Abstract
Autistic disorder (AutD) is a neurodevelopmental disorder characterized by significant disturbances in social, communicative, and behavioral functioning. A two-stage genomic screen analysis of 99 families with AutD revealed suggestive evidence for linkage to chromosome 2q (D2S116 nonparametric sib-pair LOD score [MLS] 1.12 at 198 cM). In addition, analysis of linkage disequilibrium for D2S116 showed an allele-specific P value of <.01. Recently, linkage to the same region of 2q was reported in an independent genome screen. This evidence for linkage increased when analysis was restricted to the subset of patients with AutD who had delayed onset (>36 mo) of phrase speech (PSD). We similarly classified our data set of 82 sib pairs with AutD, identifying 45 families with AutD and PSD. Analysis of this PSD subset increased our support for linkage to 2q (MLS 2.86 and HLOD 2.12 for marker D2S116). These data support evidence for a gene on chromosome 2 contributing to risk of AutD, and they suggest that phenotypic homogeneity increases the power to find susceptibility genes for AutD.
Autistic disorder (AutD [MIM 209850]) is a neurodevelopmental disorder characterized by significant disturbances in social, communicative, and behavioral functioning. Evidence from epidemiologic and genetic analyses indicates that AutD is genetically heterogeneous (reviewed by Pericak-Vance et al. 2002). Studies of both twins and isolated AutD patients have consistently shown that, even when narrowly defined and diagnosed, AutD is associated with a very wide range of clinical manifestations (Lord and Rutter 1994; Bailey et al. 1996). Recently, Leal and Ott (2000) demonstrated that stratification by clinical symptoms can lead to increased power to detect linkage if the phenotypic heterogeneity is the result of genetic heterogeneity. Thus, the identification of homogenous subgroups within the broader AutD phenotype may aid in linkage studies. The difficulty with this approach is in the identification of the appropriate phenotypic groups for stratification.
Recent studies of AutD have suggested that grouping families on the basis of reliably measurable language traits may increase power for linkage analysis (Folstein and Mankoski 2000; Bradford et al. 2001). Pragmatic language abnormality is one of the diagnostic criteria for AutD, and age at onset of phrase speech is an important clinical feature (MacLean et al. 1999; Buxbaum et al. 2001). Chromosome 2q is a region potentially harboring AutD risk genes, with several groups reporting evidence for linkage to the same region (Buxbaum et al. 2001; International Molecular Genetics Study of Autism Consortium [IMGSAC] 2001; Shao et al. 2001). Of particular interest is the analysis approach used by Buxbaum et al. with respect to chromosome 2 and AutD linkage. Buxbaum et al. (2001) reported linkage evidence for a susceptibility gene for AutD on chromosome 2. They found a maximum multipoint heterogeneity LOD score (HLOD) of 1.96 and a maximum multipoint NPL score of 2.39 on chromosome 2q (at 186 cM, for D2S364), in an analysis of 95 affected-relative–pair (ARP) families affected with AutD. Hypothesizing that a more genetically homogeneous population would increase power to identify genes and that clinical heterogeneity in AutD was due, in part, to genetic heterogeneity, Buxbaum et al. restricted their analyses to a subset of 49 ARPs with AutD with delayed onset (at age >36 mo) of phrase speech or “phrase speech delay” (PSD). Analysis of this restricted subset increased their evidence for linkage (HLOD=2.99 under the dominant model; NPL=3.32).
In the genomic-screen analysis of 99 multiplex families (both sib-pair and ARP) with AutD (Shao et al. 2001), performed by the Collaborative Autism Team (CAT), suggestive evidence for linkage to the same region of chromosome 2q (peak MLS [nonparametric sib-pair maximum LOD score] 1.12 at D2S116 [198 cM]) was found. This peak was ∼12 cM from the peak identified by Buxbaum et al. (2001). In addition, evidence for linkage disequilibrium at D2S116 (allele-specific P value <.01) was found (Bass et al. 2000). Additional markers in the region were genotyped, including D2S364 and D2S335 (at 175.9 cM), D2S2309 and D2S309 (at 198 cM), and D2S1384 (at 200.4 cM). Both D2S364 and D2S335 demonstrated an HLOD >1.0 in the study by Buxbaum et al. (2001). D2S2309 and D2S309 were near the CAT peak LOD score, and D2S1384 flanked the region (fig. 1). To assess linkage in the presence of genetic heterogeneity, HLODs were generated by use of the HOMOG program (Ott 1999). Nonparametric affected-sib-pair (ASP) analysis of the data was performed using ASPEX software. Map distances, maximum affecteds-only two-point HLODs—under both dominant and recessive models—and two-point MLS results are presented in table 1. D2S364 and D2S335 were not informative in these data.
Figure 1.
Chromosome 2q
Table 1.
