Dear Editor:
A 77-year-old woman with ischemic cardiomyopathy and COPD presented to our ER with a tender 7.5 cm thoracoabdominal aneurysm. Although the aneurysm was large, she had previously declined elective surgery given the high chance for complications. Now in the ER facing imminent rupture, she consented to an operation. After eight hours in surgery she was brought to the ICU where she went into cardiac arrest and returned to the OR for treatment of surgical bleeding. With an additional six hours of surgery and 45 units of blood she made it to the ICU mechanically ventilated and massively edematous, but not requiring vasopressors or blood products.
Her surgeons were satisfied with this outcome; given her underlying comorbidity and the magnitude of the operation, this was the best they could have hoped for. Her family, however, was horrified; they asked to have all life sustaining treatments withdrawn the following day. Although her surgeon didn't know this, their mother had for years voiced fears about the use of life support and spending her remaining life in a nursing home.
In the ER, this patient's stable condition and the uncertain value of major surgery in a frail, elderly woman with multiple comorbidities had prompted extensive discussion between the surgeon and the patient about the appropriateness of operating. Her surgeon had obtained informed consent by accurately disclosing the potential risks and benefits of surgery as well as an alternative nonoperative palliative strategy. Though it was not apparent at the time, the patient was left with significant misunderstanding about what this major operation entailed. She had an unquestionable need for postoperative intensive care, a prolonged recovery, and likely transition to a nursing home—assuming all went well. Now in hindsight it was clear that operating was not compatible with the patient's values or goals.
What could we have done differently? When we rely on the language of informed consent to make these decisions, we ask patients to choose (or refuse) treatment by comparing a procedure with a very high risk of death and complications to palliative care and certain death. It is not surprising that patients choose surgery, hoping that if the mortality rate is 80% they will be in the 20% of patients who survive. Informed consent requires that patients understand the risks, benefits, and alternatives of treatment so they can express their autonomous wishes. However, when we disclose risk by presenting multiple disarticulated risks for isolated physiologic systems (e.g., a 50% chance of renal failure or a 70% chance of respiratory failure), it is unlikely that patients will be able to associate their personal values with the likely consequences of operating.1 What seems like an autonomous decision may ultimately be a miscalculation based on inadequate information about clinical reality.
To help patients make decisions consistent with their personal preferences, doctors need to provide information about possible interventions in a way that contextualizes the medical decision into a larger personal framework.2 This is difficult, because patients rarely present to the ER with preformed preferences about specific treatments. Patients do, however, have an underlying tolerance and acceptance for certain tangible outcomes—for example, a prolonged stay in intensive care or the loss of functional status necessitating nursing home residence. When patients are given an opportunity to express personal values and preferences for outcomes, we can in turn enable the patient to make decisions that reflect these values.3
While decision aids have proven effectiveness for achieving preference-sensitive decisions, these aids have limited availability for many clinical situations and lack flexibility for in-the-moment decision making. Furthermore, decision aids often work to illustrate numerical risks and benefits without providing an example of what the clinical picture might actually look like. It's as if we've asked patients to decide on whether to take a trip to a faraway city, a place they have never been, and the only detail we provide is a street map. Wouldn't they want to know more about the journey and what they might find once they get there?
To support physicians in their efforts to help patients make decisions, we propose a decision support tool that gives patients access to the clinical picture that goes beyond the information that can be provided in the bare statistics. Our tool, called best case/worst case, can be adapted to many clinical settings and inserted in the decision making conversation before the process of informed consent.
