Abstract
Phospholipid aldehydes represent a particular subclass of lipid oxidation products. They are chemically reactive and can form Schiff bases with proteins and aminophospholipids. As chemically bound molecular entities they modulate the functional properties of biomolecules in solution and the surface of supramolecular systems including plasma lipoproteins and cell membranes. The lipid–protein and lipid–lipid conjugates may be considered the active primary platforms that are responsible for the biological effects of aldehydophospholipids, e.g. receptor binding, cell signaling, and recognition by the immune system. Despite the fact that aldehydophospholipids are covalently associated, they are subject to exchange between nucleophiles since their imine conjugates are not stable. As a consequence, aldehydophospholipids exist in a dynamic equilibrium between different “states” depending on the lipid and protein environment. Aldehydophospholipids may also contribute to the systemic administration and activity of oxidized phospholipids by inducing release of microparticles by cells. These effects are lipid-specific. Future studies should help clarify the mechanisms and consequences of these membrane-associated effects of “phospholipid stress”. This article is part of a Special Issue entitled: Oxidized phospholipids—their properties and interactions with proteins.
Abbreviations: BSA, bovine serum albumin; BY, BODIPY™; HAEC, human aortic endothelial cells; HDL, high density lipoprotein; HNE, hydroxyl-nonenal; HOdiA-PC, 1-palmitoyl-2-(5-hydroxy-8-oxo-6-octenedioyl) sn-glycero-3-phosphocholine; HOOA-PC, 1-palmitoyl-2-(5-hydroxy-8-oxooct-6-enoyl)-sn-glycero-3-phosphocholine; IL, interleukin; KDdiaPC, 1-palmitoyl-2-(9-keto-10-dodecendioyl)-sn-glycero-3-phosphocholine; KOdiA- PC, 1-palmitoyl-2-(5-keto-6-octene-dioyl)-sn-glycero-3-phosphocholine; KOOA-PC, 1-palmitoyl-(5-keto-8-oxo-6-octenoyl)-sn-glycero-3-phosphocholine; LDL, low density lipoprotein; MDA, malondialdehyde; oxLDL, oxidized low density lipoprotein; (ox)PAPC, (oxidized) 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine; oxPL, oxidized phospholipid; PAF, platelet activating factor; PazePC, 1-palmitoyl-2-azelaoyl-sn-glycero-3-phosphocholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PGPC, 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphocholine; PLA, phospholipase A; PLPC, 1-palmitoyl-sn-glycero-3-phosphocholine; PONPC, 1-palmitoyl-2-(9-oxononanayl)-sn-glycero-3-phosphocholine; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine; PoxnoPC, 1-palmitoyl-2-(9-oxo-nonanoyl)-sn-glycero-3-phosphocholine; PS, phosphatidylserine; PUFA, poly unsaturated fatty acid; ROS, reactive oxygen species; TLR, toll-like receptor; VSMC, vascular smooth muscle cells
Keywords: Oxidized phospholipids, Oxidized lipoproteins, Atherosclerosis, Lipoprotein–cell interaction, Lipid toxicity
Highlights
►The manuscript gives an overview of biophysical and biochemical properties of aldehydophospholipid–protein adducts. ►Aldehydophospholipids affect the physiological functions of atherogenic and antiatherogenic lipoproteins. ►Aldehydophospholipids target many cellular proteins apart from classical lipid and lipoprotein receptors. ►Aldehydophospholipid–protein adducts elicit autoimmune responses.
1. Introduction
The major fraction of diacyl glycerophospholipids in the phospholipid bilayers of cell membranes and the phospholipid monolayers of plasma lipoproteins and intracellular lipid droplets shows a common fatty acid pattern. In position sn-1, they contain a saturated fatty acid whereas the sn-2 hydroxy group of glycerol is esterified to a mono- or polyunsaturated fatty acid. The most abundant phospholipid classes contain ethanolamine and choline head groups. Serine and inositol phospholipids, although functionally very important and specific, are less abundant. Except for the liver, animal and human cell membranes contain large amounts of ether phospholipids [1], namely plasmalogens (1-O-alkenyl-2-acyl-sn-glycero-3-phospholipids) and lower amounts of their saturated 1-O-alkyl analogs. Only small concentrations of ether choline and ethanolamine phospholipids are found in plasma lipoproteins [1].
Under the conditions of oxidative stress, the polyunsaturated acyl chains are preferred targets of free radical attack of the bisallylic hydrogen atoms. Hydrogen abstraction is the first step in this process which is eventually followed by a series of modifications and fragmentations of the sn-2 acyl chain. As a consequence, a great variety of oxidized phospholipids is formed. Almost all of them are characterized by a saturated fatty acyl chain in position sn-1 and oxidized fatty acid residue in the sn-2 position. An exception is 1-formyl phospholipids that can be formed upon oxidation of the 1-enolether bond of plasmalogens [2].
Formation of phospholipid sn-2-aldehydes always requires fragmentation reactions. As a consequence, the respective acyl chains are shorter and more polar as compared to the native polyunsaturated counterparts. Most of the oxidized precursor phospholipids containing chiral centers in the modified sn-2 acyl residue are racemic. Therefore, it was concluded that they are predominantly generated by chemical oxidation involving transition metal ions instead of stereospecific enzymatic modification [3,4].
Many aldehydophospholipids are accompanied by their higher oxidized carboxylate derivatives in their biological environment. Although the structural difference between these compounds is small, the mechanisms underlying their activities are different because the phospholipid aldehydes are chemically reactive (Schiff base formation with proteins and aminophospholipids) whereas the carboxy-phospholipids can only physically interact with other biomolecules [4–6]. Both types of oxidized phospholipids have frequently been compared in the literature. In this review we also make an attempt to highlight the relationship between biological lipid activity and differences in lipid structure.
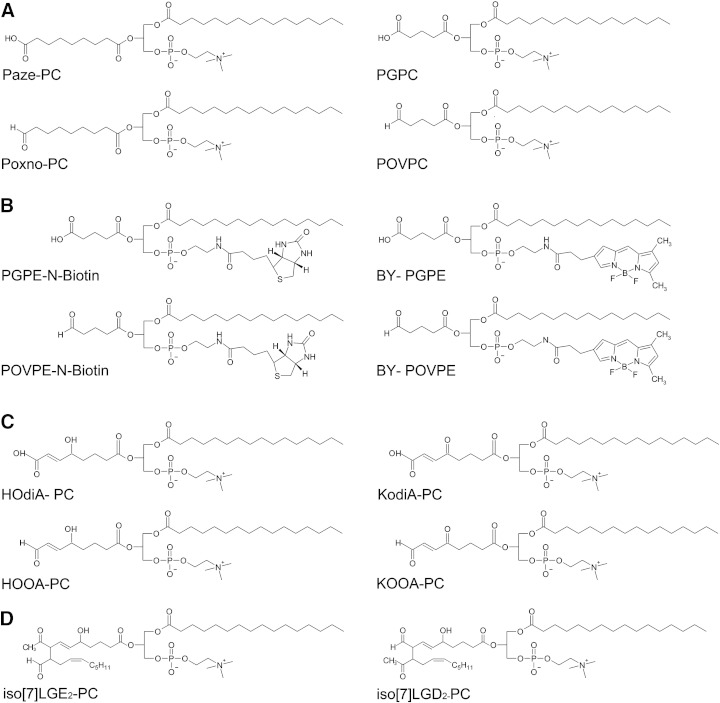
The structural motifs of oxidized phospholipids have been described in several reviews [4,7,8]. This article focuses on those compounds that contain aldehyde functions. The majority of oxidized phospholipids identified so far contain choline head groups. Representative chemical structures of aldehydo choline phospholipids and their carboxy analogs are shown in Fig. 1. The γ-hydroxyalkenal-PCs HOOA-PC/HOdiA-PC and KOOA-PC/KOdiA-PC are formed by reduction of PC hydroperoxides followed by fragmentation of the resulting hydrodienes [4]. The aldehydes HOOA-PC and KOOA-PC share the highly reactive γ-hydroxy-α,β-alkenal element with hydroxynonenal (HNE) which is released after oxidation from the ω-end of the parent fatty acid [9]. As a consequence, these compounds show a more complex reactivity towards nucleophiles such as Schiff base formation and Michael addition [9,10].
