Abstract
Aims: To define the consequences of loss of cysteine dioxygenase (CDO) on cysteine metabolism at the tissue level, we determined levels of relevant metabolites and enzymes and evidence of H2S/HS− (gaseous hydrogen sulfide and its conjugate base) toxicity in liver, pancreas, kidney, and lung of CDO−/− mice that were fed either a taurine-free or taurine-supplemented diet. Results: CDO−/− mice had low tissue and serum taurine and hypotaurine levels and high tissue levels of cysteine, consistent with the loss of CDO. CDO−/− mice had elevated urinary excretion of thiosulfate, high tissue and serum cystathionine and lanthionine levels, and evidence of inhibition and destabilization of cytochrome c oxidase, which is consistent with excess production of H2S/HS−. Accumulation of cystathionine and lanthionine appeared to result from cystathionine β-synthase (CBS)-mediated cysteine desulfhydration. Very high levels of hypotaurine in pancreas of wild-type mice and very high levels of cystathionine and lanthionine in pancreas of CDO−/− mice were observed, suggesting a unique cysteine metabolism in the pancreas. Innovation: The CDO−/− mouse model provides new insights into tissue-specific cysteine metabolism, particularly the role of pancreas in metabolism of excess cysteine by CBS-catalyzed reactions, and will be a useful model for studying the effects of excess endogenous production of H2S/HS−. Conclusion: The CDO−/− mouse clearly demonstrates that H2S/HS− production in tissues can exceed the capacity of the animal to oxidize sulfide to sulfate and demonstrates that pancreas and lung are more susceptible to toxicity from endogenous H2S/HS−production than are liver and kidney. Antioxid. Redox Signal. 19, 1321–1336.
Introduction
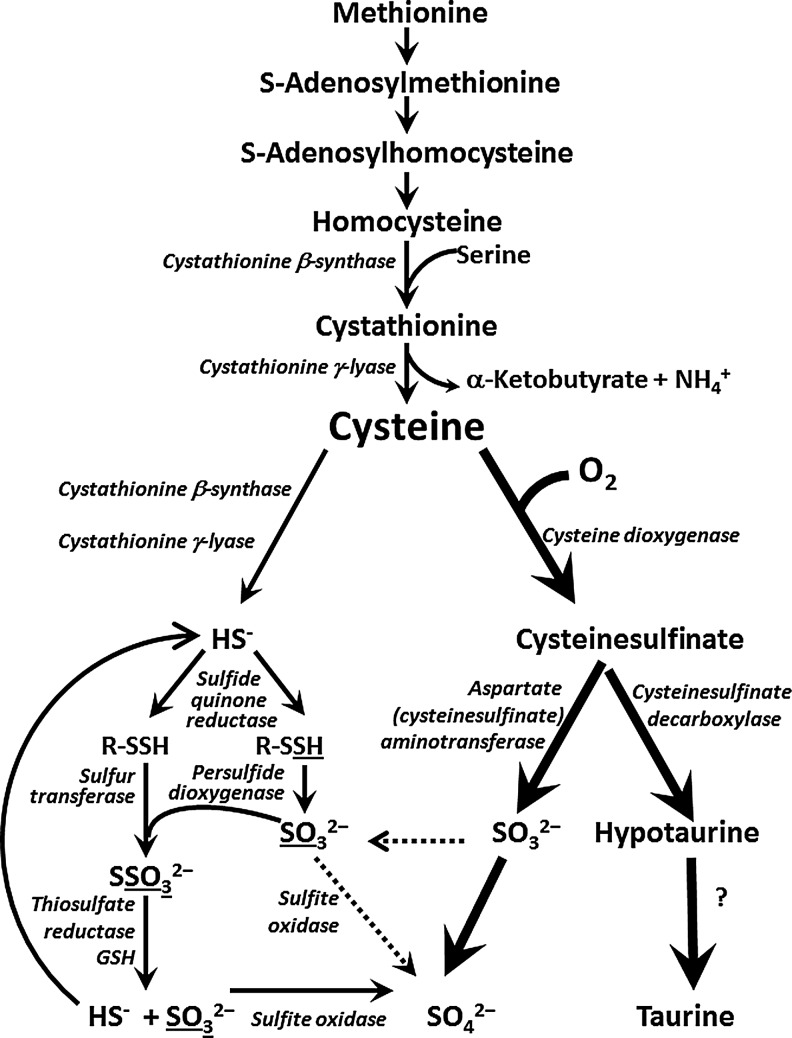
The oxidation of the sulfur atom of the sulfur amino acids, methionine and cyst(e)ine, occurs by both direct oxidation and desulfhydration-oxidation pathways in mammals (21, 46, 47). As shown in Figure 1, the major catabolic pathway for removing cysteine involves the dioxygenation of cysteine to cysteine sulfinate by cysteine dioxygenase (CDO) (42, 46, 47). Cysteine sulfinate undergoes further metabolism by decarboxylation to hypotaurine, which is further oxidized to taurine, or transamination to the putative intermediate 3-sulfinylpyruvate, which gives rise to pyruvate and sulfite (42, 46, 47). Sulfite (SO32−) is further oxidized to sulfate (SO42−) by the mitochondrial sulfite oxidase (SUOX) (10, 39). Thus, the major excretory forms of amino acid sulfur are urinary sulfate and taurine (1, 17).
