been reported to be repressed by the Myc transcription factor through a mechanism involving the direct association of Myc with pri-miRNA promoters.25 In fact, we found that of the 30 known human Myc-repressed miRNAs, 27 were down-regulated by ≥ 1.8- and ≤ 8.7-fold in high-grade versus low-grade gastric lymphomas (annotated in Figure 1A). The differential expression of 2 selected miRNAs, let-7a and miR-34a, was verified on the same panel of samples by LNA real-time RT-PCR (Figure 1B-C). To obtain more direct evidence that miRNA dysregulation is involved in high-grade transformation of gastric lymphoma, we examined let-7a and miR-34a expression in paired samples of 3 patients with gastric lymphoma for which both low- and high-grade tumor material was available. In all cases, expression of the miRNAs was significantly lower in the high-grade than in the low-grade component of the tumor (Figure 1D-E). In conclusion, we found that with the use of genomewide miRNA expression profiling high-grade transformation of gastric MALT lymphoma is accompanied by a characteristic signature of repressed miRNAs, a substantial number of which are encoded by known target genes of Myc.
A highly similar signature of Myc-repressed genes distinguishes aggressive from indolent nodal and gastric lymphomas
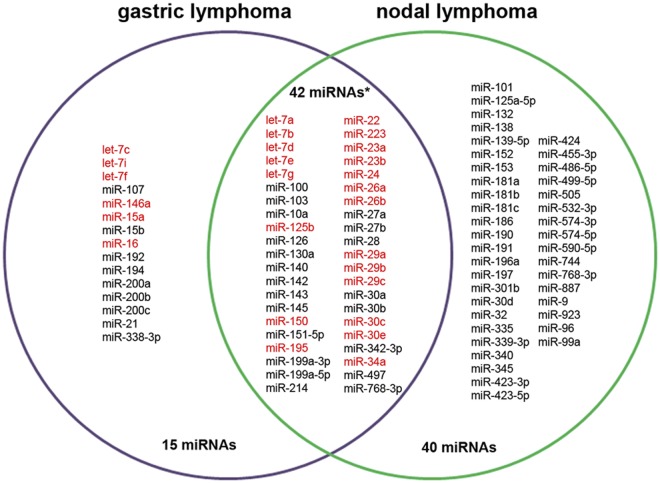
To determine whether our signature of differentially expressed miRNA genes was specific to gastric lymphoma, we compared it with an independently conducted study analyzing miRNA expression in nodal lymphomas. To this end, our lists of differentially expressed miRNA genes were compared with miRNAs that differed in expression between 4 follicular lymphomas and 4 nodal DLBCL cases as assessed by 454 deep sequencing. Surprisingly, a highly significant overlap existed among the down-regulated miRNAs; specifically, 42 of the 57 miRNAs down-regulated in gDLBCL, but not gastric MALT lymphoma, were also down-regulated in nodal DLBCL (P < .001; Figure 2). This overlap was particularly compelling because different technology platforms (microarray-based expression profiling vs deep sequencing) had been used. Down-regulation of almost the entire set of known Myc-repressed miRNAs was a shared feature of gastric and nodal DLBCL (marked in red; Figure 2). In conclusion, the result indicates that the transformation from indolent to aggressive lymphoma is accompanied by the suppression of similar sets of miRNA genes in the nodal and extranodal disease counterparts and implies that similar pathomechanisms are operative in both disease entities.
Figure 2.
Venn diagram of unique and shared miRNAs down-regulated in gastric or nodal DLBCL or both. The miRNAs differentially down-regulated in high- compared with low-grade gastric lymphoma (as assessed by microarray analysis; Figure 1) were compared with the miRNAs differentially down-regulated in aggressive compared with indolent nodal lymphoma (as assessed by 454 deep sequencing). A total of 745 miRNAs were detected in the microarray/gastric lymphoma study; 57 of these were significantly down-regulated in gastric DLBCL by > 1.5-fold compared with MALT lymphoma. A total of 298 miRNAs were detected in the deep-sequencing/nodal lymphoma study, of which 87 were down-regulated by > 1.8-fold in the aggressive nodal lymphomas compared with their indolent counterparts. Forty-two down-regulated miRNAs (*P < .001 as assessed by Fisher exact test) were found to be shared between gastric and nodal DLBCL cases compared with the respective indolent disease counterparts. miRNAs known to be repressed by Myc are highlighted in red.