Fig. 1.
Chemical structures of lipid oxidation products and labeled lipid analogs. Chemical structures of oxidized phospholipids (Panels A,C,D) and labeled lipid analogs (Panel B).
Further fragmentation of phospholipid-associated hydroxyalkenals leads to the formation of the truncated phospholipids POVPC/PGPC and PoxnoPC/PazePC. The respective compounds are oxidation products of 1-palmitoyl-2-arachidonoyl- and 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine, respectively. POVPC/PGPC contain shorter oxidized acyl chains and, as a consequence, are more polar than PoxnoPC/PazePC. The structures of the former compounds are more closely related to platelet activating factor which only contains a very short acetyl residue in position sn-2 and a long 1-O-alkyl chain in position sn-1 of glycerol.
Isolevuglandins are formed via an entirely different pathway. Nonenzymatic reaction of phospholipid-associated arachidonic acid with two oxygen molecules generates endoperoxide intermediates that are further modified by several consecutive reactions leading to isoprostanes containing a five-membered ring or isolevuglandins (no cyclisation). In contrast to the truncated phospholipids, both lipid subclasses still contain a long-chain carboxylic acid residue in position sn-2. However, these acyl chains are polar due to the presence of functional groups [4].
Biological activities of oxidized phospholipids have initially been studied using lipid mixtures obtained after oxidation of polyunsaturated phospholipids, e.g. PAPC, PLPC or biological lipid extracts. For several years, the number of biochemical and biophysical studies using chemically defined oxidized phospholipids is increasing. Most of these studies have been performed using the truncated phospholipids POVPC/PGPC and PoxnoPC/PazePC, since simple procedures are available for the preparation of these lipids. Chemical syntheses of more complex oxidized phospholipids, e.g. isoprostanes, have also been reported but are very time-consuming. Thus, the development of facile procedures for the synthesis of chemically defined phospholipid oxidation products will be a continuous challenge for the future. Chemically pure compounds will not only be needed as substrates for biochemical and biophysical investigations, but also as standards for the rapidly increasing field of lipidomics based on mass spectrometry of lipid analytes.
2. Aldehydophospholipids talk biophysics and biochemistry
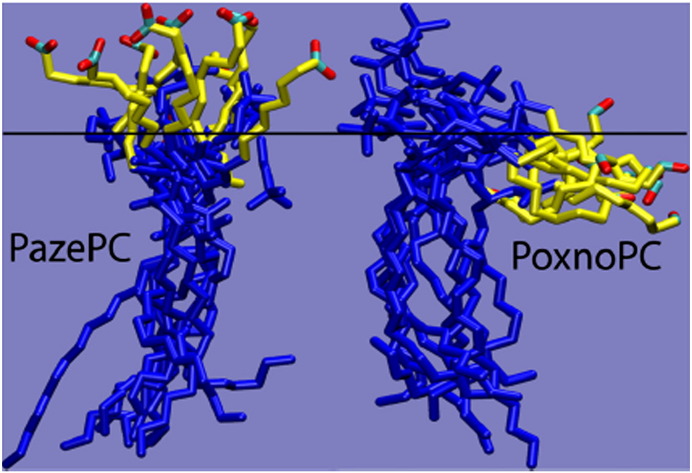
The chemical reactivity of aldehydophospholipids towards nucleophiles is intimately associated with their amphipathic nature and their association with lipid–water interfaces. Whereas the reactivity of such compounds in organic solution is largely diffusion-controlled, chemical reactions with amino (or thiol) groups of proteins and aminophospholipids in membranes and lipoproteins depend on the localization of the functional aldehyde group, the molecular lipid mobility and the steric constraints due to lateral lipid and protein packing. POVPC and Poxno-PC on the one hand and PGPC and Paze-PC on the other hand are amphiphiles that are linked to the phospholipid phase only by one long hydrophobic acyl chain. The fragmented sn-2 fatty acyl substituent in these molecules is polar and can be expected to fold back to the membrane interface or surface [11,12]. This assumption is supported by in silico studies by Wong-ekkabut [13] and by Khandelia and Mouritsen [14]. The latter authors simulated the molecular conformations of PGPC and POVPC in a phospholipid bilayer (Fig. 2). It was found that PGPC adopts an extended conformation with the negatively charged sn-2 carboxylate group stretching into the water phase. In contrast, the electroneutral but polar sn-2 aldehyde group of POVPC remains in the hydrophobic–hydrophilic membrane interface which makes it available for chemical reactions with the amino groups of membrane-associated proteins and phospholipids.
Fig. 2.
Molecular dynamics of oxidized phospholipids in bilayer membranes. Shown are snapshots of single PazePC and PoxnoPC molecules. The oxidized sn-2 chains are shown in yellow, except for the terminal functional group. The rest of the molecules are shown in blue. The horizontal black line represents the approximate average location of the phosphate groups in one bilayer leaflet. This data provides evidence that the polar sn-2 chain of oxPL pocks out to the aqueous phase (PazePC) or is located at the hydrophobic/hydrophilic interface (PoxnoPC). Therefore, it may be available for interactions with proteins from in- and outside the cells.
Figure taken from Khandelia and Mourtisen, 2009, Biophys.J.96 [14].
Several biophysical data have already been published that are in line with the model of Khandelia and Mouritsen [14]. Probing membrane properties with a set of fluorophores provided evidence that the microenvironment sensed in POVPC aggregates is less polar and less hydrated than in artificial membranes containing PGPC [15]. The dipolar surface potential of POVPC is also lower than the corresponding value for PGPC [15]. Both phenomena are in agreement with the supra-molecular properties of both oxidized phospholipids described above. The same tendencies were found in comparative studies on the longer-chain homologs Poxno-PC and Paze-PC. Fluorescence measurements again revealed lower micropolarities in the aldehydophospholipid (Poxno-PC) membranes. However, the differences in polarity measured for the slightly more hydrophobic Poxno-PC and Paze-PC are smaller than those between POVPC and PGPC. Beranova et al. [16] showed that a carboxy residue on the truncated sn-2 chain of PGPC increased lateral diffusion but hardly affected local mobility of membrane lipids. For the aldehydo-phospholipid POVPC the opposite effect was observed.
In addition to high polarity, hydration and mobility, aldehydophospholipids and other structurally related lipid oxidation products show another distinct molecular feature, namely a conical molecular shape. The polar sn-2 acyl chains become part of a huge polar head group possessing a much larger cross sectional area than the long hydrophobic sn-1 acyl chain. As a consequence [17,18], such lipids can be expected to induce the formation of positive membrane curvatures leading to a series of structural and functional consequences. First of all, the formation of membrane vesicles can be facilitated. Single molecule microscopy revealed that a fluorescent PGPC analog was rapidly enriched in lipid patches within milliseconds followed by release of fluorescent vesicles inside the cells [19]. Similar experiments using a fluorescent POVPC analog are currently under way in this laboratory. The contribution of the oxidized phospholipids to the extracellular release of membrane vesicles (membrane blebbing, e.g. in apoptotic cells) is not so clear. POVPC is less efficient in this regard than PGPC (Stemmer et al., unpublished), very likely because it does not move freely in the membrane due to chemical reaction (imine formation) with proteins and aminophospholipids. The “curvature effect” of oxidized phospholipids may also lead to functional consequences for the proteins preferring a particular membrane environment. The resultant increase in membrane heterogeneity could lead to preferred partitioning of proteins (and aminophospholipids) in particular subphases and alter their activities and reactivities. According to data obtained from a proteomic analysis, Schiff base formation of polypeptides with a fluorescent POVPC analog in living cells is very selective (see below). This effect can be due to differences in pK, transversal localization and steric accessibility of protein amino groups as well as the lateral protein partitioning in lipid phases induced by the oxidized phospholipids [20].
