Abstract
Lipid peroxidation occurs in the context of many physiological processes but is greatly increased in various pathological situations. A consequence of phospholipid peroxidation is the generation of oxidation-specific epitopes, such as phosphocholine of oxidized phospholipids and malondialdehyde, which form neo-self determinants on dying cells and oxidized low-density lipoproteins. In this review we discuss evidence demonstrating that pattern recognition receptors of the innate immune system recognize oxidation-specific epitopes as endogenous damage-associated molecular patterns, allowing the host to identify dangerous biological waste. Oxidation-specific epitopes are important targets of both cellular and soluble pattern recognition receptors, including toll-like and scavenger receptors, C-reactive protein, complement factor H, and innate natural IgM antibodies. This recognition allows the innate immune system to mediate important physiological house keeping functions, for example by promoting the removal of dying cells and oxidized molecules. Once this system is malfunctional or overwhelmed the development of diseases, such as atherosclerosis and age-related macular degeneration is favored. Understanding the molecular components and mechanisms involved in this process, will help the identification of individuals with increased risk of developing chronic inflammation, and indicate novel points for therapeutic intervention. This article is part of a Special Issue entitled: Oxidized phospholipids—their properties and interactions with proteins.
Abbreviations: 4-HNE, 4-hydroxynonenal; AMD, age-related macular degeneration; AGE(s), advanced glycation end product(s); ApoE, apolipoprotein E; BSA, bovine serum albumin; C#, complement component #; CEP, carboxyethylpyrrole; CFH, complement factor H; CFHR, complement factor H related protein; CL, cardiolipin; CHD, coronary heart disease; CPS, capsular polysaccharide; CRP, C-reactive protein; CuOx-LDL, copper-oxidized LDL; DAMP(s), damage-associated molecular pattern(s); FAAB, 2-formyl-3-(alkylamino)butanal; HBGM1, high-mobility group box 1; HSP(s), heat shock protein(s); IL-#, interleukin-#; LDL, low-density lipoprotein; MAA, malonacetaldehyde; MDA, malondialdehyde; MDHDC, 4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde; MFG-E8, Milk fat globule epidermal growth factor 8 (lactadherin); NAb(s), Natural antibodies; OSE(s), oxidation-specific epitope(s); OxCL, oxidized cardiolipin; OxLDL, oxidized LDL; OxPS, oxidized phosphatidylserine; PAMP(s), pathogen-associated molecular pattern(s); PC, phosphocholine; PE, phosphatidylethanolamine; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphcholine; PRR(s), pattern recognition receptor(s); PUFA(s), polyunsaturated fatty acid(s); RAG, recombinase activating gene; TLR#, toll-like receptor
Keywords: Lipid peroxidation, Oxidized LDL, Apoptosis, Oxidation-specific epitope, Damage-associated molecular pattern, Pattern recognition receptor
Highlights
► Phospholipid peroxidation results in the formation of oxidation-specific epitopes. ► Oxidation-specific epitopes are recognized by soluble and cellular pattern recognition receptors. ► Recognition by these receptors promotes the clearance of biological waste. ► This innate defense is challenged by increased oxidative stress, such as in chronic inflammation.
1. Introduction
Living in an aerobic environment brings along constant oxidative modification of biomolecules as part of normal tissue turn over. Our organism is usually well equipped to provide an adequate response to this physiological process and maintain homeostasis. However, during certain situations such as inflammation, this oxidative pressure is even more enhanced, which may cause the accumulation of oxidized proteins and lipids [1]. To prevent this and to ensure an appropriate response, mechanisms need to be in place that recognize and bind such modified molecules. Several proteins of the innate immune system have evolved to fulfill this function. They respond to oxidatively damaged molecules, thereby alarming the host while at the same time protecting it from overwhelming or chronic inflammation by triggering clearance mechanisms. A detailed understanding of the molecular structures that identify “biological waste” and the proteins interacting with them will provide fundamental insights into physiological house keeping mechanisms of innate immunity and help understand pathologies that may result from an impairment of these responses.
2. Innate immunity
The innate immune system represents an evolutionary old defense strategy that is present in all multicellular organisms [2]. Based on cellular and humoral responses, it provides a first line of defense against invading microbes. Following a pathogenic insult, an inflammatory response is initiated, which involves the rapid recruitment of innate immune cells, particularly neutrophils and macrophages. These cells engulf invading microbes and start producing a number of cytokines and chemokines that eventually activate lymphocytes, thereby triggering adaptive immune responses. Unlike the highly specific adaptive immunity the innate immune system typically recognizes foreign invaders by a set of pathogen-associated molecular patterns (PAMPs), which bind to various evolutionary conserved pattern recognition receptors (PRRs) and act as danger signals alarming the innate immune system. Prototypic examples for a PAMP are bacterial lipopolysaccharides that bind to the prototypic PRR toll like receptor 4 (TLR-4). Apart from cell surface receptors, PRRs can also be represented by soluble pattern recognition proteins, like the plasma reactant C-reactive protein, which binds phosphocholine (PC) conjugated to (lipo)teichoic acid on capsular polysaccharides of Streptococcus pneumoniae.
An inflammatory response can also be triggered by endogenous self-structures in the absence of microbes, and such a response is generally referred to as “sterile inflammation” [3]. Sterile inflammation is part of normal wound healing and tissue repair, which rely on an appropriate inflammatory response to the tissue damage, as the newly recruited leukocytes remove cellular debris and secrete proteases, thereby allowing tissue remodeling. If the inflammatory insult remains unresolved, this can result in chronic inflammation, inappropriate tissue destruction, or fibrosis. In analogy to PAMPs, sterile inflammation is triggered by damage-associated molecular patterns (DAMPs), which are generated by tissue injury or break down [4]. DAMPs can be either newly generated, damage-associated structures like advanced glycation end products (AGEs) or host molecules that are typically sequestered inside the cells under physiological conditions and only released as a result of cellular stress, such as necrosis following trauma, ischemia reperfusion, or chemically induced injury. Prototypic DAMPs include epitopes found on intracellular proteins like the chromatin-associated protein high-mobility group box 1 (HMGB1) or heat shock proteins (HSPs), and have also been shown to bind and/or activate PRRs.
3. Oxidation-specific epitopes are DAMPs
Research of the last years has shown that specific structures generated as a result of lipid peroxidation are recognized by various arcs of innate immunity and thereby can modulate many physiological and pathological processes [5]. The peroxidation of phospholipids occurs during a plethora of biological processes, including cellular senescence and apoptosis [6,7]. Moreover, this is greatly enhanced during inflammation. As a result a number of highly reactive phospholipid peroxidation products that can modify autologous proteins and lipids are generated. For example, phosphatidylcholine, which is present in cell membranes and low density lipoprotein (LDL) particles, contains an sn-2 polyunsaturated fatty acid (PUFA) that makes it particularly prone to oxidation, which results in the generation of highly reactive breakdown products, such as malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), and the remaining core-aldehyde, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC). These reactive aldehydes can generate covalent adducts with primary amines of proteins and amino groups of lipids such as phosphatidylethanolamine (PE), and thereby form so-called oxidation-specific epitopes (OSEs) that are recognized by different immune receptors in a hapten-specific manner. Because many OSEs possess strong pro-inflammatory properties they have been proposed as novel kind of DAMPs. A conceptual review on this topic has been recently published elsewhere [5]. This review will cover primarily OSEs that result from oxidation of PUFAs in phospholipids and novel PRRs that have recently been identified in this context.
Lipid peroxidation occurs as a physiological process that is greatly accelerated during certain pathologies. Indeed, oxidation products such as oxidized phosphatidylcholine, MDA, 4-HNE and others have been documented in virtually all inflammatory diseases including atherosclerosis, pulmonary, renal, and liver diseases, as well as diseases affecting the central nervous system like multiple sclerosis and Alzheimer's disease [8–14]. Studies on the pathogenic role of oxidized LDL (OxLDL) have been instrumental in the identification of a variety of lipid peroxidation derived structures that may act as DAMPs [5,15]. An excellent historical perspective on this has recently been published by Steinberg and Witztum [16]. Once LDL gets trapped in the intimal layer, it can undergo oxidation via enzymatic as well as non-enzymatic mechanisms. The first includes among others the modification by 12/15-lipoxygenase and myeloperoxidase. The second, non-enzymatic mechanisms are mediated by free radicals which can be indirectly generated by NADPH oxidases and nitric oxide synthases. Under physiological conditions these reactions require the catalysis by transition metal ions or hemin (reviewed in Refs. [17–21]). Both pathways can yield a variety of different adducts, residing both on the protein as well as on the lipid moiety of LDL (for review see Ref. [22]). To study the function of OxLDL, many models have been established that are used as surrogates for OxLDL occurring in vivo. One prominent example is copper-oxidized LDL (CuOx-LDL), in which LDL is oxidized by incubation with CuSO4 for given time points. Thus, CuOx-LDL contains many different OSEs, but is particularly enriched in oxidized phospholipids. Other models such as MDA-modified LDL and 4-HNE-modified LDL are generated by active derivatization of LDL with MDA or 4-HNE. Thus, these models are deliberately enriched in the two types of aldehydic adducts, respectively. The validity of these models is underscored by the fact that OxLDL found in vivo carries OSEs, which was demonstrated by immunochemical detection using specific monoclonal antibodies that recognize defined OSEs, such as MDA-adducts and PC of oxidized phosphatidylcholine [8]. Subsequently, it could be demonstrated that cells undergoing apoptosis, but not viable cells, carry the same OSEs in their membranes, which is consistent with the fact that oxidative processes are known to be triggered during apoptosis [6,7]. Therefore, OSEs do not only signify situations of increased oxidative stress, but they are also formed under physiological conditions during normal cellular turn over. In this, OSEs allow the host's immune system to identify biological waste and discriminate viable from dying cells.