Myc is overexpressed in gastric DLBCL and controls DLBCL proliferation in vitro
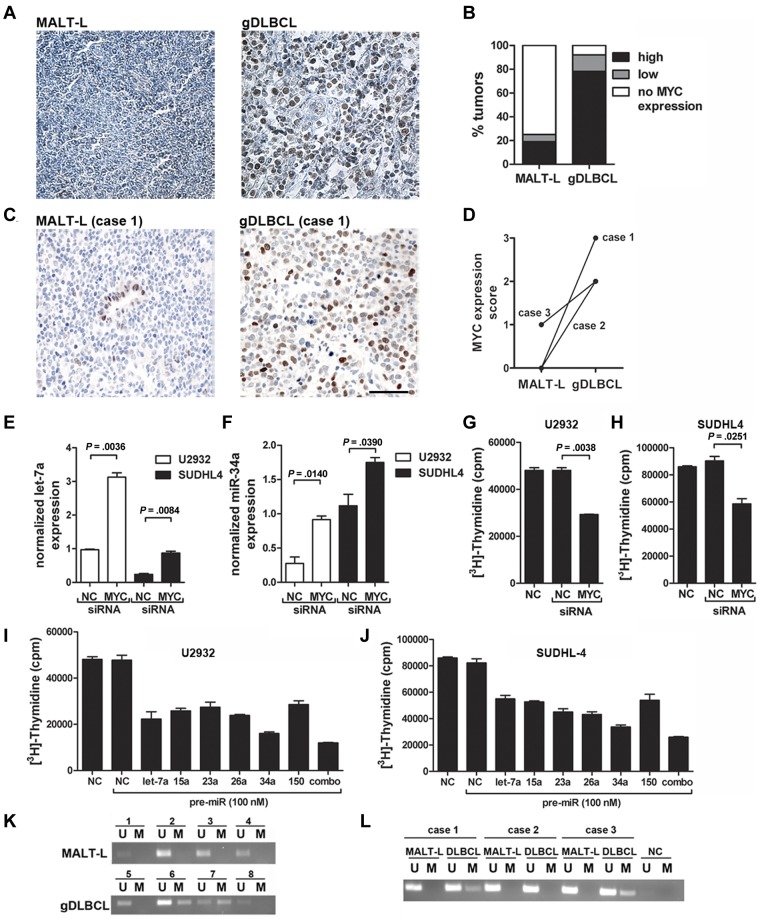
Having obtained indirect evidence of Myc overexpression in gDLBCL, but not in low-grade MALT lymphoma, we next aimed to assess the Myc expression status of a set of 37 gDLBCL and 39 low-grade lymphomas spotted onto a gastric lymphoma tissue microarray.20 Indeed, 80% of gDLBCL, but only 20% of low-grade lymphomas, showed high expression of Myc (Figure 3A-B) despite that none of the cases harbored chromosomal translocations involving the MYC genomic locus as determined by FISH analysis (supplemental Table 3). This differential expression of Myc in low- and high-grade gastric lymphoma was further substantiated in the 3 patients for whom tissue was available for both the low-grade and high-grade tumor component (Figure 3C-D). Myc expression also differed strongly in the gastric lymphoma cases used for the microRNA expression profiling shown in Figure 1; most of the gDLBCL cases, but none of the MALT lymphomas, expressed moderate or high levels of Myc (supplemental Table 4). To assess a possible causal link between Myc expression and miRNA down-regulation in DLBCL, we transiently knocked down Myc expression in 2 DLBCL lines, of which one has the characteristics of the ABC type of DLBCL (U2932) and the other has typical GCB features (SUDHL4). Neither cell line harbors chromosomal rearrangements involving the MYC genomic locus. The transient knock-down of Myc indeed increased expression of both let-7a (Figure 3E) and miR-34a (Figure 3F) in both cell lines in relation to a scrambled siRNA. Interestingly, the proliferation of both DLBCL cell lines as determined by [3H] thymidine incorporation was significantly reduced on siRNA-mediated Myc knockdown (Figure 3G-H). In contrast, the proliferation of a low-grade marginal zone lymphoma cell line, SSK41, was not reduced on knockdown of Myc (supplemental Figure 1). The combined results suggest that Myc expression drives the proliferation of transformed, but not indolent, lymphomas, possibly by down-regulation of tumor-suppressive miRNAs.
Figure 3.