Two-Point Linkage Results in the Entire Data Set and in the Subset with PSD
|
Results for All Sib-Pair Families (n=82) |
Results for Families with PSD (n=45) |
||||||
| HLOD (α) |
HLOD (α) |
||||||
| Marker | Position(cM) | Dominant | Recessive | MLS | Dominant | Recessive | MLS |
| D2S116 | 198.6 | .53 (.20) | .52 (.25) | 1.12 | 2.12 (1.0) | 2.45 (.95) | 2.86 |
| D2S2309 | 198.6 | .74 (.85) | .72 (.55) | .57 | 2.17 (.8) | 1.98 (.95) | 1.58 |
| D2S309 | 198.6 | .74 (1.00) | .86 (.20) | .75 | 1.48 (1.0) | 1.40 (.50) | 1.16 |
| D2S1384 | 200.4 | .51 (.50) | .27 (.25) | .21 | 1.56 (.6) | 1.06 (.45) | .80 |
Detailed diagnostic evaluations of the family data are as described elsewhere (Ashley-Koch et al. 1999). In brief, the Autism Diagnostic Interview-Revised (ADI-R; see Lord et al. 1994) was used to confirm the clinical diagnosis of AutD. The classification of an individual as having AutD required that an individual exceed cutoff scores in each of three areas: social behavior, communication (nonverbal or verbal), and restricted, repetitive behaviors. On the basis of the evidence for familiality of age at onset of phrase speech (MacLean et al. 1999; Buxbaum et al. 2001), the degree of resemblance among family members with respect to age at onset of phrase speech was also measured in the 82 sib-pair families in the CAT data set. The intraclass correlation coefficient (ICC) among affected siblings, calculated for the log-transformed age at onset of phase speech, was statistically significant (ICC=0.29; P=.04). A patient with AutD was classified as having PSD if he did not acquire phrase speech before age 36 mo. The concordance rate of PSD was 0.55 among siblings. Forty-five multiplex families with AutD and PSD (i.e., comprising two or more patients with AutD and PSD) and 37 families affected with AutD without PSD (i.e., comprising one or no patients with AutD and PSD) were identified. Table 2 presents the ADI-R diagnostic algorithm scores (social behavior, communication, and repetitive behavior) for these two groups. There is no significant difference with respect to the mean values of the three diagnostic algorithm scores on the ADI-R between these two subsets, except for age at onset of phrase speech.
Table 2.
Mean ADI-R Diagnostic Algorithm Scores in the PSD and Non-PSD Subsets
|
Value (SD) forSubjects in Subset |
||
| Measure | PSD(n=99) | Non-PSD(n=79) |
| ADI-R score: | ||
| Social behavior | 17.80 (9.1) | 15.7 (8.4) |
| Communication | 13.5 (4.6) | 12.5 (5.0) |
| Repetitive behavior | 5.3 (2.9) | 5.3 (3.0) |
| Age [years] at ADI-R | 8.5 (4.6) | 9.5 (4.7) |
On the basis of the results of Buxbaum et al. (2001), the chromosome 2 markers were re-evaluated by use of the same PSD stratification. The HLOD score (recessive model) increased from 0.52 to 2.45, and the HLOD score (dominant model) increased from 0.53 to 2.12 at D2S116, when the analysis was restricted to the subset of families with PSD (table 1). An additional marker, D2S2309, located at 198 cM, also gave higher scores on stratification; the HLOD score (dominant model) increased from 0.74 to 2.17. The evidence for heterogeneity was decreased in the PSD subset, since the proportion of linked families, α, increased from 0.25 to 0.95 under the recessive model and from 0.20 to 1.00 under the dominant model. The MLS at D2S116 increased from 1.12 to 2.86 in the PSD subset. For D2S2309, MLS increased from 0.57 to 1.58.
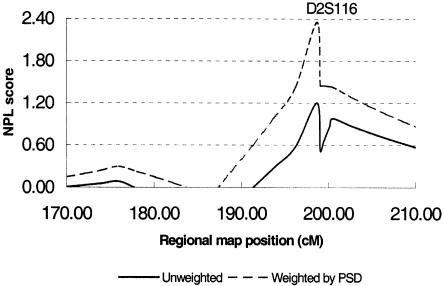
In addition, to measure the correlation, in siblings with AutD, between IBD allele sharing and PSD incidence, the GENEHUNTER-PLUS program (Kong and Cox 1997) was used to assess family-specific IBD allele sharing. A significant correlation coefficient between family-specific NPL score and incidence of PSD was observed, providing statistical evidence supporting the hypothesis that the PSD subset has increased IBD allele sharing, and, therefore, stratification enhanced the linkage evidence (correlation coefficient [r] 0.25; P=.025). Multipoint nonparametric ARP analysis, with individual weights specified for each family on the basis of the presence or absence of PSD, demonstrated an NPL score that increased, at D2S116, from 1.2 to 2.35 in the PSD-weighted analysis (fig. 2).
Figure 2.
Multipoint NPL ARP analysis
Finally, a permutation procedure was conducted to determine the significance of the increase in the NPL score at the peak marker, D2S116. Ten thousand replicates of a simulation in which 45 families (i.e., the number of families in these data classified as affected with PSD) were randomly chosen from the total number of families (82) and were analyzed, resulting in a P value of .029 for the peak NPL score, exceeding the observed NPL score of 2.35. In the permutation test conducted to determine the significance of the increase in the two-point HLOD score under the recessive model, the P value was .007. These results indicate that it is highly unlikely that the PSD findings were due to chance alone.
