Abstract
X-linked cone-rod dystrophy (COD1) is a retinal disease that primarily affects the cone photoreceptors; the disease was originally mapped to a limited region of Xp11.4. We evaluated the three families from our original study with new markers and clinically reassessed all key recombinants; we determined that the critical intervals in families 2 and 3 overlapped the RP3 locus and that a status change (from affected to probably unaffected) of a key recombinant individual in family 1 also reassigned the disease locus to include RP3 as well. Mutation analysis of the entire RPGR coding region identified two different 2-nucleotide (nt) deletions in ORF15, in family 2 (delAG) and in families 1 and 3 (delGG), both of which result in a frameshift leading to altered amino acid structure and early termination. In addition, an independent individual with X-linked cone-rod dystrophy demonstrated a 1-nt insertion (insA) in ORF15. The presence of three distinct mutations associated with the same disease phenotype provides strong evidence that mutations in RPGR exon ORF15 are responsible for COD1. Genetic heterogeneity was observed in three other families, including the identification of an in-frame 12-nt deletion polymorphism in ORF15 that did not segregate with the disease in one of these families.
X-linked cone-rod dystrophy (COD1 [MIM 304020]) is a rare, progressive visual disease primarily affecting the cone photoreceptors. Affected individuals (essentially all of whom are males) present with decreased visual acuity, myopia, photophobia, abnormal color vision, full peripheral visual fields, decreased photopic electroretinographic responses, and granularity of the macular retinal pigment epithelium (Pinckers and Timmerman 1981; Pinckers and Deutman 1987; Jacobson et al. 1989). The degree of rod-photoreceptor involvement can be variable, with degeneration increasing as the disease progresses. Although penetrance appears to be nearly 100%, there is variable expressivity with respect to age at onset, severity of symptoms, and findings (Hong et al. 1994).
X-linked retinitis pigmentosa (XLRP [MIM 268000]) is a severe form of inherited retinal degeneration that primarily affects the rod photoreceptors (Bird 1975; Fishman et al. 1988). It typically causes an early-onset night blindness and loss of peripheral vision, often causing the patients to become legally blind by the age of 30–40 years. Of the five current distinct XLRP loci, the two major ones are for retinitis pigmentosa type 2 (RP2 [MIM312600]) and type 3 (RP3 [MIM 312610]), which were mapped to Xp11.3 (Thiselton et al. 1996; Schwahn et al. 1998) and Xp21.1 (Meindl et al. 1996; Roepman et al. 1996), respectively (see the Web site of RetNet). Although RP3 accounts for >70% of XLRP (Ott et al. 1990), the original retinitis pigmentosa GTPase regulator gene (RPGR), with 19 exons (GenBank accession numbers U57629 and NM_000328) isolated from the RP3 region, was found to be mutated in only 10%–27% of families with XLRP (Meindl et al. 1996; Roepman et al. 1996; Buraczynska et al. 1997; Zito et al. 2000). Recently, this discrepancy was resolved by the discovery of a mutational hotspot in a new 3′ terminal RPGR exon, called “ORF15” (GenBank accession number AF286472), which was found to be mutated in 60% of patients with XLRP; this suggests that mutations in RPGR are the only cause of RP3-type XLRP (Vervoort et al. 2000). The RP15 locus (MIM 300029), which was previously assigned to Xp22 by linkage analysis of a single pedigree with X-linked dominant cone-rod degeneration (McGuire et al. 1995), was recently remapped to Xp11.4-p21.1, and a de novo insertion was detected in the RPGR exon ORF15 (Mears et al. 2000).