Myc is overexpressed in gDLBCL and exhibits oncogenic properties in DLBCL cell lines in vitro. (A-B) Myc expression was analyzed by immunohistochemistry on a tissue microarray comprising 37 gDLBCL and 39 low-grade MALT lymphoma (MALT-L) cases. Representative micrographs are shown (A); the fraction of MALT lymphoma and gDLBCL cases with high, low and no Myc expression is indicated (B). (C-D) Myc expression as analyzed by immunohistochemistry of matched low-grade and high-grade lymphoma material from 3 patients. Representative micrographs are shown for case 1 (C); scores on a scale of 0-3 are shown for all cases (D). The scale bar indicates 50 μm. (E-F) Quantification of let-7a (E) and miR-34a (F) expression as determined by LNA real-time RT-PCR for the indicated cells lines (U2932, SUDHL4) 48 hours after electroporation with Myc-specific or scrambled (NC) siRNA. Expression values were normalized to U6 snRNA levels. (G-H) Proliferation as assessed by [3H] thymidine incorporation of U2932 (G) and SUDHL-4 (H) cells 72 hours after electroporation with Myc-specific or scrambled (NC) siRNA. (I-J) Proliferation as assessed by [3H] thymidine incorporation of U2932 (I) and SUDHL-4 (J) cells 72 hours after electroporation with the indicated pre-miRs or scrambled negative control (NC) oligonucleotide. (K-L) miR-34a promoter methylation as determined by methylation-specific PCR of 4 unrelated cases each of MALT lymphoma (K top) and gDLBCL (K bottom) as well as 3 matched low- and high-grade lymphoma samples from the same patients (cases 1-3, L). U indicates unmethylated; and M, methylated. The Myc staining in panels A and B was performed twice with similar results; the experiments shown in panels E through J were each reproduced 3-4 times. Error bars indicate SD. Pictures were taken at room temperature with a Leica Leitz DM RB microscope (20×/0.5 NA) equipped with a Leica DFC 420C camera. Images were acquired using the Leica Application Suite 3.3.0 software.
To determine which of the Myc-repressed miRNAs have tumor-suppressive properties in DLBCL cell lines in vitro, we focused on a panel of 6 miRNAs that were consistently predicted by both the TargetScan and PicTar algorithms to target known or putative hematopoietic oncogenes such as Bcl6, Pim1, FoxP1, and Pax5. We introduced synthetic, chemically modified double-stranded precursor molecules of the 6 miRNAs (so-called pre-miRs) into U2932 and SUDHL4 cells by electroporation, either alone or in combination (Figure 3I-J). Although all Myc-suppressed miRNAs on our panel had suppressive effects on tumor-cell proliferation in relation to a scrambled negative control miRNA (Figure 3I-J) and an irrelevant miRNA not regulated by Myc (supplemental Figure 2), one candidate, miR-34a, was particularly effective in this respect. miR-34a is a known tumor suppressor miRNA in prostate and lung cancers and is a lead candidate for miRNA replacement therapy for the treatment of these malignancies.26
Because miR-34a is known to be repressed by Myc25 and to be transcriptionally activated by p53,27 we examined the p53 mutation status of the MALT lymphoma and gDLBCL cases included in our tissue microarray. Only 3 of the 37 included cases of gDLBCL and 5 of the 39 MALT lymphoma cases exhibited evidence of mutated p53; that is, they expressed high levels of p53 but low levels of its target gene p21 (supplemental Table 3). Myc expression was not correlated (directly or inversely) with p53 mutation status; both observations combined indicate that the p53 status is not a useful marker in gastric lymphomagenesis and argue against an important role of p53 inactivation in high-grade transformation.
The mechanism of gene repression by Myc remains poorly understood; one line of evidence suggests that Myc recruits the DNA CpG methyltransferase Dnmt3a to the promoters of its negatively regulated target genes, an example of which is p21.28 To investigate whether miR-34a promoter methylation may contribute to its dysregulation in gDLBCL, we performed methylation-specific PCR analyses of a CpG island that surrounds the transcriptional start site of the miR-34a pri-miRNA. The island is located > 30 kb upstream of the position of mature miR-34a and has been reported to be relevant for its regulation.29 We compared the methylation status of multiple cases each of gastric low- and high-grade lymphoma and of the low- and high-grade disease components of the 3 paired cases shown in Figure 1D-E. Interestingly, none of the 7 low-grade MALT lymphomas, but 4 of the 7 gDLBCL samples analyzed, exhibited miR-34a promoter hypermethylation (Figure 3K-L), indicating that epigenetic silencing (Myc dependently or independently) may contribute to miR-34a dysregulation in gDLBCL. In particular, 2 of the 3 paired samples exhibited miR-34a promoter methylation in the high-grade, but not the low-grade, tumor component (Figure 3L). Interestingly, we further found with the use of the Progenetix oncogenomic database (www.progenetix.net)30 that ∼ 10% of the 1096 DLBCL cases for which comparative genomic hybridization data are available show loss of the region on chromosome 1 that contains the miR-34a locus (supplemental Figure 3). In conclusion, we show here that miR-34a has tumor-suppressive activity in DLBCL cell lines and that its expression is regulated by Myc or through mechanisms that may involve epigenetic silencing of the miR-34a promoter or chromosomal deletion of the miR-34a genomic locus.
