Abstract
Background
Palliative care is increasingly viewed as a care option that should not only be offered to patients very near the end of life. An important question is whether increased use of palliative care soon after a patient's referral will improve patient functioning, an aspect of quality of life.
Objectives
The aim of this study was to determine if increased use of palliative care is associated with increased patient functioning.
Methods
The Carolinas Palliative Care Database Consortium collects palliative care encounter data from a variety of providers, settings, and patients, and it measures patient functioning, allowing us to test the hypothesis that increased use of palliative care early in a patient's palliative care experience will improve patient functioning.
Results
After controlling for other factors that could explain patient functioning, we find that each additional palliative care visit during the first month of follow-up increases patient functioning measured using an area under the curve (AUC) approach (0.008 per visit, p=0.01). However, patient functioning as measured at the initial visit is a far stronger predictor of subsequent functioning (0.52, p<0.001) than are additional palliative care visits.
Conclusions
Increased use of palliative care was associated with improved patient functioning. This held true at very low as well as very high levels of initial functioning. The strongest predictor of subsequent patient functioning is their initial status. Accounting for patient-specific differences to precisely determine the impact of palliative care on patient functioning is difficult.
Introduction
Palliative Care has typically been viewed as a care option that is offered primarily to patients with cancer, and only in the last days or weeks of life. However, palliative care has come to be viewed as an appropriate treatment option for most life-limiting illnesses, whereas cancer is becoming more of a chronic illness. Simultaneously, there is more interest in providing palliative care earlier in a patient's disease trajectory.1–3 Hospice is a subset of palliative care that is appropriate for patients believed to be in their last 6 months of life, and the median length of hospice use in the Medicare program—which finances the care for approximately 8 in 10 decedents annually—is around 15 days. If we argue that palliative care is care that focuses on ameliorating disease burden and seeks to maximize patient functioning and quality of life among patients with a life-limiting illness including but not limited to hospice, then an important question is can introduction of palliative care earlier in the disease course improve patient functioning, which could improve quality of life?
Providing palliative care earlier in the disease course is believed to offer the possibility of improving patient functioning and quality of life while potentially reducing the cost of care, a finding of a recent randomized control trial of early palliative care among patients with stage IV lung cancer.4 Determining the generalizability of this finding to broad groups of patients is a key priority. We seek to document whether increased use of palliative care has a positive impact on patient functioning, and therefore quality of life, in a community-based sample. A major benefit of our study is that it addresses this issue by using patient data from a consortium of palliative care providers who are delivering care in disparate clinical settings, including outpatient-based and hospital-based palliative care. This approach addresses the question within a group of diverse patients whose experience is expected to be similar to the broad experience of palliative care being provided to and received by patients with life-limiting illnesses across the nation.
Methods
The Carolinas Palliative Care Database Consortium5 is a community/academic partnership between Duke University and three community-based palliative care programs in North Carolina (Four Seasons, Flat Rock; Forsyth Medical Center, Winston-Salem; and Hospice of Wake County and Horizons Palliative Care, Raleigh). Since 2008, Consortium members have been systematically collecting quality improvement data to understand patient needs and inform practice change.6 Sites represent diverse practice locations (urban, suburban, and rural), patient demographics (variations in socioeconomic status and racial diversity), medical cultures (varying prevalence of medical specialists, proximity to academic medical centers), and palliative care practice models (e.g., community-based, bridging, inpatient consultative, blended with hospice).
