Abstract
Autonomic dysfunction is common in HIV. However, its clinical impact is not well understood and its protean symptoms make it difficult to diagnose. We sought to determine: (1) whether autonomic neuropathy is associated with morbidity and predicted mortality in HIV as measured by the Veterans Aging Cohort Study (VACS) index; and (2) if healthcare providers recognize the diagnosis of autonomic neuropathy. Data were obtained from 102 HIV-infected adults enrolled in a prevalence study of autonomic dysfunction from 2011–2012. Participants were predominantly minority with nearly equal numbers of men and women. Most were receiving an antiretroviral regimen with a nucleoside reverse transcriptase inhibitor backbone and a base of a non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase inhibitor. Autonomic neuropathy was defined using a laboratory-based autonomic assessment, the Composite Autonomic Severity Score (CASS). Medical records were reviewed for the year prior to the autonomic assessment. We found that the autonomic neuropathy score (CASS) was associated with the VACS index. We also found that among 53 participants with symptomatic autonomic neuropathy, the diagnosis had been considered for only one. The majority of the symptoms were either unexplained or attributed to medication side effects. This study demonstrates that autonomic neuropathy in HIV is associated with serious co-morbid illnesses known to increase mortality risk, and that HIV healthcare providers rarely consider autonomic neuropathy in their differential diagnoses. Future studies are needed to determine if autonomic neuropathy is an independent risk factor for mortality in HIV, and to raise awareness of its signs and symptoms.
Introduction
Autonomic dysfunction is common among HIV-infected individuals, usually occurring as part of a more generalized neuropathic process including distal symmetric polyneuropathy.1 Autonomic nerves are responsible for modulation of nearly all internal organ systems. Accordingly, symptoms of autonomic neuropathy are myriad and include: orthostatic dizziness or fainting, dry eyes or mouth, cold distal extremities, constipation and/or diarrhea, sexual dysfunction, nausea or vomiting especially with meals, urinary incontinence, and changes in sweating.2 When all or most of these symptoms are present, the diagnosis is fairly clear, but often patients will experience only one or two. In this case, symptoms may easily be mistaken for manifestations of systemic illnesses or medication intolerance, especially in the context of HIV.3
The full clinical impact of autonomic neuropathy in HIV is not well understood, but the clinical impact of autonomic neuropathy caused by other systemic illnesses can provide guidance. The best studied example is diabetes, which is also an issue of growing importance among HIV-infected individuals.4,5 Among diabetics, autonomic neuropathy is associated with increased morbidity,6 and mortality which is typically cardiac.7,8 There is disagreement in the literature as to whether autonomic neuropathy is the cause of these adverse outcomes or if they are simply the result of poorly controlled diabetes.9–11 Those who support a causative role for autonomic neuropathy cite impaired cardiac hemodynamics, and dysfunctional control of heart rate, including prolongation of the QT interval and cardiac arrhythmia, as possible mechanisms of action.8 If this is so, then HIV-associated autonomic neuropathy might also be associated with adverse outcomes. Morbidity and mortality in HIV is known to be influenced by age, HIV-related factors such as CD4+ count and HIV-1 viral load, and non-HIV-related factors such as liver and kidney disease. These factors have been summarized in the Veterans Aging Cohort Study (VACS) index which predicts mortality in individuals with HIV.12,13
We undertook the present study with two main goals. First, we sought to determine whether autonomic neuropathy is associated with morbidity and predicted mortality in HIV as summarized by the VACS index. Second, in light of the subtle clinical presentation and potential clinical importance of autonomic neuropathy, we sought to understand whether healthcare providers were recognizing the diagnosis of autonomic neuropathy in the clinic.
Methods
Participants
Data were obtained from 102 HIV-infected adults who were enrolled in a study of the prevalence of autonomic dysfunction in HIV, as previously described.1 Participants were recruited from the waiting room of an adult HIV clinic, in an academic medical center located in the East Harlem neighborhood of New York City. The clinic serves approximately 1500 predominantly minority, inner city patients. The investigator recruiting participants had no specific knowledge of their medical conditions, and offered an appointment to assess eligibility to any willing patient. All study visits occurred between Spring 2011 and Summer 2012. All procedures were performed according to a protocol approved by the Mount Sinai School of Medicine Institutional Review Board. All participants provided written informed consent.