3. Schiff base formation by aldehydophospholipids
Oxidized phospholipids are electrophiles. Depending on their chemical structures, they can interact with other biomolecules in an entirely physical manner or chemically, leading to a variety of covalent conjugates. Aldehydophospholipids represent a typical subclass of chemically reactive lipid oxidation products. They show the capacity of modifying free amino groups in small molecules (amino acids, aminophospholipids) as well as polypeptides by Schiff base formation [21]. As a consequence of chemical modification, localization, association and activity of enzymes and signaling proteins may be altered. If lipid–lipid adducts are formed, membrane organization may change profoundly thereby affecting lateral lipid organization, local membrane curvatures, and lipid mobilities. In the end, these lipid-associated effects may affect membrane protein function, membrane stability and the tendency to release membrane vesicles (exo- and endosomes).
A great deal of information is available about adduct formation between biological nucleophiles and hydroxynonenal (HNE) [22] which is a fragment released from the ω-end of a (phospholipid-bound) fatty acyl chain under oxidative stress. In contrast to HNE, information on conjugation of phospholipid aldehydes to biomolecules and its physiological consequences is still scarce. Inspection of the greatly different chemical structures and polarities of both compound classes shows that modification of biomolecules by HNE or oxidized phospholipids may elicit quite different effects. Modification of proteins by water-soluble HNE will change protein solubility and localization to a lesser extent as compared to modification by a phospholipid aldehyde [23]. Schiff base formation with the latter compounds leads to addition of a hydrophobic fatty acyl chain to the nucleophilic target, which represents a potential membrane anchor. This assumption is in line with data published by Kinnunen and collaborators. They found that Poxno-PC improves membrane association of phospholipase A2 and as a consequence the activity of this lipid degrading enzyme [24].
Most aldehydophospholipids contain only one long-chain fatty acid. As a consequence, they efficiently partition between the membrane lipid and the water phase. Their nucleophilic targets can be hydrophobic and water soluble proteins, membrane phospholipids as well as small polar molecules (e.g. amino acids). The following chapters describe Schiff base formation with the respective biomolecules in solution, in plasma lipoproteins, in artificial and biological membrane preparations, in live cells and in tissues. Finally, the role of these phenomena in pathophysiology is discussed. Since lipoprotein modification and lipoprotein-cell interactions play a key role in atherogenesis, this aspect of oxidized phospholipids has so far received most attention. Implications of Schiff base formation by these compounds in other diseases have also been recognized but are not so well characterized [10,22,25–29].
3.1. Modification of proteins in solution
Aldehydophospholipids react with α- and ε-amino groups of amino acids and derivatives (e.g. lysine, valine, lysine methyl ester) to form Schiff bases. Typical imines were identified after chemical reduction and analysis of the resulting amines using LC-ESI MS (see below) [21]. The same functional groups are also modified by phospholipid aldehydes in proteins (N-terminal and lysyl amino residues, respectively). For this study, a mixture of 1-palmitoyl- (70%) and 1-stearoyl-2-(9-oxo)nonanoyl-glycerophosphocholine (30%) was prepared by ozonolysis of egg yolk phosphatidylcholine. The resulting preparation containing various oxidized lipids was used to determine the modification of free amino groups in horse skeletal muscle apomyoglobin which contains one free N-terminal amino acid and 19 ε-lysyl amino group. Interestingly, only two to four phospholipid aldehydes reacted with the protein depending on reaction conditions. Obviously, there is a preference of lipid aldehydes for certain amino groups in a protein depending on accessibility and protonation of the nitrogen atom (for analysis of Hsp 90 modification by HNE, see [30]). In this context, it has to be emphasized that the Schiff bases (imines) generated from phospholipid aldehydes and amines have to be stabilized before analysis by chemical reduction with NaCNBH3 giving the corresponding stable amines.
Kinnunen and colleagues reported that modification of polypeptides by Poxno-PC improved protein binding to artificial membranes [31]. The presence of the truncated phospholipid led to better intercalation of several antimicrobial peptides in phospholipid monolayers and bilayers (liposomes). Such an effect was not observed if the lipid aldehyde was replaced by Paze-PC which contains an ω-carboxy group in the oxidized sn-2 acyl chain. This data is in agreement with the assumption that alkylation by the oxidized phospholipids generates a hydrophobic tail which anchors the peptide in the membrane [31]. Apart from possible effects of oxidized phospholipids on protein structure, membrane anchoring might also contribute to the stimulation of phospholipase A2 activity on DPPC by Poxno-PC [24]. In the absence of the lipid aldehyde, a lag phase was observed before DPPC degradation could be detected. Addition of Poxno-PC entirely abolished the initial delay and led to immediate lipid degradation [22].
Formation of covalent protein conjugates with aldehydophospholipids can be visualized if fluorescent lipid analogs are used as electrophiles. Stemmer et al.[20] prepared complexes of bovine serum albumin with a fluorescent POVPC derivative carrying BY as fluorophore at the head group (BY-POVPC). After reductive stabilization (see above), a fluorescent band was detected after SDS electrophoresis, showing the same electrophoretic mobility as the unlabeled protein. BSA can noncovalently bind one molecule of lyso-PC [32]. The complex of this protein with BY-POVPC is in addition stabilized by a covalent imine bond. Whereas amines are stable, imines are not. Exposure of BY-POVPC-labeled BSA (imine form) to other nucleophiles, e.g. proteins leads to lipid exchange between the protein competitors. This phenomenon shows that phospholipid aldehydes are exchangeable in the biological milieu despite the fact that they are covalently bound to their protein (or aminophospholipid) targets (see also lipoprotein and cell binding).
3.2. Modification of protein components in lipoproteins
The plasma lipoproteins LDL and HDL play a key role in the development and progress of atherosclerosis. Whereas LDL is proatherogenic, certain HDL subclasses exert a protective effect. Under the conditions of oxidative stress, the protein and lipid components of both particles are subject to modification by free radical-mediated and nonradical mechanisms turning the properties of both lipoproteins to the unfavorable side. From in vitro experiments with oxidized lipoprotein models, much information was obtained as to the relationship between the extent and the mode of oxidation on the one hand and the biological activities of the modified lipoproteins on the other hand. In minimally modified lipoproteins (e.g. obtained after oxidation with Fe2 + ions), mostly the lipid components are modified [33]. Phospholipid aldehydes are already present at this stage but apoB modification by these and other (e.g. HNE, MDA) electrophiles is not efficient enough, since mmLDL is still recognized by the classical apoB receptor [5]. Therefore, it is a good system to study the toxic effects of LDL lipids in vascular cells beyond receptor-mediated uptake of the entire particle. More extensive (e.g. Cu2 +-catalyzed) oxidation of lipoproteins also affects the apoproteins either by direct free radical attack or modification by oxidized lipids leading to dramatic changes in particle recognition by receptors. Copper-oxidized LDL uptake into cells is mediated by the scavenger receptor which is a hallmark in atherogenesis. It leads to unregulated cholesterol import into macrophages and eventually to the formation of foam cells [25]. The detrimental modification of apoB is due to the reaction with a series of aldehydes including MDA, HNE and core aldehydes generated from steryl esters and triacylglycerols, and last but not least oxidized phospholipids including POVPC, Poxno-PC and the keto lipid KDdia-PC (see Section 1). All these compounds mask the lysine clusters which represent the receptor docking sites in LDL. As a consequence, they render the particle more negative which makes it a good ligand for scavenger receptors [34]. Furthermore, lipid oxidation products interact with Toll-like receptors [35–39]. Recent studies provided evidence that interactions of oxidized phospholipids and oxidized LDL with TLR2 and CD36 trigger apoptosis in macrophages which suffered from ER stress induced by Thapsigargin [40].
Notably, interaction of oxLDL with cells is competitively inhibited by the POVPC-albumin complex [41,42] showing that the lipid-modified protein domains are involved in these processes. The albumin–lipid conjugate effectively competes with oxLDL uptake by macrophages [43,44]. The same modified protein also inhibits the uptake of oxLDL by cells transfected with the scavenger receptor CD36 [45]. The same effect was observed if particles prepared from a total oxLDL lipid extract were used instead of the lipoprotein.
