Summary
Background
The emergence and spread of high levels of HIV-1 drug resistance in resource-limited settings where combination antiretroviral treatment has been scaled up could compromise the effectiveness of national HIV treatment programmes. We aimed to estimate changes in the prevalence of HIV-1 drug resistance in treatment-naive individuals with HIV since initiation of rollout in resource-limited settings.
Methods
We did a systematic search for studies and conference abstracts published between January, 2001, and July, 2011, and included additional data from the WHO HIV drug resistance surveillance programme. We assessed the prevalence of drug-resistance mutations in untreated individuals with respect to time since rollout in a series of random-effects meta-regression models.
Findings
Study-level data were available for 26 102 patients from sub-Saharan Africa, Asia, and Latin America. We recorded no difference between chronic and recent infection on the prevalence of one or more drug-resistance mutations for any region. East Africa had the highest estimated rate of increase at 29% per year (95% CI 15 to 45; p=0·0001) since rollout, with an estimated prevalence of HIV-1 drug resistance at 8 years after rollout of 7·4% (4·3 to 12·7). We recorded an annual increase of 14% (0% to 29%; p=0·054) in southern Africa and a non-significant increase of 3% (–0·9 to 16; p=0·618) in west and central Africa. There was no change in resistance over time in Latin America, and because of much country-level heterogeneity the meta-regression analysis was not appropriate for Asia. With respect to class of antiretroviral, there were substantial increases in resistance to non-nucleoside reverse transcriptase inhibitors (NNRTI) in east Africa (36% per year [21 to 52]; p<0·0001) and southern Africa (23% per year [7 to 42]; p=0·0049). No increase was noted for the other drug classes in any region.
Interpretation
Our findings suggest a significant increase in prevalence of drug resistance over time since antiretroviral rollout in regions of sub-Saharan Africa; this rise is driven by NNRTI resistance in studies from east and southern Africa. The findings are of concern and draw attention to the need for enhanced surveillance and drug-resistance prevention efforts by national HIV treatment programmes. Nevertheless, estimated levels, although increasing, are not unexpected in view of the large expansion of antiretroviral treatment coverage seen in low-income and middle-income countries—no changes in antiretroviral treatment guidelines are warranted at the moment.
Funding
Bill & Melinda Gates Foundation and the European Community's Seventh Framework Programme
Introduction
In response to the global HIV epidemic, a WHO-recommended public health approach to antiretroviral therapy (ART) has been widely implemented in resource-limited countries.1,2 At the end of 2011, more than 8 million people were receiving antiretroviral therapy in low-income and middle-income countries—which was 26 times higher than the number from December, 2003.3 Successful ART rollout in resource-limited settings has used standard treatment protocols, simplified monitoring of patients, and decentralised service delivery. First-line regimens were based on non-nucleoside reverse transcriptase inhibitors (NNRTI), with protease inhibitors reserved for second-line treatment.
Although ART rollout in resource-limited settings has used highly active triple combination therapy, national health systems in such areas often have limited infrastructure, a shortage of health professionals, inconsistent supply chains, and weak enforcement of quality standards. Although HIV/AIDS-related mortality in sub-Saharan Africa has substantially fallen since the widespread distribution of ART,4 data suggest that up to 24% of patients receiving first-line ART in sub-Saharan Africa have virological failure within 12 months of initiation of first-line ART.5 Between 53% and 90% of these patients have viruses with clinically important HIV-1 drug resistance to NNRTIs and nucleoside reverse transcriptase inhibitors (NRTIs).6–9 Thus, there is concern about onward transmission of drug-resistant strains after ART scale-up. Moreover, pretreatment drug resistance, which can be transmitted or acquired through prophylactic or other ART exposure, has the potential to contribute to the increasing rates of virological failure at a population level, thus compromising long term effectiveness of recommended first-line regimens. Large studies have reported increases in the chance of virological failure of two-to-three times within 12 months of initiation of ART in populations in which resistance to components of standard first-line treatment is detected before the start of ART.10
In view of the serious public health implications associated with the emergence and transmission of drug-resistant HIV in resource-limited settings, WHO developed a global strategy to assess population-level HIV drug resistance in defined geographical areas of countries.11 As part of this strategy, standardised surveillance is done to assess resistance in recently12 and chronically infected13 populations initiating ART.
Since ART rollout in resource-limited settings, trends in prevalence and patterns of resistance in untreated patients with HIV have not been systematically assessed at a global level. We have undertaken a comprehensive assessment of available data. We aimed to estimate changes since initiation of rollout in the prevalence of HIV drug resistance in untreated HIV-infected populations worldwide, and to investigate the frequencies of drug-resistance mutations of public health importance.
