Abstract
In a previous study (Spanova et al., 2010, J. Biol. Chem., 285, 6127–6133) we demonstrated that squalene, an intermediate of sterol biosynthesis, accumulates in yeast strains bearing a deletion of the HEM1 gene. In such strains, the vast majority of squalene is stored in lipid particles/droplets together with triacylglycerols and steryl esters. In mutants lacking the ability to form lipid particles, however, substantial amounts of squalene accumulate in organelle membranes. In the present study, we investigated the effect of squalene on biophysical properties of lipid particles and biological membranes and compared these results to artificial membranes. Our experiments showed that squalene together with triacylglycerols forms the fluid core of lipid particles surrounded by only a few steryl ester shells which transform into a fluid phase below growth temperature. In the hem1∆ deletion mutant a slight disordering effect on steryl esters was observed indicated by loss of the high temperature transition. Also in biological membranes from the hem1∆ mutant strain the effect of squalene per se is difficult to pinpoint because multiple effects such as levels of sterols and unsaturated fatty acids contribute to physical membrane properties. Fluorescence spectroscopic studies using endoplasmic reticulum, plasma membrane and artificial membranes revealed that it is not the absolute squalene level in membranes but rather the squalene to sterol ratio which mainly affects membrane fluidity/rigidity. In a fluid membrane environment squalene induces rigidity of the membrane, whereas in rigid membranes there is almost no additive effect of squalene. In summary, our results demonstrate that squalene (i) can be well accommodated in yeast lipid particles and organelle membranes without causing deleterious effects; and (ii) although not being a typical membrane lipid may be regarded as a mild modulator of biophysical membrane properties.
Abbreviations: LP, lipid particles; PL, phospholipids; SQ, squalene; SE, steryl ester(s); TG, triacylglycerol(s); QM, quadruple mutant
Keywords: Squalene, Ergosterol, Lipid droplet, Plasma membrane, Fluidity, Yeast
Highlights
► Deletion of HEM1 causes squalene accumulation in yeast. ► Squalene accumulated in lipid droplets has a disordering effect. ► In biological membranes squalene modulates fluidity/rigidity.
1. Introduction
The isoprenoid squalene is an important precursor for the biosynthesis of sterols, steroids and ubiquinones. Due to its beneficial properties as antioxidant [1] and dyslipidemic properties, and its possible use for prevention from cancer and skin disorders [2–4] the biotechnological production of squalene has been studied in several organisms [5–8]. Besides the occurrence of squalene in shark liver oil, olive oil, amaranth oil and some microorganisms the yeast Saccharomyces cerevisiae was shown to accumulate squalene under certain culture conditions or/and by genetic modifications [9–14]. Under standard growth conditions squalene does not accumulate in the yeast cell but is converted to sterols. The ergosterol biosynthetic pathway is strictly aerobic and heme-dependent. Heme is necessary for the activity of the sterol-14-α-demethylase Erg11p, an NADPH-heme-dependent P450 protein [15,16]. If heme cannot be formed, e.g., in hem1∆ strains, squalene and lanosterol accumulate [13]. For studies described here we used strains deleted of HEM1 which encodes the first enzyme in heme synthesis, δ-aminolevulinic acid synthase. Such hem1∆ strains have been widely used as a model for anaerobic growth [17,18] because metabolic changes resulting from the lack of cytochromes were similar to oxygen deficiency. As a consequence of such manipulations, anaerobic yeast and hem1∆ cells become auxotrophic for sterols and unsaturated fatty acids [19–22].
In the yeast as in other cell types lipid storage occurs in lipid droplets/particles. Steryl esters (SE) and triacylglycerols (TG) are the major storage lipids of S. cerevisiae comprising roughly 50% of the lipid particle (LP) mass, each. Previous studies have shown that LP are highly flexible and dynamic organelles [23]. However, little is known about molecular processes involved in LP biogenesis. Investigations in several laboratories including our own favor a budding model [24–26]. This model is based on the hypothesis that SE and TG synthesized between the bilayer of endoplasmic reticulum (ER) form the core of nascent LP. After a certain particle size has been reached, the LP buds off the ER and becomes an independent organelle. Recently, we showed that only one of the SE and TG synthesizing enzymes of the yeast, Dga1p, Lro1p, Are1p or Are2p, is sufficient to form LP [27]. In the course of these studies a new structural model of LP was proposed suggesting that below the temperature of 18 °C a fluid core consisting of TG is surrounded by partially ordered SE shells and covered by a phospholipid [PL] monolayer with a small amount and number of proteins embedded [23,27]. Under standard growth conditions only low levels (0.5% of total mass) of squalene were found in LP [23,28]. In a hem1Δ strain accumulating squalene, approximately 70% of its cellular amount is stored in LP [13]. This result led us to speculate that in such strains the physical properties of LP might have changed. To address this question we used differential scanning calorimetry as a method to identify order–disorder transitions of SE shells in LP [27]. As will be shown in this study, squalene has indeed an effect on the structural organization of LP.
Besides its localization in LP, squalene was also detected in organelle membranes of a hem1Δ strain [13]. This result is in line with previous findings showing squalene accumulation in membranes of yeast cells grown anaerobically [29]. These findings were surprising because squalene is a highly hydrophobic molecule which lacks the polar head group usually expected for membrane bilayer forming components such as phospholipids. It has been proposed that squalene or its saturated form squalane is accommodated in the middle of a lipid bilayer thus altering structure and properties of artificial membranes [30–32].
Our biological model of organelle membranes from a hem1Δ yeast mutant which accumulated squalene enabled us to test the possible impact of squalene on biophysical and biological properties of membranes. These studies were specifically performed with membranes from a hem1Δ mutant in a dga1Δlro1Δare1Δare2Δ (quadruple mutant, QM) background. This QM is devoid of all four acyltransferases, Dga1p, Lro1p, Are1p and Are2p which catalyze the synthesis of TG and SE in the yeast [33]. As a consequence of the quadruple deletion, such strains do not form LP. A QMhem1Δ mutant turned out as a valuable tool to accumulate squalene in subcellular membranes, especially in the endoplasmic reticulum and the plasma membrane. Since the QMhem1Δ mutant lacks LP but accumulates the same amount of squalene as hem1Δ, the squalene concentration in membranes was dramatically increased [13]. Using this experimental system we demonstrated that squalene in combination with ergosterol is an important parameter to modulate the fluidity of ER and plasma membrane. Our studies also took into account the multiple alterations of membrane components in hem1Δ deletion strains which additionally affect membrane properties.
2. Materials and methods
2.1. Strains, culture conditions and subcellular fractionation
Yeast strains used in this study are listed in Table 1. Inactivation of the HEM1 gene was performed by transformation of the hem1::URA3 disruption cassette according to Gueldener et al. [34] using the following primers, HEM1 fw 5′-CTT CTC CAT TCC GTC AGC TGA TAC TCT ATT CGG TTG TGT GTT GCA CAG CTG AAG CTT CGT ACG C-3′ and HEM1rev 5′-ATA CTC ATA CGT TTC TCT CTC TTT ACT TTC TGT ACC CCC GAG GGC ATA GGC CAC TAG TGG ATC TG-3′. The disruption of HEM1 in mutants was verified by standard colony PCR and sequencing.
