Abstract
Current methods for detection of mutations by polymerase chain reaction (PCR) and sequence analysis frequently are not able to detect heterozygous large deletions. We report the successful use of a novel approach to identify such deletions, based on detection of apparent homozygosity of contiguous single-nucleotide polymorphisms (SNPs). The sequence analysis of genomic DNA PCR products containing all coding exons and flanking introns identified only a single heterozygous mutation (IVS18+2t→a) in a patient with classic infantile-onset autosomal recessive glycogen storage disease type II (GSDII). Apparent homozygosity for multiple contiguous SNPs detected by this sequencing suggested presence of a large deletion as the second mutation; primers flanking the region of homozygous SNPs permitted identification and characterization by PCR of a large genomic deletion (8.26 kb) extending from IVS7 to IVS15. The data clearly demonstrate the utility of SNPs as markers for large deletions in autosomal recessive diseases when only a single mutation is found, thus complementing currently standard DNA PCR sequence methods for identifying the molecular basis of disease.
Glycogen storage disease type II (GSDII [MIM 232300]) is an autosomal recessive disorder caused by deficiency of the lysosomal enzyme acid alpha-glucosidase (GAA [GenBank accession numbers M34424, Y00839, and NT_024915]) and the subsequent intralysosomal accumulation of glycogen, predominantly in muscle. Clinically, the disorder ranges from an infantile-onset disease, with involvement of both cardiac and skeletal muscle and death before the age of 2 years (Pompe disease), to an adult-onset, slowly progressive disease impairing only skeletal muscle.
More than 40 different mutations involving single-nucleotide changes (missense, nonsense, and splice-site mutations) or small insertions/deletions have been reported (reviewed in Hirschhorn and Reuser 2001). However, only two large deletions have been identified in patients with GSDII (Huie et al. 1994, 1999). The low frequency of reported large deletions could reflect the current extensive use and limitations of methods depending on PCR for detection of mutations.
The first reported large deletion in GSDII (extending from IVS17 to IVS18, and deleting exon 18) was initially suspected from results of non–PCR-based methods (analysis of restriction fragment sizes after digestion, electrophoresis, Southern blotting, hybridization with radiolabeled probes, and autoradiography) (Martiniuk et al. 1990). This deletion was then characterized by cloning and sequence analysis (Huie et al. 1994) and was found to be very common in Holland and relatively common in the United States (Kroos et al. 1995; Hirschhorn and Huie 1999). More recently, PCR amplification and sequence analysis of genomic DNA was used to identify and characterize a large homozygous Alu-mediated deletion extending 3′ of the gene (IVS15 to 3′ of terminal exon 20). However, the identification of this deletion and its precise boundaries was facilitated by consanguinity and, therefore, homozygosity for the deletion. Presence of the deletion was suggested by the inability to amplify a series of contiguous exons downstream of exon 14 (Huie et al. 1999). Prior knowledge of the sites of Alu elements in the acid alpha-glucosidase gene (Martiniuk et al. 1991; R.H., unpublished sequence analysis) allowed for precise definition of the boundaries of this large Alu-Alu–mediated deletion (Huie et al. 1999). However, the deletion would have been missed easily if it had been present in heterozygosity in a nonconsanguineous family. We previously suggested that, when only one heterozygous mutation is identified by sequence analysis of all exons and flanking intronic regions, apparent homozygosity for single-nucleotide polymorphisms (SNPs) extending over a large region should raise suspicion of a heterozygous large deletion (Huie et al. 1999). This hypothesis has now led to the identification of a novel large intragenic deletion (IVS7–IVS15), heteroallelic with a previously identified splice-site mutation (IVS18+2t→a) (Fernandez-Hojas et al. 2002).
The patient, of El Salvadoran heritage, presented at age 5 mo with hypotonia, cardiomegaly, hepatosplenomegaly, developmental delay, and a vacuolar myopathy. The diagnosis of GSDII was confirmed by absence of acid alpha-glucosidase activity in muscle (0 nmol vs. normal 8.13±2.1 nmol methylumbelliferyl-α-d-glucoside hydrolyzed/min/g).