COD1 was originally mapped to a region between Xp11.3 and Xp21.1, encompassing the RP3 locus (Bartley et al. 1989; Jacobson et al. 1989; Bergen et al. 1993, 1994; Hong et al. 1994; Meire et al. 1994). Subsequent linkage studies, performed by our group through analysis of three families, mapped the disease locus to a limited region of Xp11.4 between the RP2 and RP3 loci (DXS993–DXS556), on the basis of the recombinational events in families 1 and 3 and of the screening results for the original RPGR transcript (with 19 exons) that were found to be normal in family 2 (Seymour et al. 1998). The detailed clinical descriptions and the pedigrees for these three families have been described elsewhere (Jacobson et al. 1989; Hong et al. 1994; Seymour et al. 1998; Brown et al. 2000). To further narrow the COD1 interval, additional markers were used, and new dinucleotide-repeat markers were developed by the identification of the dinucleotide repeats in the sequences (through use of the RepeatMasker Web Server) from the genomic clones encompassing the DXS993–DXS556 region and by testing them for a possible polymorphism in selected key individuals. One of the dinucleotide repeats from GenBank clone AC069362 (forward primer, 5′-GCCCTTTGGATGAAAGATCC-3′; reverse primer, 5′-TTGCTTCAGCACACAAGTCA-3′) was found to be polymorphic and informative in both families 1 and 3. BLAST searches revealed that this CA repeat was previously mentioned in GenBank under the accession number Z67588. This new repeat marker (the order is tel-DXS556-DXS8042-[new marker]-DXS574-DXS993-cen) provided the first evidence that the critical intervals for families 1 and 3 were not overlapping in the original DXS993–DXS556 interval. We clinically reassessed all key recombinants and evaluated each family individually for possible heterogeneity. Since the critical intervals in families 2 and 3 were still overlapping with the RP3 locus (Seymour et al. 1998), RPGR (including the subsequently described alternative 3′ terminal exon ORF15) was reconsidered as a candidate and was screened for mutations. In family 1, it was discovered that one of the key recombinants (individual 1:IV:1 [Seymour et al. 1998]), who was initially classified as “affected” because of his symptoms of photophobia and poor color vision, was actually found to have normal central vision with no macular pathology up to his current age of 47 years. He was reclassified as “probably unaffected,” and this status change remapped the disease locus in family 1 to include RP3, as well.
All 19 original exon fragments, plus ORF15 of the RPGR gene from individuals with COD1, were amplified from leukocyte genomic DNA and screened for mutations by direct PCR sequencing, through use of either an ABI377 or ABI3700 sequencer. The intronic primers and corresponding PCR conditions for the 19 original exons have been described elsewhere (Meindl et al. 1996). ORF15 is an alternative 3′ terminal exon that contains exon 15 and extends into part of intron 15 (Vervoort et al. 2000). It has a 1,706-nucleotide (nt) coding sequence (567 amino acids with a repetitive domain rich in glutamic acid residues), and the first 152 nts of 5′ end sequence corresponds to the original RPGR exon 15. In addition to the previously published exon 15 primers, we developed and used four primer pairs to produce four overlapping fragments covering the remaining coding region of ORF15 (table 1).
Table 1.
Primers and Conditions for PCR Amplification of RPGR Exon ORF15 Coding Sequence[Note]
|
Primer(5′→3′) |
||||
| Fragment | Length(bp) | Annealing Temperature(°C) | Forward | Reverse |
| 1 | 348 | 56 | AGGAAGGAGCAGAGGATTCA | CCCTCTTCTTCCATTCTTCC |
| 2 | 444 | 56 | GGGGAGAAAGACAAGGGTAG | TCCTTTCCCCTCCTCTACTT |
| 3 | 982 | 55 | GGAAGAAGGAGACCAAGGAG | CCCATTTCCCTGTGTGTTAG |
| 4 | 414 | 56 | GCAGGATGGAGAGGAGTACA | GAGAGAGGCCAAAATTTACCA |
Note.— The first 152 nts of 5′ end sequence of ORF15 correspond to the original RPGR exon 15, and the previously described primers for exon 15 were used to amplify this part of the gene (Meindl et al. 1996). The primers in this table produce four overlapping fragments covering the remaining coding region of ORF15.
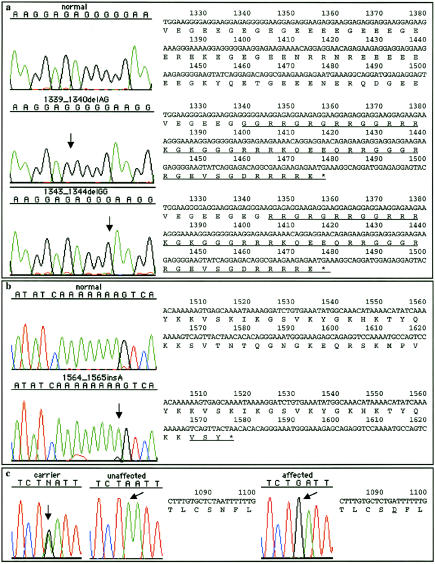
Mutation analysis identified two different 2-nt deletions in ORF15, in family 2 (1339_1340delAG) and in families 1 and 3 (1343_1344delGG), both resulting in a frameshift leading to altered amino acid structure and early termination (fig. 1a). These mutations were confirmed by segregation analysis in the original three large families. Additionally, a third, unique frameshift mutation (1564_1565insA) was identified in another individual with X-linked cone-rod dystrophy (fig. 1b). None of these COD1-related frameshift sequence alterations was detected in 100 control X chromosomes from unaffected subjects.