The convergence of linkage findings—by stratification in two independent data sets—to the same region of chromosome 2 is significant. This finding is especially interesting in light of recent evidence (Szatmari et al. 2000) that onset of phrase speech may be a crucial differential diagnostic feature within the pervasive developmental disorders. In summary, these data provide additional evidence for a gene on chromosome 2 that contributes to AutD risk, and the data also provide supporting evidence that stratification by clinical phenotype, when phenotypic heterogeneity underlies potential genetic heterogeneity, can increase the power to find susceptibility genes for AutD.
Acknowledgments
We wish to thank the patients with AutD and family members who agreed to participate in this study and the personnel of the Center for Human Genetics at Duke University Medical Center, for their input on this project. This research was supported in part by National Institutes of Health (NIH) program project grant NS26630, NIH R01 grants HD36701 and NS36768, and the National Alliance of Autism Research (NAAR), through a gift from Audrey Flack and H. Robert Marcus. The research conducted in this study complies with current U.S. laws.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AutD [MIM 209850])
References
- Ashley-Koch A, Wolpert CM, Menold MM, Zaeem L, Basu S, Donnelly SL, Ravan SA, Powell CM, Qumsiyeh MB, Aylsworth AS, Vance JM, Gilbert JR, Wright HH, Abramson RK, DeLong GR, Cuccaro ML, Pericak-Vance MA (1999) Genetic studies of autistic disorder and chromosome 7. Genomics 61:227–236 [DOI] [PubMed] [Google Scholar]
- Bailey A, Phillips W, Rutter M (1996) Autism: towards an integration of clinical, genetic, neuropsychological, and neurobiological perspectives. J Child Psychol Psychiat 37:89–126 [DOI] [PubMed] [Google Scholar]
- Bass MP, Menold MM, Joyner KL, Wolpert CM, Donnelly SL, Ravan SA, McClain C, von Wendt L, Gilbert JR, Wright HH, Abramson RK, DeLong GR, Cuccaro ML, Pericak-Vance MA (2000) Association analysis of GI candidate genes in autistic disorder. Am J Hum Genet Suppl 67:349 [Google Scholar]
- Bradford Y, Haines J, Hutcheson H, Gardiner M, Braun T, Sheffield V, Cassavant T, Huang W, Wang K, Vieland V, Folstein S, Santangelo S, Piven J (2001) Incorporating language phenotypes strengthens evidence of linkage to autism. Am J Med Genet 105:539–547 [PubMed] [Google Scholar]
- Buxbaum J, Silverman JM, Smith CJ, Kilifarsky M., Reichert J, Hollander E, Lawlor BA, Fitzgerald M, Greenberg DA, Davis K (2001) Evidence for a susceptibility gene for autism on chromosome 2 and for genetic heterogeneity. Am J Hum Genet 68:1514–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein SE, Mankoski RE (2000) Chromosome 7q: where autism meets language disorder? Am J Hum Genet 67:278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Molecular Genetics Study of Autism Consortium (IMGSAC) (2001) Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Hum Mol Genet 10:973–982 [DOI] [PubMed] [Google Scholar]
- Kong A, Cox NJ (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SM, Ott J (2000) Effects of stratification in the analysis of affected sib-pair data: benefits and costs. Am J Hum Genet 66:567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M (1994) Autism and pervasive developmental disorders. In: Rutter M, Taylor E, Hersov L (eds) Child and adolescent psychiatry: modern approaches. Blackwell Scientific, Oxford, pp 569–593 [Google Scholar]
- Lord C, Rutter M, Le Couteur A (1994) Autism Diagnostic Interview—Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685 [DOI] [PubMed] [Google Scholar]
- MacLean JE, Szatmari P, Jones MB, Bryson SE, Mahoney WJ, Bartolucci G, Tuff L (1999) Familial factors influence level of functioning in pervasive developmental disorder. J Am Acad Child Adolesc Psychiatry 38:746–753 [DOI] [PubMed] [Google Scholar]
- Ott J (1999) Analysis of human genetic linkage. 3d ed. The Johns Hopkins University Press, Baltimore, MD [Google Scholar]
- Pericak-Vance MA. The genetics of autism. In: Plomin R, DeFries J, Craig I, McGuffin P (eds) Behavioral genetics in the postgenomic era. APA Books, Washington, DC (in press) [Google Scholar]
- Shao YJ, Wolpert CM, Raiford KL, Menold MM, Donnelly SL, Ravan SA, Bass MP, McClain C, von Wendt L, Vance JM, Abramson RH, Wright HH, Ashley-Koch A, Gilbert JR, DeLong RG, Cuccaro ML, Pericak-Vance MA (2002) A genomic screen and follow-up analysis for Autism. Am J Med Genet 114:99–105 [DOI] [PubMed] [Google Scholar]
- Szatmari P, Bryson SE, Streiner DL, WIlson F, Archer L, Ryerse C (2000) Two-year outcome of preschool children with autism or Asperger's syndrome. Am J Psychiatry 157:1980–1987 [DOI] [PubMed] [Google Scholar]