Table 1.
Yeast strains used in this study.
| Yeast strains | Genotype | Source or Ref. |
|---|---|---|
| Wild type W303 | SUC2 GAL+mal mel ade2-1 can1-100 his3-11,15 leu2-3 112 trp1-1 ura3-1 | ScanBi Ltd., Alnarp, Sweden [33] |
| QM dga1Δ lro1Δ are1Δ are2Δ | W303; MATα dga1::KanMX4 lro1::TRP1are1::HIS3 are2::LEU2 ADE2 met ura3 | ScanBi Ltd., Alnarp, Sweden [33] |
| hem1Δ | W303; MATa ADE2 met hem1::URA3 his3 leu2 trp1 | This study |
| QMhem1Δ | W303; MATα dga1::KanMX4 lro1::TRP1are1::HIS3 are2::LEU2 hem1::URA3 ADE2 met | This study |
Cells were grown aerobically to the early stationary phase at 30 °C in YPD medium containing 1% yeast extract (Oxoid), 2% peptone (Oxoid) and 2% glucose (Merck). Media were inoculated with pre-cultures to the A600 of 0.1. Heme deletion strains were grown on YPD medium supplemented with 20 μg/ml ergosterol (Erg) and 0.06% Tween 80 as a source of oleic acid.
The yeast lipid particle (LP) fraction was obtained at high purity from cells grown to the stationary phase as described by Leber et al. [23]. Isolation of microsomes [ER] and plasma membrane used in this study was described by Zinser and Daum [35]. The quality of subcellular fractions was routinely tested by Western blot analysis [see below].
2.2. Protein analysis
Proteins were quantified by the method of Lowry et al. [36] using bovine serum albumin as a standard. Polypeptides were precipitated with trichloroacetic acid and solubilized in 0.1% sodium dodecyl sulfate (SDS), 0.1 M NaOH prior to quantification. Samples of the LP fraction were delipidated prior to protein quantification. Non-polar lipids were extracted with 2 volumes of diethyl ether, the organic phase was withdrawn, residual diethyl ether was removed under a stream of nitrogen, and proteins were precipitated as described above.
SDS-polyacrylamide gel electrophoresis was carried out by the method of Laemmli [37]. Samples were denatured at 37 °C to avoid hydrolysis of polypeptides. Proteins on gels were detected by staining with Coomassie Blue. Western blot analysis was performed as described by Haid and Suissa [38]. Proteins were detected by enzyme-linked immunosorbent assay (ELISA) using rabbit antiserum as primary antibody and peroxidase-conjugated goat anti-rabbit IgG as secondary antibody. Primary antibodies used in this study were from rabbits and directed against yeast Ayr1p, Por1p, Wbp1p and Gas1p. The enhanced chemiluminescent signal detection kit SuperSignal™ (Pierce Chemical Company, Rockford, IL, USA) was used to visualize immunoreactive bands which were then quantified densitometrically.
2.3. Lipid analysis
Lipids from yeast cells were extracted as described by Folch et al. [39]. Total phospholipids were quantified from lipid extracts by the method of Broekhuyse [40]. For quantification of neutral lipids, lipid extracts were applied to Silica Gel 60 plates, and chromatograms were developed in an ascending manner by a two-step developing system [41]. First, chromatograms were developed using light petroleum:diethyl ether:acetic acid (70:30:2; per vol.), and then light petroleum:diethyl ether (49:1; v/v) as solvents. To visualize separated bands, TLC plates were dipped into a charring solution consisting of 0.63 g MnCl2 × 4H2O, 60 ml water, 60 ml methanol and 4 ml concentrated sulfuric acid, briefly dried and heated at 100 °C for 20 min. Then, lipids were quantified by densitometric scanning at 400–650 nm with squalene, triolein and cholesteryl ester as standards using a Shimadzu dual-wave length chromatoscanner CS-930.
Squalene and individual sterols (free sterols and SE) from whole cells or subcellular fractions were identified and quantified by gas liquid chromatography–mass spectrometry (GLC–MS) [42]. GLC–MS was performed on an HP 5890 Gas-Chromatograph equipped with a mass selective detector HP 5972, using an HP5-MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness). Aliquots of 1 μl were injected in the splitless mode at 270 °C injection temperature with helium as carrier gas at a flow rate of 0.9 ml/min in constant flow mode. The following temperature program was used: 1 min at 100 °C, 10 °C/min to 250 °C, and 3 °C/min to 310 °C. Mass spectra were acquired in the scan mode (scan range 200–550 amu) with 3.27 scans per second. Sterols were identified based on their mass fragmentation pattern.
Fatty acids were analyzed by GLC. Lipid extracts prepared as described above were incubated with hot 2.5% sulfuric acid in methanol. After incubation, water was added, and the converted methyl esters were extracted with light petroleum. Fatty acid methyl esters were separated using a Hewlett-Packard 6890-Gas-Chromatograph equipped with a HP-INNO Wax capillary column (15 m × 0.25 mm i.d. × 0.50 μm film thicknesses) and helium as carrier gas. Fatty acids were identified by comparison to commercial fatty acid methyl ester standards (NuCheck, Inc., Elysian, MN, USA).
2.4. Preparation of phospholipid vesicles
Lipid extracts prepared as described above were taken to dryness and dispersed in 10 mM Tris/Cl, pH 7.4. Vesicles were prepared by shaking the suspension for 24 h at 30 °C. For the preparation of squalene containing vesicles, squalene was added to lipid extracts, and vesicles were prepared as described above.
2.5. Fluorescence anisotropy
Isolated microsomes and plasma membrane were resuspended in 10 mM Tris/Cl, pH 7.4, at the same phospholipid concentration. After addition of an organic diphenylhexatriene (DPH) solution at a molar ratio of 1:50 probe to phospholipid, mixtures were incubated for 5 min at 30 °C. Samples were kept in the dark as long as possible. Then, fluorescence measurements were carried out using a Shimadzu RF 540 spectrofluorimeter equipped with polarizers in the excitation and emission light path. Excitation and emission wavelengths for DPH were 350 and 452 nm, respectively (slit width 10 nm). Fluorescence intensities were corrected for background fluorescence and light scattering from the unlabelled sample. The fluorescence anisotropy r was calculated according to the equation
I║ and I┴ are measured emission intensities parallel and perpendicular to the vertical polarization plane of the excitation light [43].
2.6. Differential scanning calorimetry (DSC)
Differential scanning calorimetry experiments were performed as described previously using a VP-DSC instrument from MicroCal, Inc. Northampton, MA, USA. Samples of LP prepared from wild type and mutant strains were used undiluted. A buffer containing 0.25 M sorbitol, 10 mM MES/Tris and 0.2 mM Na2EDTA (pH 6.9) was used to fill the reference cell. Heating scans were performed at a scan rate of 15 °C/h between 5 °C and 65 °C. Pre-scan thermostating at 1 °C was allowed for 60 min. Enthalpies were calculated by integrating the peak area after baseline adjustment and normalization to SE concentrations using the MicroCal Origin software (VP-DSC version).