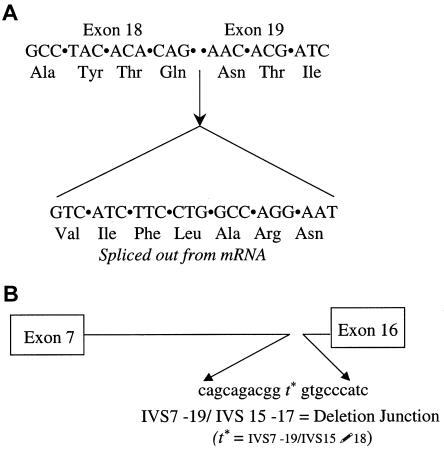
PCR amplification of genomic DNA from this patient and sequencing of all 19 coding exons and the flanking intronic regions identified only a single heterozygous mutation at a splice site (IVS18+2t→a; also reported in a second patient, of Spanish descent, with classic infantile GSDII [Fernandez-Hojas et al. 2002]). The additional studies reported herein further demonstrate that the mutation results in use of a cryptic splice-site mutation in exon 18 (fig. 1A) and splicing out of the terminal 21 nucleotides of exon 18 (nt 2627–2646, ATG=1), deleting codons 876–882 (amino acids VIFLARN). Although the translation frame is retained, splicing out of codons 876–882 deletes an asparagine (N); the sixth of seven N-linked glycosylation sites (N882) involved in localization of the enzyme to lysosomes. Prior in vitro experiments replacing the asparagine with glutamine and thus disrupting the AsnXSer/Thr motif required for N-linked glycosylation were reported to show a deleterious effect at only one of the sites (N233) (Hermans et al. 1993). By contrast, in vivo, this is the second case in which a patient with infantile-onset GSDII has been found to carry a deletion mutation at one of the seven N-linked asparagine glycosylation sites—del N882 (present report) and del N470 (Fernandez-Hojas et al. 2002). The deleterious effects of these two deletions of asparagine at N-linked glycosylation sites could result in either interference with targeting to lysosomes or an unstable protein. The in-frame deletion, in theory, predicts retention of acid alpha-glucosidase protein or cross-reacting material and nonproduction of antibody that could interfere with the efficacy of enzyme-replacement therapy.
Figure 1.
A, Splice-site mutation. The IVS18+2t→a splice-site mutation results in use of a cryptic splice site in exon 18 and deletion of the terminal 21 nucleotides of exon 18. RT-PCR of mRNA from the patient's fibroblasts (using as primers sequence contained in exon 8 and in exon 20 [5′- GACGTCCAGTGGAACGA and 5′-CAGTGCGATCTAGGGAGA]) revealed a single amplification product of apparently correct size of 1.88 kb (not shown). Direct sequence analysis of the RT-PCR product with an antisense primer located in exon 20 revealed only homogenous sequence indicating lack of detectable normally spliced mRNA and containing the abnormal splice with deletion of the terminal 21 nucleotides in exon 18 and splicing to exon 19 on the allele not bearing the large 8-kb deletion. B, Deletion mutation. Deletion junction between IVS7 and IVS15 resulting from an 8.26-kb genomic deletion extending from IVS7–19 to IVS15–17. Sequence analysis of the smaller than normal product resulting from amplification with primers in IVS5 and IVS16 (726 bp vs. 9.027 kb) revealed the site of the deletion junction, depicted graphically. The deletion extends from IVS7–19 to IVS15–17); (cagcagacggtgtgccatc).
