Abstract
Polymicrogyria is a cerebral cortical malformation that is grossly characterized by excessive cortical folding and microscopically characterized by abnormal cortical layering. Although polymicrogyria appears to have one or more genetic causes, no polymicrogyria loci have been identified. Here we describe the clinical and radiographic features of a new genetic form of polymicrogyria and localize the responsible gene. We studied two consanguineous Palestinian pedigrees with an autosomal recessive form of bilateral frontoparietal polymicrogyria (BFPP), using linkage analysis. Five affected children had moderate-to-severe mental retardation, developmental delay, and esotropia, and four of the five affected children developed seizures. Brain magnetic-resonance imaging revealed polymicrogyria that was most prominent in the frontal and parietal lobes but involved other cortical areas as well. A genomewide linkage screen revealed a single locus that was identical by descent in affected children in both families and showed a single disease-associated haplotype, suggesting a common founder mutation. The locus for BFPP maps to chromosome 16q12.2-21, with a minimal interval of 17 cM. For D16S514, the maximal pooled two-point LOD score was 3.98, and the maximal multipoint LOD score was 4.57. This study provides the first genetic evidence that BFPP is an autosomal recessive disorder and serves as a starting point for the identification of the responsible gene.
Several syndromes of bilaterally symmetrical polymicrogyria have been clinically described elsewhere (Kuzniecky et al. 1993; Guerrini et al. 1997, 2000; Kuzniecky and Barkovich 2001). Although the most commonly recognized polymicrogyria syndrome involves the perisylvian regions (MIM 260980), new forms involving the frontal lobes (Guerrini et al. 2000) or the frontal and parietal lobes (Sztriha and Nork 2000) have recently been reported. However, the genetic bases for all of these syndromes are unknown. Here we describe the clinical and genetic features of a new and strikingly stereotyped form of polymicrogyria.
The first pedigree (fig. 1A) was originally reported by Straussberg et al. (1996) and was described as having pachygyria, but improved magnetic-resonance imaging (MRI) (described below) clearly shows that the core disorder is polymicrogyria. The parents are Palestinian first cousins, with one healthy child (IV:1) and three affected children (IV:2–IV:4), who were 14, 9, and 7.5 years of age, respectively, when this was written. The affected children all had normal prenatal and perinatal history and normal head growth but showed gross developmental delay and moderate mental retardation. They can speak a few words and can walk independently. All three affected children developed medically refractory seizures: at age 5 years, individual IV:2 suffered generalized tonic-clonic and absence seizures; at age 7 years, individual IV:3 developed atonic spells and drop attacks, which were treated with topiramate, and had an electroencephalogram consistent with a diagnosis of Lennox-Gastaut syndrome; and, at age 4 years, individual IV:4 suffered generalized tonic-clonic seizures, which were treated with phenobarbital. All affected siblings have esotropia, increased muscle tone, mild truncal ataxia, and finger dysmetria, without dysmorphic features and other congenital anomalies; all show a normal karyotype.
Figure 1.
Pedigrees with BFPP, showing genotypes for eight microsatellite markers at chromosome 16q12.2-21. The marker order is cen-D16S682-D16S516-tel. The boxed regions indicate the homozygous regions in each affected individual.
The second pedigree (fig. 1B) was recently identified and is from the same village as the first, although there is no known relationship between the two families. The propositus, a 13-year-old girl, was the first child of healthy Palestinian parents who are first cousins. There is no family history of seizures or mental retardation. Pregnancy and delivery were uneventful. The child's weight at birth was 3,300 g; the mother noticed that, at birth, the child's left eye appeared to be smaller than its right eye. Global developmental delay was evident during the 1st year of life. The child sat at age 18 mo, crawled at age 24 mo, and walked at age 5 years. At age 13 years, she could say only two words apart from “mama” and “papa” and could understand a few simple commands. She communicates by gestures and is unable to dress, is not toilet trained, and cannot feed herself. Intractable epilepsy, mainly generalized tonic-clonic and atonic seizures, began at age 7 years and is treated with sodium valproate. She has a head circumference of 54 cm (60th percentile) and no dysmorphic features. Eye examination revealed left ptosis, esotropia, and normal fundi. She demonstrates increased muscle tone, brisk deep-tendon reflexes without Babinski signs, sustained ankle clonus, and a wide-based gait but no tremor. Laboratory investigations show a normal female karyotype and normal metabolism.