Definition of autonomic neuropathy
All participants underwent a standardized battery of laboratory-based assessments of autonomic function including: quantitative sudomotor axon reflex testing, heart rate response to deep breathing, Valsalva maneuver, and tilt table testing. Quantitative sudomotor axon reflex testing involves iontophoresis of acetylcholine into the skin which causes sweating. The total sweat volume is collected in a capsule, measured by an automated system and compared to standardized values. The other three autonomic tests involve noninvasive continuous monitoring of heart rate and blood pressure during respiratory and positional stimuli. The results of the testing were used to generate the Composite Autonomic Severity Score (CASS), a validated, quantitative measure of autonomic dysfunction that is scored on a scale from 0–10 (with higher numbers indicating greater impairment).14 The CASS has three subscores reflecting different aspects of autonomic function: cardiovagal (cardiac parasympathetic innervation), adrenergic (vascular sympathetic innervation), and sudomotor (sympathetic innervation of sweat glands). The authors of the CASS suggest that a CASS <2 is normal, and that a CASS of 2–3 represents mild autonomic neuropathy.15 As we have done previously,1 we chose a slightly more stringent cut-off of a CASS ≥3 to define autonomic neuropathy in acknowledgment of the medical complexity of our population.
Autonomic symptoms were assessed using the Survey of Autonomic Symptoms (SAS).16 The SAS is a validated questionnaire that elicits the following symptoms: lightheadedness, dry eyes and mouth, changes in temperature or color in the feet, changes in sweating, gastrointestinal symptoms, urinary incontinence, and erectile dysfunction (men only). Symptomatic autonomic neuropathy was defined as a CASS ≥3 and at least one symptom endorsed on the SAS.
Chart review
All records for the clinic have been housed in a single electronic medical record (EMR) since 2006. All documentation in the EMR for the year prior to the autonomic assessment was reviewed for the following information.
All patient-reported symptoms, and the differential diagnosis proposed for these symptoms. Symptoms were then classified as potentially autonomic if they corresponded to one of the items contained in the SAS.
Any mention of the word “autonomic”.
Diagnoses of diabetes and substance misuse (including abuse or dependence). These co-morbidities are considered important causes of autonomic neuropathy in the general population and are therefore possible contributors to autonomic neuropathy in HIV. They might also influence the VACS (e.g., diabetics might have more kidney disease and substance users might be more likely to have exposure to hepatitis C) and are therefore potential confounders of a relationship between autonomic neuropathy and the VACS.
The data required to calculate the VACS: CD4+ count (cells/mm3), HIV-1 RNA (copies/mL), hemoglobin (g/dL), platelets (103/μL), alanine and aspartate transaminase (U/L), creatinine (mg/dL), hepatitis C serostatus. Laboratory values had been collected within 1 month of the autonomic assessment for 78% of participants, and within 4 months for 98%.
Statistical analysis
Spearman's rank correlation was performed for the CASS and the VACS. Kendall's tau-b correlation was performed for the CASS sub-scores and the VACS. Linear regression was performed to measure the association between the VACS and the CASS, with the CASS as the independent variable, substance misuse and diabetes as co-variates, and VACS as the outcome variable. All tests were two-tailed and conducted at the α=0.05 level. We used the SPSS version 20 for all analyses.
Results
The demographic characteristics of this sample have been described previously and are summarized in (Table 1.) The participants were predominantly minority with a balanced representation of men and women. The most common mode of HIV-infection was heterosexual sex (57%), followed by intravenous drug use (20%), and men who have sex with men (18%). A small number of participants (6%) had unknown or other modes of infection (perinatal exposure, blood transfusion). Forty-two percent of participants had detectable HIV viremia (>20 copies/mL), which is somewhat lower than the general clinic population from which they were drawn, in whom 50% are detectable. However, this was mostly low level viremia. Among participants with a detectable viral load, the median viral load was 157 (IQR 50, 4123). Fifty percent of participants had peripheral neuropathy defined as ≥3 of the following: sensory symptoms, sensory signs, reduced or absent ankle reflexes, and reduced or absent sural sensory nerve amplitude. The majority of participants had longstanding HIV treated with combination antiretroviral therapy (CART). Nineteen participants had past exposure to a neurotoxic antiretroviral (stavudine, didanosine, zalcitabine) documented in the EMR, although this is likely an underestimate of actual exposure. Sixty-two participants had autonomic neuropathy (CASS ≥3), of whom 53 had symptomatic autonomic neuropathy (CASS ≥3 and ≥1 symptom on the SAS). All participants had been seen multiple times in the clinic in the preceding year (median=12 visits, IQR (6, 17.5)), including visits with physicians and allied professionals (e.g., social work, case management, nursing, psychology).
