Abstract
We report here the genetic cause of the X-linked syndrome of psychosis, pyramidal signs, and macro-orchidism (PPM-X) in a three-generation family manifesting the disorder as a mutation in the methyl-CpG binding–protein 2 (MECP2) gene in Xq28. The A140V mutation was found in all affected males and all carrier females in the family. To date, descriptions have been published of two patients with independent familial mental retardation (MR) and two patients with sporadic MR who harbor this specific mutation in the MECP2 gene. This strongly suggests that A140V is a hot spot of mutation resulting in moderate to severe MR in males. A simple and reliable PCR approach has been developed for detection of the hot spot A140V mutation to prescreen any other unexplained cases of MR before further extensive mutation analyses.
X-linked mental retardation (XLMR) is estimated to cause 14% of all MR, corresponding to ∼3 cases per 1,000 births (Stevenson 2000). Among the ∼130 XLMR syndromes described, 25 gene loci have been identified and 55 further loci mapped. Regional assignment of different X-linked forms of MR was possible by identification of large families. Lindsay et al. (1996) presented a three-generation family with MR and manic-depressive psychosis, as well as pyramidal signs, parkinsonian features, and macro-orchidism, which is classified as the PPM-X syndrome (PPMX [MIM 300055]). Extensive linkage analysis mapped the PPM-X locus to Xq28, a region harboring three known genes (RabGDI1, FMR2, and MECP2) involved in XLMR syndromes.
Following up on the first report (Amir et al. 1999), several studies have shown mutations in the MECP2 gene located in Xq28 in patients with Rett syndrome (RTT [MIM 312750]) (for review, see Amir and Zoghbi 2000; Buyse et al. 2000; Bourdon et al. 2001), a sporadic severe neurological disorder occurring almost exclusively in females. Heterozygous mutations in MECP2 are found in as many as 80% of sporadic RTT cases. Mutations in males were thought to be lethal but have been described in rare cases of neonatal encephalopathy in children born into families with RTT (Schanen and Francke 1998; Schanen et al. 1998; Sirianni et al. 1998; Villard et al. 2000) and in one case of somatic mosaicism (Clayton-Smith et al. 2000). The wide spectrum of phenotypic variability in RTT is correlated with mutation type and location in the MECP2 gene (Wan et al. 1999; Cheadle et al. 2000) and possibly the pattern of X-inactivation (Amir et al. 2000). Recently, investigations of males with severe MR identified seven different mutations (Meloni et al. 2000; Orrico et al. 2000; Couvert et al. 2001), suggesting that the MECP2 gene is a strong candidate for XLMR. In addition, an individual with mild nonspecific MR who harbors an in-frame deletion in the MECP2 gene has been described (Yntema et al. 2002).
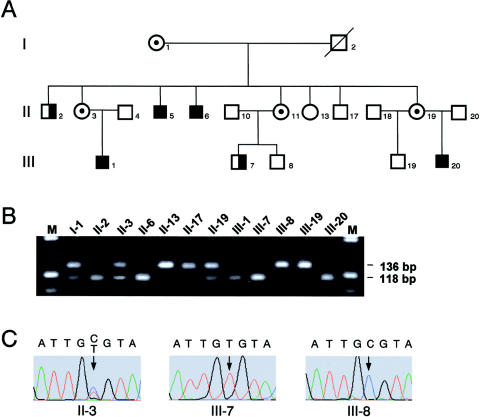
In the context of an ongoing examination of candidate genes for the PPM-X syndrome, mutation screening was performed in CLIC2, RabGDI1, and IRAK, and the results excluded these genes (data not shown). Consequently, screening of the MECP2 gene was performed by direct sequencing of six PCR fragments covering the entire coding region, using combinations of primer pairs described elsewhere (Amir et al. 1999; Buyse et al. 2000), which are available upon request. We found a C→T transition at position 419 in exon 4 (nucleotides numbered from the first base of the translation-initiation ATG codon [GenBank accession number X99686]). The transition resulted in an A140V mutation in the first two members of the family with PPM-X to be screened (the affected individuals (II-6 and III-7 in fig. 1A). This specific mutation has been described already in a family with severe MR in males (Orrico et al. 2000) and in two individuals with sporadic MR (Couvert et al. 2001). Subsequently, segregation analysis of the mutation in the family was performed on all available DNA samples, by development of a