Schiff base formation in LDL was visualized using fluorescent aldehydophospholipids [20]. After exposure of LDL to BY-POVPE (a head-group-labeled POVPC analog), a distinct fluorescent band corresponding to the imine (!) can be seen after electrophoresis on an agarose gel. The labeled particle migrates faster than native LDL since it has lost several positive charges. If the imine adduct is chemically reduced, the stable fluorescent amine adduct of apoB can be detected also after SDS electrophoresis. The fluorescent LDL imine adduct loses its labeled aldehydophospholipid if exposed to other amino nucleophiles [20]. This good exchangeability of aldehydophospholipids is physiologically relevant. Aldehydophospholipids are likely to contribute to the activatory effect of oxLDL on the prothrombinase complex [46,47]. Reductive stabilization of Schiff bases in this particle abolished this activity because the amine bound lipid components are no longer exchangeable. The stimulatory properties of oxLDL are mimicked by an ethanolamine phospholipid extract isolated from the oxidized lipoprotein. Obviously, this phospholipid fraction and proteins are nucleophiles capable of covalently binding (phospho)lipid aldehydes and removing them from other nucleophilic partners. Again, the lipid–lipid adducts are only active on the prothrombinase complex if added in the original imine form. After chemical reduction, they lose this activity.
Imine-bound POVPC is recognized in oxLDL as well as in POVPC-albumin conjugates by the antibody E06 [41,43]. This specific interaction is still maintained if the imines are reduced with NaCNBH3 [41]. From this data it can be inferred that the specific antigen-antibody binding is solely due to the choline phospholipid head group and does not depend on the type of lipid protein bonding. E06 as a protein can also undergo direct binding to POVPC by Schiff base formation, just as any other protein does.
HDL is also highly susceptible to oxidation [48]. Oxidative HDL modification is associated with changes in biophysical and immunological (see above) properties of apoAI and apoAII due to protein modification by malondialdehyde, hydroxynonenal [49], neutral core aldehydes and aldehydophospholipids. The existence of phospholipid–protein adducts in oxHDL was initially inferred from the increase in phosphorus content of isolated HDL apoproteins [50]. This effect correlated with the extent of lipoprotein oxidation and the formation of Poxno-PC and POVPC (see Section 1). Modification of HDL apoproteins by phospholipid aldehydes also elicits physiologically unfavorable effects [50]. Under physiological conditions, HDL removes cholesterol from the arterial wall to deliver it by receptor-mediated uptake into the liver. These beneficial properties are lost, if the particle is modified e.g. by Schiff base formation with lipid aldehydes. Modified ApoAI as a component of oxHDL or if isolated after incubation with Poxno-PC or POVPC was efficiently taken up by THP-1 macrophages [50]. The most likely receptor candidates for this process are CD36 and SR-BI [51]. OxHDL is a potent inhibitor of oxLDL binding to SR-BI. These results support the assumption that HDL modified by lipid aldehydes is largely recognized by the same receptors as modified LDL. They lead to an uncontrolled uptake of (oxidized) lipids by vascular cells thus contributing to foam cell formation and initiating the development of atherosclerosis [50]. Removal of cholesterol from the vascular wall (reverse cholesterol transport) is an important antiatherogenic function of HDL. This beneficial process is impaired by modification of apoAI by malondialdehyde [52] and it is likely that phospholipid aldehydes show similar effects.
Oxidation of lipoprotein (a) (Lp(a)) represents a particular aspect of vascular pathophysiology. This lipoprotein is basically an LDL particle which is associated with the apolipoprotein (a) the size of which varies depending on the Lp(a) isoform. Lp(a) has been identified as the main carrier of oxidized phospholipids in blood plasma [53,54]. These findings are interesting insofar as Lp(a) is a risk factor for atherosclerosis independent of LDL. To date it is not known how the individual lipid aldehydes influence the biological properties of this particle. Schiff base formation between phospholipids and apoB of the “LDL core” on the one hand and apo(a) on the particle surface on the other hand can be expected to induce very different effects on lipoprotein structure and activity.
3.3. Modifications of proteins in cells
Oxidized phospholipids can be delivered to cells by different donor systems including lipid–protein complexes and lipoproteins. Since oxidized phospholipids are very polar, it can be expected that a certain fraction is transferred to cells through the aqueous phase. Lipid uptake has been studied by fluorescence microscopy using suitably labeled fluorescent lipid analogs [20]. Single molecule studies showed that labeled oxidized phospholipids rapidly incorporate into the plasma membrane followed by internalization [19]. Albumin-bound PGPC and POVPC analogs (see above) are also easily available for cells. Oxidized phospholipids are abundant in oxidized lipoproteins [25]. Lipid uptake from these particles into cells is also very fast. Internalization of the entire lipoprotein via receptors facilitates but is not exclusively required for the latter process, since the highly amphipathic lipid oxidation products spontaneously exchange between proteins, lipoprotein and cell surfaces [20]. It has already been highlighted that even the covalently bound aldehydophospholipids POVPC and Poxno-PC are freely available for this exchange since Schiff bases are subject to modification by competing nucleophiles such as aminophospholipids and proteins.
Several attempts have already been made to identify the primary molecular targets of oxidized phospholipids in cells. In a proteome-wide study, cellular protein targets of hydroxynonenal have been determined [55]. To date, two proteomic approaches have been used to identify the cellular proteins that are covalently modified by labeled aldehydophospholipids. In these studies, cultured cells were incubated with the phospholipid aldehydes followed by reductive stabilization of the formed imines with NaCNBH3. After separation, the lipid-tagged proteins were detected, isolated, tryptically digested and identified by mass spectrometry.
Gugiu et al. [56] identified protein targets of oxidized phospholipid mixtures carrying biotin as affinity tag at the polar head group. These experiments were performed using human aortic endothelial cells (HAEC). The tagged proteins were isolated after binding to streptavidin beads and separated by SDS gel electrophoresis. A large number of different membrane-bound and water soluble proteins were identified, including structural and filament proteins, as well as proteins involved in stress response. It will be the goal of future studies to find out what are the functions of these targets in aldehydophospholipid toxicity (see below). The biological relevance of these data depends of course on the extent to which the labeled lipid analogs reflect the properties and interactions of their natural counterparts. Gugiu et al. [56] provide good evidence that lipid labeling does not significantly alter the biological activities of the natural compounds. From previous work, it is known that the biological activity of oxidized phospholipids depends much more on the oxidized sn-2 acyl chain than the polar head group. Oxidized phosphatidylethanolamine (PAPE) and -choline (PAPC) induce IL-8 production in HAEC. Both, oxPAPC and its labeled analog N-biotinyl-PAPE stimulated protein synthesis and mRNA expression of the same inflammatory response genes in various cells [56–58]. In addition, both compounds induced a similar inflammatory response in HAEC. Finally, it could be shown that unlabeled oxPAPC competed with the N-biotin-labeled lipid for the same protein targets [56].
Stemmer et al. [20] used a fluorescent POVPC analog carrying BODIPY as a reporter fluorophore boundto the nitrogen atom of the head group. The authors identified the protein targets of the oxidized phospholipids in cultured RAW 264.7 macrophages. The fluorescence label allowed direct sensitive detection of labeled proteins by a laser scanner on electrophoresis gels. After labeling of the live cells, tagged proteins were identified in total cell lysates after 2-D gel electrophoresis or in a total membrane fraction after separation by 1-D SDS gel electrophoresis. In both cases a large number of protein families with different functions were detected, including proteins involved in stress response, apoptosis, lipid metabolism and transport. Notably, the protein patterns on the electrophoresis gels clearly showed that protein tagging by the lipid was selective and by no means random. This phenomenon may be due to the fact that Schiff base formation in biological systems depends on several parameters including the pK of the reacting amino groups, the intracellular localization and accessibility of the affected proteins [20,59]. The selectivity of protein modification by the phospholipid aldehyde does not depend on the concentration of the oxidized phospholipid. Exposure of the cells to different lipid amounts always generated the same fluorescent protein pattern. We have evidence that fluorescent oxidized phospholipids may also be considered reliable analogs of their natural parent molecules. The fluorescent and the unlabeled lipids elicit the same apoptotic signaling in cells. In addition, the reaction of the target proteins with fluorescent and unlabeled lipid is competitive. Fluorescence tagging of cellular proteins by the latter compounds is abolished by POVPC in a concentration-dependent manner.