Quality improvement data were collected by palliative care clinicians at point of care through the Quality Data Collection Tool (QDACT) Version 1.0, a needs assessment tool developed by the Consortium.5,6 Demographic data included patient age, gender, and race. Clinicians entered the primary medical condition, and diagnoses were confirmed by comparing against submitted International Classification of Diseases, 9th Revision (ICD-9) codes for the encounter. The McCorkle7 Symptom Distress Scale, considered the first gold standard for symptom measurement in cancer patients, was the foundation for symptom queries; questions used 4-point Likert scales ranging from “not a problem” to “severe problem.” As has been previously validated,8,9 any answer choice of “moderate” or “severe” was considered clinically significant; in most analyses “moderate” and “severe” were combined. Palliative Performance Scale10 Version 2.0 (PPS) was used to document performance status and aid in prognostication; the PPS is derived from the Karnofsky Performance Scale and categories have similar meaning. Pharmacologic and nonpharmacologic symptom interventions for pain, dyspnea, depression, and constipation were documented by the palliative care provider. PPS data were used as the outcome variable in this current study, as described below, because functioning is viewed as an important aspect of patient quality of life that is not based on a patient's subjective views of a situation. Past work demonstrates the multifactoral complexity of quality of life,11 including how subjective assessments are revised by patients in response to changes in symptoms and functioning; the PPS measure is designed to measure such changes within the context of day-to-day functioning, providing tangible information to the broad concept of quality of life. Studies have confirmed the tight link between objective performance status assessment and patient-reported quality of life.12,13
Data were extracted in de-identified aggregate from the Carolinas Consortium Palliative Care Database, reflecting patient encounters between June 1, 2008 and December 31, 2011. To ensure that results reflected community-based nonacademic palliative care, any records from Duke University Health System were excluded. Duplicate records and those without complete demographics to satisfactorily identify duplicates were excluded. The study sample size is n=748. Basic descriptive statistics were used to inform multivariate models, both of which were conducted using SAS software (SAS Institute Inc., Cary, NC). Table 1 lists the variables used in this analysis.
Table 1.
Variables Used in the Analysis
| Variable | Type | Observed values | N (Total=748) |
|---|---|---|---|
| Reason for referral | Categorical | 1=Goals/Decision making | 372 |
| 2=Pain/Other symptom management | 163 | ||
| 3=Psychosocial needs | 25 | ||
| 4=EOL issues | 14 | ||
| 5=Withdrawal of life-prolonging treatments | 1 | ||
| 9=Not collected by clinician | 102 | ||
| Missing | 71 | ||
| Life expectancy | Categorical | 0=Hours to days | 4 |
| 1=Days to weeks | 17 | ||
| 2=Weeks to months | 35 | ||
| 3=4 to 6 months | 191 | ||
| 4=Greater than 6 months | 446 | ||
| 5=No change since last visits | 9 | ||
| 9=Not collected by clinician | 46 | ||
| Visit site | Categorical | 1=Forsyth | 11 |
| 2=Four Seasons | 648 | ||
| 3=Hospice of Wake | 89 | ||
| Respondent | Categorical | 3=Patient | 389 |
| 4=Family/Proxy | 190 | ||
| 5=Other | 30 | ||
| 9=Not collected by clinician | 139 | ||
| Gender | Categorical | 1=Female | 430 |
| 2=Male | 308 | ||
| Missing | 10 | ||
| Race/ethnicity | Categorical | African American/Black | 55 |
| Caucasian/White | 636 | ||
| Other ethnicity | 25 | ||
| Missing | 32 | ||
| Disease type | Categorical | Debility | 64 |
| Heart disease | 15 | ||
| Lung disease | 17 | ||
| Cancer | 30 | ||
| Others | 10 | ||
| Missing | 612 | ||
| Age at survey | Continuous | Range: 23–101 | |
| PPS at first visit | Continuous | Range: 0.1–0.9 | |
| Visit number | Continuous | Range: 1–21 |
EOL, end of life; PPS, Palliative Performance Scale.
The hypothesis tested was that more palliative care visits during the first 30 days following a referral to palliative care would be associated with improved functioning when assessed longitudinally. The index period was 30 days from the initial palliative care visit measured, and the number of palliative care visits in this period (range 1–21) was the key explanatory variable. Functioning as measured by the PPS was then assessed for as long as the patient remained in the database.