fast and reliable PCR-RFLP assay for the 419C→T (A140V) mutation using a modified forward primer 4amod and the published primer 4a-For.2 (Buyse et al. 2000), creating an AccI restriction site if the mutation is existent (fig. 1B). The A140V mutation was present in all affected individuals of the family and all female carriers under investigation, including the founder female I-1, but was absent in unaffected individuals II-13 (not included in the original published pedigree [Lindsay et al. 1996]) and II-17. To verify the results of the PCR-RFLP assay, the site of mutation was sequenced in all available DNA samples. Figure 1C shows electropherograms of carrier female II-3 and the affected male III-7 and his unaffected brother III-8. When the PCR-RFLP assay was used, the 419C→T mutation was not observed in 208 chromosomes from a German female control sample and 32 chromosomes from an appropriate British female control sample. RT-PCR spanning exons 3 and 4, including the site of mutation in the MECP2 gene, performed on RNA from lymphoblastoid cell lines from probands II-6, III-1, III-7, and III-8 (fig. 1A) did not show any difference between affected or unaffected family members (data not shown) as was expected for a missense mutation.
Figure 1.
Segregation of the mutation C419T in the family with PPM-X. A, Pedigree of part of the family. A square with the right half blackened represents an affected male with MR. A blackened square represents an affected male with MR and manic-depressive illness. A circle with a dot represents an obligate carrier female. B, PCR-RFLP assay for mutation detection. AccI-digested PCR products from exon 4, using the primers 4amod (5′-CGCCTACCTTTTCGAAGTAT-3′) and 4a-For.2 (5′-CGCTCTGCCCTATCTCTGA-3′, were separated on a 3% agarose gel; primers are modifications of those described by Buyse et al. [2000]) at an annealing temperature of 65°C. The modified forward primer 4amod, together with the C→T transition in position 419 (nucleotides numbered from the first base of the translation-initiation ATG codon; GenBank accession number X99686), creates an AccI restriction site generating two fragments of 118 bp and 18 bp from the 136-bp PCR product; because of its small size, the 18-bp digestion product is not visible on this agarose gel. M = molecular weight marker V (Roche). C, direct sequencing of the mutation 419C→T found in the family: II-3, carrier female (heterozygote 419C/T); III-7, affected male (hemizygote 419T); III-8, unaffected male (hemizygote 419C).
Comparison of the clinical features of male patients harboring the same A140V mutation in the MECP2 gene revealed similarities, besides moderate-to-severe MR, in the family described here and that described elsewhere (Orrico et al. 2000). Male patients of the family with PPM-X showed pyramidal signs and parkinsonian features (Lindsay et al. 1996), and affected males of the other family were reported to have resting tremors and slowness of movements (Orrico et al. 2000). The A140V mutation was evident in all affected males of the PPM-X family, whether they showed manic-depressive psychosis in addition to MR or not (fig. 1A). The possibility remains that such psychiatric features arose as a specific feature of the abnormal MECP2 gene or as a side effect of the MR phenotype and are not due to a mutation in a second gene causing the manic-depressive psychoses. The frequency and severity of the psychiatric features make it less likely that they are a side effect of the MR phenotype (Lindsay et al. 1996). For the two previously described sporadic cases with the A140V mutation, no clinical features other than moderate-to-severe MR have been reported (Couvert et al. 2001). It was not stated in the publication whether the patient who had additional psychiatric features harbored the A140V or one of the other three reported mutations. If the former were true, this would add to the phenotypic similarities found between carriers of this specific mutation.