The second patient in this pedigree, a 4.5-year-old brother of the propositus, was born after an uneventful pregnancy and delivery. His weight at birth was 3,150 g. At age 16 d, because of pyloric stenosis, he underwent pylorotomy. He sat at age 12 mo and crawled at age 24 mo, but, at 4.5 years, he can stand and walk only with support. He cannot understand verbal commands, reacts only to basic gestures, and says no words except “mama.” His fine-motor and play abilities are at approximately the 15-mo level. He is not toilet trained. There are no seizures yet. Physical examination reveals a head circumference of 50 cm (40th percentile), no dysmorphism, mild retrognathia, mild plagiocephaly, pectus carinatum, and bilateral 5th-finger clinodactyly. Eye examination showed esotropia and pale optic disks. On neurological examination, he demonstrated increased tone, brisk deep-tendon reflexes without Babinski signs or ankle clonus, and a wide-based gait. Laboratory investigation revealed normal male karyotype and normal metabolic studies. Electromyography and nerve-conduction studies were normal at age 6 mo. Auditory-evoked-response testing at age 6 mo revealed delayed signal transmission in the brain stem (with greater delay on the left side thereof).
Brain MRI of the affected patients from the first pedigree (figs. 2A and 2B) revealed apparent thickening of the cerebral cortex, with an irregular, “scalloped” gray-white junction, as is typically seen in polymicrogyria. These findings were bilaterally symmetric, were more severe in the frontal lobe than in the parietal lobe, and included the perirolandic regions. The temporal and occipital lobes were less severely involved. In general, there was an anterior-to-posterior gradient, as well as a superior-to-inferior gradient, in severity of the cortical malformation. The cortical surface also appeared to be mildly irregular. The perirolandic involvement resulted in only mild immaturity of the sylvian fissures, with little or no bilateral uncovering of the insula. There was a moderate overall decrease in white-matter volume, resulting in moderate enlargement of the lateral ventricles. Patchy areas of increased T2 signal, often asymmetric and relatively well defined, were evident in the periventricular and more peripheral white matter. The etiology of these lesions is unclear, but areas of demyelination are more likely than areas of dysmyelination. The corpus callosum was intact. The basal ganglia, thalami, and cerebellar hemispheres were normal. The pons appeared to be slightly decreased in size, and the superior vermian fissures were normal to mildly prominent. Brain MRI of the second pedigree (figs. 2C–2E) was similar to that of the first, except that (a) the cortical dysplasia was almost as severe in the parietal lobe as in the frontal lobe and (b) the focal areas of increased T2 signal were larger and involved the basal ganglia.
Figure 2.
Brain MRI of one affected individual from each pedigree. Shown are axial T1–weighted (A) and axial T2–weighted (B) MRI of one individual from the first pedigree and axial T1–weighted (C), axial T2–weighted (D), and sagittal T1–weighted (E) MRI of one individual from the second pedigree. Axial images in both pedigrees show apparent thickening of the cerebral cortex, with an irregular gray-white junction, which is typically seen in polymicrogyria. These findings are diffuse but more severe in the frontal and parietal lobes. In both pedigrees, there is moderate decrease in white-matter volume and moderate ventricular enlargement. Focal areas of decreased T1 and increased T2 signals are seen in the white matter of the first pedigree (A and B) and in the left lentiform nucleus in the second pedigree (C and D). Periventricular increased T2 (B) is also seen in the first pedigree. A slightly hypoplastic pons (E) is also present in both pedigrees.
The parental consanguinity and the presence of affected individuals of both sexes strong suggests autosomal recessive inheritance in both pedigrees. We studied the two pedigrees genetically after obtaining informed consent in accordance with protocols approved by the institutional review board of Beth Israel Deaconess Medical Center. DNA was obtained from peripheral-blood lymphocytes by use of standard techniques. A 10-cM genomewide linkage scan was performed on each individual of pedigree 1 at the Center for Inherited Disease Research by use of a modification of the Cooperative Human Linkage Center version 9 marker set and standard techniques. The three affected children were homozygous for the same allele for ⩾2 adjacent markers at four loci on chromosomes 2, 9, 11, and 16. Further examination by use of additional markers excluded the loci on chromosomes 2 and 9, whereas the loci on chromosomes 11p and 16q remained equally likely.
Marker analysis of the remaining two candidate loci in the second pedigree excluded chromosome 11p but also showed substantial homozygosity at chromosome 16q (figs. 1A and 1B) in all affected children, which is highly suggestive of identity by descent (Lander and Botstein 1987). Furthermore, nine consecutive microsatellite markers on chromosome 16q12.2-21 shared homozygosity for the identical marker allele among all affected children of both pedigrees (not all of whom are shown in fig. 1). The conserved disease-associated haplotype in both families is strongly suggestive of a common founder mutation, since both families came from the same village and are Palestinian.
