Abstract
We report a novel autosomal recessive disorder characterized by premature chromosome condensation in the early G2 phase. It was observed in two siblings, from consanguineous parents, affected with microcephaly, growth retardation, and severe mental retardation. Chromosome analysis showed a high frequency of prophase-like cells (>10%) in lymphocytes, fibroblasts, and lymphoblast cell lines with an otherwise normal karyotype. 3H-thymidine-pulse labeling and autoradiography showed that, 2 h after the pulse, 28%–35% of the prophases were labeled, compared with 9%–11% in healthy control subjects, indicating that the phenomenon is due to premature chromosome condensation. Flow cytometry studies demonstrate that the entire cell cycle is not prolonged, compared with that in healthy control subjects, and compartment sizes did not differ from those in healthy control subjects. No increased reaction of the cells to X-irradiation or treatments with the clastogens bleomycin and mitomycin C was observed, in contrast to results in the cell-cycle mutants ataxia telangiectasia and Fanconi anemia. The rates of sister chromatid exchanges and the mitotic nondisjunction rates were inconspicuous. Premature entry of cells into mitosis suggests that a gene involved in cell-cycle regulation is mutated in these siblings.
Mitosis is a fundamental process ensuring the proper segregation of replicated chromatids to the daughter cells. Cell entry into mitosis is under the control of a tightly regulated network of protein kinases, cyclins, and protein phosphatases. According to Pines and Rieder (2001), G2-phase and mitosis can be subdivided into five transitional phases that are characterized not only by the structural and behavioral changes of the chromosomes and the spindle but also at the molecular level, by the activation and inactivation of cell-cycle regulators such as the cyclin-dependent kinases (Cdks) and the anaphase promoting complex (APC). In vertebrates, the G2/M transition is initiated by the increase of cyclin-A-Cdk2 throughout the G2 phase of the cell cycle, resulting in chromosome condensation in the absence of significant cyclin-B1-Cdk1 activity. Subsequently, the cyclin-B1-Cdk1 complex, also known as mitosis promoting factor (MPF), is activated as a result of its dephosphorylation by Cdc25 and rapidly accumulates in the nucleus, followed by the breakdown of the nuclear envelope and the entry of the cell into metaphase (for review, see Pines and Rieder 2001).
Here, we report the first autosomal recessive cell-cycle disorder in humans, characterized by premature chromosome condensation (PCC). Two siblings, a girl aged 7 years and a boy aged 5 years, were referred to chromosome analysis because of microcephaly, growth retardation, and severe mental retardation (fig. 1). Their parents are first cousins with an otherwise unremarkable family history. The first pregnancy was uneventful; however, 3 wk before birth, microcephaly of the fetus was noted on ultrasound examination. During the second pregnancy, serial ultrasound scans indicated satisfactory head growth until the 7th mo of gestation, when microcephaly again became obvious. Delivery occurred at term in both sibs. The Apgar scores were 9/10/10 in both. Chronic nutritional placental insufficiency was histopathologically proven in the first sib; in the younger sib, however, the placenta was normal. At birth, both presented with severe microcephaly, receding foreheads, and small fontanelles. Apart from slightly upslanted palpebral fissures and small upper lips, no dysmorphic features were noted. Both showed slow developmental progress combined with profound mental retardation. At the age of 7 years, patient 1 speaks many simple words. Patient 2, presently 5 years of age, speaks only some single words; his language-perception skills have been improving recently, as has his ability to walk without support. Microcephaly was progressive in both sibs but is more striking in the boy, whose growth and psychomotor retardation are also more severe. Neurologic examination of the younger sib demonstrated spasticity, with hyperreflexia of patellar and Achilles tendon reflexes and cloni, none of which is present in the older sib. Ophthalmologic and auditory evaluation were normal in both. There were no recurrent infections and no evidence of hematoproliferative disease. Both are pleasant children with good social contacts. Their clinical features are detailed in table 1.
Figure 1.
The older and the younger sibling at the ages of 7 and 5 years, respectively.
Table 1.