A number of OSEs derived from phospholipid peroxidation have been identified, and we will subsequently discuss the best studied examples.
3.1. Phosphocholine (PC)
One of the best characterized OSEs is PC of oxidized phosphatidylcholine, whose presence has been demonstrated in atherosclerotic lesions and a number of other inflammatory settings [8,9,12]. Upon oxidation of phosphatidylcholine, its PC head group becomes exposed and available for recognition by immune receptors [6,23]. Thus, the PC head group is a cryptic epitope present in native phospholipids of LDL or viable cells that becomes revealed and accessible for binding by pattern recognition proteins as a result of conformational changes only after oxidation or when cells undergo apoptosis. Although sphingomyelin contains a PC head group as well, it is not prone to oxidative modification due to the highly saturated fatty acids it is composed of. The PC head group has also been shown to be exposed on cell membranes that have been damaged by complement or certain phospholipases [24,25]. Finally, because PC is also present as component of the capsular polysaccharide of encapsulated bacteria, such as S. pneumoniae, as well as in other gram-positive and gram-negative bacteria, protozoa and worms [26], it provides an example for molecular mimicry between oxidized phospholipids and microbial antigens.
3.2. Malondialdehyde (MDA)
MDA and its many condensation products are reliable markers for oxidative stress and have been associated with multiple disorders, including atherosclerosis, Alzheimer's disease, multiple sclerosis, acute lung injury, alcoholic hepatitis and diabetes [8–14]. MDA is typically generated as an oxidative degradation product of PUFAs containing more than two methylene-interrupted double bonds. In mammalian tissue these are represented by arachidonic acid (20:4) and docosahexaenoic acid (DHA; 22:6). MDA can also be formed from prostaglandin-substrates in an enzymatic process involving platelet thromboxane synthase [27]. In vitro, MDA forms predominantly the low reactive enolate anion at neutral and alkaline conditions. The reactivity increases with lower pH when β-hydroxyacrolein becomes the predominant species. In this state, MDA rapidly forms Michael-type 1,4-additions with nucleophilic functional groups like primary amines. Of note, MDA can form several different adducts on ε-aminogroups. For example, MDA and monofunctional aldehydes such as acetaldehyde have been shown to mutually enhance each other's reactivity towards primary amines [28], which results in the formation of hybrid adducts, the so-called malonacetaldehyde (MAA) adducts. These consist of two different modifications: One adduct is a 1:1 adduct of MDA and acetaldehyde and was identified as the 2-formyl-3-(alkylamino)butanal derivative of an amino group (FAAB adduct). The second adduct is composed of two molecules of MDA and one molecule of acetaldehyde and was identified as the 4-methyl-1,4-dihydropyridine-3,5-dicarbaldehyde derivative of an amino group (MDHDC adduct). The generation of the MDHDC-adducts seems to require, in a first step, the formation of the FAAB product, and, in a second step, the generation of an MDA-enamine. The FAAB-moiety is transferred to the nitrogen of the MDA-enamine, forming the circular MDHDC adduct [29,30]. There is increasing evidence demonstrating that the latter adduct is the biologically most relevant [31,32].
3.3. 4-Hydroxynonenal (4-HNE)
4-HNE, which is a product of oxidized ω − 6 PUFAs, such as linoleic and arachidonic acids, is another lipid peroxidation break down product that is linked to diseases such as atherosclerosis, neurodegenerative diseases, and cancer. Among the many aldehydes formed during lipid peroxidation, α,β-unsaturated hydroxyalkenals, such as 4-HNE, are of particular interest, as they are highly reactive due to three chemical functions: An aldehyde group on C1, a double bond between C2 and C3 and a secondary alcohol group on C4. The carbons C3 and C1 are electrophilic sites prone to Michael-type additions [22]. 4-HNE can form stable Michael-type adducts with a hemiacetal structure on lysines, cysteines or histidines [33]. Cysteine exhibits the highest reactivity, followed by histidine and lysine. The LDL component apolipoprotein B, however, has been reported to be mainly modified on lysine residues [34].
3.4. Carboxyethylpyrrole (CEP)
ω-(2-carboxyethyl)pyrrole (CEP)-adducts have recently gained much attention, as they have been implicated in a series of pathophysiological processes leading to age-related macular degeneration (AMD). CEP is formed upon oxidation of DHA-containing lipids, which causes the formation of reactive electrophilic phospholipid fragments like 4-hydroxy-7-oxohept-5-enoates that convert primary amino groups on bystander molecules into CEP derivatives [35]. The fact that DHA is a main component of retinal photoreceptors and highly sensitive to oxidative damage due to its six double bonds provides an explanation for the accumulation of CEP-adducts in retinal tissues as well as in the plasma of AMD patients.
3.5. Oxidized phosphatidylserine (OxPS)
Phosphatidylserine comprises approximately 10% of all cellular phospholipids. In viable cells, it is preferentially found in the inner leaflet. However, some cellular processes, most notably apoptosis, cause a loss of membrane asymmetry and the exposure of phosphatidylserine on the outer leaflet of the cell membrane [36]. During apoptosis, PS is one of the preferred peroxidation substrates that undergoes substantial derivatization with hydroxyl- and hydroperoxyl species [37,38].
3.6. Oxidized cardiolipin (OxCL)
Cardiolipin contains four unsaturated fatty acid chains. It is typically found in mitochondrial membranes, but also present in LDL [39], where it can be targeted by oxidation. OxCLs are also generated in the process of cellular apoptosis (reviewed in Ref. [40]). For a detailed review on the role of OxCL in apoptosis see Ref. [40].
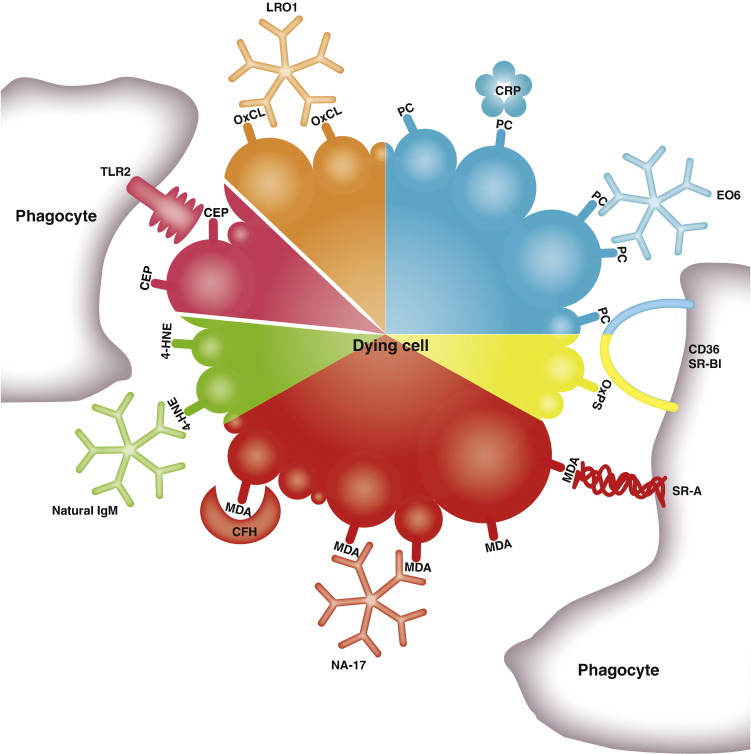
Most of these OSEs have been shown by immunohistochemical methods to occur in various inflammatory settings and during physiological processes, such as cellular aging or apoptosis [6,41]. Their recognition by receptors of innate immunity suggests an evolutionary selection for proper differentiation of damaged molecules, cells and cellular debris from intact molecules or viable cells. Both cellular and humoral components of innate immunity mediate this function (Fig. 1). It is likely that many more OSEs exist, including non-phospholipid derived structures [5].
Fig. 1.
Schematic representation of a dying cell bound by cellular and soluble PRRs that recognize different OSEs. Several OSEs are present in the membranes of dying cells, including phosphocholine (PC) of oxidized phosphatidylcholine, oxidized phosphatidylserine (OxPS), oxidized cardiolipin (OxCL), malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) adducts. Similarly, carboxyethylpyrrole (CEP) adducts are present on oxidatively damaged photoreceptors. Several PRRs of innate immunity recognize OSEs including the scavenger receptor CD36, CRP and IgM natural antibody (NAb) EO6, which bind PC; SR-A, CFH and IgM NAb NA-17, which bind MDA-adducts; the IgM NAb LRO1 that binds OxCL, CD36 binding OxPS, and TLR2 that binds CEP-adducts. B1 cell derived natural IgM with specificity for 4-HNE have also been identified.