Methods
Identification of studies
We searched for studies written in English from PubMed and Embase, and conference abstracts and presentations from Conference on Retroviruses and Opportunistic Infections, International AIDS Society Conference, and the International Drug Resistance Workshop for the 10-year period between Jan 01, 2001, and July 31, 2011. We used the following search terms: “antiretroviral therapy” AND “transmitted drug resistance”; “antiretroviral therapy” AND “(stavudine OR zidovudine OR nevirapine OR efavirenz)”; “HIV” AND “transmitted drug resistance”; “HIV” AND “antenatal”; “HIV” AND “VCT”; “genotyp*” AND “HIV” AND “naïve”; “genotyp*” AND “HIV” AND “resistance”; and “genotyp*” AND “HIV” AND “resistance” AND “primary”. We included studies in untreated adults (aged >15 years) recently or chronically infected from low-income and middle-income countries in western Pacific region, southeast Asia, sub-Saharan Africa, and Latin America and the Caribbean. We included studies if they reported at least ten genotypes in untreated patients with HIV-1 using standard population sequencing. We included studies of the prevention of mother-to-child transmission when they provided data for drug resistance before antiretroviral drug exposure in pregnant women. We excluded studies if they used non-population genotyping methods such as single-genome sequencing, allele-specific PCR, and ultra-deep sequencing. We also included data from national surveys that used WHO-recommended methods to assess resistance in both recently infected individuals and chronically infected patients initiating ART.
Data abstraction
Two reviewers (RKG, BJS) independently assessed studies for eligibility. We extracted the following data: country; year of sample collection; sex; study type; risk groups; setting (rural, peri-urban, urban); HIV-1 subtypes occurring at a prevalence of greater than 10%; timing of infection and methods (if any) used to distinguish recent versus chronic infection; baseline CD4 cell count; number of baseline genotypes; number of patients with more than one drug-resistance mutation, one or more NRTI mutation, one or more thymidine analogue mutation, one or more NNRTI mutation, and one or more protease inhibitor mutation. Most studies done after 2007 reported drug-resistance mutations in recently infected individuals according to the WHO Surveillance Drug Resistance Mutations List.14 Studies of resistance in chronically and recently infected people done before 2007 reported drug resistance mutations according to other lists such as the International AIDS Society–USA Drug Mutation list, the Stanford Drug Resistance Database list, or, in a few cases, the ANRS (French National Agency for AIDS Research) list. We classified as being recently infected individuals included in the WHO surveys of transmitted drug resistance, and individuals identified as being recently infected through serial antibody testing or a detuned antibody algorithm (table 1).12 WHO transmitted drug resistance survey methods used epidemiological or laboratory criteria, or both, to maximise the likelihood that participants will have been infected with HIV within the past 3 years and limit the likelihood of previous exposure to antiretroviral drugs. Criteria are: age younger than 25 years and, if female, no previous pregnancy; when available, documented laboratory evidence of recent infection or seroconversion or CD4 count higher than 500 cells per mm3. When studies did not follow the WHO-recommended method, definitions of recent infection were however relatively consistent with WHO criteria. Many studies assessing resistance in chronically infected individuals included patients about to initiate antiretroviral therapy. Studies were initially grouped according to WHO geographical classifications as follows: Africa, southeast Asia, western Pacific, and the Americas. Because we included studies from only resource-limited settings, we used the term Latin America and the Caribbean instead of the Americas. For the analysis presented, western Pacific and southeast Asian countries were grouped as Asia (figure 1). Individual-level data were not available for the studies and we therefore analysed study-level data only.
Table 1.
Characteristics of included studies, by region
| Number of patients (number of studies) | Median number of genotypes per study (range) | Median sampling year (range) | Studies in recently infected populations (n [%]) | Genotypes from recently infected populations (n [%]) | Number of studies using defined epidemiological criteria for recent infections (% of studies in recently infected people) | Studies in populations about to start antiretroviral treatment (% of studies in chronically infected people) | |
|---|---|---|---|---|---|---|---|
| East Africa | 4300 (33) | 78 (11–570) | 2005 (1993–2010) | 18 (55%) | 1107 (26%) | 14 (78%) | 4 (25%) |
| Southern Africa | 6251 (47) | 71 (21–570) | 2006 (1998–2009) | 22 (46%) | 2026 (32%) | 21 (95%) | 5 (20%) |
| West and central Africa | 3211 (40) | 79 (18–271) | 2005 (1998–2009) | 14 (35%) | 1090 (34) | 13 (93%) | 3 (12%) |
| Asia | 5635 (50) | 61 (11–676) | 2006 (1999–2010) | 24 (48%) | 2276 (40%) | 16 (67%) | 2 (8%) |
| Latin America and the Caribbean | 6705 (48) | 60 (16–1655) | 2004 (1995–2009) | 12 (25%) | 1346 (21%) | 2 (17%) | 0 |
Figure 1.
Countries contributing data by regions and subregions
Statistical analysis
We did an exploratory analysis and determined pooled proportions of the number of individuals with drug-resistant mutations (r) in those successfully genotyped (n). To assess heterogeneity in proportions between studies and across geographical locations, the variances of the raw proportions (p = r / n) were stabilised with the following Freeman-Tukey-type arcsine square root transformation:15,16
We assessed heterogeneity between studies by pooling studies using DerSimonian-Laird weighting and assessing the I2 statistic of the meta-analysis of the transformed proportions.