Clinical Features of the Two Patients
|
Observation in Patient |
||
| Features | 1 (Older Sibling) | 2 (Younger Sibling) |
| Term of gestation | 40 | 38 |
| Birth weight (g) | 2,780 (−1 SD) | 2,550 (−1.5 SD) |
| Length at birth (cm) | 47 (−1.5 SD) | 47 (−1.6 SD) |
| Receding forehead | + | + |
| Upslanted palpebral fissures | + | + |
| Small upper lips | + | + |
| Occipitofrontal circumference (cm) | 28.5 (−3.5 SD) | 27 (−4 SD) |
| Age at first examination (years) | 4 | 2.25 |
| Weight (g) | 11,000 (+.8 SD) | 6,300 (−3.8 SD) |
| Height (cm) | 87 (−5 SD) | 69 (−5 SD) |
| Occipitofrontal circumference (cm) | 37 (−10 SD) | 33 (−12 SD) |
| Age at second examination (years) | 7 | 5 |
| Weight (g) | 22,000 (−.8 SD) | 10,000 (−1.5 SD) |
| Height (cm) | 110 (−5 SD) | 85 (−5.7 SD) |
| Occipitofrontal circumference (cm) | 40.4 (−8 SD) | 37.8 (−10 SD) |
| Renal agenesia (unilateral) | − | 704 |
| Vesicoureterical reflux (degree III to IV; unilateral) | − | + |
| Age at sitting | 8 mo | 3 years |
| Age at unsupported walking | 15 mo | 4.75 years |
| Hyperreflexia of patellar and Achilles tendon reflexes | − | + |
| Cloni | − | + |
| Expressive language (single words) | 2 years | 4 years |
| Understanding of language | + | + |
| Cerebral MRI findings:a | ||
| Pachygyria | + | 11 |
| Frontal lobe hypoplasia | + | − |
| Agenesis of the genu of corpus callosum | + | − |
| Nodular neuronal heterotopia | Ventricular | Infratentorial and subependymal in both horns of side ventricles |
| Slight retardation of myelinization | Of cerebral medullary layer | − |
| Dilatation of side ventricles | − | Dorsal and temporal |
| Dilated external liquor space | − | + |
At age 3 mo for patient 1 and age 2 years for patient 2.
Several attempts to yield high-resolution chromosomes after methotrexate treatment were unsuccessful, yielding extremely poor metaphase resolution of 250 bands per haploid genome. Otherwise, metaphases prepared from lymphocyte cultures revealed a normal female and male karyotype in the two sibs. Surprisingly, a high rate of “prophase-like” cells were observed in the chromosome preparations from lymphocyte cultures of both patients (fig. 2a). Chromosome preparations without prior colcemid treatment yielded 10%–15% prophase-like cells in cultures of the siblings, compared with <1% in those of healthy control subjects. A similar high incidence of prophase-like cells was also observed in the patients’ fibroblast and lymphoblast cell lines, indicating a common phenomenon of dividing cells in the two children. It appeared that most of these prophase-like cells retained their nuclear membrane (fig. 2b). However, metaphase formation, anaphase lagging, chromosome segregation, and cytokinesis were perfectly normal (fig. 2c–f). Chromosome preparations from both parents showed normal karyotypes at a band resolution of ∼600 bands per haploid-chromosome complement with normal response to methotrexate treatment. The rates of prophases were in the range of healthy control subjects. These findings are consistent with an autosomal recessive trait.
Figure 2.
Premature chromosome condensation in lymphocytes and fibroblasts. a, Chromosome preparation from PHA-stimulated 72-h lymphocyte culture harvested without prior colcemid treatment. b, Chromosomes in prophase nuclei of a fibroblast line, showing an intact nuclear membrane, as observed in most of the prophase-like cells. c and d, Normal alignment of the chromosomes in metaphase plates from fibroblasts. e, Normal anaphase lagging in the fibroblasts harvested without prior colcemid treatment. f, Normal segregation of homologous chromosomes in lymphoblast cells analyzed after blocking of the cytokinesis with cytochalasin and subsequent FISH performed with two centromeric probes from chromosome 8 and chromosome 18. g, Two cells after Hoechst staining of the prematurely condensed chromosomes (arrows). h, The same cells after indirect immunostaining with lamin B antibodies, demonstrating the intact nuclear membrane.