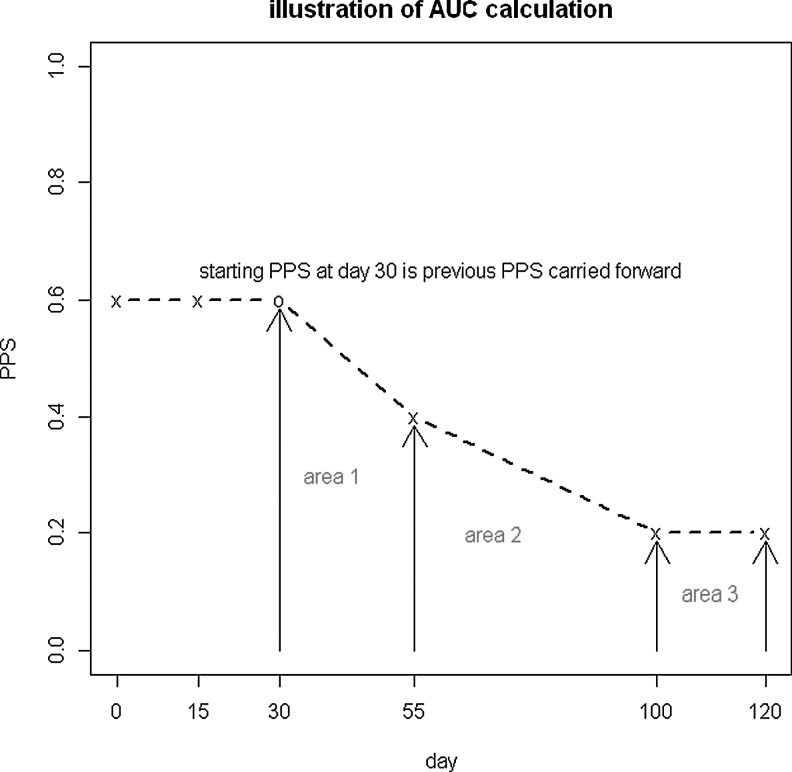
We operationalized patient functioning using an area under the curve (AUC) approach that specified PPS10 as the measure of patient functioning (see Fig. 1). This approach has previously been published by our group as a mechanism to assess the effectiveness of palliative care interventions.14,15 The PPS score theoretically ranged from 1.0 (optimal functioning) to 0.1 (10% on the PPS, because 0 is the same as dead); hence, maximum functioning would be 1.0 for the period of time a patient remained in the database (although the range of observed PPS was 0.1–0.9). The AUC was calculated using the trapezoid rule, again similar to our prior published work and normalized to AUC per day; a larger number on the AUC assessment meant better functioning. The mean AUC value was 0.5 (standard deviation [SD]=0.13, range 0.1–0.95).
FIG. 1.
Operationalizing quality of life using an area under the curve (AUC) approach. Example: This patient has 5 visits in total. He has 2 visits within the first 30 days and 3 visits after 30 days. Here is how AUC gets calculated: Area 1=(0.6+0.4)*(55−30)/2=12.5 (Trapezoidal Rule); Area 2=(0.2+0.4)*(100−55)/2=13.5 (Trapezoidal Rule); Area 3=0.2*(120−100)=4 (Rectangle); Maximum possible area after 30 days=(120−30)*1=90; AUC=(area1+area2+area3)/(maximum area)=(12.5+13.5+4)/90=30/90=0.33.
Results
A Pearson correlation coefficient shows a negative relationship between number of palliative care visits and the PPS AUC (–0.130, p value=0.0004). However, after controlling for a series of explanatory variables believed to be linked to patient functioning, the number of palliative care visits was positively (more visits, higher quality of life) related to higher functioning and statistically significant (0.008 change in the AUC per extra palliative care visit, p=0.01). This analysis controlled for a variety of variables that were believed to influence quality of life of patients who are in need of palliative care (Table 2).
Table 2.
The Impact of Palliative Care on Patient Quality of Life
| Variables | Estimate | P value | Estimate | P value |
|---|---|---|---|---|
| Intercept | 0.20 | <0.0001 | 0.20 | <0.0001 |
| Age at first visit | −0.0005 | 0.0640 | −0.0006 | 0.0183 |
| PPS at first visit | 0.52 | <0.0001 | 0.54 | <0.0001 |
| Visit number | 0.008 | 0.0137 | 0.006 | 0.0260 |
| Gender | 0.9440 | |||
| Female | −0.0003 | 0.9738 | ||
| Male (reference) | 0 | − | ||
| Visit site | <0.0001 | <0.0001 | ||
| Forsyth | −0.15 | <0.0001 | −0.13 | <0.0001 |
| Four Seasons | 0.05 | 0.0003 | 0.057 | <0.0001 |
| Hospice of Wake (reference) | 0 | − | 0 | − |
| Race | 0.2886 | |||
| African American | 0.016 | 0.3133 | ||
| Others | −0.034 | 0.2540 | ||
| White | 0 | − | ||
| Reason for referral | 0.2774 | |||
| Goals/Decision making | −0.004 | 0.8946 | ||
| Pain/Other symptom management | 0.014 | 0.6295 | ||
| Psychosocial needs | 0.019 | 0.5880 | ||
| EOL issues (reference) | 0 | − | ||
| Life expectancy | 0.7262 | |||
| Hours to days | −0.043 | 0.3616 | ||
| Days to weeks | 0.0096 | 0.7375 | ||
| Weeks to months | 0.014 | 0.4431 | ||
| 4 to 6 months | −0.0034 | 0.7234 | ||
| Greater than 6 months (reference) | 0 | − | ||
| Respondent | 0.1946 | 0.0524 | ||
| Patient | 0.023 | 0.3059 | 0.021 | 0.2250 |
| Family/Proxy | 0.007 | 0.7590 | 0.001 | 0.9525 |
| Other (reference) | 0 | − | 0 | − |
| N | 455 | 587 | ||
| R squared | 0.60 | 0.59 |
Note: Table shows full model and then model reduced to show only significantly significant predictors. N=293 missing cases due to item missing values for full model; n=161 for reduced.