Table 1.
Participant Characteristicsa
| N | 102 |
| Gender | |
| Male | 52% |
| Female | 48% |
| Ethnicity | |
| African-American | 62% |
| Hispanic | 32% |
| White | 6% |
| Age, median (IQR) | 51 (44, 57) |
| Duration of known HIV infection, median (IQR) | 16 (13, 22) |
| Antiretroviral regimens | |
| NRTI backbone/PI base | 52% |
| NRTI backbone/NNRTI base | 18% |
| NRTI backbone/integrase inhibitor base | 13% |
| Otherb | 12% |
| None | 5% |
| Current CD4, median (IQR) | 439 (288, 702) |
| Nadir CD4, median (IQR) | 154 (24, 256) |
| HIV viral load, median (IQR) | UD (UD, 253) |
| Detectable HIV viral load | 42% |
| HIV risk factor | |
| Heterosexual sex | 57% |
| Intravenous drug use (IVDU) | 20% |
| Men who have sex with men (MSM) | 18% |
| Perinatal exposure | 3% |
| Unknown | 2% |
| Blood transfusion | 1% |
NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; UD, undetectable (<20 copies/mL).
Other regimens included: NRTI only, NRTI/PI/entry inhibitor, NRTI/PI/integrase inhibitor, NRTI/integrase inhibitor/entry inhibitor, PI/integrase inhibitor.
Worsening autonomic dysfunction, as measured by higher CASS, was associated with a higher VACS (r=0.43, p<0.001). Participants meeting criteria for autonomic neuropathy (CASS ≥3) had a mean VACS of 42 (SD=25) compared to 25 (SD=17) among participants without autonomic neuropathy, which corresponds to a 5 year mortality risk of 20% versus 9%. All three CASS subscores were also modestly but significantly associated with the VACS (adrenergic: r=0.20, p=0.014; cardiovagal r=0.20, p=0.02; sudomotor r=0.25, p=0.001). The relationship between the CASS and the VACS persisted after adjustment for diabetes and substance misuse (p<0.001; standardized β=0.44).
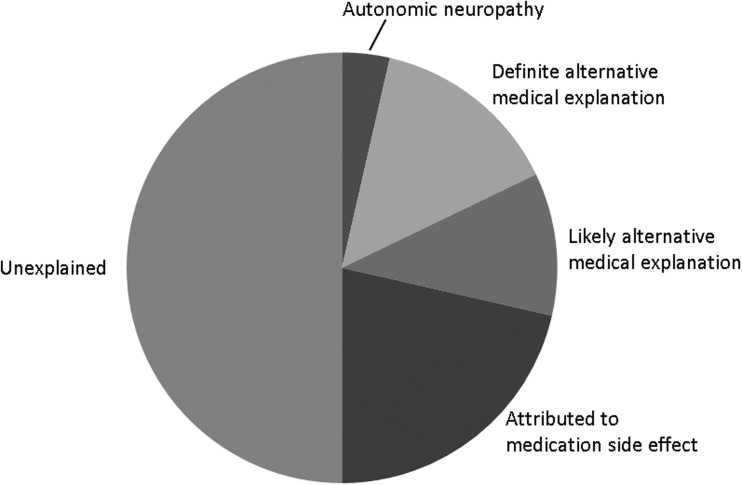
Of the 53 participants with symptomatic autonomic neuropathy, 22 (42%) had at least one autonomic-type symptom documented in the EMR. The most common were gastrointestinal symptoms (nausea, vomiting, diarrhea, constipation) which were recorded in the EMR for 11 participants, and genitourinary symptoms (erectile dysfunction, urinary hesitancy, urgency, incontinence) which were recorded for 9 participants. Less common symptoms were dizziness (n=4), changes in sweating (n=3), and dry mouth (n=1). Of these 28 symptom sets, presenting in 22 participants, a potentially autonomic cause was considered only once, in a patient complaining of orthostatic dizziness. A few other symptoms had clear medical explanations (pseudomembranous colitis causing diarrhea, a urinary tract infection causing urinary frequency, endocarditis causing diaphoresis, nausea, and vomiting), or likely medical explanations (urinary symptoms caused by presumed prostate enlargement, diabetes, or CKD). In the remainder of the cases (21 symptom sets in 16 participants) the symptoms were either attributed to medication side effects or considered unexplained (Fig. 1).