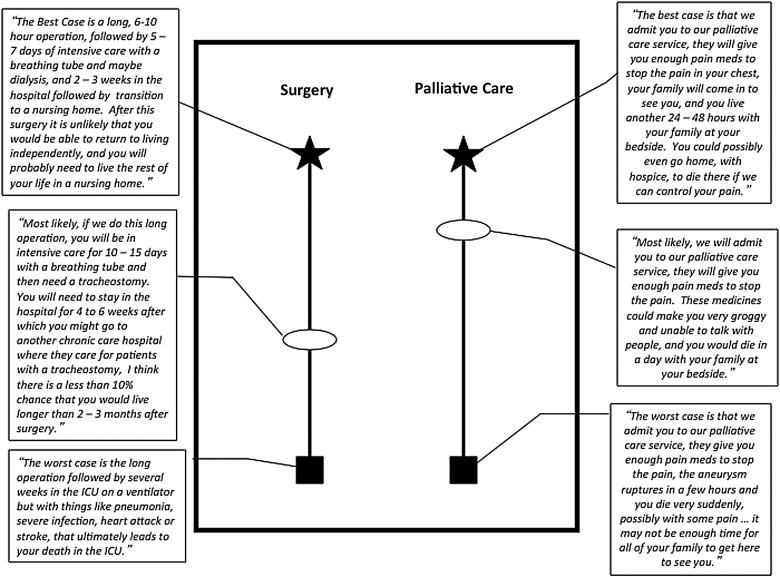
Using our patient's case as an example, the surgeon would start by describing the best case outcome and the worst case outcome, for both surgery and palliative care. The best case scenario for surgery could be described as a long operation, followed by a week in intensive care with a breathing tube, maybe dialysis, and eventual recovery enough to go to a nursing home. The worst case scenario could be described as a long operation, two to three weeks in intensive care with a breathing tube, tracheostomy, dialysis, and subsequent death despite aggressive measures. The alternative, palliative care, is described using similar language (see Figure 1). A simple graphic aid can be constructed with pen and paper contemporaneously to demonstrate the range of clinical possibilities and promote the message that there is a reasonable choice between two strategies rather than one dominant plan with a secondary “alternative.” In framing medical decisions by describing the limits of what is possible (both positive and negative), physicians allow patients to contextualize the possibilities and attach preferences or state valid fears about specific outcomes.
FIG. 1.
Dialogue and graphic aid depicting the “Best Case/Worst Case” for urgent thoracoabdominal aneurysm surgery in a frail elderly woman. The best and worst case outcomes are drawn originally at the same level but can be adjusted to reflect the patient's stated values of each outcome. For example, the surgeon can probe. “I've just told you two different best case outcomes, does one sound better to you?”—and adjust the height of the outcome accordingly.
Although this tool is vulnerable to the physician's personal assessment of the value of the outcomes presented, this subjectivity exists even in the presentation of numerical data.4 Presentation of the best and worst case scenarios with rich detail derived from personal experience and an understanding of the relevant statistics can help fill the large gaps in the story where the numbers fall short.
Using this tool the surgeon can then further synthesize his or her experience, the available evidence, and the patient's unique clinical characteristics in order to give the patient a reasonable estimation of what is most likely to occur. Using probabilistic data (if it exists) within the description of the most likely scenario, the physician can use this clinical description to help anchor the patient's decision making without suggesting any knowledge or guarantee that the most likely outcome will occur. For our patient, her frail condition preoperatively would place the most likely outcome of surgery somewhere close to the worst case—a series of aggressive interventions and poor long-term survival. Armed with this information, our patient may have been able to make a decision more in line with her values and avoid the aggressive interventions she encountered at the end of her life.
While informed consent is a necessary component for decisions that involve risk (and should be obtained subsequently for patients who choose surgery), it is a process that does not sufficiently communicate an accurate picture of clinical reality that patients need to navigate the decision making process. Best case/worst case provides a clinically feasible tool for incorporating a patient's goals and priorities into decision making5 in order to align treatment decisions with patient preferences.
Acknowledgments
We thank Bernie Lo and Mark Neuman for their comments on earlier drafts of the manuscript. We also thank the family of our patient for their permission to use her story.
Dr. Schwarze receives funding from the Greenwall Faculty Scholars Program. The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS) grant 9U54TR000021. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Neuman MD. Bosk CL. What we talk about when we talk about risk: Refining surgery's hazards in medical thought. Milbank Q. 2012;90:135–159. doi: 10.1111/j.1468-0009.2011.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quill TE. Brody H. Physician recommendations and patient autonomy: Finding a balance between physician power and patient choice. Ann Intern Med. 1996;125:763–769. doi: 10.7326/0003-4819-125-9-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 3.Barry MJ. Edgman-Levitan S. Shared decision making: Pinnacle of patient-centered care. N Engl J Med. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 4.Edwards A. Elwyn G. Covey J. Matthews E. Pill R. Presenting risk information: A review of the effects of “framing” and other manipulations on patient outcomes. J Health Commun. 2001;6:61–82. doi: 10.1080/10810730150501413. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME. Fried TR. Boyd CM. Designing health care for the most common chronic condition: Multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]