miR-34a targets the transcription factor FoxP1 in DLBCL
Bioinformatically predicted targets of miR-34a include the transcription factors Bcl6 and FoxP1, which harbor 1 and 2 putative miR-34a seed regions in their 3′-UTR, respectively. Because both have previously been linked to the pathogenesis of gDLBCL,8,31 we aimed to test their possible posttranscriptional regulation by miR-34a in the DLBCL cell lines introduced earlier. Quantitative RT-PCR of FoxP1 and Bcl6 expression after electroporation of U2932 and SUDHL4 cells with pre–miR-34a showed that FoxP1, but not Bcl6, is probably a direct target of this miRNA (Figure 4A; data not shown). Protein levels of FoxP1 were also strongly reduced on the introduction of miR-34a into U2932 cells (Figure 4B). To measure a direct effect of miR-34a binding to its seed regions in the FOXP1 gene, we cloned the wild-type sequence of the seed region, or a mutant version in which 4 of the 6 positions had been mutated, downstream of a luciferase reporter gene. Cotransfection of pre–miR-34a with the luciferase expression vector harboring the wild-type seed region, but not the mutant version, blocked reporter gene expression as assessed by luciferase activity assay (Figure 4C). Interestingly, siRNA-mediated knock-down of Myc was as efficient as pre–miR-34a introduction in inhibiting FoxP1 expression (supplemental Figure 4). In summary, our results show that FoxP1 is a target of miR-34a and (an indirect target) of Myc in DLBCL cell lines. Because Myc has been shown in a previous report to itself be targeted by miR-34a,32 we examined a possible reciprocal interaction of both factors in the context of DLBCL cells. We were indeed able to confirm that Myc expression is negatively regulated by miR-34a (supplemental Figure 5), implying that the loss of miR-34a expression in gDLBCL may further de-repress Myc and perpetuate the oncogenic consequences of Myc dysregulation.
Figure 4.
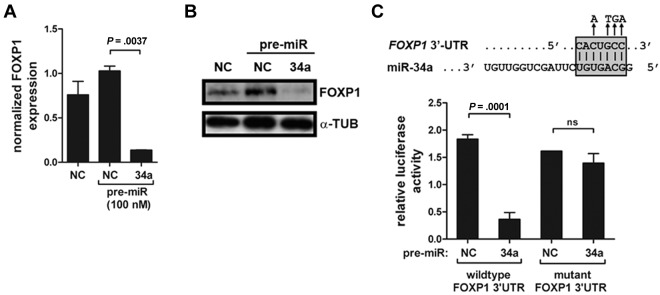
miR-34a directly targets FoxP1 in DLBCL. (A) U2932 cells were electroporated with pre-miR34a or a scrambled negative control pre-miR and analyzed with respect to FoxP1 expression 48 hours later. FoxP1 transcript levels were normalized to GAPDH expression. (B) FoxP1 protein levels of the experiment described in panel A were analyzed by Western blot. α-tubulin levels are shown to control for equal loading. (C) Dual luciferase assay of HEK293T cells cotransfected with firefly luciferase constructs containing the wild-type or mutant miR-34a target site of the FOXP1 3′-UTR downstream of the luciferase reporter. Cells were cotransfected with either pre–miR-34a or a negative control scrambled oligonucleotide and the respective luciferase construct. Data are represented as relative luciferase activity. All experiments were reproduced ≥ 3 times. Error bars indicate SD.