Ongoing studies in our laboratory aim at elucidating the identified POVPC targets as functional components of oxidized phospholipid toxicity. On the one hand, FRET experiments will be performed to determine the spatial proximity of GFP/RFP-tagged targets and the fluorescent POVPC. In addition, genes of the respective proteins will be knocked down and the influence on lipid toxicity in the cells will be measured. The functional approach is expected to improve our understanding of oxidized phospholipid toxicity in lipid-associated diseases and to help identify pharmacological targets for clinical intervention.
3.4. Consequences of protein modification by phospholipid aldehydes
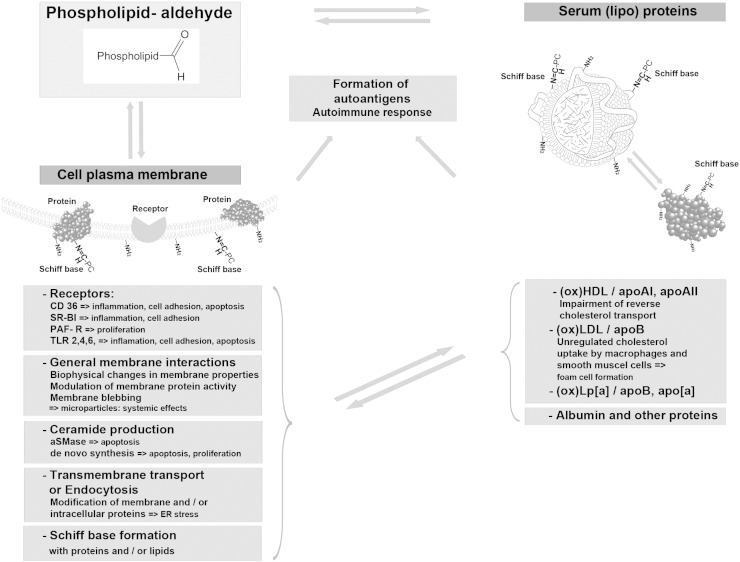
Modification by aldehydophospholipids may profoundly change the molecular properties of polypeptides, including net charge, conformation, state of association, enzymatic and signaling functions and last but not least, biological recognition, e.g. by receptors and the immune system (see Sections 2 and 3) (Fig. 3). These modifications on the molecular level lead to dramatic physiological consequences contributing to the onset or progression of diseases. Causal relationships between protein modification, e.g. in lipoproteins, and lipid-associated diseases have been the focus of numerous studies for many years. Using chemically defined oxidized phospholipids, it is possible to identify the contribution of different lipid components to the biological activities of oxidized lipoproteins. Both POVPC and and its sn-2 carboxy analog PGPC mediate harmful effects of oxidized LDL on the cells of the arterial wall [5,56]. However, the targets and pathways affected by these phospholipids are different. High amounts of POVPC are found in oxLDL and atherosclerotic plaques of mice and humans [8]. It activates the expression of specific adhesion molecules responsible for monocyte binding [8]. This effect seems to depend on Schiff base formation of POVPC with its primary targets because it is abolished if the phospholipid aldehyde is chemically reduced before addition to the cells [60]. Sustained exposure of vascular cells to POVPC or PGPC leads to apoptosis of vascular cells [5]. Again, the mechanisms of toxicity are different. The aldehydophospholipid activates an acid sphingomyelinase in cultured vascular smooth muscle cells [5] and macrophages (Stemmer et al. unpublished) within minutes. As a consequence, ceramide is formed which mediates the apoptotic signal. PGPC leads to a slow but sustained ceramide formation over hours. The enzymes contributing to this phenomenon are still subject to identification. According to recent work, ER stress and its biological consequences in macrophages are a major component of oxidized phospholipid toxicity [40].
Fig. 3.
Schematic representation of aldehydo-phospholipid exchange and its biological consequences. Phospholipid aldehydes form covalent Schiff bases with free amino groups of proteins and aminophospholipids. The phospholipid electrophiles are exchangeable between proteins, lipoproteins and cell surfaces, since the imines are subject to reaction with competing nucleophiles. (Lipo-)Proteins modified by aldehydo-phospholipids interact with receptors on the cell surface thereby triggering intracellular signaling cascades in a concentration- and time-dependent manner. Cooperative interactions of lipid-modified proteins with different receptors, e.g. CD 36 and TLRs, may lead to specific consequences (apoptosis or cell proliferation) depending on the cell surface components involved [38,40]. In addition, the toxic lipids may alter the organization of the membrane lipid bilayer and as a consequence unspecifically modify the activities of signaling and enzyme proteins. Finally, aldehydo-phospholipids can enter the intracellular space and modify protein localization and activities after Schiff base formation and lipidation. The biological consequences of the latter processes are currently subject to investigation [20]. In summary, the toxicity of oxidized phospholipids is due to a multifactorial phenomenon which is based both on the lipophilic character of these compounds and their selective interactions of proteins. If the target cells are components of the vascular wall, all these molecular events will contribute to the initiation and progression of atherosclerosis depending on the individual physiological circumstances. Modification of soluble proteins, lipoproteins and cellular proteins by aldehydophospholipids generates autoantigens which elicit an autoimmune response. The biological implications of this phenomenon are discussed in this special issue (see the chapter by C. Binder). Finally, oxidized phospholipids give rise to the formation of membrane vesicles. The resulting microparticles are spread through the circulation to various organs and can elicit toxic effects far distant from their site of origin.
Proteins conjugated to aldehyde (choline) phospholipids on lipoproteins and cell surfaces induce the formation of autoantibodies which can be detected in blood plasma [44]. The same immune response is likely to be triggered by so-called microparticles which are released from apoptotic cells and contain high amounts of oxidized phospholipids [43,61–63] (see Section 3.5 and the chapter by C. Binder in this special issue). Wang et al. [64] performed a well designed experiment twenty years ago to prove the formation of specific autoantibodies against a chemically defined aldehydophospholipid. They prepared Schiff bases from bovine thyroglobulin and a PAF analog containing an ω-aldehydoalkyl chain in position sn-1. After chemical stabilization by reduction, this complex was used for antibody production. The antibody was specific for the protein-associated PAF analog and showed only minor activity towards lyso-PAF, plasmalogens and other phospholipids.
Monoclonal antibodies against oxidized phospholipid-specific epitopes have been generated for analytical and diagnostic purposes. The anti-oxLDL antibody FOH1a/DLH3 recognizes modified apoB in oxidized LDL [65]. This antibody does not react with MDA-treated, acetylated or native LDL. However, it specifically binds to a series of polypeptides associated with POVPC or Poxno-PC [65]. Hörkkö et al. [44] produced monoclonal IgM antibodies from apoE-deficient mice (EO-autoantibodies), which bind to oxLDL. A widely used antibody is EO6 which specifically recognizes the polar head groups of oxidized but not native choline phospholipids [44]. Consistently, it inhibits the binding of copper-oxidized LDL as well as POVPC-serum albumin adducts to mouse macrophages [44]. Epitopes for EO-autoantibodies have also been found in human arteriosclerotic lesions and in circulating human LDL [42] providing evidence that aldehydophospholipid-associated proteins also exist in vivo. It has to be emphasized that covalent modification of apoB by oxidized phospholipids is not a requirement for oxLDL recognition by CD36 [66]. On the one hand, ApoB may not only be modified by lipid electrophiles but also by direct free radical attack by reactive oxygen, nitrogen or chlorine species. The result would be the same, namely a loss of lysines and an increase in net negative charge. On the other hand, negatively charged oxidized phospholipids such as PGPC or Paze-PC ae also inducers of CD36 binding. They do not covalently modify (apolipo)proteins but add electrostatic charges to the phospholipid monolayer of the lipoprotein particle.