4. Cellular PRRs recognize OSEs
4.1. Scavenger receptors (SRs)
The notion that OSEs are recognized by receptors of innate immunity stems from the initial search for a scavenger receptor for OxLDL. A number of SRs have been identified, including CD36, SRA-1 and A-2, SR-BI, MARCO, LOX-1, and others (for review see Ref. [42]). Although they all have been found to bind OxLDL, they recognize a variety of different patterns including structures identical to microbial PAMPs [42,43]. CD36 has been identified as a major SR for OxLDL [16,44] and it is now clear that it accounts for most of the binding and uptake of OxLDL by macrophages [45]. Binding of OxLDL to CD36 has been shown to be mediated by oxidized phospholipids, such as POVPC. In fact, this binding is in part mediated by the PC head group of oxidized phosphatidylcholine, while PC of native PL does not mediate recognition [46]. Both, PC-epitopes of oxidized phosphatidylcholine conjugated to proteins such as bovine serum albumin (BSA) or lipids mediate binding and uptake by the class B scavenger receptors, CD36 and SR-B1. This mode of interaction is also supported by studies, in which the PC-specific monoclonal natural IgM EO6 (see below) inhibited the binding of POVPC-modified BSA to CD36 and SR-B1 [6,46,47]. Importantly, both POVPC-BSA and EO6 were shown to completely inhibit the uptake of apoptotic cells by macrophages in vitro [6]. These data identify PC on OxLDL as well as apoptotic cells as critical DAMP for recognition by macrophage scavenger receptors. Interestingly, PC seems to be recognized by CD36 only as part of oxidized phosphatidylcholine present in OxLDL or on apoptotic cells, indicating the importance of the context in which the epitope is presented [46]. Additionally, oxidized PC-containing phospholipids have been shown to activate platelets via CD36 thereby promoting a prothrombotic phenotype [48]. The interaction of oxidized phospholipids with CD36 has also been suggested to be mediated by oxidized moieties on the sn-2 side chain of oxidized phospholipids, irrespective of the head group [49,50]. These aspects of oxidized phospholipid recognition are reviewed in Ref. [51].
Consistent with the identity of CD36 as PRR, multiple other ligands for it have been described [52]. Early studies identified an interaction between CD36 and phosphatidylserine [53,54]. In these experiments, however, no measures were taken to prevent oxidation and it has eventually become evident, that CD36 binds OxPS, rather than phosphatidylserine, on the surface of apoptotic cells [55]. The uptake of apoptotic cells could be competed in vitro by addition of excess amount of OxPS, but not native PS, indicating OxPS as another ligand for CD36 on the surface of apoptotic cells [55].
In analogy to the interaction of the OSEs PC and OxPS with CD36, other SRs may also bind specific OSEs. For example, SR-B1 and SR-A have also been shown to bind OxLDL [56] and the latter may serve as a receptor for MDA epitopes [57].
4.2. Toll-like receptors (TLRs)
TLRs represent classic PRRs of innate immunity, as they typically sense microbial PAMPs and initiate a host response to fight an infection. In the last years, however it became apparent that certain TLRs can also be activated by endogenous ligands such as heat shock proteins, hyaluronan, and heparan sulphate (reviewed in Ref. [3]). Oxidized 1-palmitoyl-2-arachidonyl-sn-glyero-3-phosphocholine (OxPAPC), which contains many different oxidized phospholipid species, has been shown to alter TLR4 complex formation with CD14 on endothelial cells [58,59]. This has been found to involve binding of oxidized phospholipids to CD14 and an altering of caveolar membranes [58]. For a detailed review on these aspects see Refs. [60,61]. Importantly, the binding to CD14 in its soluble form and to other lipoprotein-binding proteins seems to be responsible for the ability of OxPAPC to dampen LPS-induced TLR4 activation [59]. On the other hand, OxPAPC was shown to induce an inflammatory response in alveolar macrophages via TLR4 [9]. Although the exact epitope in OxPAPC responsible for this has not been identified, there is evidence supporting a role for PC, as the PC-specific IgM EO6 blocked IL-6 secretion induced by oxidized lung surfactant in macrophages [9]. Furthermore, the binding of OxLDL to CD36 has been shown to trigger an inflammatory response through the assembly of a TLR4/TLR6 heterodimer [62]. Only future studies using specific oxidized phospholipids and other lipids of OxLDL will help define the exact interactions.
Another evidence for the involvement of TLRs in recognizing OSEs has been recently demonstrated by West et al. In their elegant study they could show that CEP-modifications are bound by TLR2 in vitro, and that TLR2, but not TLR4 are required for a CEP-induced angiogenic response in endothelial cells in vivo [63].
Thus SR and TLR are PRRs that recognize OSEs. Despite their promiscuity for several ligands any given receptor or combination of receptors seems to have specificity for one certain epitope. This specificity as well as the cooperation of a given repertoire of receptors on the surface of a cell transmits signals that link oxidative stress with innate immunity.
5. Soluble PRRs recognize OSEs
Similar to cellular PRRs, there are also soluble pattern recognition proteins that can bind molecular patterns. Some of these soluble PRRs are the secreted forms of cell surface PRRs, and may either act as decoy receptors or as vehicles to deliver the ligand to signaling receptors. Other soluble PRRs include members of the pentraxin family, such as C-reactive protein (CRP) or complement proteins, as well as germ line encoded natural antibodies, with various effector functions that will be discussed below.
5.1. C-reactive protein
CRP is a highly conserved pentameric pattern recognition molecule that is mainly produced by hepatocytes [64]. The plasma concentration of CRP rises rapidly in response to inflammation, making it a prototypical acute phase reactant and suggesting that it contributes to host defense. Due to its upregulation in bacterial infections, it is commonly used as a diagnostic marker for clinical purposes. Because CRP is moderately elevated even in chronic inflammatory states, it has also become a valuable biomarker for inflammation and cardiovascular disease [65].
CRP was originally identified because of its ability to bind PC covalently attached to the capsular polysaccharide of S. pneumoniae. The recruitment of CRP to the surface of pneumococci and other pathogens that carry PC is calcium dependent and can promote their clearance [66–69]. However, CRP also binds to the PC moiety of oxidized phosphatidylcholine present in OxLDL and apoptotic cells, where CRP triggers the early steps of the classical complement pathway, while limiting the amount of membrane attack complex [70,71]. Thereby, CRP binding improves opsonization and phagocytosis of apoptotic cells by macrophages, and promotes their silent clearance [71]. The impact of CRP on the uptake of OxLDL was tested by exogenously adding a mix of OxLDL and CRP to macrophages in vitro. These studies suggest that CRP facilitates the uptake of OxLDL [72,73], though the in vivo relevance of this is still unknown.
5.2. Complement factor H
CFH is a 155 kDa glycoprotein that is made up of 20 complement control protein (CCP) modules and is the major inhibitor of the alternative pathway of complement activation [74]. It binds to C3b, thereby accelerating the decay of the alternative pathway C3-convertase while at the same time acting as a cofactor for the factor I-mediated proteolytic inactivation of C3b to iC3b. CFH regulates complement both in fluid phase and on cellular surfaces. However, while CFH binds and inactivates promptly C3b in fluid phase, the inactivation of surface-bound C3b by CFH is dependent on the chemical composition of the surface to which C3b is bound. The affinity of CFH for C3b is strongly increased in the presence of sialic acid and other polyanionic molecules typically present on host cells [74].
We recently discovered that MDA epitopes are bound by CFH. This binding seems to require the presence of advanced MAA-adducts such as MDHDC and is Ca2+ and Mg2+ independent. It is mediated via CCP 7 and 20 within the CFH molecule, which could cooperatively mediate the interaction of CFH with MDA. We could further demonstrate that the common CFH SNP rs1061170, which leads to a Y > H exchange in amino acid 402 of CCP 7, results in decreased binding to MDA. MDA epitopes represent a major ligand for CFH on the surface of apoptotic and necrotic cells as well as apoptotic blebs. CFH exerts its cofactor activity on MDA-decorated surfaces, leading to the local generation of the anti-inflammatory iC3b fragment. Furthermore, CFH directly neutralizes MDA-induced IL-8 production by macrophages as well as retinal pigment epithelium cells [75]. In analogy to this, CFH has been previously shown to inhibit TNFα production by endothelial cells that were stimulated with apoptotic blebs [76]. We therefore speculate that this pro-inflammatory response is mediated by MDA epitopes on the surface of apoptotic blebs.