EOL, end of life; PPS, Palliative Performance Scale.
The key variable in explaining patient functioning was their level of function at the initial palliative care visit as measured by the PPS score (0.52, p<0.001). To provide a clearer sense of the relative impact of initial functioning of patients as compared with the effect of another palliative care visit on functioning, we used the model shown in Table 2 to predict AUC while varying initial quality of life and number of palliative care visits, with other variables held constant at the sample mean; results of this analysis are shown in Table 3. For example, someone with very poor functioning at the initial visit (0.1 on PPS) would have the AUC increase from 0.29 to 0.32 if he or she had 5 instead of 1 palliative care visit. Conversely, someone with a very good functioning at the initial visit (PPS=0.9 at initial visit) had AUC increase from 0.72 with 1 palliative care visit to 0.75 with 5 such visits.
Table 3.
The Relative Impact of an Additional Palliative Care Visit versus Baseline Quality of Life, on Subsequent Quality of Life
| |
Number of visits |
||||
|---|---|---|---|---|---|
| PPS at baseline | 1 | 2 | 3 | 4 | 5 |
| 0.1 | 0.29 | 0.30 | 0.31 | 0.31 | 0.32 |
| 0.3 | 0.40 | 0.41 | 0.41 | 0.42 | 0.43 |
| 0.5 | 0.51 | 0.52 | 0.52 | 0.53 | 0.53 |
| 0.7 | 0.62 | 0.62 | 0.63 | 0.64 | 0.64 |
| 0.9 | 0.72 | 0.73 | 0.74 | 0.74 | 0.75 |
Note: Values are predicted value of area under the curve (AUC) given the PPS as baseline and number of palliative care visits with other variables shown in the pared-down model held at the sample mean, using ordinary least squares (OLS) regression; n=587.
PPS, Palliative Performance Scale.
Discussion
We found that increased use of palliative care improved patient functioning, a tangible measure that is linked with patient quality of life, after controlling for other factors. However, the initial functioning measure identified was far more important in predicting the subsequent level of functioning and improvements gained through involvement with palliative care. This demonstrates a positive value of palliative care on functioning, which presumably translates to better quality of life, while also showing that patient characteristics likely limit what may be achieved via such care in an absolute sense.
This study documents community palliative care across a variety of settings (inpatient, community provided) and shows that there is a positive impact of increased palliative care on functioning, although the impact of baseline functioning and quality of life are far more important. An important limitation in an analysis such as this one is accounting for patient differences that could confound any relationship between use of palliative care and quality of life, and our inability to account for all possible confounders is a limitation. For example, it is easy to imagine someone seeking more palliative care due to unmitigated pain and lack of symptom control, whereas a patient who was doing better would use less care. This points out the fact that there remains a great deal of patient-level variation that cannot be accounted for in this study, especially information that could signify some patients as being more able to benefit from palliative care. Further, there is a question about whether the improvements in the PPS measure are clinically meaningful, although they were found to be statistically significant.
In this study we attempted to deal with the issues of patient heterogeneity as best we could in an observational setting by controlling for observed characteristics believed to be linked to functioning and quality of life, but questions such as these cannot be fully addressed in such an observational analysis. Our findings should therefore be understood as preliminary, and need to be replicated and tested in other settings with even richer covariates, or perhaps in clinical trials. Further work to document the link between the receipt of palliative care and patient quality of life that had a richer set of covariates could better parse the ability of patients to benefit from palliative care that could yield important confirmatory and extending analyses showing where investments in palliative care might be expected to clearly benefit patients by improving patient quality of life.