Because this preliminary pooled analysis showed much heterogeneity within and between continents, we divided Africa into regions (west and central, southern, and east) and aimed to further explore whether the following covariates were potential causes: duration of infection (recent vs chronic), risk group (ie, men who have sex with men, heterosexual contact, intravenous drug users, female sex workers); differences in genotyping methods and methods used to interpret the effect of mutations on drug resistance, and population-based measures of ART availability. We defined two population-based metrics of ART availability: time since rollout and ART coverage. The appendix provides the rollout dates by country. ART coverage in a particular country was defined as the number of people recieving ART in a given year divided by the WHO estimate of people with HIV (diagnosed or undiagnosed) in the same year in that country. Heterogeneity in study level mutation estimates resulting from location (urban vs rural) could not be explored because of the small number of studies undertaken exclusively in rural settings. Furthermore, we did not explore heterogeneity by the proportion of women in each study because of its strong relation with risk group, as well as the fact that a quarter of studies did not report sex ratios. Although a range of genotyping methods was used across studies, published data suggest that commercially available genotyping methods and in-house reference laboratory methods give similar results.17,18 Furthermore, laboratories contributing data to WHO surveys have undergone a standardised external quality assurance programme.19 Finally, because there are subtle differences between mutation lists used to identify mutations in the studies included (eg, some lists call a change at a specific position a mutation whereas others do not) a possibility exists that bias was introduced, although, as shown previously,20 this bias is likely to be negligible. Accordingly, only duration of infection, time since rollout, and ART coverage were assessed further.
To assess these potential causes of heterogeneity, we used logistic regression models with a random effect at study level to allow for between-study heterogeneity previously shown to perform well for meta-analyses of binomial data with few events.21 In studies with no mutations, the proportion was estimated as: 1÷(4×n). Meta-regression models indicated that duration of infection was not related to the proportion of drug-resistant mutations (p=0·92). Because the prevalence of drug-resistance mutations was not different between chronic or recently infected populations, we considered these groups together in subsequent analyses. Analyses involving metrics of ART availability indicated that associations between time since ART rollout and prevalence of drug-resistance mutations varied across geographical regions (ie, there was a significant interaction between region and time since rollout; p<0·0004). Therefore, we analysed associations between ART availability metrics and prevalence of mutation separately for each geographical region. In Asia, there was an interaction at the country-level (India, China, Vietnam, Thailand; p<0·0001); there were too few studies to do a country-level meta-regression. Therefore, to describe drug resistance at the country level we derived pooled estimates using the Freeman-Tukey-type arcsine square root transformation—we used the DerSimonian-Laird random effects method to pool the transformed proportions. The outcome of the meta-regression model was the proportional change in resistance from one year to the next, reported as an odds ratio (the odds ratio is very similar to the proportional percentage change at low prevalence; therefore, the odds ratio was reported as percentage change per year for ease of interpretation).
We also did the above analyses to estimate the prevalence of drug-class-specific mutations (thymidine analogue mutations, NNRTI, and protease inhibitor mutations) by region and in respect to its variation with ART availability (time since rollout and ART coverage).
Prevalence of thymidine analogue, NNRTI, and protease inhibitor mutations, as well as prevalence of selected individual mutations as a proportion of all people with drug resistance were pooled with random-effects meta-analysis, with the Tukey transformation and DerSimonian-Laird weightings, as previously described. We used Stata (version 11.2) and SAS (version 9.1.3) for all statistical analyses.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MRJ had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We analysed 191 datasets from 162 individual reports spanning 42 countries (figure 2), and an additional 27 datasets from the WHO HIV drug resistance surveillance programme. Overall, study level data were available for 26 102 patients—there were similar numbers of studies reporting drug resistance data for recently and chronically infected individuals in all regions except Latin America and the Caribbean and west and central Africa, where only about 30% of studies were reported to be in recently infected individuals (table 1). 14 (7% of 191) studies recruited chronically infected individuals attending ART clinics initiating patients on first-line treatment—the remaining studies reported data derived from voluntary counselling and testing facilities, hospitals, blood donation centres, or pathology laboratories. Of the studies examining recent infection, 33 studies (37% of 90) studies recruited from antenatal clinic sites and 47 studies (52% of 90) recruited from voluntary counselling and testing sites—the remaining studies reported data derived from hospitals, blood donation centres, or pathology laboratories. Our analysis showed no difference between chronic and recent infection in the prevalence of one or more drug-resistance mutation for any region. The vast majority of studies were in individuals with heterosexual risk or in mixed populations (only four [2%] studies were done in men who have sex with men and, with two [1%] done in intravenous drug users). Similarly, the vast majority of studies were done in urban, peri-urban, or a mixture of urban and rural populations (only seven [4%] studies were undertaken in purely rural populations). Because data for ART coverage were available from only 2003 onwards, 53 studies (28% of all eligible studies) were not included in this analysis and therefore results are not directly comparable with the primary analysis based on time since rollout. Drug resistance data for both measures of ART availability are summarised in the appendix.
Figure 2.