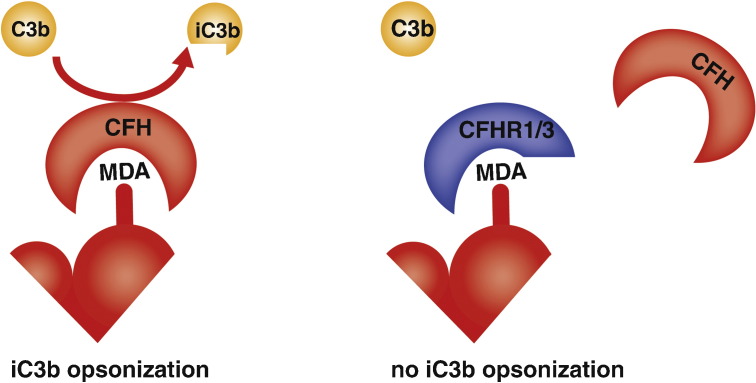
CFH is one representative of a family of complement regulators, whose other members, the so-called CFH-related proteins do not have a complement regulatory function, but share high sequence homology with the MDA-binding domains of CFH [74]. Competition assays suggested that CFHRs may interfere with the complement regulatory activity of CFH on MDA-decorated surfaces [75]. This is of particular interest, as this observation may provide an explanation for the fact that deficiencies in CFHR1 and CFHR3 were found to protect from age-related macular degeneration. Both CFHRs could compete with CFH for the binding to MDA epitopes in vivo, thereby preventing CFH to mediate its complement regulatory activity at these sites (Fig. 2) [77].
Fig. 2.
Model of competition between CFH and CFHR1/3 on MDA-decorated surfaces. (A) CFH binds to MDA on the surface of apoptotic cells, where it promotes factor I-mediated cleavage of C3b into iC3b. (B) CFHR1 and 3 lack a complement regulatory function but contain potential MDA-binding motifs and may therefore compete with CFH for MDA binding, leading to reduced anti-inflammatory iC3b generation on MDA-decorated surfaces.
5.3. Natural antibodies
NAbs represent another humoral component of innate immunity. The main source of NAbs, which are predominantly of the IgM class, is a specific B-cell subtype called B-1 cells [78,79]. The latter can be distinguished from the conventional B-2 cells by their surface marker expression, anatomical localization and activation requirements. Because their antigen binding sites are typically generated by the recombination of germ line encoded variable genes, these Abs have only a limited repertoire of binding specificities for diverse structures, such as (phospho)lipids and carbohydrates [80]. NAbs have specificity for both microbial and self antigens, which enables them to act in the first line defense against infections but also to provide house keeping functions by promoting the clearance of cellular debris. It is believed that both functions have led to a positive selection of certain specificities during evolution [80]. Recently, we found that OSEs are major targets of IgM NAbs in mice and humans [81]. The prototypic example of a NAb with specificity for OSEs is T15/EO6, which has specificity for PC. EO6 was originally cloned as an anti-OxLDL mAb from the spleens of atherosclerotic ApoE−/− mice, which have very high titers of IgM Abs against epitopes of OxLDL [8]. Subsequent characterization of its variable regions of both heavy and light chain revealed a 100% sequence identity with the prototypic anti-PC NAb T15 [82]. T15 has been studied for over 30 years for its ability to confer optimal protection from lethal infections with S. pneumoniae by binding the PC of capsular polysaccharide (CPS) [83]. Thus, T15/EO6 Abs recognize PC as part of oxidized phosphatidylcholine as well as PC directly conjugated to (lipo)teichoic acid of CPS. This dual specificity allows it to act in the first line defense against microbial infections as well as in mediating homeostatic house keeping functions.
Besides PC, IgM NAbs against other OSEs have also been described. For example, another anti-OxLDL monoclonal IgM Ab, LRO1, that was cloned from the spleens of hypercholesterolemic LDLR–/– mice was found to have specificity for oxidized, but not native cardiolipin. The variable region of this mAb is also encoded by germ line genes, and also binds to the surface of apoptotic but not viable cells [84].
To study the repertoire of NAbs with specificity for OSEs, we have selectively reconstituted immunodeficient recombinase activating gene (RAG)-1−/− mice with B-1 cells. Thus, by definition all immunoglobulins found in plasma of these mice are B-1 cell derived NAbs. Plasma of these mice contained primarily immunoglobulins of the IgM class and of all IgM NAbs that showed specificity to OSEs, including CuOx-LDL, MDA-LDL, and 4-HNE-LDL. IgM against MDA-adducts were the most prevalent. In fact, ~ 15% of all IgM NAbs were found to bind to MDA-type adducts, suggesting a great need to defend against this specific modification [81]. Indeed, MDA and MDA-modified proteins are known to induce inflammatory responses and are recognized by innate immunity [31,57]. We also cloned an IgM NAb with specificity for MDA-LDL from the spleens of B-1 cell reconstituted mice. This clone, termed NA-17, displayed complete germ line usage except for one nucleotide insertion in the variable region of the light chain. Importantly, consistent with the presence of MDA epitopes on the surface of dying cells, NA-17 strongly stained apoptotic cells and accelerated their in vivo clearance by macrophages [81]. The notion that OSEs are dominant targets of NAbs is also supported by studies using umbilical cord plasma as source of human NAbs. Unlike IgG, IgM Abs do not pass the placental barrier and therefore are exclusively of fetal origin, representing the human equivalent for NAbs. Consistent with the data obtained in mice, human umbilical cord blood contained high titers of IgM Abs against MDA-LDL and CuOx-LDL, but not against native LDL or an irrelevant protein [81].
Thus, OSEs represent major DAMPs for both cellular and soluble PRRs that recognize specific epitopes in a hapten-specific manner. Because the generation of OSEs is associated with cellular death or oxidative damage of molecules, these epitopes represent critical tags that allow innate immunity to distinguish between viable and damaged or dying cells on the other hand. For example PC of oxidized phosphatidylcholine present in OxLDL and membranes of dying cells is recognized by the SR CD36, CRP, and the IgM NAb T15/EO6. In analogy, MDA-adducts, also present in membranes of dying cells, are recognized by SR-A, CFH, and NAbs such as NA-17. Similarly, other OSEs such as OxCL or 4-HNE adducts are recognized by IgM NAbs, and we hypothesize that they too are also bound by a set of other PRRs. In addition, OxPS has been suggested to be bound by milk fat globule epidermal growth factor 8 (MFG-E8), which promotes the clearance of apoptotic cells by macrophages [85]. The interaction of OSEs with cellular and humoral PRRs is summarized in Table 1. The fact that such a high variety of PRRs is directed at specific OSEs underlines the need and importance to respond to them. Each of these PRRs fulfills an important physiological and non-redundant role in tissue homeostasis, which becomes even more critical under situations of increased oxidative stress. In analogy to PC of oxidized phosphatidylcholine and pneumococci, it is hypothesized that for many if not all OSEs identical or equivalent structures on microbial antigens exist.
Table 1.
Innate immune receptors interacting with OSEs and major functional consequences thereof.
| OSE | Humoral PRRs | Cellular PRRs |
|---|---|---|
| PC |
CRP: Ca2 +-dependent interaction; facilitates clearance of apoptotic cells and OxLDL in vitro [70,71] EO6/T15 IgM: blocks binding of OxLDL to CD36 [46] and SR-B1 [47] in vitro [6]; inhibits proinflammatory effects of oxidized phospholipids and apoptotic cells [9,109]; promotes clearance of apoptotic cells in vivo [107]; protects from atherosclerosis [110,111] |
CD36: major SR responsible for OxLDL uptake [44]; binding to OxLDL triggers assembly of TLR4/6 heterodimer [62]; mediates uptake of oxidatively damaged photoreceptors [116] SR-B1: mediates OxLDL uptake, which can be competed by EO6/T15 [48] |
| MDA |
CFH: binds via CCP 7 and 20, neutralizes proinflammatory properties of MDA, mediates iC3b generation, implications for age-related macular degeneration [75] NA-17 IgM: promotes clearance of apoptotic cells in vivo [81] |
SR-A: may serve as a receptor for MDA-LDL [57] |
| 4-HNE | IgM NAbs[81] | ? |
| OxCL | LRO1 IgM[84] | ? |
| CEP | ? | TLR2: mediates CEP-induced angiogenic response [63] |
| OxPS | MFG-E8: mediates clearance of apoptotic cells by macrophages [85] | CD36: important for the uptake of apoptotic cells via oxidized phosphatidylserine |
6. Disease implications
The described innate responses to OSEs have critical physiological house keeping functions as they protect from the negative consequences of the accumulation of cellular debris and oxidized membranes. If this process is intrinsically impaired or once it becomes overwhelmed, critical house keeping functions cannot be maintained which may trigger various pathologies. Chronic inflammatory processes in particular can be the consequence of either inefficient responses to oxidative stress and/or the accumulation of oxidation products beyond the immune system's capacity.
6.1. Atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the vascular wall and the underlying cause for heart attacks and a majority of strokes. It is a prototypic disease of increased oxidative stress with a complex pathogenesis that is influenced by many variables. One of the main factors contributing to the development of atherosclerotic lesions is an increased level of LDL cholesterol. The oxidation of LDL in the intimal space is one of the initiating events in the development of atherosclerotic lesions. Oxidative neoepitopes present on OxLDL facilitate SR-mediated uptake of OxLDL by macrophages. Once the levels of intimal OxLDL get too high, macrophages start to accumulate OxLDL resulting in foam cell formation. This is a key event in atherosclerotic lesion formation. In vitro experiments have demonstrated that up to 90% of OxLDL is taken up by macrophages via CD36 and SR-A [45]. Comparing human CD36 deficient monocyte/macrophages with normal monocyte/macrophages confirmed that about 50% of the OxLDL uptake can be attributed to CD36 [86]. Because this uptake also enhances the clearance of pro-inflammatory OxLDL in the presence of a functional cholesterol efflux activity, it may well be regarded initially as a protective response. In this way SRs fulfill a homeostatic function, and may therefore be also beneficial during early stages of atherosclerotic lesion formation. However, once OxLDL levels exacerbate in the atherosclerotic intima, SRs contribute to foam cell formation and thereby lesion progression. On the one hand they do so by binding of OSE-modified proteins, which triggers inflammatory responses [62,75]. On the other hand, owing to the cholesterol overload, foam cells eventually undergo apoptosis resulting in the accumulation of apoptotic cells that compete for the uptake by macrophages. If not swiftly cleared they further contribute to the pool of pro-inflammatory OSEs within atherosclerotic lesions [87]. In line with the quality of this response, atherosclerosis studies performed using SR-deficient mice have yielded different results depending on the anatomical site, stage of atherosclerosis and sex of the animal [88–90].