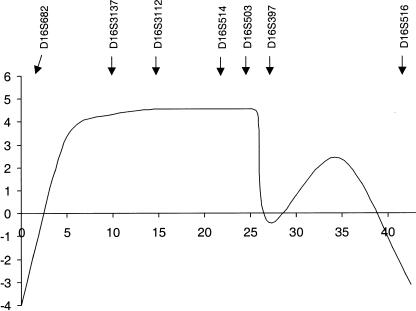
Statistical analysis provided strong evidence for linkage of bilateral frontoparietal polymicrogyria (BFPP) to chromosome 16q12.2-21 (table 1). Two-point analysis was performed using MLINK, from the LINKAGE programs (Lathrop et al. 1984), and multipoint analysis was performed using the Allegro program (Gudbjartsson et al. 2000). We assumed a susceptibility allele with frequency 0.001 and a recessive mode of inheritance with penetrance 0.99. Marker-allele frequencies are taken from the Genome Database, or were determined by the genotyping of 23 unrelated individuals (17 of European descent, 2 of Asian descent, and 4 of Arabic descent) who are not affected with BFPP. Allele frequencies for D16S397 were assumed to be equal. Several markers yielded pooled two-point LOD scores >3.0 (table 1). Multipoint analysis provided a maximal LOD score 4.57 (fig. 3), without assuming a common founder mutation. These data and analysis of homozygosity indicated a minimal candidate interval for BFPP of ∼17 cM, between D16S3137 and D16S397.
Table 1.
Two-Point LOD Scores for Markers on Chromosome 16q12-21[Note]
|
LOD Score at θ = |
|||||||
| MarkerandPedigree | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D16S682: | |||||||
| Ped 1 | −4.91 | .05 | .59 | .68 | .57 | .36 | .16 |
| Ped 2 | −8.18 |
−2.35 |
−1.11 |
−.62 |
−.23 |
−.07 |
−.01 |
| Zmax | −13.09 | −2.30 | −.52 | .06 | .35 | .29 | .14 |
| D16S3137: | |||||||
| Ped 1 | .97 | .95 | .86 | .75 | .52 | .31 | .13 |
| Ped 2 | 1.40 |
1.36 |
1.21 |
1.02 |
.66 |
.34 |
.12 |
| Zmax | 2.37 | 2.31 | 2.07 | 1.77 | 1.18 | .65 | .25 |
| D16S3112: | |||||||
| Ped 1 | 2.00 | 1.95 | 1.77 | 1.55 | 1.09 | .65 | .26 |
| Ped 2 | .39 |
.38 |
.33 |
.26 |
.15 |
.06 |
.01 |
| Zmax | 2.39 | 2.33 | 2.10 | 1.81 | 1.24 | .71 | .27 |
| D16S489: | |||||||
| Ped 1 | 1.91 | 1.87 | 1.69 | 1.47 | 1.03 | .61 | .23 |
| Ped 2 | 1.30 |
1.27 |
1.11 |
.92 |
.56 |
.25 |
.05 |
| Zmax | 3.22 | 3.13 | 2.80 | 2.39 | 1.59 | .85 | .28 |
| D16S3089: | |||||||
| Ped 1 | 2.00 | 1.96 | 1.78 | 1.55 | 1.10 | .65 | .26 |
| Ped 2 | 1.26 |
1.22 |
1.06 |
.86 |
.48 |
.17 |
−.01 |
| Zmax | 3.26 | 3.17 | 2.83 | 2.41 | 1.58 | .82 | .25 |
| D16S514: | |||||||
| Ped 1 | 2.26 | 2.21 | 2.02 | 1.78 | 1.30 | .81 | .36 |
| Ped 2 | 1.73 |
1.68 |
1.51 |
1.30 |
.89 |
.50 |
.19 |
| Zmax | 3.98 | 3.90 | 3.54 | 3.09 | 2.18 | 1.32 | .55 |
| D16S503: | |||||||
| Ped 1 | 1.19 | 1.16 | 1.05 | .92 | .64 | .38 | .16 |
| Ped 2 | .72 |
.70 |
.62 |
.53 |
.35 |
.20 |
.08 |
| Zmax | 1.91 | 1.86 | 1.68 | 1.44 | .99 | .58 | .24 |
| D16S3043: | |||||||
| Ped 1 | 2.36 | 2.31 | 2.12 | 1.88 | 1.38 | .89 | .41 |
| Ped 2 | 1.06 |
1.04 |
.94 |
.82 |
.58 |
.35 |
.15 |
| Zmax | 3.42 | 3.35 | 3.06 | 2.70 | 1.96 | 1.24 | .57 |
| D16S397: | |||||||
| Ped 1 | 1.82 | 1.78 | 1.61 | 1.40 | .98 | .56 | .21 |
| Ped 2 | −1.82 |
−1.12 |
−.53 |
−.29 |
−.10 |
−.04 |
−.01 |
| Zmax | .00 | .66 | 1.09 | 1.11 | .87 | .53 | .20 |
| D16S516: | |||||||
| Ped 1 | .0 | .0 | .0 | .0 | .0 | .0 | .0 |
| Ped 2 | −1.13 |
−.44 |
.10 |
.26 |
.29 |
.18 |
.06 |
| Zmax | −1.13 | −.44 | .10 | .26 | .29 | .18 | .06 |
Note.— The marker order is cen-D16S682-D16S516-tel. “Ped 1” refers to the first pedigree, and “Ped 2” refers to the second pedigree; Zmax is the total of the scores for both pedigrees.