EO6 has also been found to bind equimolar amounts of POVPC in protein-free form and this is plausible, too [41]. The free lipid aldehyde may also give rise to Schiff base formation with the antibody without any need of specific immune recognition of the lipid in protein-bound form. In the case of protein-conjugated aldehydophospholipids, EO6 recognizes the choline phospholipid head group irrespective of peptide sequence.
Proteins are subject to modification by (phospho)lipid aldehydes not only on (lipo)protein and cell surfaces. There is evidence that intracellular proteins also form lipid–protein Schiff bases [56]. Such lipid–protein adducts have been identified by proteomic analysis after affinity tagging with labeled lipid analogs (see Section 3.3). In addition, protein-bound choline phospholipid aldehydes have been detected using the specific antibody E06 [44].
It has been shown that modification of intracellular proteins by hydroxynonenal and malondialdehyde leads to alterations of intermolecular interactions and biochemical functions [9,10,23,29,67–70]. The physiological consequences of protein conjugation to phospholipid aldehydes inside cells will be subject to future investigations. A first step in this direction is the identification of the primary target candidates (see Section 3.3).
3.5. Particular aspects of oxidized phospholipids. Membrane blebbing and formation of microparticles
Uptake of oxidized phospholipids into cells as components of lipoproteins or other carrier systems (see Section 3.2) elicit cytotoxic effects [5,40]. Oxidative (lipid) stress may in turn stimulate the intracellular formation of such compounds and potentiate their toxicity in cells. The toxic effects of oxidized phospholipids are not only restricted to the site of their formation. Especially the truncated and highly modified derivatives are so polar that they readily exchange between cells through the aqueous phase and therefore can partition within a certain area of the affected tissue. A much more efficient way is the systemic administration of phospholipid oxidation products by vesicle (microparticle) transport through the circulation [61–63]. Sustained exposure to oxidized phospholipids can induce apoptosis or even necrosis [5]. Both types of cell death are accompanied by the release of membrane fragments either in a very controlled process leaving the parent cell intact (apoptotic blebs) or in a less defined way leading to cell lysis and formation of cell debris (necrosis). Membrane vesicles secreted from apoptotic cells contain high amounts of oxidized phospholipids [61–63] and as a consequence possess the capacity of transmitting the toxic compounds within tissues and the entire organism. Yang et al. [71] recently showed that levels of peroxidized phospholipids and their truncation products including HpODE-PC, Paze-PC and PGPC are elevated in blood plasma of rats and humans with steatohepatitis induced by chronic exposure to ethanol. The respective compounds contain sn-2 carboxyacyl chains. The analogous sn-2 aldehydoacyl phospholipids were not mentioned in this study though it seems likely that the latter compounds are also formed under oxidative stress in the liver. Data from independent studies using the antibody EO6 are in line with this assumption. They have provided evidence that protein-associated choline phospholipid head groups are components of microparticles isolated from blood plasma (C. Binder, personal communication).
The mechanism of (apoptotic) membrane blebbing may be based on different lipid and protein effects. First of all, the oxidized phospholipids themselves can increase the tendency of a biological membrane to release vesicles and these effects depend on minor differences in phospholipid structures to a large extent. In a time-resolved fluorescence microscopy study, Rhode et al. [19] showed that fluorescent PGPC induces the formation of endosomes within seconds [19]. In addition, this phospholipid gives rise to massive formation of exocytic plasma membrane vesicles (apoptotic blebs) (Stemmer et al. unpublished). Both effects can be explained in terms of phospholipid geometry. PGPC is conically shaped since it contains a large head group and only a narrow hydrophobic tail (sn-1 acyl chain) [18].
The aldehydophospholipid POVPC behaves entirely different as compared to its sn-2 carboxyacyl counterpart. It is internalized by cultured cells very slowly and the amount of exocytic particles released by this lipid is much smaller. In addition, the protein pattern of the respective microparticles is entirely different. Obviously, membrane blebbing induced by either phospholipid is a very specific process which does not only affect the amount but also the protein composition of microparticles (Stemmer et al. unpublished). It remains to be clarified to what extent the lipidomes of such membrane fragments are influenced. As discussed already in the previous chapters, membrane properties of the carboxylate lipids are entirely biophysical and entirely bound to the phospholipid bilayer. In contrast, POVPC can form Schiff bases with proteins and aminophospholipids (phosphatidylcholine and phosphatidylserine). Especially the lipid–lipid adducts are likely to be compounds that can destabilize bilayer structures. Khandelia and collaborators performed molecular dynamics studies and found that accumulation of such lipid condensation products leads to breakdown of phosphatidylcholine bilayers (H. Khandelia, personal communication.)
Apart from direct effects on microparticle formation, secondary effects of oxidized phospholipids may also come into play. POVPC rapidly activates a transient increase of acid sphingomyelinase activity within minutes (see above) which generates ceramide and thereby mediates cytotoxic signaling. It remains to be clarified whether this effect is due to direct lipidation of the enzyme by the phospholipid aldehyde. In contrast to POVPC, PGPC induces a slow but sustained increase in ceramide levels. The enzymes responsible for the latter effect are still subject to identification. In an elegant experiment, Kinnunen and colleagues demonstrated that ceramide shows a high capacity of inducing vesicle release from membranes [72]. If ceramide was generated from sphingomyelin by sphingomyelinase in large unilamellar vesicles, exo- or endocytosis was observed depending on the localization of the sphingolipid hydrolase (outside and inside, respectively). The time scale for ceramide formation under the influence of PGPC and POVPC is very different. As a consequence both lipids might contribute to the apparent microparticle profiles, e.g. in plasma, to a different extent with respect to time dependence, amount and composition of lipid particles.
Finally, cell death is associated with the externalization of phosphatidylserine. The respective phospholipid fraction contains oxidized fatty acyl chains. In addition, it can form lipid–lipid adducts with aldehydophospholipid and generate new toxic compounds. It will be a challenge of future studies to find out whether and to what extent Schiff base formation by aldehydophospholipids with this and other membrane components plays a role in the formation and the toxic effects of microparticles.
4. Perspectives
Aldehydophospholipids are covalently bound components of proteins in solution, in lipoproteins and in cells. Their biological activities have mainly been studied with emphasis on their role as components of oxidized lipoproteins and their contribution to atherogenic processes. Evidence has also been provided that these compounds and other oxidized phospholipids, are associated with various other diseases although it is not clear whether they are causally involved in the development or are generated as a consequence of the pathological conditions. A particular facet of the pathophysiology of aldehydophospholipids is their role in microparticle formation that is lipid-specific and contributes to their systemic cytotoxicity. The role of this lipid class in the formation and activity of microparticles is unknown. A priori, their biological activities need not always to be detrimental. It remains to be elucidated whether and under what circumstances aldehydophospholipids and their Schiff base adducts exert beneficial effects in the organism.
Acknowledgement
This work was financially supported by the Austrian Science Fund FWF (project F3006-B05 — special research program SFB Lipotox) and ESF EuroMEMBRANE CRP OXPL (project I308-B12).
Footnotes
This article is part of a Special Issue entitled: Oxidized phospholipids—their properties and interactions with proteins.