A similar house keeping function could be carried out by humoral PRRs that recognize OSEs. For example, CRP has been reported to promote the clearance of apoptotic cells in vitro [71]. Elevated levels of the CRP are a robust and independent biomarker for inflammation and the development of CVD. Although it is intriguing to speculate that the ability of CRP to bind PC of oxidized phosphatidylcholine could mediate a protective effect in atherosclerosis, there is little evidence supporting such a role. In fact initially many in vitro data suggested a pro-inflammatory function of CRP on vascular cells [65]. A number of studies have been conducted to assess the role of human CRP (mice do not express CRP) in various mouse models of atherosclerosis. Both passively administered human CRP and transgenically expressed CRP showed no conclusive effect on atherosclerosis development in mice [91–96].
In analogy to CRP, which binds PC of oxidized phosphatidylcholine, we have recently identified CFH as major MDA-binding protein in plasma. We and others have demonstrated the presence of CFH in atherosclerotic lesions [75,97,98] and CFH has been suggested to inhibit complement activation during atherogenesis [99]. Intriguingly, efficient clearance of CRP-labeled apoptotic cells requires the presence of CFH [71]. Therefore, by carrying CRP-binding PC- as well as CFH-binding MDA epitopes on their surface, apoptotic cells provide the ideal platform for complement mediated opsonization and clearance. Recently, a number of studies have evaluated the association of the CFH Y402H polymorphism, which impairs MDA binding, with the risk of coronary heart disease (CHD), ischemic stroke, and venous thromboembolism [99–101]. In a prospective cohort, the CFH Y402H polymorphism was associated with an increased risk for myocardial infarction. However, these results have not been consistent in multiple smaller cross-sectional or case-control studies, and no study has included African Americans [101,102]. In the multiethnic ARIC cohort, the CFH 402H allele was associated with an increased risk for incident CHD and ischemic stroke in whites, with the strength and significance of the association depending on the presence of hypertension [103]. A comprehensive meta-analysis published in 2010 based on eight different patient cohorts, however, did not find any association of the CFH Y402H variant with CHD [104].
There is now accumulating evidence demonstrating that plasma levels of IgM antibodies directed against models of OxLDL, such as MDA-LDL and CuOx-LDL are inversely correlated with the risk for coronary artery disease or carotid atherosclerosis. In fact, LDLR–/– mice deficient in secreted IgM develop accelerated atherosclerosis [105]. Moreover, transfer of B-1 cells into splenectomized ApoE–/– mice conferred atheroprotection, but this effect was lost when B-1 cells from sIgM–/– ko mice were transferred [106]. Because, a large part of natural IgM has specificity for OSEs it can be hypothesized that IgM NAb with these specificities mediate atheroprotection in part by promoting the clearance of cellular debris and neutralizing inflammatory activities of OSEs. IgM antibodies are important for complement mediated clearance of apoptotic cells [107]. This has been shown for the PC-specific IgM EO6, which both accelerates the clearance of apoptotic cells in vivo and neutralizes the pro-inflammatory effects of apoptotic blebs and oxidized phospholipids on endothelial cells and macrophages, respectively [9,108]. Furthermore, EO6 has been shown to inhibit the upregulation of IL-8 in endothelial cells after stimulation with apoptotic cells [109]. Similarly, the MDA-specific IgM NAb NA-17 has been shown to significantly enhance the uptake of apoptotic cells by macrophages in vivo [81]. We propose that NAbs inhibit SR-mediated uptake while at the same time promoting the clearance of OxLDL and apoptotic cells by other mechanisms involving complement.
Important insights for a role of OSE-specific IgM in atherogenesis came from experiments that exploited the molecular mimicry between pneumococci and OxLDL. We could show that immunizing mice with pneumococcal extracts resulted in robust production of anti-OxLDL IgM Abs. This increase in PC-specific IgM titers was nearly exclusively due to the expansion of EO6/T15id+IgM, and cholesterol-fed LDLR–/– mice immunized with this protocol resulted in significantly decreased lesion formation [110]. Faria-Neto et al. subsequently corroborated the atheroprotective role of this IgM clone in demonstrating that passive transfer of T15id+IgM reduced atherosclerosis in a vein graft atherosclerosis model [111]. Because IgM titers against other OSEs, most prominently MDA, are manifold higher than PC-specific titers, MDA-specific IgM NAb may provide even greater production. We speculate that the combined response of multiple OSE-specific IgM NAb has the capacity to provide optimal protection from atherosclerotic lesion formation.
6.2. Age-related macular degeneration (AMD)
AMD is the leading cause of irreversible blindness in older adults in industrialized countries [112,113]. The pathogenesis of AMD, a degenerative disorder affecting the retina that leads to irreversible vision loss, has been linked to increased oxidative stress [114,115]. Intense light exposure has been shown to generate oxidized phospholipids on the surface of outer photoreceptor segments, which leads to their uptake by retinal pigment epithelium cells in a CD36-dependent manner [116]. A hallmark of developing AMD is the accumulation of extracellular deposits, termed drusen, which have been shown to contain 4-HNE-, MDA-, as well as CEP-modified proteins [117]. Mice that were sensitized by immunization with CEP-adducted proteins display complement deposition in the eyes and develop signs of AMD [114]. On top of this, CEP-adducts have been shown to directly promote angiogenesis in a VEGF-dependent manner during laser-induced choroidal neovascularization, a model for the neovascular form of AMD [118]. In ischemia reperfusion and wound healing models, CEP induced VEGF secretion via a MyD88-dependent TLR2 signaling [63].
Several risk factors increase the susceptibility to AMD. Apart from advanced age, these include environmental factors like smoking as well as genetic factors [113]. In 2005, several independent research groups reported that the single nucleotide polymorphism rs1061170 in the CFH gene substantially increases the risk for developing AMD [119–122]. This variant has a frequency of 35% and may be responsible for more than 50% of all AMD cases [123,124]. Because we demonstrated that the amino acid substitution caused by this polymorphism substantially impairs the ability of CFH to bind to MDA epitopes, our findings provide a direct connection between oxidative stress and AMD pathogenesis. Furthermore, the risk variant of CFH has strongly impaired cofactor activity on MDA-decorated surfaces, such as represented by apoptotic cell membranes, and this may result in increased inflammatory responses due to inefficient clearance of cellular debris. Indeed, we showed that intravitreal injections of MDA-modified BSA in mice trigger IL-8 production in the retinal pigment epithelium, which was neutralized by simultaneous injection of CFH. The disease-associated variant of CFH may have reduced efficiency in neutralizing the pro-inflammatory effects of MDA. Future studies will show whether other SNPs predisposing for AMD also result in impaired MDA-binding capacity.
7. Conclusions
In this article we reviewed the existing evidence that identifies OSEs as important targets of innate immunity. Because of their capacity to induce inflammatory responses, OSEs represent endogenous danger signals that qualify as DAMPs. A variety of cell-associated as well as soluble PRRs have been found to target specific OSEs and provide critical house keeping functions of innate immunity. These include TLRs, SRs, as well as plasma proteins of innate immunity, such as CRP and CFH, and NAbs. All of these recognize OSEs as structural motifs that are generated as a consequence of lipid peroxidation. OSEs are generated in virtually all inflammatory processes and therefore ubiquitously present in various pathologies. The most abundant and relevant source of OSEs are lipoproteins as well as apoptotic cells, blebs, and cellular debris. Because programmed cell death occurs throughout life, it is clear that the need to respond to and remove dying cells efficiently represents a profound evolutionary pressure for the innate immune system. In this regard, OSEs are an intriguing target for PRRs, as they are only present in dying cells but not viable cells. Thereby, both cell-associated and soluble PRRs have the ability to distinguish between—potentially dangerous—dying cells, and viable healthy cells on the other hand. The fact that the same innate responses also target equivalent microbial PAMPs may provide an additional selection pressure.
The recognition of OSEs by innate immune receptors is highly relevant to various diseases, including atherosclerosis and AMD. The swift removal of dying cells is particularly imperative in these diseases, and several layers of immunity need to ensure this vital process. Once this system fails or becomes overwhelmed, cellular debris starts to accumulate resulting in inflammation, adverse immune activation, and disease. This can be a consequence of genetically impaired functions of the players involved or a chronically increased generation of oxidized waste. The latter is the case for diseases of increased oxidative stress. OxLDL in atherosclerotic lesions, as well as oxidatively damaged photoreceptors in the retina contain OSEs and therefore compete for the same clearance mechanisms as dying cells. Thus, in these sites of increased oxidative stress the threshold at which these homeostatic house keeping functions of innate immunity fail is much lower compared to other tissues, which increases their vulnerability. A better understanding of the identity and function of the molecular players involved will ultimately help identify individuals that possess a higher risk for disease. Moreover, this may also lead to novel therapeutic strategies that exploit endogenous protective mechanisms.