3. Results
3.1. Squalene influences the structure of yeast lipid particles
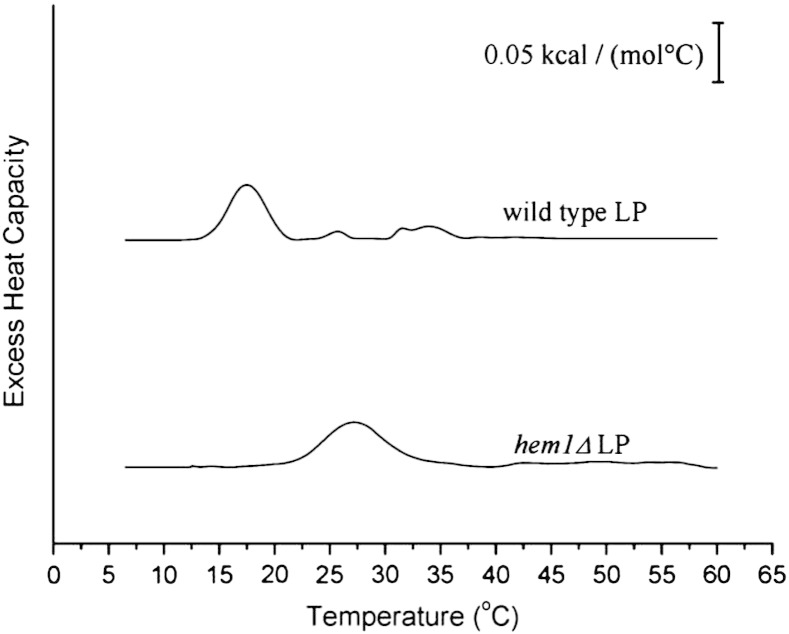
In a recent study we proposed a new model of LP structure based on results obtained by differential scanning calorimetry (DSC) and small angle X-ray scattering [27]. It was shown that below the phase transition temperature SE of LP form shell like layers which surround a core of TG. In another recent investigation we showed that squalene can become a prominent LP component when accumulated in a hem1Δ yeast strain [13]. We speculated that the presence of squalene in LP may affect the order and the structural organization of the droplet. To test this hypothesis we performed differential scanning calorimetry with LP variants isolated from a hem1Δ mutant and compared the data to LP from wild type. Thermograms of these measurements are displayed in Fig. 1, and the thermodynamic parameters are shown in Table 2. LP from wild type exhibited two distinct endothermic transitions, one at 17.5 °C and one at 33.9 °C (Fig. 1, top). This finding is in agreement with our previous measurements [27]. These transitions were attributed to the melting of acyl chains from SE, since no such signals were obtained in a negative control, i.e. in a LP variant unable to produce SE [27].
Fig. 1.
Differential scanning calorimetry of lipid particles. Thermograms showing the second heating scans of LP preparations from wild type W303 (top) and from a hem1Δ mutant (bottom). The thermograms are displaced on the y-axis by arbitrary units for the sake of clarity. The excess heat capacity was normalized to mole SE of the respective samples. Scan rate was 15 °C/h. Analyzed data are listed in Table 2.
Table 2.
Thermodynamic parameters of lipid particles from S. cerevisiae wild type W303 and from mutant hem1Δ. Tm1 and Tm2: temperature transition of steryl esters (SE); ΔHcal1 and ΔHcal2: calorimetric enthalpy of SE transitions; enthalpy is given per mole SE. n.d., not detectable.
|
Tm1 °C |
ΔHcal1 kcal/mol |
Tm2 °C |
ΔHcal2 kcal/mol |
|
|---|---|---|---|---|
| W303 | 17.5 | 0.37 | 33.9 | 0.1 |
| hem1Δ | 27.2 | 0.35 | n.d. | n.d. |
In LP isolated from hem1Δ only one phase transition at 27.2 °C was detected (Fig. 1, bottom; Table 2). This observation looks striking at a first glance and has to be interpreted with caution. As can be seen from Table 3, differences in the composition of wild type and hem1Δ LP are manifold. Compared to wild type, the amount of squalene was dramatically increased in LP from hem1Δ, mostly at the expense of SE. In the mutant, only one tenth of the wild type SE is present suggesting that less SE layers will be formed in this strain. Considering that the endothermic transition is derived only from SE, the unchanged enthalpy must be attributed to still highly ordered SE layers in hem1Δ. This is feasible as the degree of fatty acid saturation in SE and even more dramatically in TG was much higher in the mutant than in wild type thus contributing to a higher thermal stability of the rigid SE layers. In addition, it can be assumed that TG is less intercalated into the rigid SE layers perturbing their order, but rather mixes with the highly flexible squalene molecules forming the fluid core of the LP. Although the exact nature of the phase transition, e.g. smectic-like to fluid, cannot be deduced from the calorimetric experiment, the data suggest that the internal structure of the mutant differs from wild type insofar as fewer though more ordered SE shells are formed which are, however, already in a fluid phase below the growth temperature.
Table 3.
Lipid composition of wild type and hem1Δ lipid particles. Lipid particles were isolated and analyzed as described under Materials and methods. SQ, squalene; SE, steryl esters; SFA, saturated fatty acids; UFA, unsaturated fatty acids.
| % of total mass in LP | UFA/SFA | ||
|---|---|---|---|
| W303 | SQ | 1.28 ± 0.37 | |
| SE | 63.24 ± 3.66 | 4.28 ± 1.35 | |
| TG | 35.48 ± 3.29 | 9.26 ± 4.47 | |
| hem1Δ | SQ | 23.81 ± 2.78 | |
| SE | 6.80 ± 1.92 | 2.22 ± 0.16 | |
| TG | 69.41 ± 0.86 | 0.76 ± 0.48 |
3.2. Squalene affects yeast organelle membranes
Our previous studies had shown that in the dga1Δlro1Δare1Δare2Δhem1Δ (QMhem1Δ) mutant which is unable to produce TG and SE and does not contain LP, squalene formed at large amounts due to the deletion of HEM1 is stored in the ER and plasma membrane at substantial concentrations [13]. When we performed growth tests with wild type, hem1Δ, QM and QMhem1Δ we realized that strains accumulating squalene exhibited growth defects at pH 4.0, pH 8.0, in the presence of 0.7 M NaCl and in the presence of 2% DMSO (Fig. 2). We took these results as a hint that membranes of hem1Δ strains, especially the plasma membrane may suffer from the presence of squalene. It has to be noted, however, that the observed defects may also be the result of ergosterol depletion in these strains. Nevertheless, these observations led us to study a possible effect of squalene in microsomes and in plasma membrane preparations from the respective strains in some more detail. For this purpose, microsomes and plasma membrane from the respective strains and from the corresponding controls were isolated. Western blot analysis using markers for plasma membrane (Gas1p), microsomes (Wbp1p), lipid particles (Ayr1p) and mitochondria (Por1p) revealed that all preparations were obtained at high quality and purity (Table 4) in agreement with previously published results [35].