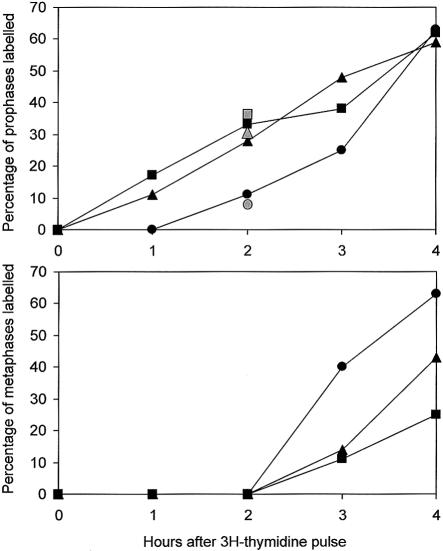
To elucidate the phenomenon, we analyzed the cell-cycle progression of logarithmically growing lymphoblast cell lines from both siblings by autoradiography after pulse-labeling with 3H-thymidine for 10 min. There was a striking difference between patients and control subjects in the percentage of labeled pro- and metaphases within the first 4 h after 3H-thymidine application. As soon as 1 h after 3H-thymidine pulse-labeling, the cells of the siblings showed 11% and 17% labeled prophases, compared with 0% in the control subjects, and, after 2 h, 28% and 33%, compared with 11% in the control (fig. 3). We repeated this experiment with a second control cell line at the 2-h interval after 3H-thymidine pulse-labeling. Again, a percentage as high as 32% (n=113) and 35% (n=166) of labeled prophases was found in both patients, compared with 9% (n=34) in the healthy control. Thus, chromosome condensation in the patients’ cells starts as soon as 1 h after the end of the S-phase or earlier. Within the 1–2-h period after adding 3H-thymidine of the cultures, no labeled metaphases were detected, either in the patients or in the control subjects. In the 3–4-h interval, the number of labeled metaphases increased in the control cell line, whereas this effect was slightly retarded in both patient cell lines (fig. 3). This finding could indicate a transitional prophase delay.
Figure 3.
Cell-cycle analysis after 3H-thymidine-pulse-labeling of logarithmically growing lymphoblast cell lines from both siblings. The cells were treated for 10 min with 1 μCi/ml 3H-thymidine (104, 7 Ci/mmol; specific activity: 104 Ci/mM) and were harvested for chromosome preparation at 1-h intervals. The numbers of labeled and unlabeled pro- and metaphase cells was determined after autoradiography for patient 1 (triangle), patient 2 (square), and a control (circle). Gray symbols give the percentage determined in an independent second experiment 2 h after pulse labeling.
To determine whether the G2/M compartment of the cell cycle was extended as a consequence of a potentially elongated prophase stage, cell-cycle analysis was performed using the 5-bromo-2′-deoxyuridine (BrdU)–Hoechst 33258 method (Kubbies et al. 1989). The principle of the BrdU-Hoechst assay is based on the incorporation of the halogenated base analogue during DNA replication; however, the assay makes use of the fact that BrdU-substituted chromatin quenches the fluorescence of the dye Hoechst 33258. This method differentiates not only between cycling and noncycling cells in a given culture but also between the distributions of the cycling cells in as many as four consecutive cycles. The cell-cycle distribution of primary lymphocytes from patient 2 after 72 h of culture showed perfectly normal scattering (table 2). This result indicates normal cell-cycle progression and virtually rules out G2/M prolongation.
Table 2.
Cell-Cycle Distribution in Native 72-h Cultures of PHA-Stimulated Lymphocytes from Patient 2 and 12 Age-Matched Healthy Control Subjects[Note]
|
Proportion(%) |
||||||||||
| First Cycle |
Second Cycle |
Third Cycle |
Fourth Cycle |
|||||||
| Source | G0/G1 | S | G2 | G1 | S | G2 | G1 | S | G2 | G1 |
| Patient 2 | 35.6 | 14.7 | 6.2 | 14.1 | 6.5 | 5.2 | 7.7 | 7.2 | 1.9 | .8 |
| Control subjects: | ||||||||||
| Mean | 37.5 | 12.8 | 5.4 | 10.9 | 12.1 | 5.3 | 8.1 | 5.8 | 1.2 | .7 |
| 1 SD | 11.5 | 2.5 | 3.0 | 3.0 | 5.4 | 2.5 | 2.9 | 3.1 | 1.1 | 1.3 |
| Range | 14.1–53.0 | 8.2–18.9 | 1.8–11.1 | 8.2–16.3 | 3.2–19.8 | 2.3–9.3 | 5.1–14.9 | 2.4–12.0 | .1–3.6 | 0–4.5 |
Note.— The distribution was assessed using BrdU-Hoechst flow cytometry for cell-cycle analysis (Kubbies et al. 1989). A correction for cells having divided once, twice, or more times was introduced to truly reflect the fate of the cells initially placed in culture. The control subjects had an age range of 4–6 years.