Figure 1.
ORF15 mutations and RPGR exon 9 sequence alteration, with the corresponding predicted translation products. The locations for sequence alterations are indicated by arrows. The partial sequences of ORF15 (a and b) and RPGR exon 9 (c) are shown on the right side of each chromatogram, with the resulting changes in the open reading frame underlined. a, The 2-nt deletions identified in the original three families, which cause a frameshift resulting in very similar truncated protein products (46 or 44 novel amino acids, and premature truncation resulting in the loss of 76 amino acids). b, The 1-nt insertion identified in another individual with X-linked cone-rod dystrophy, which results in three novel amino acids and in premature truncation resulting in the loss of 43 amino acids. c, A nonconservative amino acid change (Asn345Asp) in RPGR exon 9 (A1092G), which segregated with the mutation in families 1 and 3 and suggests a shared disease haplotype.
A nonconservative amino acid change (Asn345Asp) in RPGR exon 9 (A1092G) also segregated with the mutation in families 1 and 3 (fig. 1c). These results are consistent with the shared disease haplotype that we observed among families 1 and 3 during the linkage analysis (Seymour et al. 1998). The affected males from families 1 and 3 share the same haplotype for RP3, [RPGR exon 9 (A1092G); ORF15 (1343_1344delGG)]-DXS1068-DXS8025-DXS1058-DXS977-DXS556-DXS8042, corresponding to a region that is not shared by recombinant unaffected individuals. These results suggest that it is highly likely that these two families are related. The same RPGR exon 9 missense change was previously reported in one patient with XLRP and was not detected in 150 control X chromosomes (Sharon et al. 2000). However, Sharon et al. (2000) considered this change to be a nonpathogenic variant, since the patient’s affected brother did not carry this sequence alteration. This missense change was detected in 2 of 100 control X chromosomes in the unaffected population we screened, which supports the previous consideration that it is a rare sequence variant.
We reconfirmed the existence of genetic heterogeneity for X-liked cone-rod dystrophy (Bergen and Pinckers 1997) on the basis of linkage data and the lack of RPGR-ORF15 mutations in three other families, including the observance of an in-frame 12-nt deletion polymorphism that did not segregate with the disease in one of these families. This same polymorphism (1307_1318del12) was also observed in the individual COD1 sample for which we have found the third mutation (1564_1565insA) that prematurely terminates the protein. This polymorphism was detected in 6 of 100 control X chromosomes from unaffected subjects.
Our results indicate that mutations in the RPGR exon ORF15 are the primary cause of COD1, on the basis of the three independent mutations identified in the set of families considered here (two of these mutations segregated perfectly with the disease in the three large pedigrees of five to six generations) that give rise to similar C-terminal truncations in the RPGR-ORF15 protein. In addition to the already established role of RPGR in XLRP (a disease that predominantly affects the rod photoreceptors), the mutations that we identified in the same gene appear to primarily affect the cone photoreceptors and to cause a cone dystrophy phenotype. To the best of our knowledge, these results represent the first definitive evidence that mutations in RPGR are associated with COD1. All three COD1 mutations that we identified were in the last 369-nt 3′-end coding sequence of ORF15. Interestingly, only one XLRP mutation has been reported in this region (1344delG [Vervoort et al. 2000]), and the remaining 31 published ORF15 mutations were detected within the preceding 5′ region (Mears et al. 2000; Vervoort et al. 2000; Yokoyama et al. 2001). Affected males in the RP15 family (with the 173_174insA mutation [Mears et al. 2000]) were reported to have early cone involvement, and the diagnosis for patient 55 (with the 689_692del4 mutation) from Vervoort's series (Vervoort et al. 2000) was “probable” X-linked cone dystrophy. These patients may represent intermediate phenotypes within the broad spectrum (i.e., between typical XLRP and typical COD1) of retinal disease caused by RPGR mutations. Further studies will be necessary to determine the role of the RPGR exon ORF15 in both rod and cone photoreceptor functions, as well as its impact on the disease phenotype.