Fig. 2.

Stress sensitivity of hem1Δ mutants. Cells were grown on YPD plates supplemented with ergosterol and unsaturated fatty acids either at pH 4.0, in the presence of 0.7 M NaCl, or in the presence of 2% dimethylsulfoxide for 72 h. On plates, serial dilutions (steps of 1:10) of cultures are shown starting with a sample of OD600 = 1. Growth at pH 8.0 was monitored after 1 week. YPD Erg + UFA, YPD medium with ergosterol and unsaturated fatty acids; DMSO, dimethylsulfoxide.
Table 4.
Quality control of yeast subcellular fractions. Subcellular fractions were isolated as described under Materials and methods. The relative enrichment of markers in the homogenate was set as 1. Marker proteins: Gas1p, β-1,3-glucanosyltransferase (marker for plasma membrane); Wbp1p, β-subunit of the oligosaccharyl transferase glycoprotein complex (marker for microsomes); Por1p, porin (marker for mitochondria); Ayr1p, acyldihydroxyacetone phosphate acyltransferase (marker for lipid particles). Fractions of mitochondria were used as positive control for marker Por1p. n.d., not detectable; –, not measured.
| Relative enrichment (fold) |
|||||
|---|---|---|---|---|---|
| Microsomes 30,000 g | Plasma membrane | Mitochondria | Lipid particles | ||
| Wild type | Gas1p | n.d. | ~ 100 | – | n.d. |
| Wbp1p | 2.3 ± 0.3 | 0.02 ± 0.03 | – | n.d. | |
| Por1p | 0.35 ± 0.11 | 0.22 ± 0.09 | 2.9 ± 0.2 | n.d. | |
| Ayr1p | n.d. | n.d. | – | ~ 100 | |
| hem1Δ | Gas1p | n.d. | ~ 100 | – | n.d. |
| Wbp1p | 5.4 ± 0.9 | 0.2 ± 0.2 | – | n.d. | |
| Por1p | 0.1 ± 0.07 | 1.3 ± 0.2 | 2.9 ± 0.2 | n.d. | |
| Ayr1p | n.d. | n.d. | – | ~ 100 | |
| QM | Gas1p | n.d. | ~ 100 | – | |
| Wbp1p | 3.5 ± 0.1 | 1.8 ± 0.2 | – | ||
| Por1p | n.d. | 1.1 ± 0.2 | 2.9 ± 0.2 | ||
| QMhem1Δ | Gas1p | n.d. | ~ 100 | – | |
| Wbp1p | 1.7 ± 0.2 | 0.32 ± 0.19 | – | ||
| Por1p | n.d. | 0.15 ± 0.03 | 2.9 ± 0.2 | ||
To test membrane fluidity/rigidity of biological membranes we employed steady-state fluorescent anisotropy. The physical state of membrane samples was tested by using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH), a non-polar compound inserting into the hydrophobic region of the bilayer [44]. Based on previous results it was assumed that squalene may be inserted in the bilayer [30,32].
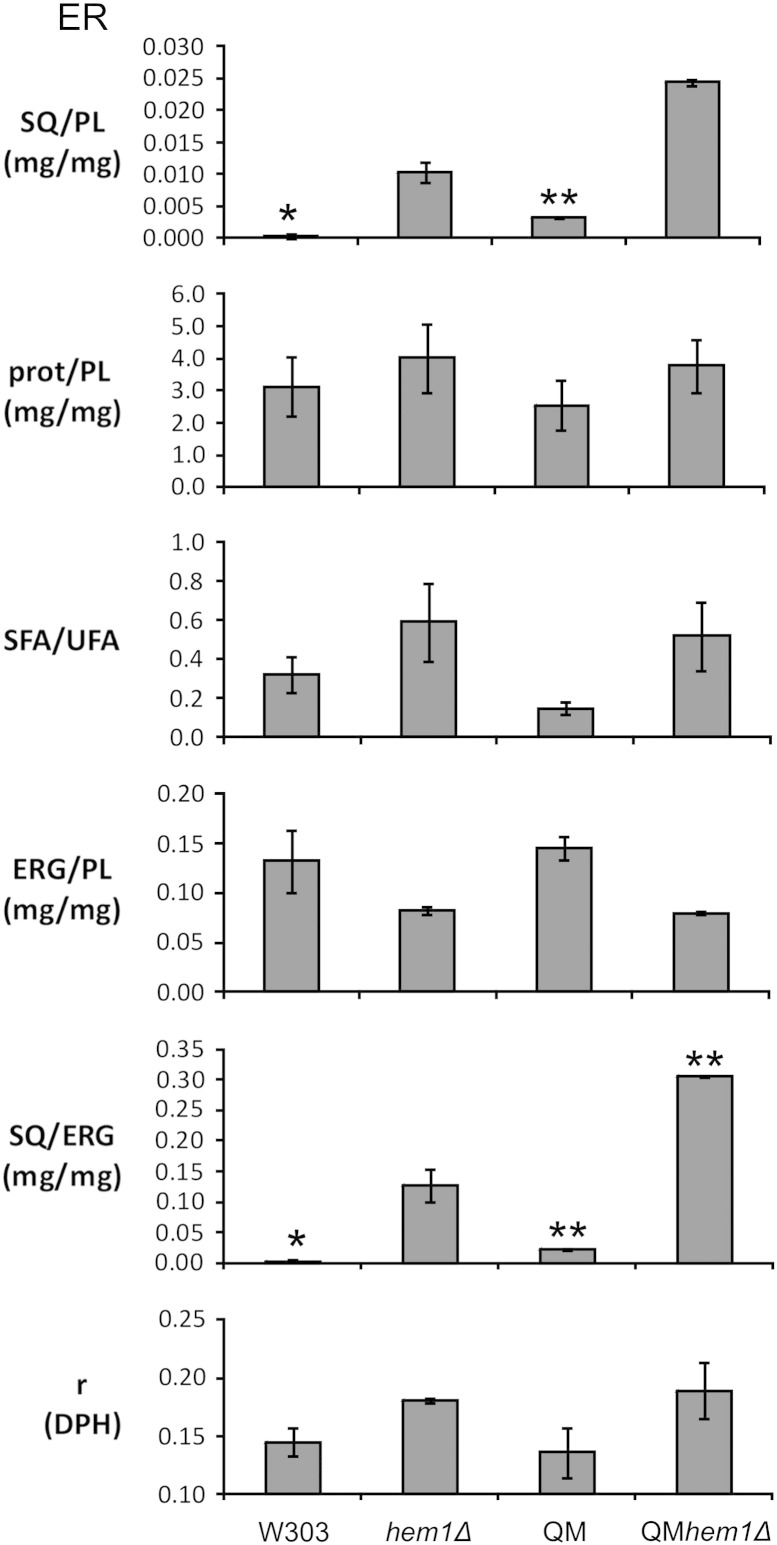
In contrast to artificial membranes consisting of a defined multi-component system, data obtained with biological membranes are more difficult to explain and need to be interpreted with caution. The fluidity of a membrane depends on various components such as the amount of sterols, the degree of fatty acid saturation and the concentration of polypeptides present in a lipid bilayer. Since yeast ER and plasma membrane samples used for anisotropy measurements were derived from four different strains, namely wild type, hem1Δ, QM and QMhem1Δ, we analyzed the respective samples for the above mentioned parameters (Figs. 3 and 4). Numerical values of these measurements are shown in Table 5. These data were included in the interpretation of anisotropy measurements.