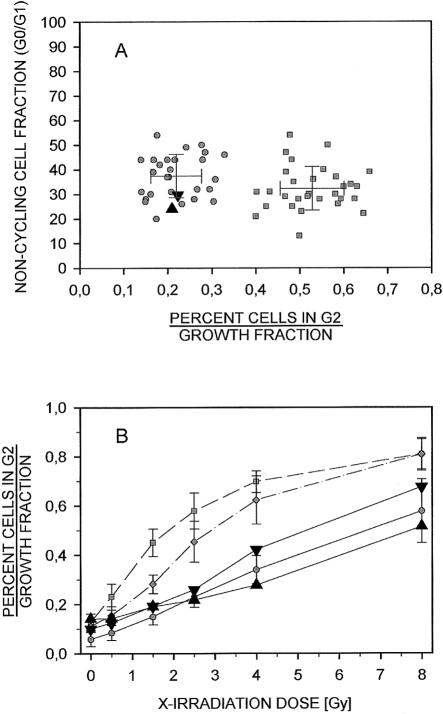
Cells affected by DNA damage are thought to become delayed in the G2 phase of the cell cycle to allow for repair of their DNA lesions (Seyschab et al. 1993). To test whether the global DNA damage recognition and repair pathways were intact in these siblings, we again used BrdU-Hoechst flow cytometry. We first investigated the effect of ionizing irradiation on the percentage of cells in G2 phase in the lymphoblast lines from both siblings. At a single-dose level (1.5 gray [Gy]), the percentage of cells in the G2 phases relative to the growth fractions of patient 1 and patient 2 were 0.21 and 0.22, respectively (fig. 4A). These ratios fell perfectly within the range of normal control lines. Ataxia telangiectasia (AT) lines served as positive controls. These radiosensitive cell lines were distinctly different in their G2 phase proportions 48 h after irradiation, with no overlap with the control lines (Seyschab et al. 1992). The noncycling fractions (percent G0/G1 phase) were similar in all cultures, indicating comparable growth. Radiosensitivity of the patients' lymphoblastoid lines was further explored for a broader range of radiation doses. Increasing the levels of irradiation from 0.5 to 8.0 Gy, the percentages of cells in G2, relative to the corresponding growth fractions, increased in a dose-dependent fashion, as was expected (fig. 4B). The dose-response curves of patient 1 and patient 2 closely resemble those of healthy control lines—particularly if the mean of the two sibling lines is considered. By contrast, the dose-response curve of the AT lines indicates G2 phase to growth fraction rates much higher than those in the control subjects. Lines from patients with Nijmegen breakage syndrome (NBS) also showed increased G2 phase proportions, although not to the extent of AT lines. These results do not suggest increased radiosensitivity of the lines from both patients. The sensitivity of the patients' cells towards the bifunctional DNA alkylating clastogen mitomycin C (MMC) was also assayed by flow cytometry. Neither primary lymphocytes from patient 2, after 72 h of culture (fig. 5A), nor lymphoblasts from both patients, after 48 h of culture (fig. 5B), showed increased sensitivity towards this clastogen, compared with samples from healthy control subjects. The cytogenetic analysis of chromosome breaks after treatment of peripheral lymphocytes with 1 μg/ml and 10 μg/ml bleomycin exhibited no difference from healthy control subjects. There was no increase in spontaneous chromosomal aberrations nor in the rate of sister chromatid exchanges (data not shown). We conclude from these studies that the ATM/ATR-mediated G2 phase checkpoint, and other ATM/ATR-independent checkpoints, are functional and that their impairment cannot be the reason for the increased rate of prophase-like cells.
Figure 4.
Normal response of the lymphoblast cell lines from patient 1 (upright triangle) and patient 2 (inverted triangle) to ionizing radiation. A, The G2-phase proportions of these lines, relative to their corresponding growth fractions at 1.5 Gy of irradiation, were 0.21 and 0.22, respectively. These ratios were within the range of 28 normal control lines (mean ± 1 SD = 0.22 ± 0.06; range 0.14–0.33), as opposed to 30 radiosensitive A-T lines (mean ± 1 SD = 0.53 ± 0.07; range 0.40–0.66). All cultures revealed similar growth fractions (noncycling cells: patient 1, 24%; patient 2, 30%; mean ± 1 SD of the healthy control subjects: 37.2% ± 8.7%, range: 20%–54%; of the A-T lines: 32.7 ± 8.9 %, range: 13%–54%). B, The G2 phase proportions, relative to the corresponding growth fractions of the lines of both patients at a broader range of irradiation (0.5–8.0 Gy) resemble the dose-response curve of eight healthy control subjects (blackened circles, mean ± 1 SD) but are distinct from those of five A-T lines (blackened squares, mean ± 1 SD) and of three NBS lines (unblackened circles, mean ± 1 SD).