The miR-34a target FoxP1 is a bona fide oncoprotein in DLBCL
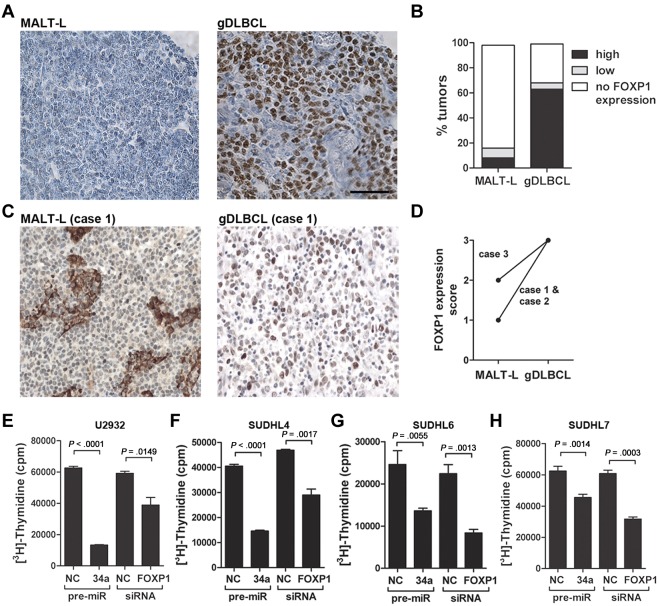
Because FoxP1 is a direct target of miR-34a, we asked whether FoxP1 is differentially expressed in gDLBCL and low-grade MALT lymphoma. Indeed, most of the gDLBCL cases but very few of the low-grade lymphoma cases spotted onto our tissue microarray showed reactivity with a FoxP1-specific antibody (Figure 5A-B). Interestingly, all FoxP1-positive cases also expressed Myc, irrespective of whether they were classified as low-grade or high-grade lymphomas (supplemental Table 3). However, FoxP1 expression was not significantly correlated with expression of Bcl6, which is often used to distinguish between GCB- and ABC-type DLBCL: similar proportions of FoxP1-positive cases were Bcl6-positive and -negative (supplemental Table 3). Chromosomal rearrangements involving the FOXP1 genomic locus were not detected by FISH in any of the MALT lymphoma or gDLBCL cases spotted onto our tissue microarray (supplemental Table 3), ruling out that FoxP1 dysregulation is caused by genomic events. Interestingly, the differential expression of FoxP1 in gastric low- and high-grade lymphoma could be confirmed on the 3 pairs of low-grade and high-grade tumor components harvested from the same patients (Figure 5C-D). FoxP1 expression analysis of the gastric lymphoma cases used for microRNA expression profiling also showed higher FoxP1 expression in the high-grade than in the low-grade cases (supplemental Table 4). Having shown that FoxP1 is overexpressed in gDLBCL, we postulated that the siRNA-mediated knockdown of FoxP1 should have similar antiproliferative effects in DLBCL cell lines as the delivery of miR-34a. This was indeed the case; knockdown of FoxP1 by ∼ 64% (supplemental Figure 6) blocked the proliferation of 4 GCB- and ABC-type DLBCL cell lines at similar levels and roughly as efficiently as the re-introduction of miR-34a (Figure 5E-H). In contrast, the proliferation of SSK41 low-grade marginal zone lymphoma cells was not reduced on knockdown of FoxP1 and was only modestly reduced by miR-34a (supplemental Figure 7). In conclusion, our results suggest that the transcription factor FoxP1 is a direct target (yet probably not the only target) of miR-34a that is overexpressed in gDLBCL, but not in low-grade lymphoma, and represents a bona fide oncoprotein in DLBCL. Taken together, our results establish a mechanistic link between overexpression of Myc, the concomitant repression of Myc-regulated miRNA genes, and the deregulation of FoxP1 (see schematic in Figure 6).
Figure 5.
FOXP1 is a bone fide oncoprotein in DLBCL. (A-B) FoxP1 expression was analyzed by immunohistochemistry with the use of the tissue microarray described in Figure 3. Representative micrographs are shown (A); the fraction of MALT lymphoma and gDLBCL cases with high, low, and no FoxP1 expression is shown (B). The scale bar indicates 50μm. (C-D) FoxP1 expression as analyzed by immunohistochemistry of matched low-grade and high-grade lymphoma material from the same patients. Representative micrographs are shown for case 1 (C); scores on a scale of 0-3 are shown for all 3 cases (D). (E-H) Proliferation as determined by [3H] thymidine incorporation of U2932 (E), SUDHL4 (F), SUDHL6 (G), and SUDHL7 (H) cells 72 hours after electroporation with pre-miR34a, a FoxP1-specific siRNA or the respective negative control (NC) scrambled oligonucleotides. The FoxP1 staining in panels A through D was performed twice with similar results; the experiments shown in panels E through H were reproduced ≥ 3 and ≤ 5 times. Error bars indicate SD. Pictures were taken at room temperature with a Leica Leitz DM RB microscope (20×/0.5 NA) equipped with a Leica DFC 420C camera. Images were aquired using the Leica Application Suite 3.3.0 software.
Figure 6.