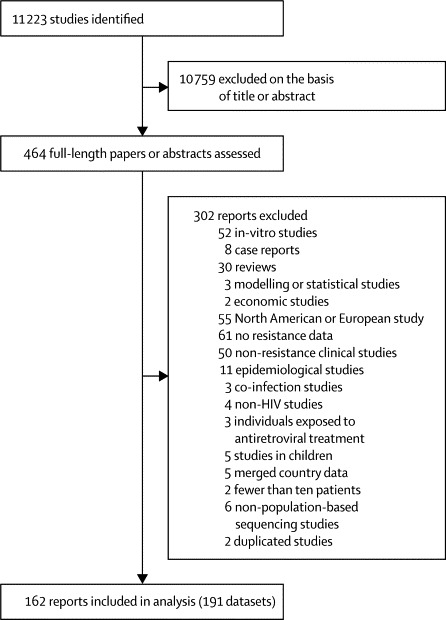
Study selection
East Africa had the highest average modelled rate of increase in prevalence of any drug-resistance mutations at 29% per year (95% CI 15 to 45; p=0·0001) since rollout, with a strong association between prevalence of any drug-resistance mutation and ART coverage (p=0·0013) (figure 3 and appendix). Our modelled estimates suggest that the prevalence of any drug resistance in east Africa at rollout was 1·0% (0·6 to 1·9), and 7·4% (4·3 to 12·7) 8 years later. The estimated annual rate of increase was 14% (0 to 29; p=0·054) in southern Africa and 3% (–9 to 16; p=0·618) in west and central Africa (figure 3), with estimated prevalences of resistance at rollout of 1·4% (0·8 to 2·3) and 3·1% (1·7 to 5·6), respectively. ART coverage did not seem to be associated with the prevalence of drug resistance in southern (p=0·88) or west and central Africa (p=0·55).
Figure 3.
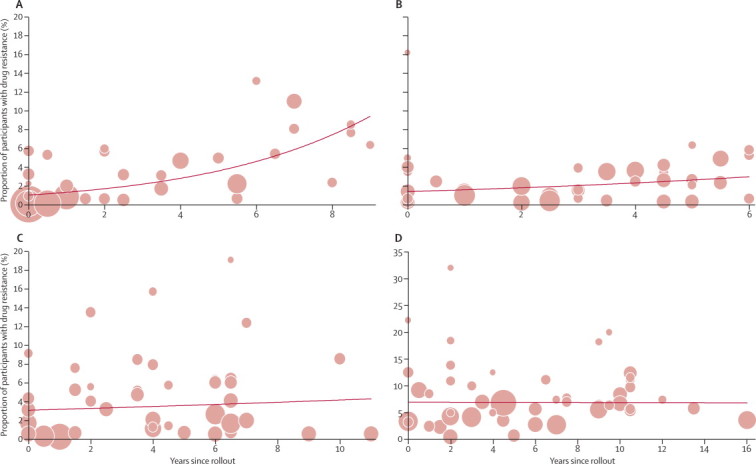
Prevalence of drug resistance in treatment-naive trial participants with HIV-1, by time since antiretroviral rollout
(A) East Africa. (B) Southern Africa. (C) West and central Africa. (D) Latin America and the Caribbean. Every circle is a study and the size of the circle is proportional to the precision of the estimate from the individual study, with sizes comparable within individual graphs only. The trend line is predicted prevalence.
We did a sensitivity analysis of changes in drug resistance prevalence over time. We calculated inverse variance weighted prevalence of any drug-resistance mutation for each region in blocks of 2 years after rollout. This sensitivity analysis showed increasing drug resistance since rollout in east Africa and southern Africa (p=0·0006 for both), but not in west and central Africa (p=0·43) or in Latin America (p=0·50; table 2).
Table 2.
Sensitivity analysis showing proportion of individuals with one or more drug-resistance mutation, by region and years after rollout of antiretroviral treatment (ART)
| 0–2 years after ART rollout | 3–4 years after ART rollout | 5–7 years after ART rollout | 8–9 years after ART rollout | p value | |
|---|---|---|---|---|---|
| East Africa | 0·9 (0·5–1·6) | 3·5 (2·1–5·7) | 5·1 (2·6–9·9) | 7·4 (4·2–12·9) | 0·0006 |
| Southern Africa | 2·1 (1·6–2·6) | 2·3 (1·6–3·3) | 3·7 (2·5–5·4) | .. | 0·0006 |
| West and central Africa | 1·8 (1·1–3·1) | 5·7 (4·0–8·0) | 3·5 (2·5–5·0) | .. | 0·43 |
| Latin America and the Caribbean | 5·9 (4·1–8·4) | 6·5 (4·7–9·0) | 3·9 (2·7–5·8) | 7·6 (4·8–12·2) | 0·50 |
Data are % of population with with one more mutation as defined by the WHO surveillance drug resistance mutations list (95% CI). ··=no studies done 8–9 years after rollout.
Because of country-level heterogeneity in Asia, regional analysis was inappropriate. Furthermore, there were too few studies from Asian countries to do formal country-level meta-regression analyses. Of Asian countries, most studies were from Thailand, China, India, and Vietnam (table 3). Thailand had the lowest overall prevalence of any drug resistance. Studies done in Vietnam reported the highest prevalence of drug-resistance mutations. There were also pronounced differences in the prevalence of resistance at or before the start of rollout between countries (appendix).
Table 3.
Characteristics of studies from four Asian countries
| Number of study groups | Median sampling year (range) | Mean time since scale-up (years [range]) | Weighted prevalence of drug-resistance mutations (95% CI) | |
|---|---|---|---|---|
| Thailand | 12 | 2005 (2000–09) | 4·3 (0·5–9·0) | 0·5% (0·1–1·4) |
| China | 15 | 2006 (2001–10) | 4·0 (0–7·5) | 2·6% (1·4–4·1) |
| India | 10 | 2007 (1999–10) | 2·7 (0–6·0) | 2·7% (1·1–4·7) |
| Vietnam | 9 | 2008 (2006–09) | 2·5 (1·0–4·0) | 4·5% (3·3–6·0) |
Data are given for only those countries with five or more studies. Not included in this table are Indonesia (n=1) and Cambodia (n=3).