Figure 5.
Normal response of the cells from patient 1 (upright triangle) and patient 2 (inverted triangle) to MMC. A, The G2-phase proportion of primary lymphocytes of patient 2, relative to their corresponding growth fraction at 10 ng/ml MMC, was 0.30. This ratio was within the limits of 23 normal control lymphocyte cultures (mean ± 1 SD = 0.25 ± 0.05; range 0.18–0.34), as opposed to 35 MMC-sensitive FA cultures (mean ± 1 SD = 0.63 ± 0.06; range 0.47–0.73). All cultures revealed similar growth fractions (percent noncycling cells, patient 2: 40%; mean ± 1 SD of the healthy control subjects 31.8 ± 13.1%; range 13.4%–71.2%; mean ± 1 SD of the FA lymphocytes: 39.7% ± 15.7%; range 13.4%–81.1%). B, The G2-phase proportions of the lymphoblast lines of both patients relative to their corresponding growth fractions at 10 ng/ml MMC were 0.24 and 0.27, respectively. These ratios were within the range of 12 normal control lines (mean ± 1 SD = 0.23 ± 0.04; range 0.18–0.31) as opposed to 5 MMC-sensitive FA lines (mean ± 1 SD = 0.45 ± 0.04; range 0.41–0.51). The lines of both patients revealed similar growth fractions (percent noncycling cells: patient 1, 15.9%; patient 2, 18.1%) as the normal control lines (mean ± 1 SD = 23.0% ± 8.9%; range 4.7%–38.5%).
To investigate whether the premature chromosome condensation has an effect on chromosome segregation at anaphase, we analyzed the malsegregation rate in binucleated lymphocytes of both patients and in four healthy individuals, by FISH. Binucleated lymphocytes were prepared after treatment with the cytokinesis inhibitor cytochalasin for 44 h. The FISH and the criteria of scoring the slides were as described elsewhere (Zijno et al. 1994; Shi et al. 2000). FISH was performed with two centromeric probes from chromosome 8 and chromosome 18 (Vysis: CEP-8, SpectrumOrange; CEP-18, SpectrumGreen). The mean number of binucleated cells showing nondisjunction of chromosome 8 or 18 was 0.556% for both patients (n=1,977; SD ± 0.086) compared to a mean number of 0.584% in four healthy control subjects (n=3,427; SD ± 0.343). The statistical analysis by the χ2 test for homogeneity revealed no significant difference in the rate of malsegregation between the two patients and the control subjects (χ2=0.517; 1 df; P>.05) (table 3). This is in agreement with our observation of normal anaphase configurations of the chromosomes in fibroblast cultures harvested in situ without colcemid treatment (fig. 2e, f).
Table 3.
Malsegregation of Chromosomes 8 and/or 18 in Binucleated Lymphocytes from Both Patients and from Four Control Subjects, Analyzed after Blocking of the Cytokinesis with Cytochalasin and Subsequent FISH with Two Centromeric Probes
| Individual | No. of Cells Scored | No. (%) of CellsShowing Nondisjunctionof Chromosomes 8 or 18 |
| Patient 1 | 1,006 | 5 (.497) |
| Patient 2 | 971 | 6 (.618) |
| Control subject 1 | 596 | 5 (.839) |
| Control subject 2 | 931 | 9 (.966) |
| Control subject 3 | 948 | 3 (.316) |
| Control subject 4 | 952 | 3 (.315) |
| Both patients | 1,977 | 11 (.556 ± .086)a |
| All control subjects | 3,427 | 20 (.584 ± .343)a |
Values in parenthesis are the mean percentage ± SD of cells showing nondisjunction.
Furthermore, we investigated whether the nuclear envelope is retained in the prophase-like cells or whether premature chromosome condensation is paralleled by premature disassembly of the nuclear membrane. Altogether, 50 prophase cells of the fibroblast cell line from patient 1 were analyzed after the chromatin was stained with Hoechst 33258, followed by in situ fixation in 100% methanol and indirect immunofluorescence with a lamin B antibody (Oncogene Research Products). All 50 prophase cells showed an intact nuclear membrane, compared with 100 interphase cells on the same slide (fig. 2g, h). This is in agreement with the data of Georgatos et al. (1997) who demonstrated, also by indirect immunofluorescence with a lamin B antibody, that the disassembly of the nuclear envelope takes place in late prophase/prometaphase in normal mammalian cells. Furthermore, our results indicate that chromosome condensation and the disassembly of the nuclear membrane in late prophase are independent processes.