The inability to detect a second mutation despite clear heterozygosity of the splice-site mutation in IVS18, combined with observation of homozygosity for all 14 SNPs from exon 8 to exon 17, suggested that the second chromosome carried a large deletion that was missed by standard DNA PCR-based detection methods. Heterozygosity for SNPs in IVS5 and exon 18 suggested approximate boundaries for a large intragenic deletion. (These SNPs, their location [for exons, numbers = cDNA from the start of translation], and the average heterozygosity determined in 15–76 chromosomes are as follows: IVS5 +12 g/a [0.49]; 1203 A/G in exon 8 [0.49]; 1374 C/T in exon 9 [0.18]; IVS9–19 c/g [0.48]; IVS9–7 t/c [0.16]; IVS10–52 a/c [0.49]; 1581 A/G in exon 11 [0.42]; IVS13 +21 g/a [0.08]; IVS14–64 g/a [0.39]; IVS14–5 t/g [0.09]; 2065G/A in exon 15 [0.16]; 2133 A/G in exon 15 [0.34]; 2338 G/C in exon 17 [0.42]; and 2553 A/G in exon 18 [0.50]) (modified from the summary, table, and diagram of gene structure in Hirschhorn and Reuser 2001 [except IVS14–64 g/a, described herein]). PCR amplification with primers in IVS5 and IVS16, flanking the presumed deletion, resulted in a smaller than expected product (0.767 vs. 9.027 kb), consistent with a large deletion (8.26 kb). Sequence analysis of the smaller-than-expected fragment identified the deletion junction at IVS7–19 and IVS15–17 (fig. 1B). Computer analysis did not reveal any obvious repetitive or homologous sequence(s) that would suggest a mechanism for the deletion. The 8.26-kb deletion includes the enzyme catalytic site in exon 10–11 and at least one evolutionarily conserved region in exon 14 (Huie et al. 1998). Analysis of parental DNA revealed that the large deletion was paternally inherited, whereas the IVS18 splice-site mutation was maternally inherited.
Use of PCR and automated sequence analysis has allowed for detection of a large spectrum of mutations at an accelerated rate. However, some mutations, particularly large deletions, may be missed by these methods. For example, we previously were able to identify a large Alu-Alu–mediated deletion only because of consanguinity and homozygosity in the proband, leading to failure to amplify a series of contiguous exons (Huie et al. 1999). The currently described IVS7–IVS15 deletion was suspected after only one clearly heterozygous mutation was identified, despite complete sequence analysis, and homozygosity for multiple contiguous SNPs was noted 5′ of the heterozygous mutation. Conversely, large deletions on the second allele overlapping the locus of a detected mutation may be missed because the identified mutation is erroneously presumed to be homozygous. This emphasizes the need for confirmation of homozygosity in the proband by identification of the mutation in both parents.
Although our laboratory has been able to identify both mutations, in unpublished results, on 100% of the 40 chromosomes studied by these DNA PCR-based methods, others have reported identification of mutations detected by PCR-based methods in only 29 (69%) of 42 chromosomes (Laforet et al. 2000) and 17 (77%) of 22 chromosomes (Ko et al. 1999). These latter results could reflect technical problems or mutations in the promoter and other noncoding regions, but they could also reflect high frequency of either the large deletions we have identified or as yet unidentified deletion(s) in the two ethnic populations (French and Taiwanese) studied by these authors.
In summary, large deletions should be considered in autosomal recessive disorders when one mutation is present in heterozygosity but the second mutation is not detected, despite sequence analysis of all coding exons and their flanking intronic regions, and multiple contiguous SNPs are present in apparent homozygosity. In addition, when mutations and SNPs are present in apparent homozygosity, despite reports of nonconsanguinity, a large deletion should ideally be ruled out by studies of parents, to allow for effective counseling (unless the mutation is a frequent mutation from a common founder). The same principles could apply to autosomal dominant disorders when there is failure to identify a mutation in an affected individual.