FIG. 1.
Ascribed etiologies for autonomic-type symptoms in participants subsequently found to have HIV-associated autonomic neuropathy.
Discussion
There are preliminary data indicating that autonomic neuropathy is common in HIV, however its clinical impact is not well understood.1 In this study, we found that autonomic neuropathy is associated with a greater burden of illness and higher predicted mortality as measured by the VACS index, which includes age, CD4+ count, HIV-1 viral load, hemoglobin, and markers of liver and kidney disease. We previously reported that autonomic neuropathy was associated with HIV-associated distal symmetric polyneuropathy.1 Thus a clinical phenotype of the HIV-patient likely to have autonomic neuropathy emerges: an older patient with peripheral neuropathy and one or more major co-morbid illnesses. Although this phenotype may be helpful in identifying patients at risk for autonomic neuropathy, arriving at a diagnosis is still complicated by the nonspecific nature of autonomic symptoms which makes them difficult to distinguish from the effects of end organ disease or medication toxicity. In our sample, 73% of participants with autonomic neuropathy who reported autonomic-type symptoms to their healthcare provider did not receive a specific diagnosis; the symptoms were unexplained or attributed to medication side effects.
Our data suggest that this may be an important oversight. We found that autonomic neuropathy was associated with morbidity and higher predicted mortality. Since we do not have longitudinal data from which to gather actual mortality rates, these data must be interpreted with caution. However, the theory that HIV-associated autonomic neuropathy could predict morbidity and mortality has a precedent in the diabetic autonomic neuropathy literature. In diabetes, autonomic neuropathy is associated with other end-organ complications, such as nephropathy and retinopathy,6 but is also an independent risk factor for mortality, largely due to cardiac events.7,8 This may also prove true in HIV. In addition there may also be other, more HIV-specific complications of autonomic neuropathy. Some authors have hypothesized that the lipodystrophy syndrome may be a selective form of autonomic neuropathy.17 Others have shown a complicated interaction between the sympathetic innervation of lymph nodes and viral replication, with an overall decrease in sympathetic innervation of lymph nodes in the rhesus macaque following SIV infection, but enhanced viral replication adjacent to the remaining sympathetic varicosities.18,19
This study has limitations. The data are cross-sectional, so a determination of whether there is a causal relationship between autonomic neuropathy and morbidity cannot be made, and actual (as opposed to predicted) mortality rates cannot be known. The laboratory values used to calculate the VACS index were chart derived and so were not exactly contemporaneous with the autonomic assessment. All participants receive care in a single clinic in an academic medical center, serving a predominantly inner city minority population. Thus, our clinic patients may be more medically complex than most HIV patients. It should also be noted that our sampling method within the clinic was not random. Since we approached patients from the waiting area, those who were in the clinic more often were more likely to be selected. Indeed, the median number of visits annually to the clinic per patient is six, whereas the median for our patients was 12. Patients come to the clinic for medical, psychiatric, and social needs, and so our sample is likely to over-represent the most challenging patients from our clinic. This may limit the generalizability of our findings. However, it also provided the benefit of a sample with adequate representation of higher VACS scores, which would not have been seen in a sample of lower medical complexity.
It is also possible that the finding of under-diagnosis of autonomic neuropathy is unique to our institution. However we consider this unlikely. If anything, we would expect our providers to be more aware of the neurologic complications of HIV, since this is an area of clinical and research interest at our center.
In summary, our data show that autonomic neuropathy in HIV is associated with a higher burden of illness, and accordingly, with a higher predicted mortality risk. Furthermore, autonomic neuropathy is infrequently considered in the differential diagnosis of autonomic-type symptoms. Further research is needed to know how these observations might be incorporated into clinical care. At present it may be prudent for the HIV care provider simply to be alert to the symptoms of autonomic neuropathy, particularly in older patients with distal symmetric polyneuropathy and co-morbid illnesses. This increased awareness may help inform medication management, for example, slower titration of antihypertensives to avoid orthostatic hypotension, or institution of a pro-kinetic agent for symptoms of gastroparesis. Further research will be needed to determine the utility of such approaches. Longitudinal studies of autonomic dysfunction in HIV are also needed to determine its natural history, including whether it is an independent risk factor for mortality.