For Latin America and the Caribbean, there was no effect of time since rollout of ART on prevalence of drug resistance (p=0·960) nor was there an association between ART coverage and prevalence of drug resistance in the 25 studies done from 2003 onwards that had coverage data available (p=0·052; figure 3 and appendix). The estimated prevalence of any drug-resistance mutation in Latin America and the Caribbean 8 years after rollout was 6·9% (5·6 to 8·4), with the prevalence of drug-resistance mutations at the start of rollout being 6·9% (5·0 to 9·5; figure 3).
With respect to class of antiretroviral drugs, in east Africa, there was a substantial increase in NNRTI resistance over time since rollout—36% per year (21 to 52; p<0·0001; figure 4). Southern Africa showed an increase of 23% per year (7 to 42%; p=0·0049); west and central Africa had a non-significant increase of 15% per year (–1 to 32; p=0·0646; appendix), and the estimated prevalence of NNRTI resistance was 5·1% (3·1–8·2) 8 years after rollout in east Africa. The prevalence of thymidine analogue mutations, which can reduce the effectiveness of second-line NRTI drugs, seemed to increase with time since rollout in east Africa (31% per year [4–66]; p=0·021), but no significant changes were noted in other regions (appendix).
Figure 4.
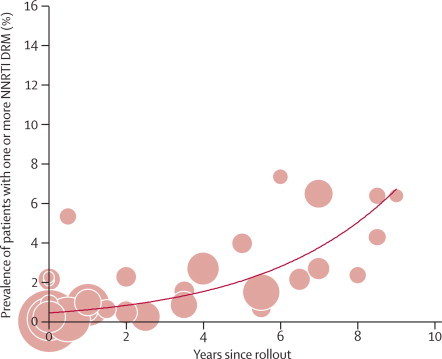
Prevalence of major drug-resistance mutations that confer resistance to non-nucleoside reverse transcriptase inhibitors, by time since antiretroviral rollout in east Africa
Size of circle is proportional to the precision of the estimate from the individual study, with sizes comparable within individual graphs only. The trend line is predicted prevalence. DRM=drug-resistance mutation. NNRTI=non-nucleoside reverse transcriptase inhibitor.
In view of the increasing prevalence of HIV drug resistance in parts of sub-Saharan Africa, the identification of signature mutations for surveillance purposes might be useful in the future. NNRTI mutations were the most commonly seen mutations, occurring in 40–60% of patients with one or more drug-resistance mutation, and showed some variation by region (appendix). For NNRTI resistance, the weighted prevalence of the K103N/S mutation was about 60% and 25% for Y181C/I/V in individuals with any DRM. The weighted prevalence of M184V/I, which confers high-level lamivudine resistance, was 8% (appendix).
Discussion
Our findings suggest a significant increase in prevalence of drug resistance over time since antiretroviral rollout in regions of sub-Saharan Africa; this rise is driven by NNRTI resistance in studies from east and southern Africa. In east Africa, resistance increased at almost 30% per year. Despite the steep increase in east Africa, we do not recommend extrapolation of this trend beyond the period covered by the data. Our estimate for increase in drug resistance in southern Africa was 14% per year, and no increase over time was noted in west and central Africa. We saw no change over time in Latin America and the Caribbean. Because of heterogeneity between countries in Asia, we were unable to assess time trends in this region and there were too few data at country level to do formal meta-regression analysis. The increasing prevalence of resistance is not unexpected in view of the large expansion of ART coverage seen in low-income and middle-income countries.
The most important transmitted drug-resistance mutations in the context of ART rollout are single amino acid mutations conferring high-level resistance to NNRTIs, in view of the fact that NNRTIs are the foundation of available first-line ART and prevention of mother-to-child transmission regimens. These mutations have been associated with treatment failure in cases in which they exist before the initiation of first-line treatment.10,22,23 We estimated a 36% increase in prevalence of NNRTI mutations per year in east Africa and 23% per year in southern Africa. Our findings show that more than half of HIV-infected African patients with NNRTI resistance harbour viruses with the K103N mutation, and, therefore, that detection of this sentinel mutation might be useful in expanded surveillance activities for estimation of the prevalence of NNRTI resistance. Studies from east Africa that reported high prevalence of NNRTI resistance in recently infected adults excluded women with previous pregnancy,24,25 or enrolled patients infected within the past year,26 discounting the possibility that the NNRTI resistance is a consequence of the use of single-dose nevirapine in mothers for the prevention of transmission to their child.
Several possible explanations exist for the regional differences in our modelled time trends. The scale-up of ART has occurred at variable rates, and with variable country coverage,27 both of which will determine transmission of resistant viruses. ART coverage itself is associated with the proportion of individuals with HIV who have been diagnosed, which might also vary between regions of sub-Saharan Africa. ART programme functioning (as assessed by indicators such as patient adherence, drug supply, drug regimens used, and treatment success) could contribute to the differences seen between regions. For example, the achievement of high levels of ART coverage in the context of suboptimum programme functioning might result in the greatest increases in HIV drug resistance over time. Finally, the availability of ART was inconsistent outside the public sector already in the pre rollout era in most countries, and this variability is shown by the detection of some level of resistance close to or at the start of rollout (figure 3). West and central Africa had a higher prevalence of drug resistance at the time of rollout than did other parts of sub-Saharan Africa (figure 3) and this uneven distribution might affect the dynamics of drug resistance over time.