Phenotypically, both patients have some features (e.g., microcephaly and growth retardation) that are also common in Seckel syndrome and in chromosome instability disorders such as NBS, Fanconi anemia, and Bloom syndrome. However, patients with Seckel syndrome have a different facial aspect, their fetal growth retardation is more severe (Majewski and Goecke 1982), and they do not show the cytological peculiarities observed here. The presence of a chromosome-instability disorder in our patients was excluded by the normal sensitivity of their cells towards radiomimetic and alkylating agents, ionizing radiation, and the normal rate of sister chromatid exchanges. To the best of our knowledge, there is only one phenotype description that resembles some phenotypic and karyological manifestations in our patients; this girl, who presented with microcephaly and moderate mental retardation, was the only daughter of a consanguineous marriage. A high percentage of prophase-like cells was observed, in combination with normal cell-cycle progression (Tommerup et al. 1993). The condensed chromosomes were apparently retained within the nuclear membrane. However, in contrast with our patients, the cells of this patient exhibited a high proportion of endomitoses and endoreduplications, a high spontaneous chromosomal-breakage rate, and an increased sensitivity towards both alkylating agents and X-rays. Furthermore, the structure of the condensed metaphases of this girl was abnormal, showing spontaneous coiling and banding patterns, even in metaphase spreads where the nuclear membrane was absent. This is not the case in our patients, in whom the chromosome morphology at metaphase is normal. Another striking difference from the case reported by Tommerup et al. is the obvious fact that the prophase-like cells did not show labeling when 3H-thymidine was added 3 h prior to harvesting. This indicates that the phenomenon in this girl might be due to prophase delay rather than to premature chromosome condensation. In conclusion, all these significant distinctions substantiate the idea that the cellular phenotype in the case described by Tommerup et al. differs from that in our patients.
Thus, the combination of premature chromosome condensation with microcephaly and mental retardation described here is unique and points to a new autosomal recessive disorder affecting a gene that is involved in the regulation of mitosis. To date, germline mutations resulting in the phenomenon of premature chromosome condensation have not been described, either in man or in other mammals. The term “premature chromosome condensation” was introduced by Johnson and Rao in 1970. They observed that, ⩽30 min after fusion of mitotic with interphase cells, the interphase chromatin condenses into distinct chromosomes. The accompanying morphological events—such as breakdown of the nuclear membrane, depolymerization of nuclear lamins, and formation of the spindle apparatus—generally mimic the normal entry of cells into mitosis. It soon came to light that the underlying “factor” is universal in both the animal and plant kingdoms and also is responsible for the induction of chromosome condensation in meiosis (von der Haar et al. 1981; for review, see Sperling and Rao 1974). The biochemical analysis of this “factor,” in combination with the analysis of fission yeast mutants that prematurely advance into mitosis, led to its identification as an evolutionarily highly conserved protein complex cyclin-B1-Cdk1, also known as MPF.
In contrast to yeast, in vertebrates, cyclin-A-Cdk2 increases throughout G2 phase, peaking in late prophase in the absence of significant cyclin-B1-Cdk1 activity. It has been demonstrated that exogenous cyclin-A-Cdk2 microinjected into G2 HeLa cells rapidly induces chromosome condensation and that inhibitors of cyclin-A-Cdk2 prevent cells from chromosome condensation in early prophase (Furuno et al. 1999). Thus, in mammalian cells, events that prepare G2 cells for mitosis and initiate chromosome condensation in early prophase seem to be mediated not by cyclin-B1-Cdk1 but by cyclin-A-Cdk2. During G2 phase, cyclin-B1-Cdk1 is localized in the cytoplasm and is held in its inactive state by phosphorylation at the residues Thr14 and Tyr15 (Takizawa and Morgan 2000). The activation of cyclin-B1-Cdk1 is mediated by the removal of these phosphate groups through phosphatase Cdc25 and the phosphorylation of Thr161 by the Cdk-activating kinase (CAK). In terminal prophase, activated cyclin-B1-Cdk1 is rapidly translocated to the nucleus, pushing the cell past the “point of no return,” where the cell has lost the ability to inactivate cyclin-B1-Cdk1 and return to G2 phase (Rieder and Cole 1998; Hagting et al. 1999; Karlsson et al. 1999; O’Connell et al. 2000; Goldstone et al. 2001). Since Cdk1 is capable of phosphorylating and thereby activating Cdc25, the activation of Cdk1 depends, in part, on a positive-feedback loop. In addition, Cdc25 is regulated by at least two other phosphatases (PPP1 and PPP2A) and kinases (Chk1 and Cds1). Thus, initiation of mitosis is regulated through complex feedback systems that are far from being understood in detail (Rieder and Cole 1998; Ohi and Gould 1999; Smits and Medema 2001).