References
- 1.Marathe G.K., Davies S.S., Harrison K.A., Silva A.R., Murphy R.C., Castro-Faria-Neto H., Prescott S.M., Zimmerman G.A., McIntyre T.M. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J. Biol. Chem. 1999:28395–28404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 2.Khaselev N., Murphy R.C. Structural characterization of oxidized phospholipid products derived from arachidonate-containing plasmenyl glycerophosphocholine. J. Lipid Res. 2000:564–572. [PubMed] [Google Scholar]
- 3.Connelly P.W., Draganov D., Maguire G.F. Paraoxonase-1 does not reduce or modify oxidation of phospholipids by peroxynitrite. Free Radic. Biol. Med. 2005:164–174. doi: 10.1016/j.freeradbiomed.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Fruhwirth G.O., Loidl A., Hermetter A. Oxidized phospholipids: from molecular properties to disease. Biochim. Biophys. Acta. 2007:718–736. doi: 10.1016/j.bbadis.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Loidl A., Sevcsik E., Riesenhuber G., Deigner H.P., Hermetter A. Oxidized phospholipids in minimally modified low density lipoprotein induce apoptotic signaling via activation of acid sphingomyelinase in arterial smooth muscle cells. J. Biol. Chem. 2003:32921–32928. doi: 10.1074/jbc.M306088200. [DOI] [PubMed] [Google Scholar]
- 6.Moumtzi A., Trenker M., Flicker K., Zenzmaier E., Saf R., Hermetter A. Import and fate of fluorescent analogs of oxidized phospholipids in vascular smooth muscle cells. J. Lipid Res. 2007:565–582. doi: 10.1194/jlr.M600394-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Subbanagounder G., Watson A.D., Berliner J.A. Bioactive products of phospholipid oxidation: isolation, identification, measurement and activities. Free Radic. Biol. Med. 2000:1751–1761. doi: 10.1016/s0891-5849(00)00233-1. [DOI] [PubMed] [Google Scholar]
- 8.Watson A.D., Leitinger N., Navab M., Faull K.F., Horkko S., Witztum J.L., Palinski W., Schwenke D., Salomon R.G., Sha W., Subbanagounder G., Fogelman A.M., Berliner J.A. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 9.Schaur R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 2003:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 10.Uchida K., Toyokuni S., Nishikawa K., Kawakishi S., Oda H., Hiai H., Stadtman E.R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg M.E., Li X.M., Gugiu B.G., Gu X., Qin J., Salomon R.G., Hazen S.L. The lipid whisker model of the structure of oxidized cell membranes. J. Biol. Chem. 2008:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 12.Podrez E.A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P.J., Shan L., Gugiu B., Fox P.L., Hoff H.F., Salomon R.G., Hazen S.L. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 13.Wong-Ekkabut J., Xu Z., Triampo W., Tang I.M., Tieleman D.P., Monticelli L. Effects of lipid peroxidiation on the properties of lipid bilayers: a molecular dynamics study. Biophys. J. 2007:4225–4236. doi: 10.1529/biophysj.107.112565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandelia H., Mouritsen O.G. Lipid gymnastics: evidence of complete acyl chain reversal in oxidized phospholipids from molecular simulations. Biophys. J. 2009:2734–2743. doi: 10.1016/j.bpj.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pande A.H., Kar S., Tripathy R.K. Oxidatively modified fatty acyl chain determines physicochemical properties of aggregates of oxidized phospholipids. Biochim. Biophys. Acta. 2010:442–452. doi: 10.1016/j.bbamem.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Beranova L., Cwiklik L., Jurkiewicz P., Hof M., Jungwirth P. Oxidation changes physical properties of phospholipid bilayers: fluorescence spectroscopy and molecular simulations. Langmuir. 2010:6140–6144. doi: 10.1021/la100657a. [DOI] [PubMed] [Google Scholar]
- 17.Epand R.F., Mishra V.K., Palgunachari M.N., Anantharamaiah G.M., Epand R.M. Anti-inflammatory peptides grab on to the whiskers of atherogenic oxidized lipids. Biochim. Biophys. Acta. 2009:1967–1975. doi: 10.1016/j.bbamem.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith H.L., Howland M.C., Szmodis A.W., Li Q., Daemen L.L., Parikh A.N., Majewski J. Early stages of oxidative stress-induced membrane permeabilization: a neutron reflectometry study. J. Am. Chem. Soc. 2009:3631–3638. doi: 10.1021/ja807680m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhode S., Grurl R., Brameshuber M., Hermetter A., Schutz G.J. Plasma membrane fluidity affects transient immobilization of oxidized phospholipids in endocytotic sites for subsequent uptake. J. Biol. Chem. 2009:2258–2265. doi: 10.1074/jbc.M807591200. [DOI] [PubMed] [Google Scholar]
- 20.Stemmer U., Ramprecht C., Zenzmaier E., Stojčić B., Rechberger G., Kollroser M., Hermetter A. Uptake and protein targeting of fluorescent oxidized phospholipids in cultured RAW 264.7 macrophages. Biochim. Biophys. Acta. 2012:706–718. doi: 10.1016/j.bbalip.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravandi A., Kuksis A., Shaikh N., Jackowski G. Preparation of Schiff base adducts of phosphatidylcholine core aldehydes and aminophospholipids, amino acids, and myoglobin. Lipids. 1997:989–1001. doi: 10.1007/s11745-997-0129-6. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000:1685–1696. doi: 10.1016/s0891-5849(00)00226-4. [DOI] [PubMed] [Google Scholar]
- 23.Levonen A.L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G., Morrow J.D., rley-Usmar V.M. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Code C., Mahalka A.K., Bry K., Kinnunen P.K. Activation of phospholipase A2 by 1-palmitoyl-2-(9′-oxo-nonanoyl)-sn-glycero-3-phosphocholine in vitro. Biochim. Biophys. Acta. 2010:1593–1600. doi: 10.1016/j.bbamem.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Glass C.K., Witztum J.L. Atherosclerosis. the road ahead. Cell. 2001:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 26.Palinski W., Rosenfeld M.E., Yla-Herttuala S., Gurtner G.C., Socher S.S., Butler S.W., Parthasarathy S., Carew T.E., Steinberg D., Witztum J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. U. S. A. 1989:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayre L.M., Zelasko D.A., Harris P.L., Perry G., Salomon R.G., Smith M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J. Neurochem. 1997:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 28.Selley M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson's disease. Free Radic. Biol. Med. 1998:169–174. doi: 10.1016/s0891-5849(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 29.West J.D., Marnett L.J. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- 30.Connor R.E., Marnett L.J., Liebler D.C. Protein-selective capture to analyze electrophile adduction of hsp90 by 4-hydroxynonenal. Chem. Res. Toxicol. 2011:1275–1282. doi: 10.1021/tx200157t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattila J.P., Sabatini K., Kinnunen P.K. Oxidized phospholipids as potential molecular targets for antimicrobial peptides. Biochim. Biophys. Acta. 2008:2041–2050. doi: 10.1016/j.bbamem.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Brown S.D., Baker B.L., Bell J.D. Quantification of the interaction of lysolecithin with phosphatidylcholine vesicles using bovine serum albumin: relevance to the activation of phospholipase A2. Biochim. Biophys. Acta. 1993:13–22. doi: 10.1016/0005-2760(93)90260-g. [DOI] [PubMed] [Google Scholar]
- 33.Salvayre R., Auge N., Benoist H., Negre-Salvayre A. Oxidized low-density lipoprotein-induced apoptosis. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2002:213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 34.Gillotte K.L., Horkko S., Witztum J.L., Steinberg D. Oxidized phospholipids, linked to apolipoprotein B of oxidized LDL, are ligands for macrophage scavenger receptors. J. Lipid Res. 2000:824–833. [PubMed] [Google Scholar]
- 35.Bochkov V.N., Kadl A., Huber J., Gruber F., Binder B.R., Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 36.Erridge C., Kennedy S., Spickett C.M., Webb D.J. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 2008:24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J.S., Slutsky A.S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C.J., Penninger J.M. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A., Lacy-Hulbert A., El Khoury J., Golenbock D.T., Moore K.J. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walton K.A., Cole A.L., Yeh M., Subbanagounder G., Krutzik S.R., Modlin R.L., Lucas R.M., Nakai J., Smart E.J., Vora D.K., Berliner J.A. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 2003:1197–1203. doi: 10.1161/01.ATV.0000079340.80744.B8. [DOI] [PubMed] [Google Scholar]