We used country-level ART coverage data as an alternative measure of ART availability. Although data were available for only 72% of studies (those done before 2003 did not have coverage data), this analysis showed a strong relation for increase in HIV drug resistance in east Africa with coverage (appendix).
Data included in this analysis are unlikely to represent national prevalence of HIV drug resistance because most studies were done in urban areas where ART coverage (and pre rollout ART use) is likely to be substantially higher than are national averages. However, over time, levels of HIV drug resistance in regions within a country can be expected to be more evenly distributed because of greater penetration of ART into rural areas and because cross-transmission between rural and urban populations is likely to occur.
Hamers and colleagues28 reported the results of a multicentre cohort study of 2590 patients done in South Africa, Zambia, Zimbabwe, Uganda, and Kenya. Their pooled-analysis of data from areas surveyed in these countries showed a correlation between the time since start of ART rollout and the prevalence of at least one drug-resistance mutation and NNRTI resistance. Findings from small observational studies have suggested an increase in the prevalence of HIV drug resistance over time in Kampala, Uganda,24 and Yaounde, Cameroon.29 Added to the findings of these studies, the data reported here provide region-specific quantification of changes in prevalence of drug resistance after the rollout of ART.
The consequences of increasing HIV drug resistance in patients who are starting ART are substantial, and include: increased treatment failure rates with greater morbidity and mortality, increased need for more expensive second-line regimens, and costs associated with further transmission of more highly resistant and potentially untreatable viruses.
A crucial outcome of this analysis is that countries in resource-limited settings should routinely do population-level resistance surveillance as part of national treatment programmes. Although a full cost-effectiveness analysis of strategies to limit HIV drug resistance is not available, the cost of implementing a survey of transmitted drug resistance in a specific geographical area with WHO methods is inexpensive, ranging from US$30 000 to $60 000 per survey when compared with the total cost of ART rollout (Bertagnolio S, unpublished). However, the biggest barriers to the surveillance of HIV drug resistance are the restricted capacity of local laboratories, inadequate general infrastructure and human resources, and, possibly, a restricted understanding of the value of surveillance information and how it can be used to guide policy. A cheaper and simpler resistance-testing platform potentially involving point mutation assay methods designed to identify key NNRTI mutations of public health interest with dried blood spots might encourage countries to routinely monitor resistance at the population level.30,31
In addition to heightened surveillance, continued monitoring and adjustment of programmatic factors that lead to the emergence and spread of resistance to ART are needed.13 Bennett and colleagues32 assessed data from more than 2000 clinics in 50 countries worldwide between 2004 and 2009 by means of WHO-defined early warning indicators for drug resistance. This study documented stock-outs of antiretroviral drugs in about 40% of monitored sites in sub-Saharan Africa.32 This problem could be addressed through improved supply management systems that would ensure uninterrupted availability of ART. 40% of ART programmes in sub-Saharan Africa were reported to have more than 20% loss to follow-up. Problems with retention of patients are further barriers to successful, sustained viral suppression at the population level.32
Other potential strategic implications of our findings include the rapid scale-up of routine viral-load testing to enable timely detection and management of treatment failure to limit the emergence of HIV drug resistance.6,33 The prevalence of thymidine analogue mutations is explained by the extensive use of thymidine analogues in resource-limited settings. Accumulation of thymidine analogue mutations might reduce the effectiveness of second-line tenofovir-containing combinations.7,34 Better-tolerated first-line regimens (containing tenofovir) could become more widely used in the future and might contribute to the lowering of HIV drug resistance through improved adherence.35
In areas where pre-treatment NNRTI drug resistance exceeds an as yet undetermined threshold, a change from NNRTI-based first-line treatment to boosted protease inhibitors might improve rates of viral suppression and reduce treatment-emergent drug resistance.36,37 Economic and programmatic assessment would inform decision making regarding the population prevalence level at which change in first-line regimen, individualised pretreatment genotyping, or intensified viral load monitoring with early regimen switch would become cost effective.
Our study had several limitations. Our search strategy was restricted to only English-language articles. There were also limitations in our analysis of summary data from different groups of patients over time—eg, the samples of patients might differ in ways not controlled for in the analysis despite allowing for random effects in the analyses. Furthermore, although heterogeneity has been extensively considered and assessed in our analysis, there might be unexplained sources of heterogeneity in the dataset. The paucity of studies from rural settings means that the analysis shows transmitted drug resistance in only urban and peri-urban areas. However, as access to ART increases in sub-Saharan Africa, similar trends might be expected in rural areas under similar ART programmatic conditions. Despite intending to select studies of drug-naive populations, our dataset might have included data for women who had previously received treatment to prevent transmission to their children, and for individuals who had previous undisclosed antiretroviral exposure. However, pre-existing HIV drug resistance, whether due to transmission or undisclosed previous treatment, will adversely affect the success of ART rollout programmes. Finally, differences in regimen (zidovudine vs stavudine, or protease inhibitor vs NNRTI in some parts of Latin America), programme functioning and adherence could not be controlled for in the analysis and might play a part in regional differences. No difference in the prevalence of mutations was noted between recently and chronically infected populations, which might be because of the heterogeneity of the criteria used to assess recent versus chronic infection in the studies included in the Article, leading to potential misclassification bias. Additional research based on standardised definitions of duration of infection is needed to confirm this finding.