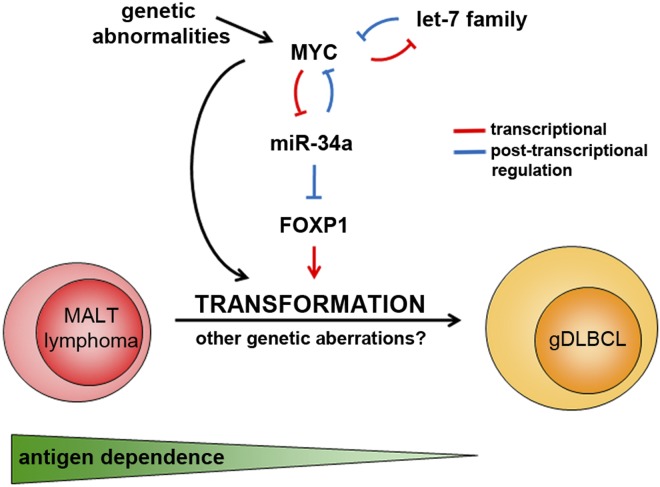
Schematic representation of the interplay between Myc, miR-34a, and FoxP1 during malignant transformation of low-grade gastric MALT lymphoma to gDLBCL. Myc overexpression can be caused by chromosomal translocations involving MYC and IG loci or other genetic abnormalities or by posttranscriptional regulation through miRNAs such as members of the let7 family and miR-34a (as first shown by Christoffersen et al32). Antigen dependence is lost during malignant transformation.
Discussion
Gastric DLBCL is an aggressive fast-growing disease with inferior survival rates compared with MALT lymphoma. The potential contribution of miRNA deregulation to high-grade transformation of MALT lymphoma is unknown. Here, we used a genomewide microarray approach to compare the miRNA expression profiles of gastric MALT lymphoma and gDLBCL. Of the substantial number of miRNAs differentially expressed between the 2 disease entities, a large majority were down-regulated in the gDLBCL cases analyzed. Global reduction of miRNA expression is a hallmark of cancer progression and is thought to contribute to neoplastic transformation by allowing increased expression of oncoproteins.33 The Myc oncoprotein is known to directly suppress the expression of a large number of human miRNAs, thereby contributing to the phenomenon of reduced abundance of miRNAs in cancer cells.25,34,35 A Myc-repressed miRNA signature was found to characterize both gastric and nodal DLBCL; Myc overexpression was much more common in gDLBCL than in low-grade MALT lymphoma. Chromosomal aberrations involving the MYC locus are reported in the literature for a subset of gDLBCL but are never detected in gastric low-grade lymphomas and rarely found in nodal DLBCL.22,36,37 We did not detect chromosomal translocations involving MYC in any of our 37 gDLBCL cases analyzed. Myc is itself known to be posttranscriptionally regulated by miRNAs.32 Interestingly, several Myc-regulating miRNAs (including miR-34a and let7a) are also targets of Myc repression,32 establishing a feedback loop that aggravates and perpetuates the effects of Myc overexpression and may thus contribute to cancer progression.
Several lines of evidence argue that the Myc-regulated miRNA miR-34a acts as a strong tumor suppressor in solid cancers. It is located on chromosome 1p36.22 in a region that has previously been associated with various malignancies, including lung cancer17; it is transcriptionally induced by the tumor suppressor p53, and its overexpression inhibits growth of various cancer types in vitro.27 The first proof of principle for the success of miR-34a “replacement therapy” in cancer treatment was recently reported with a preclinical model of non–small cell lung cancer; the local and systemic delivery of chemically synthesized miR-34a was achieved in this model by formulation with a lipid-based delivery reagent.26 We demonstrate here for the first time that miR-34a has tumor-suppressive properties in DLBCL. It is down-regulated in the highly malignant compared with the indolent form of gastric and nodal lymphomas, and its expression is directly regulated by Myc, which we show here to possess oncogenic properties in various DLBCLs, but not low-grade lymphoma cell lines. Ectopic miR-34a re-expression strongly inhibits DLBCL proliferation; in line with another recent report, we demonstrate here that miR-34a directly targets a suspected hematopoietic oncogene, FOXP1, by binding to ≥ 1 of 2 predicted seed regions in the FOXP1 3′-UTR.38 In addition, we found that FoxP1 is overexpressed in the high-grade form of gastric lymphoma and indeed possesses bona fide oncogenic properties in DLBCL cell lines, suggesting that it represents a relevant, but probably not an exclusive, target of miR-34a in this disease entity.