Acknowledgments
We are grateful to the families who have participated in this study. We thank Erica Fong for helping us with AB3700 sequencing in the Core Laboratories of the Center for Human Genetics and Integrative Biology at the University of Pittsburgh. We also thank Dr. Robert E. Ferrell for aiding us with our control samples. This research was supported by National Institutes of Health (NIH) grant EY13130 (to M.B.G.); by NIH Core Grant for Vision Research EY08098; by the Eye and Ear Foundation of Pittsburgh (support to M.B.G.); by Fight For Sight, the Research Division of Prevent Blindness America (support to F.Y.K.D.); and by Research to Prevent Blindness, New York.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for RPGR [accession numbers U57629 and NM_000328] and ORF15 [accession number AF286472])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for COD1 [MIM 304020], RP2 [MIM 312600], RP3 [MIM 312610], RP15 [MIM 300029], and XLRP [MIM 268000])
- RepeatMasker Web Server, http://ftp.genome.washington.edu/cgi-bin/RepeatMasker
- RetNet, http://www.sph.uth.tmc.edu/Retnet/
References
- Bartley J, Gies C, Jacobson D (1989) Cone dystrophy (X-linked) (COD1) maps between DXS7 (L1.28) and DXS206 (Xj1.1) and is linked to DXS84 (754). Cytogenet Cell Genet 51:959 [Google Scholar]
- Bergen AA, Meire F, Schuurman EJ, Delleman JW (1994). DNA carrier detection in X-linked progressive cone dystrophy. Clin Genet 45:236–240 [DOI] [PubMed] [Google Scholar]
- Bergen AA, Meire F, ten Brink J, Schuurman EJ, van Ommen GJ, Delleman JW (1993) Additional evidence for a gene locus for progressive cone dystrophy with late rod involvement in Xp21.1-p11.3. Genomics 18:463–464 [DOI] [PubMed] [Google Scholar]
- Bergen AA, Pinckers AJ (1997) Localization of a novel X-linked progressive cone dystrophy gene to Xq27: evidence for genetic heterogeneity. Am J Hum Genet 60:1468–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird AC (1975) X-linked retinitis pigmentosa. Br J Ophthalmol 59:177–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J Jr, Kimura AE, Gorin MB (2000) Clinical and electroretinographic findings of female carriers and affected males in a progressive X-linked cone-rod dystrophy (COD-1) pedigree. Ophthalmology 107:1104–1110 [DOI] [PubMed] [Google Scholar]
- Buraczynska M, Wu W, Fujita R, Buraczynska K, Phelps E, Andreasson S, Bennett J, Birch DG, Fishman GA, Hoffman DR, Inana G, Jacobson SG, Musarella MA, Sieving PA, Swaroop A (1997) Spectrum of mutations in the RPGR gene that are identified in 20% of families with X-linked retinitis pigmentosa. Am J Hum Genet 61:1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman GA, Farber MD, Derlacki DJ (1988) X-linked retinitis pigmentosa: profile of clinical findings. Arch Ophthalmol 106:369–375 [DOI] [PubMed] [Google Scholar]
- Hong H-K, Ferrell RE, Gorin MB (1994) Clinical diversity and chromosomal localization of X-linked cone dystrophy (COD1). Am J Hum Genet 55:1173–1181 [PMC free article] [PubMed] [Google Scholar]
- Jacobson DM, Thompson HS, Bartley JA (1989) X-linked progressive cone dystrophy: clinical characteristics of affected males and female carriers. Ophthalmology 96:885–895 [DOI] [PubMed] [Google Scholar]