Figure 3.
Multipoint-LOD-score analysis of BFPP in chromosome 16q12.2-21. Analysis was performed using seven informative markers. D16S682 was arbitrarily set as the zero point on the map. The relative map positions are shown along the X-axis, and multipoint LOD score is shown on the Y-axis. The locations of the markers used in the multipoint analysis are indicated. The maximal multipoint LOD score is 4.57 in the region of D16S514.
The five patients described here share a congenital syndrome of developmental delay, moderate-to-severe mental retardation, seizures, esotropia, pyramidal signs, cerebellar impairment, and bilateral polymicrogyria, which is more prominent in the frontal and parietal lobes. The identification of the cortical abnormality as polymicrogyria is based on the characteristic scalloped appearance of the border between gray and white matter, which appears thus because of the numerous small folds of the polymicrogyria (Raybaud et al. 1996; Barkovich et al. 1999). Although lower-resolution scans and thick MRI sections sometimes suggest merely a thickened cortex and would thus demonstrate pachygyria (Straussberg et al. 1996), the finer cuts clearly demonstrate polymicrogyria. Therefore, we identify the syndrome as BFPP. Genetic-mapping data confirmed that this syndrome is transmitted as an autosomal recessive condition.
The etiology of polymicrogyria is unclear, and both neuronal migration defects and ischemia have been postulated as possible mechanisms (Barth 1987; Barkovich et al. 1995). The absence of radiographic evidence for widespread perfusion failure, which is usually associated with vascular-induced polymicrogyria, supports a direct genetic abnormality of neuronal migration and/or subsequent cortical lamination. Microscopically, two forms of polymicrogyria are recognized—namely, unlayered and four-layered, depending on whether the cortex is completely disorganized or abnormally laminated, respectively. The lack of neuropathological material makes it impossible to establish which histological type of polymicrogyria is present in the patients presented here. However, the preferential involvement of frontal and parietal lobes, rather than the occipital lobe, further suggests that the gene for BFPP may be critically involved in forming the region-specific cytoarchitectonic patterns that characterize the human cerebral cortex.
Bilateral polymicrogyria syndromes are likely to be genetically heterogeneous, since examples of autosomal dominant (Guerreiro et al. 2000) and X-linked inheritance (Yoshimura et al. 1998; Borgatti et al. 1999) have been suggested. Moreover, another syndrome with certain related features, which is referred to as “bilateral frontal polymicrogyria” (BFP), has been described elsewhere (Guerrini et al. 2000). Patients with BFP demonstrate a variety of MRI patterns, some of which are similar to those of patients with BFPP and others of which are not. Therefore, we have used different terminology, to suggest that BFP and BFPP are probably genetically distinct. Although patients with BFP have not been studied genetically, 2 of 13 patients were of Arabic descent and were born to consanguineous parents, suggesting autosomal recessive inheritance and, thus, that they actually may have BFPP. Two patients reported to have BFPP (Sztriha and Nork 2000) were also of Arabic descent. Further analysis may reveal a more widespread Arabic founder mutation that could allow fine mapping of the gene for BFPP. Further clarification of the genetics and clinical findings on BFPP, however, should help identify additional pedigrees, and, in turn, should facilitate identification of the responsible gene.