Fig. 3.
Analysis of isolated microsomal membranes. Cells were cultivated to the stationary phase with/without supplements, and microsomes were isolated as described by Zinser and Daum [35]. Proteins were estimated by the method of Lowry et al. [36], total phospholipids were quantified by the method of Broekhuyse [40], and sterols were quantified by GLC–MS from aliquots containing a defined amount of protein. Anisotropy was measured as described in Materials and methods. Standard deviation indicated by one asterisk (*) is < 0.0005, and by two asterisks (**) < 0.0025. SQ, squalene; PL, phospholipids; prot, proteins; SFA, saturated fatty acids; UFA, unsaturated fatty acids; ERG, ergosterol; DPH, diphenylhexatriene; r, anisotropy.
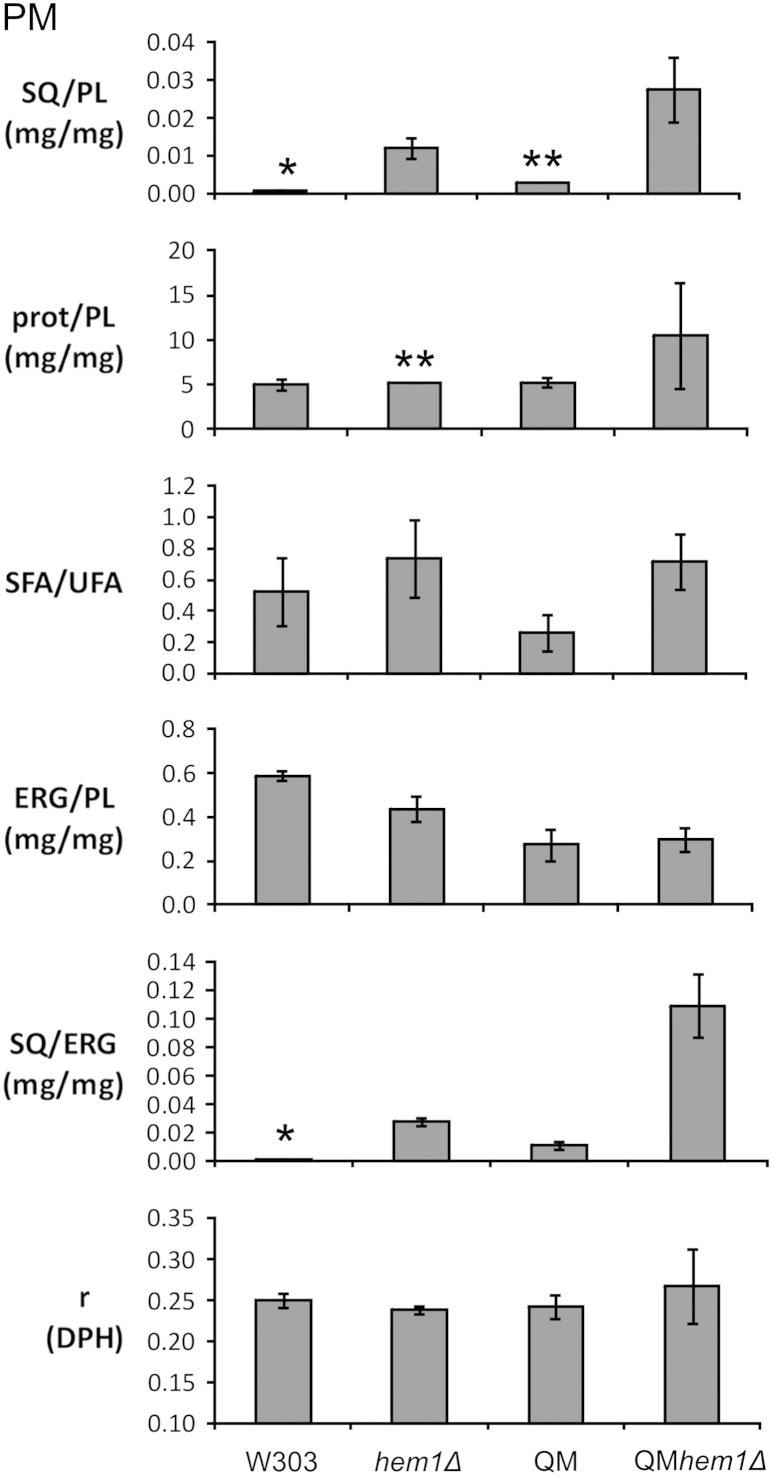
Fig. 4.
Analysis of isolated plasma membranes. Cells were cultivated to stationary phase with/without supplements, and plasma membrane was isolated as described by Zinser and Daum [35]. For abbreviations see legend to Fig. 3.
Table 5.
Analysis of isolated membranes. For the abbreviations see legend to Fig. 3.