- McGuire RE, Sullivan LS, Blanton SH, Church MW, Heckenlively JR, Daiger SP (1995) X-linked dominant cone-rod degeneration: linkage mapping of a new locus for retinitis pigmentosa (RP15) to Xp22.13-p22.11. Am J Hum Genet 57:87–94 [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Hiriyanna S, Vervoort R, Yashar B, Gieser L, Fahrner S, Daiger SP, Heckenlively JR, Sieving PA, Wright AF, Swaroop A (2000) Remapping of the RP15 locus for X-linked cone-rod degeneration to Xp11.4-p21.1, and identification of a de novo insertion in the RPGR exon ORF15. Am J Hum Genet 67:1000–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl A, Dry K, Herrmann K, Manson F, Ciccodicola A, Edgar A, Carvalho MR, Achatz H, Hellebrand H, Lennon A, Migliaccio C, Porter K, Zrenner E, Bird A, Jay M, Lorenz B, Wittwer B, D'Urso M, Meitinger T, Wright A (1996) A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat Genet 13:35–42 [DOI] [PubMed] [Google Scholar]
- Meire FM, Bergen AA, De Rouck A, Leys M, Delleman JW (1994) X linked progressive cone dystrophy: localisation of the gene locus to Xp21-p11.1 by linkage analysis. Br J Ophthalmol 78:103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J, Bhattacharya SS, Chen JD, Denton MJ, Donald J, Dubay C, Farrar GJ, Fishman GA, Frey D, Gal A, Humphries P, Jay B, Jay M, Litt M, Machler M, Musarella M, Neugebauer M, Naussbaum RL, Terwilliger JD, Weleber RG, Wirth B, Wong F, Worton RG, Wright AF (1990) Localizing multiple X chromosome-linked retinitis pigmentosa loci using multilocus homogeneity tests. Proc Natl Acad Sci USA 87:701–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinckers A, Deutman AF (1987) X-linked cone dystrophy: an overlooked diagnosis? Int Ophthalmol 10:241–243 [DOI] [PubMed] [Google Scholar]
- Pinckers A, Timmerman GJ (1981) Sex-difference in progressive cone dystrophy. I. Ophthalmol Paediatr Genet 1:17–24 [Google Scholar]
- Roepman R, van Duijnhoven G, Rosenberg T, Pinckers AJ, Bleeker-Wagemakers LM, Bergen AA, Post J, Beck A, Reinhardt R, Ropers HH, Cremers FP, Berger W (1996) Positional cloning of the gene for X-linked retinitis pigmentosa 3: homology with the guanine-nucleotide-exchange factor RCC1. Hum Mol Genet 5:1035–1041 [DOI] [PubMed] [Google Scholar]
- Schwahn U, Lenzner S, Dong J, Feil S, Hinzmann B, van Duijnhoven G, Kirschner R, Hemberger M, Bergen AA, Rosenberg T, Pinckers AJ, Fundele R, Rosenthal A, Cremers FP, Ropers HH, Berger W (1998) Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nat Genet 19:327–332 [DOI] [PubMed] [Google Scholar]
- Seymour AB, Dash-Modi A, O'Connell JR, Shaffer-Gordon M, Mah TS, Stefko ST, Nagaraja R, Brown J, Kimura AE, Ferrell RE, Gorin MB (1998) Linkage analysis of X-linked cone-rod dystrophy: localization to Xp11.4 and definition of a locus distinct from RP2 and RP3. Am J Hum Genet 62:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon D, Bruns GA, McGee TL, Sandberg MA, Berson EL, Dryja TP (2000) X-linked retinitis pigmentosa: mutation spectrum of the RPGR and RP2 genes and correlation with visual function. Invest Ophthalmol Vis Sci 41:2712–2721 [PubMed] [Google Scholar]
- Thiselton DL, Hampson RM, Nayudu M, Van Maldergem L, Wolf ML, Saha BK, Bhattacharya SS, Hardcastle AJ (1996) Mapping the RP2 locus for X-linked retinitis pigmentosa on proximal Xp: a genetically defined 5-cM critical region and exclusion of candidate genes by physical mapping. Genome Res 6:1093–1102 [DOI] [PubMed] [Google Scholar]
- Vervoort R, Lennon A, Bird AC, Tulloch B, Axton R, Miano MG, Meindl A, Meitinger T, Ciccodicola A, Wright AF (2000) Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet 25:462–466 [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Maruiwa F, Hayakawa M, Kanai A, Vervoort R, Wright AF, Yamada K, Niikawa N, Naoi N (2001) Three novel mutations of the RPGR gene exon ORF15 in three Japanese families with X-linked retinitis pigmentosa. Am J Med Genet 104:232–238 [PubMed] [Google Scholar]
- Zito I, Gorin MB, Plant C, Bird AC, Bhattacharya SS, Hardcastle AJ (2000) Novel mutations of the RPGR gene in RP3 families. Hum Mutat 15:386 [DOI] [PubMed] [Google Scholar]