Recent attention has focused on FoxP1 and its potential role in tumorigenesis because of its up-regulation in a variety of B-cell neoplasias. Our results confirm and extend previous reports linking FoxP1 overexpression to poor prognosis in MALT lymphoma and DLBCL.21,39 The posttranscriptional regulation of FoxP1 by miR-34a further provides a plausible explanation for the conundrum that FoxP1 is highly expressed in many lymphomas not harboring the rare chromosomal translocation t(3;14)(p13;q32), which juxtaposes the FOXP1 and IGH gene loci in certain nongastric MALT lymphomas and extranodal DLBCL.40,41 In conclusion, we propose here that miR-34a replacement therapy should be considered not only for the treatment of solid cancers but may also be beneficial in patients with miR-34a–negative, FoxP1-overexpressing hematopoietic malignancies such as gastric DLBCL. The beneficial therapeutic effects of miR-34a are anticipated because of the strong tumor-suppressive properties of this miRNA, which are exerted by regulation of its oncogene target FoxP1.
Supplementary Material
Acknowledgments
We thank David Nadal and Joe Jiricny for helpful discussions and Friedrich Grässer for co-supervising the lymphoma sequencing project. We thank Andrea Patrignani and Sabina Hafen for help with microarray experiments.
This work was supported by Oncosuisse (OCS-02 099-08-2007), the Zurich University Research Priority Program in Systems Biology, and Deutsche Krebshilfe (grant 107166). S.B.C. was supported by the Swiss Group for Clinical Cancer Research (SAKK).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.J.C. and A.M. designed and performed the research, analyzed data, and wrote the manuscript; S.B.C. provided patient samples and performed IHC; J.I. and C.R. performed deep sequencing of nodal lymphomas; S.N., H.R., and R.S. helped with microarray experimentation and data analysis; and S.D. and A.T. constructed the tissue array and performed FISH and IHC analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Müller, Institute of Molecular Cancer Research, University of Zürich, Winterthurerstr 190, 8057 Zürich, Switzerland; e-mail: mueller@imcr.unizh.ch.
References
- 1.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330(18):1267–1271. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 2.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4(8):644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 3.Qin Y, Greiner A, Trunk MJ, Schmausser B, Ott MM, Muller-Hermelink HK. Somatic hypermutation in low-grade mucosa-associated lymphoid tissue-type B-cell lymphoma. Blood. 1995;86(9):3528–3534. [PubMed] [Google Scholar]
- 4.Craig VJ, Arnold I, Gerke C, et al. Gastric MALT lymphoma B cells express polyreactive, somatically mutated immunoglobulins. Blood. 2010;115(3):581–591. doi: 10.1182/blood-2009-06-228015. [DOI] [PubMed] [Google Scholar]
- 5.Craig VJ, Cogliatti SB, Arnold I, et al. B-cell receptor signaling and CD40 ligand-independent T cell help cooperate in Helicobacter-induced MALT lymphomagenesis. Leukemia. 2010;24(6):1186–1196. doi: 10.1038/leu.2010.76. [DOI] [PubMed] [Google Scholar]
- 6.Chan JK, Ng CS, Isaacson PG. Relationship between high-grade lymphoma and low-grade B-cell mucosa-associated lymphoid tissue lymphoma (MALToma) of the stomach. Am J Pathol. 1990;136(5):1153–1164. [PMC free article] [PubMed] [Google Scholar]
- 7.Peng H, Du M, Diss TC, Isaacson PG, Pan L. Genetic evidence for a clonal link between low and high-grade components in gastric MALT B-cell lymphoma. Histopathology. 1997;30(5):425–429. doi: 10.1046/j.1365-2559.1997.5450786.x. [DOI] [PubMed] [Google Scholar]
- 8.Starostik P, Patzner J, Greiner A, et al. Gastric marginal zone B-cell lymphomas of MALT type develop along 2 distinct pathogenetic pathways. Blood. 2002;99(1):3–9. doi: 10.1182/blood.v99.1.3. [DOI] [PubMed] [Google Scholar]
- 9.Du M, Peng H, Singh N, Isaacson PG, Pan L. The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood. 1995;86(12):4587–4593. [PubMed] [Google Scholar]
- 10.Omonishi K, Yoshino T, Sakuma I, Kobayashi K, Moriyama M, Akagi T. bcl-6 protein is identified in high-grade but not low-grade mucosa-associated lymphoid tissue lymphomas of the stomach. Mod Pathol. 1998;11(2):181–185. [PubMed] [Google Scholar]