Acknowledgements
D.W. and C.J.B. were supported by the Austrian Academy of Sciences, a BRIDGE grant from the Austrian Research Promotion Agency (FFG), the SFB Lipotox F30 of the Austrian Science Fund (FWF), and the Fondation Leducq.
Footnotes
This article is part of a Special Issue entitled: Oxidized phospholipids—their properties and interactions with proteins.
References
- 1.Chou M.Y., Hartvigsen K., Hansen L.F., Fogelstrand L., Shaw P.X., Boullier A., Binder C.J., Witztum J.L. Oxidation-specific epitopes are important targets of innate immunity. J. Intern. Med. 2008;263:479–488. doi: 10.1111/j.1365-2796.2008.01968.x. [DOI] [PubMed] [Google Scholar]
- 2.Janeway C., Travers P., Walport M., Shlomchik M. 5th edition. 2001. Immunobiology. [Google Scholar]
- 3.Chen G.Y., Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 5.Miller Y.I., Choi S.H., Wiesner P., Fang L., Harkewicz R., Hartvigsen K., Boullier A., Gonen A., Diehl C.J., Que X., Montano E., Shaw P.X., Tsimikas S., Binder C.J., Witztum J.L. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang M.K., Bergmark C., Laurila A., Horkko S., Han K.H., Friedman P., Dennis E.A., Witztum J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan V.E., Borisenko G.G., Tyurina Y.Y., Tyurin V.A., Jiang J., Potapovich A.I., Kini V., Amoscato A.A., Fujii Y. Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic. Biol. Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Palinski W., Horkko S., Miller E., Steinbrecher U.P., Powell H.C., Curtiss L.K., Witztum J.L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G., Ermolaeva M., Veldhuizen R., Leung Y.H., Wang H., Liu H., Sun Y., Pasparakis M., Kopf M., Mech C., Bavari S., Peiris J.S., Slutsky A.S., Akira S., Hultqvist M., Holmdahl R., Nicholls J., Jiang C., Binder C.J., Penninger J.M. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horie K., Miyata T., Maeda K., Miyata S., Sugiyama S., Sakai H., van Ypersole C., de Strihou V.M., Monnier J.L., Witztum K. Kurokawa. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Invest. 1997;100:2995–3004. doi: 10.1172/JCI119853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez Perez M.J., Gonzalez-Reimers E., Santolaria-Fernandez F., de la Vega-Prieto M.J., Martinez-Riera A., Gonzalez P.A., Rodriguez Rodriguez E., Duran-Castellon M.C. Lipid peroxidation and serum cytokines in acute alcoholic hepatitis. Alcohol Alcohol. 2006;41:593–597. doi: 10.1093/alcalc/agl077. [DOI] [PubMed] [Google Scholar]
- 12.Haider L., Fischer M.T., Frischer J.M., Bauer J., Hoftberger R., Botond G., Esterbauer H., Binder C.J., Witztum J.L., Lassmann H. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134:1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dei R., Takeda A., Niwa H., Li M., Nakagomi Y., Watanabe M., Inagaki T., Washimi Y., Yasuda Y., Horie K., Miyata T., Sobue G. Lipid peroxidation and advanced glycation end products in the brain in normal aging and in Alzheimer's disease. Acta Neuropathol. 2002;104:113–122. doi: 10.1007/s00401-002-0523-y. [DOI] [PubMed] [Google Scholar]
- 14.Neale T.J., Ojha P.P., Exner M., Poczewski H., Ruger B., Witztum J.L., Davis P., Kerjaschki D. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J. Clin. Invest. 1994;94:1577–1584. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berliner J.A., Subbanagounder G., Leitinger N., Watson A.D., Vora D. Evidence for a role of phospholipid oxidation products in atherogenesis. Trends Cardiovasc. Med. 2001;11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D., Witztum J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 17.Miller Y.I., Choi S.H., Fang L., Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell. Biochem. 2010;51:229–251. doi: 10.1007/978-90-481-8622-8_8. [DOI] [PubMed] [Google Scholar]
- 18.Podrez E.A., Abu-Soud H.M., Hazen S.L. Myeloperoxidase-generated oxidants and atherosclerosis. Free Radic. Biol. Med. 2000;28:1717–1725. doi: 10.1016/s0891-5849(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 19.Shao B., Heinecke J.W. HDL, lipid peroxidation, and atherosclerosis. J. Lipid Res. 2009;50:599–601. doi: 10.1194/jlr.E900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chisolm G.M., Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic. Biol. Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 21.Steinbrecher U.P., Parthasarathy S., Leake D.S., Witztum J.L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc. Natl. Acad. Sci. U. S. A. 1984;81:3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Binder C.J., Chang M.K., Shaw P.X., Miller Y.I., Hartvigsen K., Dewan A., Witztum J.L. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 24.Narkates A.J., Volanakis J.E. C-reactive protein binding specificities: artificial and natural phospholipid bilayers. Ann. N. Y. Acad. Sci. 1982;389:172–182. doi: 10.1111/j.1749-6632.1982.tb22135.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y.P., Mold C., Du Clos T.W. Sublytic complement attack exposes C-reactive protein binding sites on cell membranes. J. Immunol. 1994;152:2995–3005. [PubMed] [Google Scholar]
- 26.Harnett W., Harnett M.M. Phosphorylcholine: friend or foe of the immune system? Immunol. Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 27.Hecker M., Ullrich V. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J. Biol. Chem. 1989;264:141–150. [PubMed] [Google Scholar]
- 28.Ohya T. Formation of a new 1,1,1 adduct in the reaction of malondialdehyde, n-hexylamine and alkanal under neutral conditions. Biol. Pharm. Bull. 1993;16:137–141. doi: 10.1248/bpb.16.137. [DOI] [PubMed] [Google Scholar]
- 29.Tuma D.J., Thiele G.M., Xu D., Klassen L.W., Sorrell M.F. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 30.Xu D., Thiele G.M., Kearley M.L., Haugen M.D., Klassen L.W., Sorrell M.F., Tuma D.J. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem. Res. Toxicol. 1997;10:978–986. doi: 10.1021/tx970069t. [DOI] [PubMed] [Google Scholar]
- 31.Thiele G.M., Duryee M.J., Willis M.S., Sorrell M.F., Freeman T.L., Tuma D.J., Klassen L.W. Malondialdehyde-acetaldehyde (MAA) modified proteins induce pro-inflammatory and pro-fibrotic responses by liver endothelial cells. Comp. Hepatol. 2004;3(Suppl. 1):S25. doi: 10.1186/1476-5926-2-S1-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duryee M.J., Klassen L.W., Schaffert C.S., Tuma D.J., Hunter C.D., Garvin R.P., Anderson D.R., Thiele G.M. Malondialdehyde-acetaldehyde adduct is the dominant epitope after MDA modification of proteins in atherosclerosis. Free Radic. Biol. Med. 2010;49:1480–1486. doi: 10.1016/j.freeradbiomed.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaur R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 34.Jurgens G., Lang J., Esterbauer H. Modification of human low-density lipoprotein by the lipid peroxidation product 4-hydroxynonenal. Biochim. Biophys. Acta. 1986;875:103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- 35.Gu X., Sun M., Gugiu B., Hazen S., Crabb J.W., Salomon R.G. Oxidatively truncated docosahexaenoate phospholipids: total synthesis, generation, and Peptide adduction chemistry. J. Org. Chem. 2003;68:3749–3761. doi: 10.1021/jo026721t. [DOI] [PubMed] [Google Scholar]
- 36.Fadok V.A., Bratton D.L., Frasch S.C., Warner M.L., Henson P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998;5:551–562. doi: 10.1038/sj.cdd.4400404. [DOI] [PubMed] [Google Scholar]
- 37.Tyurina Y.Y., Tyurin V.A., Zhao Q., Djukic M., Quinn P.J., Pitt B.R., Kagan V.E. Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis? Biochem. Biophys. Res. Commun. 2004;324:1059–1064. doi: 10.1016/j.bbrc.2004.09.102. [DOI] [PubMed] [Google Scholar]
- 38.Tyurin V.A., Tyurina Y.Y., Feng W., Mnuskin A., Jiang J., Tang M., Zhang X., Zhao Q., Kochanek P.M., Clark R.S., Bayir H., Kagan V.E. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J. Neurochem. 2008;107:1614–1633. doi: 10.1111/j.1471-4159.2008.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deguchi H., Fernandez J.A., Hackeng T.M., Banka C.L., Griffin J.H. Cardiolipin is a normal component of human plasma lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1743–1748. doi: 10.1073/pnas.97.4.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagan V.E., Bayir H.A., Belikova N.A., Kapralov O., Tyurina Y.Y., Tyurin V.A., Jiang J., Stoyanovsky D.A., Wipf P., Kochanek P.M., Greenberger J.S., Pitt B., Shvedova A.A., Borisenko G. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic. Biol. Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrano G.R., Parthasarathy S., Steinberg D. Recognition of oxidatively damaged erythrocytes by a macrophage receptor with specificity for oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U. S. A. 1994;91:3265–3269. doi: 10.1073/pnas.91.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greaves D.R., Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 2009;50 Suppl.:S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger M., Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu. Rev. Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 44.Endemann G., Stanton L.W., Madden K.S., Bryant C.M., White R.T., Protter A.A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 45.Kunjathoor V.V., Febbraio M., Podrez E.A., Moore K.J., Andersson L., Koehn S., Rhee J.S., Silverstein R., Hoff H.F., Freeman M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 46.Boullier A., Friedman P., Harkewicz R., Hartvigsen K., Green S.R., Almazan F., Dennis E.A., Steinberg D., Witztum J.L., Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J. Lipid Res. 2005;46:969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Gillotte-Taylor K., Boullier A., Witztum J.L., Steinberg D., Quehenberger O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001;42:1474–1482. [PubMed] [Google Scholar]