In mammals, there is only one description of an in vitro mutation, in the hamster cell line BHK21, that undergoes premature chromosome condensation at a nonpermissive temperature (Kai et al. 1986; Uchida et al. 1990). The underlying tsBN2 mutation is complemented by the human RCC1 gene, which encodes a guanine-nucleotide–exchange factor for the nuclear Ras homologue Ran (Renault et al. 1998). RCC1 binds directly to the histones H2A and H2B and is essential for mitotic spindle assembly and nuclear envelope formation (Nemergut et al. 2001). On the basis of homozygosity mapping with highly polymorphic microsatellite DNA markers flanking RCC1 in 1q36.1, we excluded the possibility that RCC1 is the candidate of the premature chromosome disorder described here. On the basis of our observations in the two affected children, whose cells progress prematurely in chromosome condensation, it is quite unlikely that the underlying mechanism affects cyclin-B1-Cdk1 or regulators of it, since late prophase and entry into metaphase mediated by cyclin-B1-Cdk1 appears to be normal. Nevertheless, we excluded these candidate genes by homozygosity mapping (cyclin B1, at 5q12, and Cdk1, at 10q21.1), as well as the three mammalian Cdc25 genes (Cdc25A, at 3p21; Cdc25B, at 20p13; and Cdc25C, at 5q31).
Much more probable is an underlying defect affecting the regulation of early prophase, presumably the premature activation of the cyclin-A-Cdk2 complex. However, for these loci as well (cyclin A, at 4q27, and Cdk-2, at 12q13), the two patients inherited different parental alleles, indicating that these genes might not be responsible for the observed cellular phenotype. Thus, our data indicate that another gene is mutated in this family. A systematic genome scan with tightly linked microsatellites in combination with functional complementation of candidate genes is in progress and may pave the way to identification of the underlying gene defect.
Acknowledgments
We are indebted to the family for their cooperation. We thank Marianne Plieth, Marlies Schwanke, and Britta Teubner for their excellent technical assistance. The work was supported by the grant NE 531/2-1 from the Deutsche Forschungsgemeinschaft.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
References
- Furuno N, den Elzen N, Pines J (1999) Human cyclin A is required for mitosis until mid prophase. J Cell Biol 147:295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos SD, Pyrpasopoulou A, Theodoropoulos PA (1997) Nuclear envelope breakdown in mammalian cells involves stepwise lamina disassembly and microtubule-drive deformation of the nuclear membrane. J Cell Sci 110:2129–2140 [DOI] [PubMed] [Google Scholar]
- Goldstone S, Pavey S, Forrest A, Sinnamon J, Gabrielli B (2001) Cdc25-dependent activation of cyclin A/cdk2 is blocked in G2 phase arrested cells independently of ATM/ATR. Oncogene 20:921–932 [DOI] [PubMed] [Google Scholar]
- Hagting A, Jackman M, Simpson K, Pines J (1999) Translocation of cyclin B1 to the nucleus at prophase requires a phosphorylation-dependent nuclear import signal. Curr Biol 9:680–689 [DOI] [PubMed] [Google Scholar]
- Johnson RT, Rao PN (1970) Mammalian cell fusion: induction of premature chromosome condensation in interphase nuclei. Nature 226:717–722 [DOI] [PubMed] [Google Scholar]
- Kai R, Ohtsubo M, Sekiguchi T, Nishimoto T (1986) Molecular cloning of a human gene that regulates chromosome condensation and is essential for cell proliferation. Mol Cell Biol 6:2027–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Katich S, Hagting A, Hoffmann I, Pines J (1999) Cdc25B and Cdc25C differ markedly in their properties as initiators of mitosis. J Cell Biol 146:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbies M, Hoehn H, Schindler D, Chen Y, Rabinovitch PS (1989) Cell cycle analysis via BrdU-Hoechst flow cytometry—principles and applications. In: Yen A (ed) Flow cytometry: advanced research and clinical applications, vol 2. CRC Press, Boca Raton, FL, pp 6–28 [Google Scholar]