- 11.Kondo T, Oka T, Sato H, et al. Accumulation of aberrant CpG hypermethylation by Helicobacter pylori infection promotes development and progression of gastric MALT lymphoma. Int J Oncol. 2009;35(3):547–557. doi: 10.3892/ijo_00000366. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 13.Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101(32):11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Yanaihara N, Caplen N, Bowman E, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9(3):189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Volinia S, Bonome T, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2008;105(19):7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9(6):435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Imig J, Motsch N, Zhu JY, et al. microRNA profiling in Epstein-Barr virus-associated B-cell lymphoma. Nucleic Acids Res. 2011;39(5):1880–1893. doi: 10.1093/nar/gkq1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernasconi B, Karamitopoulou-Diamantis E, Tornillo L, et al. Chromosomal instability in gastric mucosa-associated lymphoid tissue lymphomas: a fluorescent in situ hybridization study using a tissue microarray approach. Hum Pathol. 2008;39(4):536–542. doi: 10.1016/j.humpath.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Hoeller S, Schneider A, Haralambieva E, Dirnhofer S, Tzankov A. FOXP1 protein overexpression is associated with inferior outcome in nodal diffuse large B-cell lymphomas with non-germinal centre phenotype, independent of gains and structural aberrations at 3p14.1. Histopathology. 2010;57(1):73–80. doi: 10.1111/j.1365-2559.2010.03600.x. [DOI] [PubMed] [Google Scholar]
- 22.Obermann EC, Csato M, Dirnhofer S, Tzankov A. Aberrations of the MYC gene in unselected cases of diffuse large B-cell lymphoma are rare and unpredictable by morphological or immunohistochemical assessment. J Clin Pathol. 2009;62(8):754–756. doi: 10.1136/jcp.2009.065227. [DOI] [PubMed] [Google Scholar]
- 23.Chim CS, Wong KY, Qi Y, et al. Epigenetic inactivation of the miR-34a in hematological malignancies. Carcinogenesis. 2010;31(4):745–750. doi: 10.1093/carcin/bgq033. [DOI] [PubMed] [Google Scholar]
- 24.Frommer M, McDonald LE, Millar DS, et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci U S A. 1992;89(5):1827–1831. doi: 10.1073/pnas.89.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang TC, Yu D, Lee YS, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40(1):43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiggins JF, Ruffino L, Kelnar K, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70(14):5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447(7148):1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner C, Deplus R, Didelot C, et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24(2):336–346. doi: 10.1038/sj.emboj.7600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle. 2008;7(16):2591–2600. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 30.Baudis M, Cleary ML. Progenetix.net: an online repository for molecular cytogenetic aberration data. Bioinformatics. 2001;17(12):1228–1229. doi: 10.1093/bioinformatics/17.12.1228. [DOI] [PubMed] [Google Scholar]
- 31.Chen YW, Hu XT, Liang AC, et al. High BCL6 expression predicts better prognosis, independent of BCL6 translocation status, translocation partner, or BCL6-deregulating mutations, in gastric lymphoma. Blood. 2006;108(7):2373–2383. doi: 10.1182/blood-2006-05-022517. [DOI] [PubMed] [Google Scholar]
- 32.Christoffersen NR, Shalgi R, Frankel LB, et al. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2010;17(2):236–245. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- 33.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39(5):673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 34.Gao P, Tchernyshyov I, Chang TC, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sander S, Bullinger L, Klapproth K, et al. MYC stimulates EZH2 expression by repression of its negative regulator miR-26a. Blood. 2008;112(10):4202–4212. doi: 10.1182/blood-2008-03-147645. [DOI] [PubMed] [Google Scholar]
- 36.van Krieken JH, Raffeld M, Raghoebier S, Jaffe ES, van Ommen GJ, Kluin PM. Molecular genetics of gastrointestinal non-Hodgkin's lymphomas: unusual prevalence and pattern of c-myc rearrangements in aggressive lymphomas. Blood. 1990;76(4):797–800. [PubMed] [Google Scholar]
- 37.Kramer MH, Hermans J, Wijburg E, et al. Clinical relevance of BCL2, BCL6, and MYC rearrangements in diffuse large B-cell lymphoma. Blood. 1998;92(9):3152–3162. [PubMed] [Google Scholar]
- 38.Rao DS, O'Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity. 2010;33(1):48–59. doi: 10.1016/j.immuni.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sagaert X, de Paepe P, Libbrecht L, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(16):2490–2497. doi: 10.1200/JCO.2006.05.6150. [DOI] [PubMed] [Google Scholar]
- 40.Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19(4):652–658. doi: 10.1038/sj.leu.2403644. [DOI] [PubMed] [Google Scholar]
- 41.Haralambieva E, Adam P, Ventura R, et al. Genetic rearrangement of FOXP1 is predominantly detected in a subset of diffuse large B-cell lymphomas with extranodal presentation. Leukemia. 2006;20(7):1300–1303. doi: 10.1038/sj.leu.2404244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.