- 40.Seimon T.A., Nadolski M.J., Liao X., Magallon J., Nguyen M., Feric N.T., Koschinsky M.L., Harkewicz R., Witztum J.L., Tsimikas S., Golenbock D., Moore K.J., Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friedman P., Horkko S., Steinberg D., Witztum J.L., Dennis E.A. Correlation of antiphospholipid antibody recognition with the structure of synthetic oxidized phospholipids. Importance of Schiff base formation and aldol condensation. J. Biol. Chem. 2002:7010–7020. doi: 10.1074/jbc.M108860200. [DOI] [PubMed] [Google Scholar]
- 42.Palinski W., Horkko S., Miller E., Steinbrecher U.P., Powell H.C., Curtiss L.K., Witztum J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 1996:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang M.K., Bergmark C., Laurila A., Horkko S., Han K.H., Friedman P., Dennis E.A., Witztum J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U. S. A. 1999:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horkko S., Bird D.A., Miller E., Itabe H., Leitinger N., Subbanagounder G., Berliner J.A., Friedman P., Dennis E.A., Curtiss L.K., Palinski W., Witztum J.L. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid–protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J. Clin. Invest. 1999:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boullier A., Gillotte K.L., Horkko S., Green S.R., Friedman P., Dennis E.A., Witztum J.L., Steinberg D., Quehenberger O. The binding of oxidized low density lipoprotein to mouse CD36 is mediated in part by oxidized phospholipids that are associated with both the lipid and protein moieties of the lipoprotein. J. Biol. Chem. 2000:9163–9169. doi: 10.1074/jbc.275.13.9163. [DOI] [PubMed] [Google Scholar]
- 46.Guichardant M., Taibi-Tronche P., Fay L.B., Lagarde M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med. 1998:1049–1056. doi: 10.1016/s0891-5849(98)00149-x. [DOI] [PubMed] [Google Scholar]
- 47.Zieseniss S., Zahler S., Muller I., Hermetter A., Engelmann B. Modified phosphatidylethanolamine as the active component of oxidized low density lipoprotein promoting platelet prothrombinase activity. J. Biol. Chem. 2001:19828–19835. doi: 10.1074/jbc.M007506200. [DOI] [PubMed] [Google Scholar]
- 48.Bowry V.W., Stanley K.K., Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl. Acad. Sci. U. S. A. 1992:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greilberger J., Jurgens G. Oxidation of high-density lipoprotein HDL3 leads to exposure of apo-AI and apo-AII epitopes and to formation of aldehyde protein adducts, and influences binding of oxidized low-density lipoprotein to type I and type III collagen in vitro1. Biochem. J. 1998:185–191. doi: 10.1042/bj3310185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed Z., Ravandi A., Maguire G.F., Kuksis A., Connelly P.W. Formation of apolipoprotein AI-phosphatidylcholine core aldehyde Schiff base adducts promotes uptake by THP-1 macrophages. Cardiovasc. Res. 2003:712–720. doi: 10.1016/s0008-6363(03)00257-8. [DOI] [PubMed] [Google Scholar]
- 51.Gillotte-Taylor K., Boullier A., Witztum J.L., Steinberg D., Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001:1474–1482. [PubMed] [Google Scholar]
- 52.Shao B., Pennathur S., Pagani I., Oda M.N., Witztum J.L., Oram J.F., Heinecke J.W. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J. Biol. Chem. 2010:18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bergmark C., Dewan A., Orsoni A., Merki E., Miller E.R., Shin M.J., Binder C.J., Horkko S., Krauss R.M., Chapman M.J., Witztum J.L., Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 54.Edelstein C., Pfaffinger D., Hinman J., Miller E., Lipkind G., Tsimikas S., Bergmark C., Getz G.S., Witztum J.L., Scanu A.M. Lysine-phosphatidylcholine adducts in kringle V impart unique immunological and potential pro-inflammatory properties to human apolipoprotein(a) J. Biol. Chem. 2003:52841–52847. doi: 10.1074/jbc.M310425200. [DOI] [PubMed] [Google Scholar]
- 55.Vila A., Tallman K.A., Jacobs A.T., Liebler D.C., Porter N.A., Marnett L.J. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem. Res. Toxicol. 2008:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gugiu B.G., Mouillesseaux K., Duong V., Herzog T., Hekimian A., Koroniak L., Vondriska T.M., Watson A.D. Protein targets of oxidized phospholipids in endothelial cells. J. Lipid Res. 2008:510–520. doi: 10.1194/jlr.M700264-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Gargalovic P.S., Imura M., Zhang B., Gharavi N.M., Clark M.J., Pagnon J., Yang W.P., He A., Truong A., Patel S., Nelson S.F., Horvath S., Berliner J.A., Kirchgessner T.G., Lusis A.J. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc. Natl. Acad. Sci. U. S. A. 2006:12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeh M., Leitinger N., de Martin R., Onai N., Matsushima K., Vora D.K., Berliner J.A., Reddy S.T. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001:1585–1591. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 59.Sheves M., Albeck A., Friedman N., Ottolenghi M. Controlling the pKa of the bacteriorhodopsin Schiff base by use of artificial retinal analogues. Proc. Natl. Acad. Sci. U. S. A. 1986:3262–3266. doi: 10.1073/pnas.83.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subbanagounder G., Leitinger N., Schwenke D.C., Wong J.W., Lee H., Rizza C., Watson A.D., Faull K.F., Fogelman A.M., Berliner J.A. Determinants of bioactivity of oxidized phospholipids. Specific oxidized fatty acyl groups at the sn-2 position. Arterioscler. Thromb. Vasc. Biol. 2000:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 61.Distler J.H., Huber L.C., Hueber A.J., Reich C.F., III, Gay S., Distler O., Pisetsky D.S. The release of microparticles by apoptotic cells and their effects on macrophages. Apoptosis. 2005:731–741. doi: 10.1007/s10495-005-2941-5. [DOI] [PubMed] [Google Scholar]
- 62.Huber J., Vales A., Mitulovic G., Blumer M., Schmid R., Witztum J.L., Binder B.R., Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 2002:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 63.Huber L.C., Jungel A., Distler J.H., Moritz F., Gay R.E., Michel B.A., Pisetsky D.S., Gay S., Distler O. The role of membrane lipids in the induction of macrophage apoptosis by microparticles. Apoptosis. 2007:363–374. doi: 10.1007/s10495-006-0622-7. [DOI] [PubMed] [Google Scholar]
- 64.Wang C.J., Tai H.H. A facile synthesis of an aldehydic analog of platelet activating factor and its use in the production of specific antibodies. Chem. Phys. Lipids. 1990:265–273. doi: 10.1016/0009-3084(90)90164-m. [DOI] [PubMed] [Google Scholar]
- 65.Itabe H., Takeshima E., Iwasaki H., Kimura J., Yoshida Y., Imanaka T., Takano T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J. Biol. Chem. 1994:15274–15279. [PubMed] [Google Scholar]
- 66.Podrez E.A., Hoppe G., O'Neil J., Hoff H.F. Phospholipids in oxidized LDL not adducted to apoB are recognized by the CD36 scavenger receptor. Free Radic. Biol. Med. 2003:356–364. doi: 10.1016/s0891-5849(02)01294-7. [DOI] [PubMed] [Google Scholar]
- 67.Chen J., Petersen D.R., Schenker S., Henderson G.I. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol. Clin. Exp. Res. 2000:544–552. [PubMed] [Google Scholar]
- 68.Niemela O., Parkkila S., Pasanen M., Iimuro Y., Bradford B., Thurman R.G. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol. Clin. Exp. Res. 1998:2118–2124. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 69.Schlorff E.C., Husain K., Somani S.M. Dose- and time-dependent effects of ethanol on plasma antioxidant system in rat. Alcohol. 1999:97–105. doi: 10.1016/s0741-8329(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 70.West J.D., Marnett L.J. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem. Res. Toxicol. 2006:173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 71.Yang L., Latchoumycandane C., McMullen M.R., Pratt B.T., Zhang R., Papouchado B.G., Nagy L.E., Feldstein A.E., McIntyre T.M. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J. Biol. Chem. 2010:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holopainen J.M., Angelova M.I., Kinnunen P.K. Vectorial budding of vesicles by asymmetrical enzymatic formation of ceramide in giant liposomes. Biophys. J. 2000:830–838. doi: 10.1016/S0006-3495(00)76640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]