- 48.Podrez E.A., Byzova T.V., Febbraio M., Salomon R.G., Ma Y., Valiyaveettil M., Poliakov E., Sun M., Finton P.J., Curtis B.R., Chen J., Zhang R., Silverstein R.L., Hazen S.L. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Podrez E.A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P.J., Shan L., Febbraio M., Hajjar D.P., Silverstein R.L., Hoff H.F., Salomon R.G., Hazen S.L. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J. Biol. Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 50.Podrez E.A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., Finton P.J., Shan L., Gugiu B., Fox P.L., Hoff H.F., Salomon R.G., Hazen S.L. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol. Chem. 2002;277:38503–38516. doi: 10.1074/jbc.M203318200. [DOI] [PubMed] [Google Scholar]
- 51.Hazen S.L. Oxidized phospholipids as endogenous pattern recognition ligands in innate immunity. J. Biol. Chem. 2008;283:15527–15531. doi: 10.1074/jbc.R700054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silverstein R.L., Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryeom S.W., Silverstein R.L., Scotto A., Sparrow J.R. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J. Biol. Chem. 1996;271:20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- 54.Fadok V.A., Warner M.L., Bratton D.L., Henson P.M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J. Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 55.Greenberg M.E., Sun M., Zhang R., Febbraio M., Silverstein R., Hazen S.L. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeman M., Ekkel Y., Rohrer L., Penman M., Freedman N.J., Chisolm G.M., Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc. Natl. Acad. Sci. U. S. A. 1991;88:4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shechter I., Fogelman A.M., Haberland M.E., Seager J., Hokom M., Edwards P.A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J. Lipid Res. 1981;22:63–71. [PubMed] [Google Scholar]
- 58.Walton K.A., Hsieh X., Gharavi N., Wang S., Wang G., Yeh M., Cole A.L., Berliner J.A. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 2003;278:29661–29666. doi: 10.1074/jbc.M300738200. [DOI] [PubMed] [Google Scholar]
- 59.Bochkov V.N., Kadl A., Huber J., Gruber F., Binder B.R., Leitinger N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 60.Berliner J.A., Gharavi N.M. Endothelial cell regulation by phospholipid oxidation products. Free Radic. Biol. Med. 2008;45:119–123. doi: 10.1016/j.freeradbiomed.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bochkov V.N., Oskolkova O.V., Birukov K.G., Levonen A.L., Binder C.J., Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A., Lacy-Hulbert A., El Khoury J., Golenbock D.T., Moore K.J. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West X.Z., Malinin N.L., Merkulova A.A., Tischenko M., Kerr B.A., Borden E.C., Podrez E.A., Salomon R.G., Byzova T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Volanakis J.E. Human C-reactive protein: expression, structure, and function. Mol. Immunol. 2001;38:189–197. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 65.Verma S., Szmitko P.E., Ridker P.M. C-reactive protein comes of age. Nat. Clin. Pract. Cardiovasc. Med. 2005;2:29–36. doi: 10.1038/ncpcardio0074. quiz 58. [DOI] [PubMed] [Google Scholar]
- 66.Szalai A.J., Briles D.E., Volanakis J.E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in transgenic mice. J. Immunol. 1995;155:2557–2563. [PubMed] [Google Scholar]
- 67.Mold C., Nakayama S., Holzer T.J., Gewurz H., Du Clos T.W. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J. Exp. Med. 1981;154:1703–1708. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szalai A.J., VanCott J.L., McGhee J.R., Volanakis J.E., Benjamin W.H., Jr. Human C-reactive protein is protective against fatal Salmonella enterica serovar typhimurium infection in transgenic mice. Infect. Immun. 2000;68:5652–5656. doi: 10.1128/iai.68.10.5652-5656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lysenko E., Richards J.C., Cox A.D., Stewart A., Martin A., Kapoor M., Weiser J.N. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol. Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 70.Chang M.K., Binder C.J., Torzewski M., Witztum J.L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gershov D., Kim S., Brot N., Elkon K.B. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 2000;192:1353–1364. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Tits L., de Graaf J., Toenhake H., van Heerde W., Stalenhoef A. C-reactive protein and annexin A5 bind to distinct sites of negatively charged phospholipids present in oxidized low-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2005;25:717–722. doi: 10.1161/01.ATV.0000157979.51673.2c. [DOI] [PubMed] [Google Scholar]
- 73.Singh U., Dasu M.R., Yancey P.G., Afify A., Devaraj S., Jialal I. Human C-reactive protein promotes oxidized low density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. J. Lipid Res. 2008;49:1015–1023. doi: 10.1194/jlr.M700535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zipfel P.F., Skerka C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 75.Weismann D., Hartvigsen K., Lauer N., Bennett K.L., Scholl H.P., Charbel Issa P., Cano M., Brandstatter H., Tsimikas S., Skerka C., Superti-Furga G., Handa J.T., Zipfel P.F., Witztum J.L., Binder C.J. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mihlan M., Stippa S., Jozsi M., Zipfel P.F. Monomeric CRP contributes to complement control in fluid phase and on cellular surfaces and increases phagocytosis by recruiting factor H. Cell Death Differ. 2009;16:1630–1640. doi: 10.1038/cdd.2009.103. [DOI] [PubMed] [Google Scholar]
- 77.Hughes A.E., Orr N., Esfandiary H., Diaz-Torres M., Goodship T., Chakravarthy U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat. Genet. 2006;38:1173–1177. doi: 10.1038/ng1890. [DOI] [PubMed] [Google Scholar]
- 78.Berland R., Wortis H.H. Origins and functions of B-1 cells with notes on the role of CD5. Annu. Rev. Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 79.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 80.Baumgarth N., Tung J.W., Herzenberg L.A. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin. Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 81.Chou M.Y., Fogelstrand L., Hartvigsen K., Hansen L.F., Woelkers D., Shaw P.X., Choi J., Perkmann T., Backhed F., Miller Y.I., Horkko S., Corr M., Witztum J.L., Binder C.J. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J. Clin. Invest. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw P.X., Horkko S., Chang M.K., Curtiss L.K., Palinski W., Silverman G.J., Witztum J.L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Briles D.E., Forman C., Hudak S., Claflin J.L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J. Exp. Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tuominen A., Miller Y.I., Hansen L.F., Kesaniemi Y.A., Witztum J.L., Horkko S. A natural antibody to oxidized cardiolipin binds to oxidized low-density lipoprotein, apoptotic cells, and atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2006;26:2096–2102. doi: 10.1161/01.ATV.0000233333.07991.4a. [DOI] [PubMed] [Google Scholar]
- 85.Borisenko G.G., Iverson S.L., Ahlberg S., Kagan V.E., Fadeel B. Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine: implications for macrophage clearance of apoptotic cells. Cell Death Differ. 2004;11:943–945. doi: 10.1038/sj.cdd.4401421. [DOI] [PubMed] [Google Scholar]
- 86.Nozaki S., Kashiwagi H., Yamashita S., Nakagawa T., Kostner B., Tomiyama Y., Nakata A., Ishigami M., Miyagawa J., Kameda-Takemura K. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J. Clin. Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moore K.J., Kunjathoor V.V., Koehn S.L., Manning J.J., Tseng A.A., Silver J.M., McKee M., Freeman M.W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suzuki H., Kurihara Y., Takeya M., Kamada N., Kataoka M., Jishage K., Ueda O., Sakaguchi H., Higashi T., Suzuki T., Takashima Y., Kawabe Y., Cynshi O., Wada Y., Honda M., Kurihara H., Aburatani H., Doi T., Matsumoto A., Azuma S., Noda T., Toyoda Y., Itakura H., Yazaki Y., Kodama T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 90.Kuchibhotla S., Vanegas D., Kennedy D.J., Guy E., Nimako G., Morton R.E., Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ortiz M.A., Campana G.L., Woods J.R., Boguslawski G., Sosa M.J., Walker C.L., Labarrere C.A. Continuously-infused human C-reactive protein is neither proatherosclerotic nor proinflammatory in apolipoprotein E-deficient mice. Exp. Biol. Med. (Maywood) 2009;234:624–631. doi: 10.3181/0812-RM-347. [DOI] [PubMed] [Google Scholar]