In addition, we examined the X inactivation status in DNA from blood cells of the obligate carrier females (I-1, II-3, II-19) and female II-13, who is not carrying the mutation. One of the primers—either RS-6 or RS-7 (Meloni et al. 2000)—was fluorescently labeled, and the PCR products of the CAG repeat of the androgen receptor gene from HpaII digested and nondigested DNA (Allen et al. 1992) were run on an ABI 377 automatic sequencer (Applied Biosystems). The founder female I-1 was not informative for the polymorphism, but all other females showed a random pattern of X inactivation in peripheral blood leukocytes (data not shown). Therefore, the random pattern of X inactivation and the mild form of MR in the female heterozygous mutation carriers are further similarities between both familial cases harboring the A140V mutation. Additional phenotypic abnormalities in females of the family reported by Orrico et al. (2000)—such as microcephaly, asternic habitus with poorly muscled build, speech difficulties, genu valgum, and gait disturbance—have not been found in the family with PPM-X. This raises the possibility that skewed X inactivation might have occurred during developmental processes and/or in tissues other than peripheral blood lymphocytes. In addition, the healthy phenotype and random X-inactivation pattern in peripheral blood lymphocytes of the mothers of the two sporadic cases, who also carry the A140V mutation (Couvert al. (2001), point to the possibility of neuronal mosaicism resulting from skewed MECP2 expression. Overall, the mutated amino acid 140, located in the middle of the methyl-CpG binding–domain (MBD), would shorten the predicted α-helix length by half (Orrico et al. 2000). The shortened α-helix could explain the harmful effect of this mutation in hemizygous males. In addition, solution structure modeling of the MECP2 MBD-DNA complex located A140 relatively near the DNA backbone, and the A140V mutation was considered to cause reduced DNA-binding affinity through the introduction of valine’s bulky side chain (Ohki et al. 2001).
In conclusion, we report here the genetic cause of the PPM-X syndrome as a mutation in the MECP2 gene. This is the second family with MR that included affected individuals harboring the A140V mutation in the gene and showing additional similar clinical features in the affected males. The discovery of two further sporadic MR cases with the same mutation strongly suggests that A140V is a hot spot of mutation resulting in MR. Accordingly, the occurrence of several mutations in MECP2 resulting in MR in males should lead to a systematic screening of this gene in all unexplained cases of MR. The results also suggest that the A140V mutation in MECP2 has a role in the manic-depressive psychosis in this family, and such a role would be worth investigating further in patients with similar unexplained psychiatric features. A simple and reliable PCR approach has been developed for detection of the hot spot A140V mutation, which is here recommended as the first screening step before any further exhaustive mutation analyses of the coding region of the MECP2 gene.
Acknowledgments
We thank the family reported here for their help and cooperation. In addition, we thank Tatjana Kraus and Sabine Epp, for excellent technical assistance, Ute Ernst and Sara Burmester, for sequencing, and Ruth Sutton, for mutation screening in the British control sample.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PPMX [MIM 300055] and Rett [MIM 312750])
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for translation-initiation ATG codon [accession number X99686])
References
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW (1992) Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239 [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Schultz R, Malicki DM, Tran CQ, Dahle EJ, Philippi A, Timar L, Percy AK, Motil KJ, Lichtarge O, Smith EO, Glaze DG, Zoghbi HY (2000) Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann Neurol 47:670–679 [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188 [DOI] [PubMed] [Google Scholar]