The data on which this report is based were collected from a unique palliative care data collection consortium that includes patients receiving palliative care from a variety of clinical settings (inpatient palliative care units, community-based palliative care). This increases the heterogeneity of patient conditions, which makes identifying the precise impact of palliative care on patient quality of life more difficult as noted above. However, this is the reality into which palliative care must be delivered—diverse patients in many settings who are suffering from the sequelae of life-limiting illness(es). Future work should link information on cost of care along with quality in order to allow for a complete assessment of the value of palliative care to community-based patients. Further, the assessment of overall quality of life for persons with life-limiting illness is very important, and should be at the forefront of deciding what treatment options are preferred and should be incentivized. We used an objective measure of functioning (the PPS score) that is believed to be linked to overall quality of life. However, quality of life is a broad concept that includes subjective aspects that have been shown to change in response to objective changes in health and functioning.11 Better understanding of how these concepts relate to one another among patients with life-limiting illness is a top research priority.
Acknowledgments
This research was supported in part by the Changes in Health Care Financing and Organization (HCFO) initiative, a program of the Robert Wood Johnson Foundation grant no. 68708 and the Agency for Healthcare Research and Quality grant no. 5R01HS018360.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.(CDC) CfDCaP. Cancer survivors–United States, 2007. MMWR. 2011;60:269–272. [PubMed] [Google Scholar]
- 2.Jemal A. Ward E. Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers FJ. Linder J. Simultaneous care: Disease treatment and palliative care throughout illness. J Clin Oncol. 2003;21:1412–415. doi: 10.1200/JCO.2003.01.104. [DOI] [PubMed] [Google Scholar]
- 4.Temel JS. Greer JA. Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 5.Bull J. Zafar SY. Wheeler JL, et al. Establishing a regional, multisite database for quality improvement and service planning in community-based palliative care and hospice. J Palliat Med. 2010;13:1013–1020. doi: 10.1089/jpm.2010.0017. [DOI] [PubMed] [Google Scholar]
- 6.Kamal AH BJ. Downey W. Abernethy AP. National Institute of Nursing Research, Science of Compassion: Future Directions in Palliative and End-of-Life Care [abstract 55]; Bethesda, MD: Aug 10–11, 2011. Development, implementation of the QDACT (Quality Data Collection Tool) for real-time quality monitoring, reporting in palliative care. [Google Scholar]
- 7.McCorkle R. Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 8.Degner LF. Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Sarna L. Lindsey AM. Dean H, et al. Weight change and lung cancer: Relationships with symptom distress, functional status, and smoking. Res Nurs Health. 1994;17:371–379. doi: 10.1002/nur.4770170508. [DOI] [PubMed] [Google Scholar]
- 10.Anderson F. Downing GM. Hill J, et al. Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 11.Ubel PA. Jankovic A. Smith D, et al. What is perfect health to an 85-year-old?: Evidence for scale recalibration in subjective heatlh ratings. M Care. 2005;43:1054–1057. doi: 10.1097/01.mlr.0000178193.38413.70. [DOI] [PubMed] [Google Scholar]
- 12.Dahele M. Ung Y. Meharchand J, et al. Integrating regional and communit lung cancer services to improve patient care. Curr Oncol. 2007;14:234–237. doi: 10.3747/co.2007.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izquierdo-Porrera AM. Gardner AW. Bradham DD, et al. Relationships between objectives measures of peripheral arterial disease severity to self-report quality of life in older adults with inermittent cladication. J Vasc Surg. 2005;41:625–630. doi: 10.1016/j.jvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Abernethy AP. Currow DC. Hunt R, et al. A pragmatic 2x2x2 factorial cluster randomized controlled trial of eduational outreach visiting and cas conferencing in palliative care-methodology of the Palliative Care Trial [ISRCTN 81117481] Contemp Clin Trials. 2006;21:83–100. doi: 10.1016/j.cct.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Abernethy AP. Currow DC. Shelby-James T, et al. Delivery strategies to optimize resource utilization and performance status for patients with advanced life-limiting illness: Results from the “Palliative Care Trial” [SRC TN 81117481] J Pain Symptom Manage. 2013;45:188–505. doi: 10.1016/j.jpainsymman.2012.02.024. [DOI] [PubMed] [Google Scholar]