Continued commitment remains essential to reach the goal of universal access and improve the quality of HIV care and treatment in resource-limited settings. We have shown that HIV drug resistance in untreated patients has been increasing in some areas of sub-Saharan Africa since ART rollout. In view of these concerning findings, urgent strategic action is clearly needed to maximise the long-term effectiveness of available first-line regimens, and efforts should be focused on the optimisation of the functioning of national HIV treatment programmes. This optimisation would involve the establishment of robust supply chains to prevent drug stock-outs and treatment interruptions, scale-up of routine viral load monitoring to detect early failures, expanded access to alternative drug regimens, and the development of meaningful and sustainable strategies to overcome structural barriers to adherence and routine defaulter tracing to maximise retention.
Now, more than ever, investment and political will are urgently needed to sustain and expand global surveillance efforts. Such investments are essential to maximise the effectiveness of treatment scale-up. Although still within expected levels, further increases in the prevalence of HIV drug resistance might jeopardise the global HIV response and curb a decade-long trend of decreasing HIV-related morbidity and mortality in low-income and middle-income countries. However, no changes in antiretroviral treatment guidelines are warranted at the moment.
Acknowledgments
Acknowledgments
SB is a staff member at WHO, but the views expressed in this paper do not necessarily represent the decisions or stated policies of WHO. This study largely funded by the Bill & Melinda Gates Foundation (grant number 38180 to the WHO), and funded in part by the European Community's Seventh Framework Programme (FP7/2007-2013) under the project “Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN)”—grant agreement number 223131. RKG and DD received funding from the Wellcome Trust (WT093722MA and WT090661/Z/09/Z, respectively), and MRJ from the National Institutes of Health (K23 AI074423-05). JG is funded by a joint MRC and GlaxoSmithKline industrial CASE studentship. We thank the ministries of health who have contributed data for this analysis.
Contributors
SB had the idea for the study. RKG, BJS, AH, SB, and MRJ designed the study. RKG wrote the first draft. SB, MRJ, RH, and NN provided data. JG, DD, and WS did the statistical analyses. RKG, BJS, WS, DD, JG, AH, NN, DP, RH, SB, and MRJ analysed and interpreted data and contributed to the writing of subsequent drafts of the paper.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
References
- 1.Gilks CF, Crowley S, Ekpini R. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 2.WHO Antiretroviral therapy for HIV infection in adults and adolescents—recommendations for a public health approach: 2010 revision. http://www.who.int/hiv/pub/arv/adult2010/en/index.html (accessed July 1, 2011). [PubMed]
- 3.Together we will end AIDS. Joint United Nations Programme on HIV/AIDS. 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/20120718_togetherwewillendaids_en.pdf (accessed July 18, 2012).
- 4.Jahn A, Floyd S, Crampin AC. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth RE, van der Loeff MF, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RK, Hill A, Sawyer AW. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:409–417. doi: 10.1016/S1473-3099(09)70136-7. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RK, Pillay D, Ranopa M, et al. Rapid accumulation of thymidine-analog mutations and virologic implications in the absence of viral load monitoring. 18th conference on retroviruses and opportunistic infections; Boston, USA; Feb 27 to March 2, 2011. abstract 618.
- 8.Hamers RL, Sigaloff KC, Wensing AM. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54:1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 9.Hosseinipour MC, van Oosterhout JJ, Weigel R. The public health approach to identify antiretroviral therapy failure: high-level nucleoside reverse transcriptase inhibitor resistance among Malawians failing first-line antiretroviral therapy. AIDS. 2009;23:1127–1134. doi: 10.1097/QAD.0b013e32832ac34e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wittkop L, Günthard HF, de Wolf F. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): a European multicohort study. Lancet Infect Dis. 2011;377:1580–1587. doi: 10.1016/S1473-3099(11)70032-9. [DOI] [PubMed] [Google Scholar]
- 11.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(suppl 2):1–13. [PubMed] [Google Scholar]
- 12.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther. 2008;13(suppl 2):25–36. [PubMed] [Google Scholar]
- 13.Jordan MR, Bennett DE, Bertagnolio S, Gilks CF, Sutherland D. World Health Organization surveys to monitor HIV drug resistance prevention and associated factors in sentinel antiretroviral treatment sites. Antivir Ther. 2008;13(suppl):15–23. [PubMed] [Google Scholar]
- 14.Bennett DE, Camacho RJ, Otelea D. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One. 2009;4:e4724. doi: 10.1371/journal.pone.0004724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaynes ET. Confidence intervals vs Bayesian intervals. Foundations of probability theory, statistical inference, and statistical theories of science. Dordrecht, Netherlands: Reidel, 1976.