- Amir RE, Zoghbi HY (2000) Rett syndrome: methyl-CpG-binding protein 2 mutations and phenotype-genotype correlations. Am J Med Genet 97:147–152 [DOI] [PubMed] [Google Scholar]
- Bourdon V, Philippe C, Labrune O, Amsallem D, Arnould C, Jonveaux P (2001) A detailed analysis of the MECP2 gene: prevalence of recurrent mutations and gross DNA rearrangements in Rett syndrome patients. Hum Genet 108:43–50 [DOI] [PubMed] [Google Scholar]
- Buyse IM, Fang P, Hoon KT, Amir RE, Zoghbi HY, Roa BB (2000) Diagnostic testing for Rett syndrome by DHPLC and direct sequencing analysis of the MECP2 gene: identification of several novel mutations and polymorphisms. Am J Hum Genet 67:1428–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle JP, Gill H, Fleming N, Maynard J, Kerr A, Leonard H, Krawczak M, Cooper DN, Lynch S, Thomas N, Hughes H, Hulten M, Ravine D, Sampson JR, Clarke A (2000) Long-read sequence analysis of the MECP2 gene in Rett syndrome patients: correlation of disease severity with mutation type and location. Hum Mol Genet 9:1119–1129 [DOI] [PubMed] [Google Scholar]
- Clayton-Smith J, Watson P, Ramsden S, Black GCM (2000) Somatic mutation in MECP2 as a non-fatal neurodevelopmental disorder in males. Lancet 356:830–832 [DOI] [PubMed] [Google Scholar]
- Couvert P, Bienvenu T, Aquaviva C, Poirier K, Moraine C, Gendrot C, Verloes A, Andrès C, Le Fevre AC, Souville I, Steffann J, des Portes V, Ropers H-H, Yntema HG, Fryns J-P, Briault S, Chelly J, Cherif B (2001) MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet 10:941–946 [DOI] [PubMed] [Google Scholar]
- Lindsay S, Splitt M, Edney S, Berney TP, Knight SJ, Davies KE, O'Brien O, Gale M, Burn J (1996) PPM-X: a new X-linked mental retardation syndrome with psychosis, pyramidal signs, and macro-orchidism maps to Xq28. Am J Hum Genet 58:1120–1126 [PMC free article] [PubMed] [Google Scholar]
- Meloni I, Bruttini M, Longo I, Mari F, Rizzolio F, D'Adamo P, Denvriendt K, Fryns JP, Toniolo D, Renieri A (2000) A mutation in the Rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males. Am J Hum Genet 67:982–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki I, Shimotake N, Fujita N, Jee J-G, Ikegami T, Nakao M, Shirakawa M (2001) Solution structure of the methyl-CpG binding domain of the human MBD1 in complex with methylated DNA. Cell 105:487–497 [DOI] [PubMed] [Google Scholar]
- Orrico A, Lam C, Galli L, Dotti MT, Hayek G, Tong SF, Poon PM, Zappella M, Federico A, Sorrentino V (2000) MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett 481:285–288 [DOI] [PubMed] [Google Scholar]
- Schanen C, Francke U (1998) A severely affected male born into a Rett syndrome kindred supports X-linked inheritance and allows extension of the exclusion map. Am J Hum Genet 63:267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanen NC, Kurczynski TW, Brunelle D, Woodcock MM, Dure LS, Percy AK (1998) Neonatal encephalopathy in two boys in families with recurrent Rett syndrome. J Child Neurol 13:229–231 [DOI] [PubMed] [Google Scholar]
- Sirianni N, Naidu S, Pereira J, Pillotto RF, Hoffman EP (1998) Rett syndrome: confirmation of X-linked dominant inheritance, and localization of the gene to Xq28. Am J Hum Genet 63:1552–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE (2000) Splitting and lumping in the nosology of XLMR. Am J Med Genet 97:174–182 [DOI] [PubMed] [Google Scholar]
- Villard L, Kpebe A, Cardoso C, Chelly J, Tardieu M, Fontes M (2000) Two affected boys in a Rett syndrome family. Neurology 55:1188–1193 [DOI] [PubMed] [Google Scholar]
- Wan M, Lee SS, Zhang X, Houwink-Manville I, Song HR, Amir RE, Budden S, Naidu S, Pereira JL, Lo IFM, Zoghbi HY, Schanen NC, Francke U (1999) Rett syndrome and beyond: recurrent spontaneous and familial MECP2 mutations at CpG hot spots. Am J Hum Genet 65:1520–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema HG, Oudakker AR, Kleefstra T, Hamel BCJ, van Bokhoven H, Chelly J, Kalscheuer VM, Fryns J-P, Raynaud M, Moizard M-P, Moraine C (2002) In-frame deletion in MECP2 causes mild nonspecific mental retardation. Am J Med Genet 107:81–83 [DOI] [PubMed] [Google Scholar]