Acknowledgment
This publication was supported by a grant (K23 NS066789) from the National Institute of Neurological Disorders and Stroke (NINDS) to Dr. Robinson-Papp.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Robinson-Papp J. Sharma S. Simpson DM. Morgello S. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. J Neurovirol. 2013;19:172–180. doi: 10.1007/s13365-013-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sletten DM. Suarez GA. Low PA. Mandrekar J. Singer W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87:1196–1201. doi: 10.1016/j.mayocp.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson-Papp J. Elliott KJ. Pizzirusso M. Morgello S. Autonomic neuropathy in HIV: A case report and review of potential symptoms in an advanced-stage, HIV cohort. WJA. 2012;2:265–269. [Google Scholar]
- 4.Satlin MJ. Hoover DR. Glesby MJ. Glycemic control in HIV-infected patients with diabetes mellitus and rates of meeting American Diabetes Association management guidelines. AIDS Patient Care STDS. 2011;25:5–12. doi: 10.1089/apc.2010.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alencastro PR. Fuchs SC. Wolff FH. Ikeda ML. Brandao AB. Barcellos NT. Independent predictors of metabolic syndrome in HIV-infected patients. AIDS Patient Care STDS. 2011;25:627–634. doi: 10.1089/apc.2010.0360. [DOI] [PubMed] [Google Scholar]
- 6.Voulgari C. Psallas M. Kokkinos A. Argiana V. Katsilambros N. Tentolouris N. The association between cardiac autonomic neuropathy with metabolic and other factors in subjects with type 1 and type 2 diabetes. J Diabetes Complic. 2011;25:159–167. doi: 10.1016/j.jdiacomp.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Maser RE. Mitchell BD. Vinik AI. Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: A meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 8.Kuehl M. Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 2012;8:405–416. doi: 10.1038/nrendo.2012.21. [DOI] [PubMed] [Google Scholar]
- 9.Astrup AS. Tarnow L. Rossing P. Hansen BV. Hilsted J. Parving HH. Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29:334–339. doi: 10.2337/diacare.29.02.06.dc05-1242. [DOI] [PubMed] [Google Scholar]
- 10.Pop-Busui R. Evans GW. Gerstein HC, et al. Effects of cardiac autonomic dysfunction on mortality risk in the action to control cardiovascular risk in diabetes (ACCORD) trial. Diabetes Care. 2010;33:1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez GA. Clark VM. Norell JE, et al. Sudden cardiac death in diabetes mellitus: Risk factors in the Rochester diabetic neuropathy study. J Neurol Neurosurg Psychiatry. 2005;76:240–245. doi: 10.1136/jnnp.2004.039339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate JP. Justice AC. Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27:563–572. doi: 10.1097/QAD.0b013e32835b8c7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justice AC. McGinnis KA. Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: The role of 'non-HIV' biomarkers. HIV Med. 2010;11:143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low PA. Composite Autonomic Scoring Scale for laboratory quantification of generalized autonomic failure. Mayo Clin Proc. 1993;68:748–752. doi: 10.1016/s0025-6196(12)60631-4. [DOI] [PubMed] [Google Scholar]
- 15.Low PA. Benrud-Larson LM. Sletten DM, et al. Autonomic symptoms and diabetic neuropathy: A population-based study. Diabetes Care. 2004;27:2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 16.Zilliox L. Peltier AC. Wren PA, et al. Assessing autonomic dysfunction in early diabetic neuropathy: The Survey of Autonomic Symptoms. Neurology. 2011;76:1099–1105. doi: 10.1212/WNL.0b013e3182120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fliers E. Sauerwein HP. Romijn JA, et al. HIV-associated adipose redistribution syndrome as a selective autonomic neuropathy. Lancet. 2003;362:1758–1760. doi: 10.1016/s0140-6736(03)14858-1. [DOI] [PubMed] [Google Scholar]
- 18.Sloan EK. Nguyen CT. Cox BF. Tarara RP. Capitanio JP. Cole SW. SIV infection decreases sympathetic innervation of primate lymph nodes: The role of neurotrophins. Brain Behav Immun. 2008;22:185–194. doi: 10.1016/j.bbi.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloan EK. Tarara RP. Capitanio JP. Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]