| mg/g organelle protein |
% |
SFA/UFA | SQ/PL | ERG/PL | SQ/ERG | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SQ | ERG | Total PL | UFA | SFA | ||||||
| ER | W303 | 0.128 ± 0.118 | 50.27 ± 5.34 | 336.66 ± 90.79 | 75.84 ± 5.10 | 24.16 ± 5.10 | 0.32 ± 0.09 | 0.0003 ± 0.0004 | 0.132 ± 0.031 | 0.0024 ± 0.0021 |
| hem1Δ | 3.052 ± 0.864 | 23.87 ± 2.00 | 259.32 ± 64.12 | 63.93 ± 10.23 | 36.07 ± 10.23 | 0.59 ± 0.27 | 0.0103 ± 0.0016 | 0.082 ± 0.004 | 0.127 ± 0.026 | |
| QM | 1.222 ± 0.280 | 54.34 ± 14.29 | 410.16 ± 122.30 | 86.85 ± 2.07 | 13.15 ± 2.07 | 0.15 ± 0.03 | 0.0032 ± 0.0001 | 0.145 ± 0.012 | 0.023 ± 0.001 | |
| QMhem1Δ | 6.643 ± 1.570 | 21.81 ± 5.26 | 271.60 ± 59.26 | 66.14 ± 7.60 | 33.86 ± 7.60 | 0.52 ± 0.18 | 0.0244 ± 0.0005 | 0.080 ± 0.002 | 0.305 ± 0.002 | |
| PM | W303 | 0.198 ± 0.027 | 119.16 ± 10.36 | 202.45 ± 24.14 | 66.34 ± 9.44 | 33.66 ± 9.44 | 0.52 ± 0.22 | 0.0010 ± 0.00001 | 0.590 ± 0.019 | 0.0017 ± 0.0001 |
| hem1Δ | 2.389 ± 0.537 | 84.76 ± 11.11 | 194.14 ± 0.21 | 58.53 ± 10.30 | 41.47 ± 10.30 | 0.73 ± 0.31 | 0.0123 ± 0.0028 | 0.437 ± 0.057 | 0.028 ± 0.003 | |
| QM | 0.561 ± 0.047 | 52.12 ± 8.68 | 191.90 ± 19.21 | 79.74 ± 7.38 | 20.26 ± 7.38 | 0.26 ± 0.12 | 0.0029 ± 0.0001 | 0.275 ± 0.073 | 0.011 ± 0.003 | |
| QMhem1Δ | 3.169 ± 0.511 | 34.070 ± 3.446 | 113.19 ± 63.82 | 58.85 ± 6.44 | 42.08 ± 5.12 | 0.72 ± 0.18 | 0.028 ± 0.008 | 0.301 ± 0.054 | 0.110 ± 0.022 | |
In microsomes (ER), the anisotropy measured with DPH was significantly higher in samples derived from hem1Δ and QMhem1Δ mutants than in the corresponding control strains (Fig. 3). These values indicated that mutant membranes were more rigid than wild type. As mentioned above, this result represents an overall effect which is caused by various parameters. As can be seen from Fig. 3, the ratio of ergosterol to phospholipids decreased in hem1Δ and QMhem1Δ, but the degree of fatty acid saturation, the protein to phospholipid ratio and the squalene to phospholipid ratio were increased. It is noteworthy that ergosterol and unsaturated fatty acids were derived from external supplementation. Whereas the decrease of the ergosterol content in microsomes from hem1Δ would increase membrane fluidity, the enhanced amount of proteins and saturated fatty acids present in the membrane causes the opposite effect. Thus, the level of ergosterol as a possible reason for higher rigidity in hem1Δ microsomes was excluded. However, the question as to the effects of proteins and saturated fatty acids remained. To clarify this point we performed anisotropy measurements with artificial membrane vesicles consisting of lipids extracted from microsomes of wild type, hem1Δ, QM and QMhem1Δ (Table 6). To these samples, squalene was added at an amount corresponding to the squalene concentration in hem1Δ samples, i.e. ~ 0.01 mg/mg PL in wild type samples (wt + SQ) and ~ 0.02 mg/mg PL in QM samples. The anisotropy measured with DPH increased significantly after addition of squalene to lipid extracts from wild type membranes as can be seen from Table 6. No additional effect on the anisotropy was observed in samples from hem1Δ where the initial amount of squalene was high. We assumed that squalene present in this extract led to a defined level of rigidity which was not further increased. The situation was different in the QM. The anisotropy value was already as high as in hem1Δ and addition of squalene to both QM and QMhem1Δ had no further effect on the anisotropy. These results indicate that incorporation of squalene increased rigidity of microsomal membranes which are rather fluid. If membranes were rigid already, e.g. by the presence of squalene in hem1Δ or by other effects in QM, further attenuation was not observed.
Table 6.
Anisotropy of artificial membrane vesicles from lipid extracts of isolated membranes. Organelles were isolated as described in Figs. 2 and 3. Vesicles from lipid extracts with or without addition of squalene were formed, and anisotropy was measured as described in Materials and methods. + SQ, amount of squalene added as found in hem1Δ; DPH, diphenylhexatriene.
| Organelle | Strain | Additive | Concentration of SQ (mg/mg PL) | Anisotropy (DPH) |
|---|---|---|---|---|
| Microsomes | W303 | – | 0.0003 | 0.108 ± 0.005 |
| + SQ | 0.0103 | 0.124 ± 0.018 | ||
| hem1Δ | – | 0.0103 | 0.145 ± 0.005 | |
| + SQ | 0.0206 | 0.148 ± 0.009 | ||
| QM | – | 0.0032 | 0.146 ± 0.003 | |
| + SQ | 0.0244 | 0.147 ± 0.005 | ||
| QMhem1Δ | – | 0.0244 | 0.130 ± 0.006 | |
| + SQ | 0.0488 | 0.128 ± 0.032 | ||
| Plasma membrane | W303 | – | 0.0010 | 0.289 ± 0.005 |
| + SQ | 0.0123 | 0.264 ± 0.006 | ||
| hem1Δ | – | 0.0123 | 0.253 ± 0.055 | |
| + SQ | 0.0246 | 0.250 ± 0.025 | ||
| QM | – | 0.0029 | 0.160 ± 0.006 | |
| + SQ | 0.0967 | 0.193 ± 0.009 | ||
| QMhem1Δ | – | 0.0967 | 0.222 ± 0.012 | |
| + SQ | 0.1934 | 0.222 ± 0.001 |
Anisotropy measurements with plasma membrane revealed that initial anisotropy values in wild type were already higher than in microsomes (see Fig. 4). This effect can be attributed to the high ergosterol to phospholipid ratio and the large amount of saturated fatty acids present in the plasma membrane. In plasma membrane preparations from hem1Δ and QMhem1Δ, respectively, the anisotropy was not significantly increased over controls. Again, the specific effect of squalene in these samples was difficult to explain, and experiments with lipid extracts from different samples were performed. In lipid vesicles derived from wild type and hem1Δ plasma membrane addition of squalene did not increase the anisotropy (see Table 6). It has to be noted that in these samples the anisotropy was already initially high due to effects described above. Thus, the squalene effect is less pronounced compared to the more dominant effects of other membrane components. In vesicles from QM and QMhem1Δ plasma membrane, the anisotropy was significantly lower than in wild type (see Table 6). This effect was attributed to the markedly decreased ergosterol to phospholipid ratio (see Fig. 4) in QM and QMhem1Δ. In QM samples, the addition of squalene increased anisotropy reaching the value of QMhem1. In line with our previous observations, the excess of squalene in vesicles from QMhem1Δ plasma membrane did not exhibit any additional change in rigidity. This result indicates that in rigid membranes squalene had rather a softening than a rigidifying effect.
In summary, squalene appears to increase rigidity in membranes which are rather fluid such as microsomes (see Fig. 3). In membranes with a high level of basal rigidity the influence of squalene is minor (see Fig. 4). These results suggest that the ratio of squalene to sterol may contribute to the regulation of membrane fluidity/rigidity.