- 16.Stuart A, Ord J. Kendall's advanced theory of statistics. 6th edn. Arnold Publishers; London, England: 1994. [Google Scholar]
- 17.Erali M, Page S, Reimer LG, Hillyard DR. Human immunodeficiency virus type 1 drug resistance testing: a comparison of three sequence-based methods. J Clin Microbiol. 2001;39:2157–2165. doi: 10.1128/JCM.39.6.2157-2165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rangel HR, Garzaro DJ, Torres JR. Prevalence of antiretroviral drug resistance among treatment-naive and treated HIV-infected patients in Venezuela. Mem Inst Oswaldo Cruz. 2009;104:522–525. doi: 10.1590/s0074-02762009000300020. [DOI] [PubMed] [Google Scholar]
- 19.Parkin N, Bremer J, Bertagnolio S. Genotyping External Quality Assurance in the World Health Organization HIV Drug Resistance Laboratory Network During 2007–2010. Clin Infect Dis. 2012;54(suppl 4):S266–S272. doi: 10.1093/cid/cir992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green H, Tilston P, Fearnhill E, Pillay D, Dunn DT, UK Collaborative Group on HIV Drug Resistance The impact of different definitions on the estimated rate of transmitted HIV drug resistance in the United Kingdom. J Acquir Immune Defic Syndr. 2008;49:196–204. doi: 10.1097/QAI.0b013e318185725f. [DOI] [PubMed] [Google Scholar]
- 21.Stijnen T, Hamza TH, Ozdemir P. 2010. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046–3067. doi: 10.1002/sim.4040. [DOI] [PubMed] [Google Scholar]
- 22.Goodman DD, Zhou Y, Margot NA. Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS. 2011;25:325–333. doi: 10.1097/QAD.0b013e3283427dcb. [DOI] [PubMed] [Google Scholar]
- 23.Li JZ, Paredes R, Ribaudo HJ. Relationship between minority nonnucleoside reverse transcriptase inhibitor resistance mutations, adherence, and the risk of virologic failure. AIDS. 2012;26:185–192. doi: 10.1097/QAD.0b013e32834e9d7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndembi N, Hamers RL, Sigaloff KC. Transmitted antiretroviral drug resistance among newly HIV-1 diagnosed young individuals in Kampala. AIDS. 2011;25:905–910. doi: 10.1097/QAD.0b013e328346260f. [DOI] [PubMed] [Google Scholar]
- 25.Sigaloff KC, Mandaliya K, Hamers RL. High prevalence of transmitted antiretroviral drug resistance among newly HIV type 1 diagnosed adults in Mombasa, Kenya. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/AID.2011.0348. published online Feb 2. PMID 22149307. [DOI] [PubMed] [Google Scholar]
- 26.Price MA, Wallis CL, Lakhi S. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retroviruses. 2011;27:5–12. doi: 10.1089/aid.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNAIDS UN General Assembly special session country reports. http://www.unaids.org/en/regionscountries/countries/ (accessed Sept 21, 2011).
- 28.Hamers RL, Wallis CL, Kityo C. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis. 2011;11:750–759. doi: 10.1016/S1473-3099(11)70149-9. [DOI] [PubMed] [Google Scholar]
- 29.Aghokeng AF, Kouanfack C, Laurent C. Scale-up of antiretroviral treatment in sub-Saharan Africa is accompanied by increasing HIV-1 drug resistance mutations in drug-naive patients. AIDS. 2011;25:2183–2188. doi: 10.1097/QAD.0b013e32834bbbe9. [DOI] [PubMed] [Google Scholar]
- 30.Bertagnolio S, Parkin NT, Jordan M, Brooks J, García-Lerma JG. Dried blood spots for HIV-1 drug resistance and viral load testing: a review of current knowledge and WHO efforts for global HIV drug resistance surveillance. AIDS Rev. 2010;12:195–208. [PubMed] [Google Scholar]
- 31.Hamers RL, Smit PW, Stevens W, Schuurman R, Rinke de Wit TF. Dried fluid spots for HIV type-1 viral load and resistance genotyping: a systematic review. Antivir Ther. 2009;14:619–629. [PubMed] [Google Scholar]
- 32.Bennett DE, Jordan MR, Bertagnolio S. HIV drug resistance early warning indicators in cohorts of individuals starting antiretroviral therapy between 2004 and 2009: World Health Organization global report from 50 countries. Clin Infect Dis. 2012;54(suppl 4):S280–S289. doi: 10.1093/cid/cis207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffmann CJ, Charalambous S, Sim J. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49:1928–1935. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MD, Margot N, Lu B. Genotypic and phenotypic predictors of the magnitude of response to tenofovir disoproxil fumarate treatment in antiretroviral-experienced patients. J Infect Dis. 2004;189:837–846. doi: 10.1086/381784. [DOI] [PubMed] [Google Scholar]
- 35.von Wyl V, Cambiano V, Jordan MR. Cost-effectiveness of tenofovir instead of zidovudine for use in first-line antiretroviral therapy in settings without virological monitoring. PloS One. 2012 doi: 10.1371/journal.pone.0042834. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta RK, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis. 2008;47:712–722. doi: 10.1086/590943. [DOI] [PubMed] [Google Scholar]
- 37.von Wyl V, Yerly S, Böni J. Emergence of HIV-1 drug resistance in previously untreated patients initiating combination antiretroviral treatment: a comparison of different regimen types. Arch Intern Med. 2007;167:1782–1790. doi: 10.1001/archinte.167.16.1782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