FIG. 1.
Metabolism of methionine sulfur and cysteine by direct oxidation and desulfhydration-oxidation pathways. The direct oxidation pathway is usually the major route for disposal of excess cysteine, as indicated by the heavy lines and arrows. Little is known about the reaction by which hypotaurine is converted to taurine.
Innovation.
Cysteine dioxygenase (CDO) is expressed in liver, pancreas, kidney, lung, and white and brown adipose tissues, allowing these tissues to metabolize excess cysteine to sulfate and taurine. Disruption of CDO expression leads to abnormal accumulation of sulfide/sulfane sulfur products and evidence of H2S/HS− (hydrogen sulfide and its conjugate base) toxicity. H2S/HS− production in the CDO−/− mouse clearly exceeds the animal's capacity to oxidize sulfide to sulfate. Pancreas and lung are particularly susceptible to endogenous H2S/HS− toxicity, whereas liver and kidney appear to be somewhat protected. Furthermore, the potential usefulness of elevated serum cystathionine and lanthionine levels as biomarkers for excess cysteine desulfhydration by cystathionine β-synthase is established.
Cysteine can also undergo desulfhydration, which is mainly catalyzed by the transsulfuration enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CTH) (6, 21, 40, 41). Desulfhydration pathways result in release of the sulfur atom of cyst(e)ine as hydrogen sulfide (H2S/HS−); the major form of hydrogen sulfide in cells and physiological fluids is HS−, which is in equilibrium with H2S(aq). Desulfhydration of the keto acid of cysteine is catalyzed by 3-mercaptopyruvate sulfur transferase (MPST), although this reaction requires a reducing agent such as thioredoxin or dihydrolipoic acid to release H2S/HS− from the MPST-bound persulfide formed from the substrate (33). The importance of cysteine transamination/MPST in cysteine desulfhydration is probably limited by the extent of cysteine transamination (42, 43). H2S/HS− formed as a result of these desulfhydration pathways is considered an important “gaseous” signaling molecule, such as nitric oxide and carbon monoxide (29, 34, 36). The sulfide released in these pathways is further oxidized to sulfate in the mitochondria, perhaps with thiosulfate (SSO32−) as an obligate intermediate (14, 19, 30, 52). Two molecules of hydrogen sulfide are converted to one molecule of thiosulfate, and thiosulfate is subsequently cleaved by GSH-dependent thiosulfate reductase to yield sulfite+H2S (42, 52). The sulfite is oxidized to sulfate by SUOX, as for sulfite generated in the CDO-mediated pathway of cysteine catabolism, but the outer sulfur atom released as H2S must undergo another cycle of sulfide oxidation (42, 52).
Studies of CDO function in cells and animals (46, 50, 51) indicate that the most important functions of the CDO pathway are its role in maintaining low cysteine levels, which, in turn, prevents excess metabolism of cysteine to H2S/HS− by the alternative desulfhydration pathways, and its role in taurine synthesis. In our initial characterization of CDO knockout mice (51), we found that loss of CDO activity resulted in very low plasma and hepatic taurine levels (i.e., 2% to 7% of wild-type control levels, respectively) and elevated plasma and hepatic cysteine levels (i.e., 200% and 150% of wild-type control levels, respectively). We also observed that taurine supplementation had little effect on the clinical and metabolic phenotype other than returning hepatic and plasma taurine levels to normal, indicating that the phenotype may be dependent on excess metabolism of cysteine by the alternative desulfhydration pathways.