4. Discussion
Storage of non-polar lipids in all types of cells including yeast occurs in a compartment named lipid particle (LP) or lipid droplet [45]. In previous days, LP were regarded as an inert subcellular structure. This view has changed insofar as we know by now that certain enzymes of LP may play an important metabolic role [46]. More recently, we were also able to demonstrate an internal structure of LP [27]. These studies were extended by investigations presented here. The structure of LP from S. cerevisiae wild type cells is rather simple. A core consisting of TG is surrounded by SE shells and the surface is covered by a phospholipid monolayer with a small amount of proteins embedded. In previous studies with the yeast S. cerevisiae [13], we also showed that squalene accumulated in hem1Δ strains and localized mainly to LP. Squalene is highly hydrophobic and expected to mix with other non-polar lipids of the LP such as TG and SE. The influence of squalene on the order and arrangement of LP was seen with samples from hem1Δ where only one phase transition was detected in contrast to wild type. The transition was characterized by a similar enthalpy suggesting that the fewer SE layers present in this droplet exhibit the same order as wild type, most likely due to the highly increased degree of fatty acid saturation. Consequently, the majority of squalene has to be localized to the center of the droplet forming the fluid core together with TG. It has to be considered that in contrast to wild type, SE layers of hem1Δ LP melt below the growth temperature and thereby may confer better access to stored sterols, which are rare in this strain and thus important for cell viability.
Our previous studies [13] also had shown that due to genetic manipulations squalene can be accommodated at substantial amount in yeast subcellular membranes. We hypothesized that the presence of squalene in membranes, especially in the plasma membrane, may affect growth of the yeast and/or cause sensitivity to external stress. Hauss et al. [31] had advocated such a model using squalane in artificial membranes. These authors showed, however, that proton flux was affected by the presence of squalane in the membrane bilayer. Growth studies presented here (see Fig. 2) showed that hem1Δ mutants become sensitive to low and high pH, high salt concentration and to dimethylsulfoxide. In biological membranes, however, this effect cannot be attributed to a single component, and alteration of the membrane composition from different mutants had to be included in the interpretation.
Also anisotropy measured with ER and plasma membrane samples is a result of overall effects caused by different components. To pinpoint the squalene influence in detail, we performed in parallel studies with artificial membranes. It appears that in soft membranes squalene has some stabilizing effect making the membranes more rigid. These observations are in line with experiments using model system membranes [30–32]. In such model membranes, squalene is most likely not localized axially in the membrane, but in the interior of the bilayer.
Interestingly, anisotropy effects observed with microsomal membranes and plasma membrane, and with lipid extracts from both samples differed. In soft microsomal membranes, squalene had a clear stabilizing effect, whereas in the more rigid plasma membrane the influence of squalene was less pronounced. We conclude that the ratio of ergosterol to squalene is crucial for a possible stabilizing effect of squalene. In contrast to our observation, no effect of the squalene in artificial membranes was observed by Simon et al. [32] when they studied interaction of squalene with liposomes and monolayers of dipalmitoyl phosphatidylcholine. These authors used large amounts of squalene (10%–50% mol) and thus studied most likely monolayer covered droplets instead of bilayers with a thick layer of squalene in between. In our experiments, we observed minor changes, since we used only squalene concentrations up to 0.4 mol%. In agreement with our experiments, Lohner et al. [30] showed that squalene can be incorporated up to a concentration of about 6 mol% in pure phospholipid vesicles altering the lamellar-to-inverse-hexagonal phase transition with increasing the size of inverse hexagonal tubes. These authors argued that squalene must be located in a most disordered region of the bilayer, evoking that squalene rather exists in a coiled than extended conformation and localizes to the interior of the bilayer. Our experiments using biological and model membranes extend this model insofar as squalene in the ER may rather adapt to a conformation close to ergosterol, whereas in the plasma membrane squalene might prefer the coiled conformation. In any case, the membrane modulating role of squalene is a novel facet of biophysical properties of this lipid.
Acknowledgements
This work was financially supported by the Fonds zur Förderung der wissenschaftlichen Forschung in Österreich (projects 18857 and 23029 to G.D.). The authors wish to thank Sten Stymne for providing yeast strains.
References
- 1.Kohno Y., Egawa Y., Itoh S., Nagaoka S., Takahashi M., Mukai K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Biophys. Acta. 1995;1256 doi: 10.1016/0005-2760(95)00005-w. 1256, 52–56. [DOI] [PubMed] [Google Scholar]
- 2.Desai K.N., Wei H., Lamartiniere C.A. The preventive and therapeutic potential of the squalene-containing compound, Roidex, on tumor promotion and regression. Cancer Lett. 1996;101:93–96. doi: 10.1016/0304-3835(96)04122-5. [DOI] [PubMed] [Google Scholar]
- 3.Ikekawa T., Umeji M., Manabe T., Yanoma S., Irinoda K., Mizunuma H., Ikekawa N. Studies on antitumor activity of squalene and its related compounds. Yakugaku Zasshi. 1986;106:578–582. doi: 10.1248/yakushi1947.106.7_578. [DOI] [PubMed] [Google Scholar]
- 4.Smith T.J. Squalene: potential chemopreventive agent. Expert Opin. Investig. Drugs. 2000;9:1841–1848. doi: 10.1517/13543784.9.8.1841. [DOI] [PubMed] [Google Scholar]
- 5.Catchpole O.J., von Kamp J.C., Grey J.B. Extraction of squalene from shark liver oil in a packed column using supercritical carbon dioxide. Ind. Eng. Chem. Res. 1997;36:4318–4324. [Google Scholar]
- 6.Bondioli P., Mariani C., Lanzani A., Fedeli E., Muller A. Squalene recovery from olive oil deodorizer distillates. J. Am. Oil Chem. Soc. 1993;70:763–766. [Google Scholar]
- 7.Bhattacharjee P., Singhal R.S. Extraction of squalene from yeast by supercritical carbon dioxide. World J. Microbiol. Biotechnol. 2003;19:605–608. [Google Scholar]
- 8.Chen G.Q., Fan K.W., Lu F.P., Li Q.A., Aki T., Chen F., Jiang Y. Optimization of nitrogen source for enhanced production of squalene from thraustochytrid Aurantiochytrium sp. N. Biotechnol. 2010;27:382–389. doi: 10.1016/j.nbt.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Polakowski T., Stahl U., Lang C. Overexpression of a cytosolic hydroxymethylglutaryl-CoA reductase leads to squalene accumulation in yeast. Appl. Microbiol. Biotechnol. 1998;49:66–71. doi: 10.1007/s002530051138. [DOI] [PubMed] [Google Scholar]