Acknowledgments
We thank the families who kindly consented to join the study; Dr. L. Kornreich and Professor C. Legum, for invaluable help with sample collection and MRI analysis; Timothy Cherry, for helping to determine marker-allele frequencies; Adria Bodell, for coordinating patient studies; and other members of the Walsh lab, for their help. This work was supported by National Institutes of Health program grant HD07466 (to X.P.), by National Institute of Neurological Disorders and Stroke grant R01 NS 35129 (to C.A.W.), and by the March of Dimes. The Center for Inherited Disease Research is fully funded through a federal contract (N01-HG-65403) from the National Institutes of Health to Johns Hopkins University.
Electronic-Database Information
The accession number and URLs for data in this report are as follows:
- Genome Database, The, http://www.gdb.org/ (for marker-allele frequencies)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for perisylvian polymicrogyria [MIM 260980])
References
- Barkovich AJ, Hevner R, Guerrini R (1999) Syndromes of bilateral symmetrical polymicrogyria. AJNR Am J Neuroradiol 20:1814–1821 [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Rowley H, Bollen A (1995) Correlation of prenatal events with the development of polymicrogyria. AJNR Am J Neuroradiol 16:822–827 [PMC free article] [PubMed] [Google Scholar]
- Barth PG (1987) Disorders of neuronal migration. Can J Neurol Sci 14:1–16 [DOI] [PubMed] [Google Scholar]
- Borgatti R, Triulzi F, Zucca C, Piccinelli P, Balottin U, Carrozzo R, Guerrini R (1999) Bilateral perisylvian polymicrogyria in three generations. Neurology 52:1910–1913 [DOI] [PubMed] [Google Scholar]
- Gudbjartsson DF, Jonasson K, Frigge ML, Kong A (2000) Allegro, a new computer program for multipoint linkage analysis. Nat Genet 25:12–13 [DOI] [PubMed] [Google Scholar]
- Guerreiro MM, Andermann E, Guerrini R, Dobyns WB, Kuzniecky R, Silver K, Van Bogaert P, Gillain C, David P, Ambrosetto G, Rosati A, Bartolomei F, Parmeggiani A, Paetau R, Salonen O, Ignatius J, Borgatti R, Zucca C, Bastos AC, Palmini A, Fernandes W, Montenegro MA, Cendes F, Andermann F (2000) Familial perisylvian polymicrogyria: a new familial syndrome of cortical maldevelopment. Ann Neurol 48:39–48 [PubMed] [Google Scholar]
- Guerrini R, Barkovich AJ, Sztriha L, Dobyns WB (2000) Bilateral frontal polymicrogyria: a newly recognized brain malformation syndrome. Neurology 54:909–913 [DOI] [PubMed] [Google Scholar]
- Guerrini R, Dubeau F, Dulac O, Barkovich AJ, Kuzniecky R, Fett C, Jones-Gotman M, Canapicchi R, Cross H, Fish D, Bonanni P, Jambaque I, Andermann F (1997) Bilateral parasagittal parietooccipital polymicrogyria and epilepsy. Ann Neurol 41:65–73 [DOI] [PubMed] [Google Scholar]
- Kuzniecky R, Andermann F, Guerrini R (1993) Congenital bilateral perisylvian syndrome: study of 31 patients. The CBPS Multicenter Collaborative Study. Lancet 341:608–612 [DOI] [PubMed] [Google Scholar]
- Kuzniecky RI, Barkovich AJ (2001) Malformations of cortical development and epilepsy. Brain Dev 23:2–11 [DOI] [PubMed] [Google Scholar]
- Lander ES, Botstein D (1987) Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science 236:1567–1570 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci USA 81:3443–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybaud CGN, Canto-Moreira N, Poncet M (1996) High-definition magnetic resonance imaging identification of cortical dysplasias: micropolygyria versus lissencephaly. In: Guerrini R, Canapicchi R, Zifkin BG, Andermann R, Roger J, Pfanner P (eds) Dysplasias of cerebral cortex and epilepsy. Lippincott-Raven, Philadelphia, pp 131–143 [Google Scholar]
- Straussberg R, Gross S, Amir J, Gadoth N (1996) A new autosomal recessive syndrome of pachygyria. Clin Genet 50:498–501 [DOI] [PubMed] [Google Scholar]
- Sztriha L, Nork M (2000) Bilateral frontoparietal polymicrogyria and epilepsy. Pediatr Neurol 22:240–243 [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Hamada F, Tomoda T, Wakiguchi H, Kurashige T (1998) Focal pachypolymicrogyria in three siblings. Pediatr Neurol 18:435–438 [DOI] [PubMed] [Google Scholar]