Acknowledgments
This work was supported by a grant from the March of Dimes (to R.H.) and in part by the General Clinical Research Center, National Center for Research Resources, National Institutes of Health (grant M01RR00096, to the New York University School of Medicine).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank, http://www.ncbi.nih.nlm.nih.gov/Genbank/ (for accession numbers M34424, Y00839, and NT_024915 )
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for GSDII [MIM 23230])
References
- Fernandez-Hojas R, Huie ML, Navarro C, Dominguez C, Roig M, Lopez-Coronas D, Teijeira S, Kwame, A-Y, Hirschhorn R (2002) Identification of six novel mutations in the acid alpha-glucosidase gene in three Spanish patients with infantile onset glycogen storage disease type II. Neuromusc Disord 12:159–166 [DOI] [PubMed] [Google Scholar]
- Hermans MM, Wisselaar HA, Kroos MA, Oostra BA, Reuser AJ (1993) Human lysosomal alpha-glucosidase: functional characterization of the glycosylation sites. Biochem J 289:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R, Huie ML (1999) Frequency of mutations for glycogen storage disease type II in different populations: the delta 525T and delta exon 18 mutations are not generally “common” in Caucasian populations. J Med Genet 36:85–86 [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R, Reuser AJJ (2001) Glycogen storage disease type II: acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Valle D, Sly WS (eds) The metabolic and molecular bases of inherited disease, 8th ed. McGraw-Hill, New York, pp 3389–3420 [Google Scholar]
- Huie ML, Chen A, Grix A, Sklower-Brooks S, Hirschhorn R (1994) A de novo 13 nt deletion, a missense mutation and a deletion of exon 18 in two patients with infantile onset GSD II. Hum Mol Genet 3:1081–1087 [DOI] [PubMed] [Google Scholar]
- Huie ML, Shanske AL, Kasper JS, Marion RW, Hirschhorn R (1999) A large Alu-mediated deletion, identified by PCR, as the molecular basis for glycogen storage disease type II (GSDII). Hum Genet 104:94–98 [DOI] [PubMed] [Google Scholar]
- Huie ML, Tsujino S, Sklower Brooks S, Engel A, Elias E, Bonthron DT, Bessle C, Shanske S, DiMauro S, Goto YI, Hirschhorn R (1998) Glycogen storage disease type II: identification of four novel missense mutations (D645N, G648S, R672W, R672Q) and two insertion/deletions in the acid alpha glucosidase locus of patients of differing phenotype. Biochem Biophys Res Comm 244:921–927 [DOI] [PubMed] [Google Scholar]
- Ko TM, Hwu WL, Lin YW, Tsen, LH, Hwa HL, Wang TR, Chuang SM (1999) Molecular genetic study of Pompe disease in Chinese patients in Taiwan. Hum Mutat 13:380–384 [DOI] [PubMed] [Google Scholar]
- Kroos MA, van der Kraan M, van Diggelen OP, Kleijer WP, Reuser AJJ, Van den Boogaard MJ, Ausems MG, Ploos van Amstel HK, Poenaru L, Nicolino M (1995) Glycogen storage disease type II: frequency of three common mutant alleles and their associated clinical phenotypes studied in 121 patients. J Med Genet 32:836–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforet P, Nicolino M, Eymard B, Puech JP, Caillaud C, Poenaru L, Fardeau M (2000) Juvenile and adult onset acid maltase deficiency in France: genotype-phenotype correlation. Neurology 55:1122–1128 [DOI] [PubMed] [Google Scholar]
- Martiniuk F, Bodkin M, Tzall S, Hirschhorn R (1991) Isolation and characterization of the structural gene for human acid alpha glucosidase. DNA Cell Biol 10:283–292 [DOI] [PubMed] [Google Scholar]
- Martiniuk F, Mehler M, Tzall S, Meredith G, Hirschhorn R (1990) Extensive genetic heterogeneity in patients with acid alpha glucosidase deficiency as detected by abnormalities of DNA and mRNA. Am J Hum Genet 47:73–78 [PMC free article] [PubMed] [Google Scholar]