- 10.Donald K.A., Hampton R.Y., Fritz I.B. Effects of overproduction of the catalytic domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase on squalene synthesis in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1997;63:3341–3344. doi: 10.1128/aem.63.9.3341-3344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahnke L., Klein H.P. Oxygen requirements for formation and activity of the squalene epoxidase in Saccharomyces cerevisiae. J. Bacteriol. 1983;155:488–492. doi: 10.1128/jb.155.2.488-492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorenz R.T., Casey W.M., Parks L.W. Structural discrimination in the sparking function of sterols in the yeast Saccharomyces cerevisiae. J. Bacteriol. 1989;171:6169–6173. doi: 10.1128/jb.171.11.6169-6173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spanova M., Czabany T., Zellnig G., Leitner E., Hapala I., Daum G. Effect of lipid particle biogenesis on the subcellular distribution of squalene in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2010;285:6127–6133. doi: 10.1074/jbc.M109.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantzouridou F., Tsimidou M.Z. Observations on squalene accumulation in Saccharomyces cerevisiae due to the manipulation of HMG2 and ERG6. FEMS Yeast Res. 2010;10:699–707. doi: 10.1111/j.1567-1364.2010.00645.x. [DOI] [PubMed] [Google Scholar]
- 15.Kalb V.F., Loper J.C., Dey C.R., Woods C.W., Sutter T.R. Isolation of a cytochrome P-450 structural gene from Saccharomyces cerevisiae. Gene. 1986;45:237–245. doi: 10.1016/0378-1119(86)90021-1. [DOI] [PubMed] [Google Scholar]
- 16.Bard M., Lees N.D., Turi T., Craft D., Cofrin L., Barbuch R., Koegel C., Loper J.C. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids. 1993;28:963–967. doi: 10.1007/BF02537115. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira T., Regnacq M., Alimardani P., Moreau-Vauzelle C., Berges T. Lipid dynamics in yeast under haem-induced unsaturated fatty acid and/or sterol depletion. Biochem. J. 2004;378:899–908. doi: 10.1042/BJ20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiner S., Micolod D., Zellnig G., Schneiter R. A genomewide screen reveals a role of mitochondria in anaerobic uptake of sterols in yeast. Mol. Biol. Cell. 2006;17:90–103. doi: 10.1091/mbc.E05-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreasen A.A., Stier T.J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J. Cell. Physiol. 1954;43:271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- 20.Gollub E.G., Liu K.P., Dayan J., Adlersberg M., Sprinson D.B. Yeast mutants deficient in heme biosynthesis and a heme mutant additionally blocked in cyclization of 2,3-oxidosqualene. J. Biol. Chem. 1977;252:2846–2854. [PubMed] [Google Scholar]
- 21.Bard M., Woods R.A., Haslam J.M. Porphyrine mutants of Saccharomyces cerevisiae: correlated lesions in sterol and fatty acid biosynthesis. Biochem. Biophys. Res. Commun. 1974;56:324–330. doi: 10.1016/0006-291x(74)90845-6. [DOI] [PubMed] [Google Scholar]
- 22.Andreasen A.A., Stier T.J. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Physiol. 1953;41:23–36. doi: 10.1002/jcp.1030410103. [DOI] [PubMed] [Google Scholar]
- 23.Leber R., Zinser E., Zellnig G., Paltauf F., Daum G. Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast. 1994;10:1421–1428. doi: 10.1002/yea.320101105. [DOI] [PubMed] [Google Scholar]
- 24.Czabany T., Athenstaedt K., Daum G. Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta. 2007;1771:299–309. doi: 10.1016/j.bbalip.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Rajakumari S., Grillitsch K., Daum G. Synthesis and turnover of non-polar lipids in yeast. Prog. Lipid Res. 2008;47:157–171. doi: 10.1016/j.plipres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Walther T.C., Farese R.V., Jr. The life of lipid droplets. Biochim. Biophys. Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., Ingolic E., Daum G. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:17065–17074. doi: 10.1074/jbc.M800401200. [DOI] [PubMed] [Google Scholar]
- 28.Milla P., Athenstaedt K., Viola F., Oliaro-Bosso S., Kohlwein S.D., Daum G., Balliano G. Yeast oxidosqualene cyclase (Erg7p) is a major component of lipid particles. J. Biol. Chem. 2002;277:2406–2412. doi: 10.1074/jbc.M104195200. [DOI] [PubMed] [Google Scholar]
- 29.Blagovic B., Rupcic J., Mesaric M., Maric V. Lipid analysis of the plasma membrane and mitochondria of brewer's yeast. Folia Microbiol. (Praha) 2005;50:24–30. doi: 10.1007/BF02931290. [DOI] [PubMed] [Google Scholar]
- 30.Lohner K., Degovics G., Laggner P., Gnamusch E., Paltauf F. Squalene promotes the formation of non-bilayer structures in phospholipid model membranes. Biochim. Biophys. Acta. 1993;1152:69–77. doi: 10.1016/0005-2736(93)90232-o. [DOI] [PubMed] [Google Scholar]
- 31.Hauss T., Dante S., Dencher N.A., Haines T.H. Squalane is in the midplane of the lipid bilayer: implications for its function as a proton permeability barrier. Biochim. Biophys. Acta. 2002;1556:149–154. doi: 10.1016/s0005-2728(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 32.Simon S.A., Lis L.J., MacDonald R.C., Kauffman J.W. The noneffect of a large linear hydrocarbon, squalene, on the phosphatidylcholine packing structure. Biophys. J. 1977;19:83–90. doi: 10.1016/S0006-3495(77)85570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandager L., Gustavsson M.H., Ståhl U., Dahlqvist A., Wiberg E., Banas A., Lenman M., Ronne H., Stymne S. Storage lipid synthesis is non-essential in yeast. J. Biol. Chem. 2002;277:6478–6482. doi: 10.1074/jbc.M109109200. [DOI] [PubMed] [Google Scholar]
- 34.Gueldener U., Heinisch J., Koehler G.J., Voss D., Hegemann J.H. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res. 2002;30:e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinser E., Daum G. Isolation and biochemical characterization of organelles from the yeast, Saccharomyces cerevisiae. Yeast. 1995;11:493–536. doi: 10.1002/yea.320110602. [DOI] [PubMed] [Google Scholar]
- 36.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 37.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Haid A., Suissa M. Immunochemical identification of membrane proteins after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Methods Enzymol. 1983;96:192–205. doi: 10.1016/s0076-6879(83)96017-2. [DOI] [PubMed] [Google Scholar]
- 39.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 40.Broekhuyse R.M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta. 1968;152:307–315. doi: 10.1016/0005-2760(68)90038-6. [DOI] [PubMed] [Google Scholar]
- 41.Schneiter R., Daum G. Analysis of yeast lipids. Methods Mol. Biol. 2006;313:75–84. doi: 10.1385/1-59259-958-3:075. [DOI] [PubMed] [Google Scholar]
- 42.Quail M.A., Kelly S.L. The extraction and analysis of sterols from yeast. Methods Mol. Biol. 1996;53:123–131. doi: 10.1385/0-89603-319-8:123. [DOI] [PubMed] [Google Scholar]
- 43.Lakowicz J.R. Plenum Press; New York: 1983. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 44.Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J. Biol. Chem. 1974;249:2652–2657. [PubMed] [Google Scholar]
- 45.Zweytick D., Athenstaedt K., Daum G. Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta. 2000;1469:101–120. doi: 10.1016/s0005-2736(00)00294-7. [DOI] [PubMed] [Google Scholar]
- 46.Athenstaedt K., Zweytick D., Jandrositz A., Kohlwein S.D., Daum G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1999;181:6441–6448. doi: 10.1128/jb.181.20.6441-6448.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]