Since CDO is expressed in certain nonhepatic tissues and can be up-regulated in those tissues in the absence of hepatic CDO expression (45, 50), we further investigated the metabolism of cysteine in various tissues known to express CDO using both wild-type and CDO−/− mice and taurine-free and taurine-supplemented diets, with an emphasis on evidence for changes in the extent of cysteine catabolism via desulfhydration pathways. In addition, since the literature is confusing with regard to the localized expression of CDO in mouse tissues, we determined the expression of CDO in murine tissues, taking advantage of CDO−/− tissues to use as a control for potential false positives from nonspecific antibody binding.
Results
The localization of CDO in murine tissues
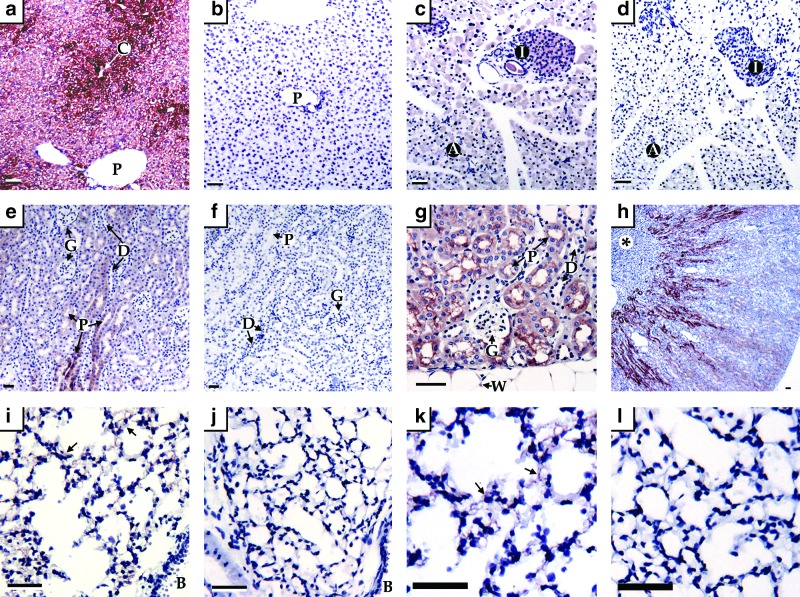
CDO is expressed in hepatocytes, with expression being greater in perivenous than in periportal cells (Fig. 2a). CDO is expressed throughout both the exocrine and endocrine pancreas (Fig. 2c), in the loop of Henle and proximal convoluted tubules of the kidney (Fig. 2e, g, h) and in the parenchymal cells of the lungs (Fig 2i, k). CDO is also expressed in the cytoplasm of white (Fig. 2g) and brown adipocytes (not shown).
FIG. 2.
Immunohistochemical detection of CDO in wild-type mouse tissues using tissues of CDO knockout mice as controls for nonspecific staining. (a) CDO+/+ liver shows the presence of CDO throughout the parenchyma, with more intense staining surrounding the central vein (C) than the portal area (P), the latter identified by the presence of two or more vessels that comprise the portal complex (hepatic artery, bile duct, lymphatic vessel, and portal vein). (c) CDO+/+ pancreas with diffuse staining throughout exocrine acini (A) and within the endocrine islets (I). (e, g, h) CDO+/+ kidney with intense CDO staining in both convoluted and straight portions of the proximal tubule (P) as identified by cells with central euchromatic nuclei and brush border. The proximal tubules of juxtamedullary nephrons show the most intense staining, followed by less intense staining in the cortical nephron proximal tubules. Significantly less staining is seen in the distal tubule (D) as characterized by its euchromatic eccentric nuclei, small cytoplasm, and larger lumen. No staining was observed in glomeruli (G) or inner medulla, including the medullary papilla (*). (i, k) CDO+/+ lung shows punctate CDO staining throughout the alveolar pneumocytes (arrows), but no specific staining was seen in the bronchiolar epithelium (B). Specificity of the CDO antibody is clearly demonstrated by lack of CDO staining in the CDO−/− liver (b), pancreas (d), kidney (f), and lung (j, l). All sections were counterstained with hematoxalin (blue). All scale bars represent 50 μm. CDO, cysteine dioxygenase. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The effects of CDO knockout and taurine supplementation on body weight and feed intake
Animals (pups and dams) had free access to a semipurified diet with or without supplemental taurine from birth until postnatal day (PND) 42. At PND42, pups were switched to diets enriched in cystine and methionine but otherwise similar to the taurine-free or taurine-supplemented diets received before PND42. These higher sulfur amino acid diets were fed from PND42 to the end of the experiment at PND56. As shown in Table 1, CDO−/− mice were smaller than CDO+/+ mice. Mice lost between 1% and 7% of their initial body weight during the 2-week period on the higher sulfur amino acid diets, with male mice losing more weight than female mice and taurine-supplemented mice losing more weight than unsupplemented mice. Total feed intake and feed intake per unit body weight were not affected by genotype or taurine supplementation.