- Majewski F, Goecke T (1982) Studies of microcephalic primordial dwarfism. I: Approach to a delineation of the Seckel syndrome. Am J Med Genet 12:7–21 [DOI] [PubMed] [Google Scholar]
- Nemergut ME, Mizzen CA, Stukenberg T, Allis CD, Macara IG (2001) Chromatin docking and exchange activity enhancement of RCC1 by histones H2A and H2B. Science 292:1540–1543 [DOI] [PubMed] [Google Scholar]
- O'Connell MJ, Walworth NC, Carr AM (2000) The G2-phase DNA-damage checkpoint. Trends Cell Biol 10:296–303 [DOI] [PubMed] [Google Scholar]
- Ohi R, Gould KL (1999) Regulating the onset of mitosis. Curr Opin Cell Biol 11:267–273 [DOI] [PubMed] [Google Scholar]
- Pines J, Rieder CL (2001) Re-staging mitosis: a contemporary view of mitotic progression. Nat Cell Biol 3:E3–E6 [DOI] [PubMed] [Google Scholar]
- Renault L, Nassar N, Vetter I, Becker J, Klebe C, Roth M, Wittinghofer A (1998) The 1.7 A crystal structure of the regulator of chromosome condensation (RCC1) reveals a seven-bladed propeller. Nature 392:97–101 [DOI] [PubMed] [Google Scholar]
- Rieder CL, Cole RW (1998) Entry into mitosis in vertebrate somatic cells is guarded by a chromosome damage checkpoint that reverses the cell cycle when triggered during early but not late prophase. J Cell Biol 142:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyschab H, Schindler D, Friedl R, Barbi G, Boltshauser E, Fryns JP, Hanefeld F, Korinthenberg R, Krägeloh-Mann I, Scheres JMJC, Schinzel A, Seemanová E, Tommerup N, Hoehn H (1992) Simultaneous measurement, using flow cytometry, of radiosensitivity and defective mitogen response in ataxia telangiectasia and related syndromes. Eur J Pediatr 151:756–760 [DOI] [PubMed] [Google Scholar]
- Seyschab H, Sun Y, Friedl R, Schindler D, Höhn H (1993) G2 phase cell cycle disturbance as a manifestation of genetic cell damage. Hum Genet 92:61–68 [DOI] [PubMed] [Google Scholar]
- Shi Q, Adler ID, Zhang J, Zhang X, Shan X, Martin R (2000) Incidence of mosaic cell lines in vivo and malsegregation of chromosome 21 in lymphocytes in vitro of trisomy 21 patients: detection by fluorescence in situ hybridization on binucleated lymphocytes. Hum Genet 106:29–35 [DOI] [PubMed] [Google Scholar]
- Smits VA, Medema RH (2001) Checking out the G(2)/M transition. Biochim Biophys Acta 1519:1–12 [DOI] [PubMed] [Google Scholar]
- Sperling K, Rao PN (1974) The phenomenon of premature chromosome condensation: its application to basic and applied research. Humangenetik 23:235–258 [DOI] [PubMed] [Google Scholar]
- Takizawa CG, Morgan DO (2000) Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol 12:658–665 [DOI] [PubMed] [Google Scholar]
- Tommerup N, Mortensen E, Morten H, Nielsen H, Wegner R-D, Schindler D, Mikkelsen M (1993) Chromosomal breakage, endomitosis, endoreduplication, and hypersensitivity toward radiomimetric and alkylating agents: a possible new autosomal recessive mutation in a girl with craniosynostosis and microcephaly. Hum Genet 92:339–346 [DOI] [PubMed] [Google Scholar]
- Uchida S, Sekiguchi T, Nishitani H, Miyauchi K, Ohtsubo M, Nishimoto T (1990) Premature chromosome condensation is induced by a point mutation in the hamster RCC1 gene. Mol Cell Biol 10:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Haar B, Sperling K, Gregor D (1981) Maturing Xenopus oocytes induce chromosome condensation in somatic plant nuclei. Exp Cell Res 134:477–481 [DOI] [PubMed] [Google Scholar]
- Zijno A, Marcon F, Leopardi P, Crebelli R (1994) Simultaneous detection of X-chromosome loss and non-disjunction in cytokinesis-blocked human lymphocytes by in situ hybridization with a centromeric DNA probe: implications for the human lymphocyte in vitro micronucleus assay using cytochalasin B. Mutagenesis 9:225–232 [DOI] [PubMed] [Google Scholar]