- 92.Tennent G.A., Hutchinson W.L., Kahan M.C., Hirschfield G.M., Gallimore J.R., Lewin J., Sabin C.A., Dhillon A.P., Pepys M.B. Transgenic human CRP is not pro-atherogenic, pro-atherothrombotic or pro-inflammatory in apoE−/− mice. Atherosclerosis. 2008;196:248–255. doi: 10.1016/j.atherosclerosis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 93.Schwedler S.B., Amann K., Wernicke K., Krebs A., Nauck M., Wanner C., Potempa L.A., Galle J. Native C-reactive protein increases whereas modified C-reactive protein reduces atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2005;112:1016–1023. doi: 10.1161/CIRCULATIONAHA.105.556530. [DOI] [PubMed] [Google Scholar]
- 94.Trion A., de Maat M.P., Jukema J.W., van der Laarse A., Maas M.C., Offerman E.H., Havekes L.M., Szalai A.J., Princen H.M., Emeis J.J. No effect of C-reactive protein on early atherosclerosis development in apolipoprotein E*3-leiden/human C-reactive protein transgenic mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:1635–1640. doi: 10.1161/01.ATV.0000171992.36710.1e. [DOI] [PubMed] [Google Scholar]
- 95.Reifenberg K., Lehr H.A., Baskal D., Wiese E., Schaefer S.C., Black S., Samols D., Torzewski M., Lackner K.J., Husmann M., Blettner M., Bhakdi S. Role of C-reactive protein in atherogenesis: can the apolipoprotein E knockout mouse provide the answer? Arterioscler. Thromb. Vasc. Biol. 2005;25:1641–1646. doi: 10.1161/01.ATV.0000171983.95612.90. [DOI] [PubMed] [Google Scholar]
- 96.Hirschfield G.M., Gallimore J.R., Kahan M.C., Hutchinson W.L., Sabin C.A., Benson G.M., Dhillon A.P., Tennent G.A., Pepys M.B. Transgenic human C-reactive protein is not proatherogenic in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8309–8314. doi: 10.1073/pnas.0503202102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oksjoki R., Jarva H., Kovanen P.T., Laine P., Meri S., Pentikainen M.O. Association between complement factor H and proteoglycans in early human coronary atherosclerotic lesions: implications for local regulation of complement activation. Arterioscler. Thromb. Vasc. Biol. 2003;23:630–636. doi: 10.1161/01.ATV.0000057808.91263.A4. [DOI] [PubMed] [Google Scholar]
- 98.Seifert P.S., Hansson G.K. Complement receptors and regulatory proteins in human atherosclerotic lesions. Arteriosclerosis. 1989;9:802–811. doi: 10.1161/01.atv.9.6.802. [DOI] [PubMed] [Google Scholar]
- 99.Kardys I., Klaver C.C., Despriet D.D., Bergen A.A., Uitterlinden A.G., Hofman A., Oostra B.A., Van Duijn C.M., de Jong P.T., Witteman J.C. A common polymorphism in the complement factor H gene is associated with increased risk of myocardial infarction: the Rotterdam Study. J. Am. Coll. Cardiol. 2006;47:1568–1575. doi: 10.1016/j.jacc.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 100.Goverdhan S.V., Lotery A.J., Cree A.J., Ye S. Complement factor H Y402H gene polymorphism in coronary artery disease and atherosclerosis. Atherosclerosis. 2006;188:213–214. doi: 10.1016/j.atherosclerosis.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 101.Zee R.Y., Diehl K.A., Ridker P.M. Complement factor H Y402H gene polymorphism, C-reactive protein, and risk of incident myocardial infarction, ischaemic stroke, and venous thromboembolism: a nested case–control study. Atherosclerosis. 2006;187:332–335. doi: 10.1016/j.atherosclerosis.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 102.Giannakis E., Jokiranta T.S., Male D.A., Ranganathan S., Ormsby R.J., Fischetti V.A., Mold C., Gordon D.L. A common site within factor H SCR 7 responsible for binding heparin, C-reactive protein and streptococcal M protein. Eur. J. Immunol. 2003;33:962–969. doi: 10.1002/eji.200323541. [DOI] [PubMed] [Google Scholar]
- 103.Volcik K.A., Ballantyne C.M., Braun M.C., Coresh J., Mosley T.H., Boerwinkle E. Association of the complement factor H Y402H polymorphism with cardiovascular disease is dependent upon hypertension status: the ARIC study. Am. J. Hypertens. 2008;21:533–538. doi: 10.1038/ajh.2007.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sofat R., Casas J.P., Kumari M., Talmud P.J., Ireland H., Kivimaki M., Marmot M., Hughes A.D., Thom S., Ebrahim S., Whittaker J.C., Smeeth L., Lawlor D.A., Humphries S.E., Hingorani A.D. Genetic variation in complement factor H and risk of coronary heart disease: eight new studies and a meta-analysis of around 48,000 individuals. Atherosclerosis. 2010;213:184–190. doi: 10.1016/j.atherosclerosis.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 105.Lewis M.J., Malik T.H., Ehrenstein M.R., Boyle J.J., Botto M., Haskard D.O. Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kyaw T., Tay C., Krishnamurthi S., Kanellakis P., Agrotis A., Tipping P., Bobik A., Toh B.H. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ. Res. 2011;109:830–840. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 107.Ogden C.A., Kowalewski R., Peng Y., Montenegro V., Elkon K.B. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 108.Huber J., Vales A., Mitulovic G., Blumer M., Schmid R., Witztum J.L., Binder B.R., Leitinger N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 109.Chang M.K., Binder C.J., Miller Y.I., Subbanagounder G., Silverman G.J., Berliner J.A., Witztum J.L. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J. Exp. Med. 2004;200:1359–1370. doi: 10.1084/jem.20031763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Binder C.J., Horkko S., Dewan A., Chang M.K., Kieu E.P., Goodyear C.S., Shaw P.X., Palinski W., Witztum J.L., Silverman G.J. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat. Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 111.Faria-Neto J.R., Chyu K.Y., Li X., Dimayuga P.C., Ferreira C., Yano J., Cercek B., Shah P.K. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis. 2006;189:83–90. doi: 10.1016/j.atherosclerosis.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 112.Pascolini D., Mariotti S.P., Pokharel G.P., Pararajasegaram R., Etya'ale D., Negrel A.D., Resnikoff S. global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2002;11(2004):67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- 113.Congdon N., O'Colmain B., Klaver C.C., Klein R., Munoz B., Friedman D.S., Kempen J., Taylor H.R., Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 114.Hollyfield J.G., Bonilha V.L., Rayborn M.E., Yang X., Shadrach K.G., Lu L., Ufret R.L., Salomon R.G., Perez V.L. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Suzuki M., Kamei M., Itabe H., Yoneda K., Bando H., Kume N., Tano Y. Oxidized phospholipids in the macula increase with age and in eyes with age-related macular degeneration. Mol. Vis. 2007;13:772–778. [PMC free article] [PubMed] [Google Scholar]
- 116.Sun M., Finnemann S.C., Febbraio M., Shan L., Annangudi S.P., Podrez E.A., Hoppe G., Darrow R., Organisciak D.T., Salomon R.G., Silverstein R.L., Hazen S.L. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 2006;281:4222–4230. doi: 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schutt F., Bergmann M., Holz F.G., Kopitz J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- 118.Ebrahem Q., Renganathan K., Sears J., Vasanji A., Gu X., Lu L., Salomon R.G., Crabb J.W., Anand-Apte B. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: implications for age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13480–13484. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haines J.L., Hauser M.A., Schmidt S., Scott W.K., Olson L.M., Gallins P., Spencer K.L., Kwan S.Y., Noureddine M., Gilbert J.R., Schnetz-Boutaud N., Agarwal A., Postel E.A., Pericak-Vance M.A. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 120.Hageman G.S., Anderson D.H., Johnson L.V., Hancox L.S., Taiber A.J., Hardisty L.I., Hageman J.L., Stockman H.A., Borchardt J.D., Gehrs K.M., Smith R.J., Silvestri G., Russell S.R., Klaver C.C., Barbazetto I., Chang S., Yannuzzi L.A., Barile G.R., Merriam J.C., Smith R.T., Olsh A.K., Bergeron J., Zernant J., Merriam J.E., Gold B., Dean M., Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Edwards A.O., Ritter R., III, Abel K.J., Manning A., Panhuysen C., Farrer L.A. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 122.Klein R.J., Zeiss C., Chew E.Y., Tsai J.Y., Sackler R.S., Haynes C., Henning A.K., SanGiovanni J.P., Mane S.M., Mayne S.T., Bracken M.B., Ferris F.L., Ott J., Barnstable C., Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thakkinstian A., Han P., McEvoy M., Smith W., Hoh J., Magnusson K., Zhang K., Attia J. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum. Mol. Genet. 2006;15:2784–2790. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 124.Donoso L.A., Vrabec T., Kuivaniemi H. The role of complement Factor H in age-related macular degeneration: a review. Surv. Ophthalmol. 2010;55:227–246. doi: 10.1016/j.survophthal.2009.11.001. [DOI] [PubMed] [Google Scholar]