Table 1.
Body Weight and Feed Intake of CDO+/+ and CDO−/− Mice Fed a Sulfur Amino-Acid-Enriched Diet With or Without Taurine
| |
Wild-type |
Null |
Wild-type |
Null |
Wild-type |
Null |
Wild-type |
Null |
P value; three-way ANOVA |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
−Taurine diet |
+Taurine diet |
−Taurine diet |
+Taurine diet |
|
|
|
|
||||
| Female | Male | Genotype (G) | Diet (D) | Sex (S) | Inter-actions | |||||||
| Body weighta g/mouse | 17.1±0.2 | 16.1±0.6 | 17.9±0.7 | 16.8±0.9 | 23.1±0.6 | 19.9±0.9 | 22.8±0.9 | 21.1±1.3 | <0.01 | <0.0001 | ||
| Change in weight over 2-week diet perioda g/mouse | −0.6±0.1 | −0.1±0.3 | −1.0±0.4 | −1.2±0.1 | −1.0±0.1 | −0.6±0.3 | −1.3±0.4 | −1.6±0.2 | <0.01 | <0.05 | ||
| Daily feed intakeb g/mouse | 2.06±0.02 | 1.86±0.14 | 2.27±0.01 | 2.03±0.01 | 2.44±0.03 | 2.08±0.26 | 2.42±0.07 | 2.39±0.24 | <0.05 | |||
| Daily feed intakeb g/g body weight | 0.120±0.003 | 0.119±0.004 | 0.131±0.004 | 0.123±0.005 | 0.108±0.001 | 0.122±0.004 | 0.110±0.001 | 0.125±0.003 | <0.05 | G×S <0.01 | ||
Values for body weight are means±SEM for seven mice.
Values for feed intake are based on measurements for three to six cages of mice, with each cage containing one to four mice.
CDO, cysteine dioxygenase.
The effects of CDO knockout and taurine supplementation on taurine and hypotaurine levels
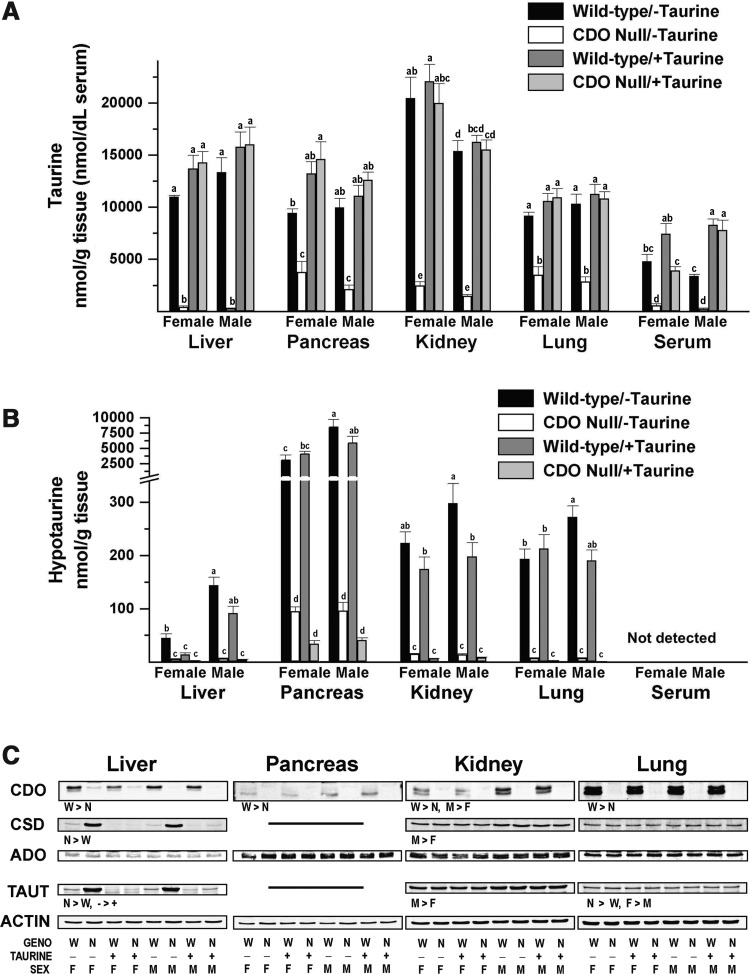
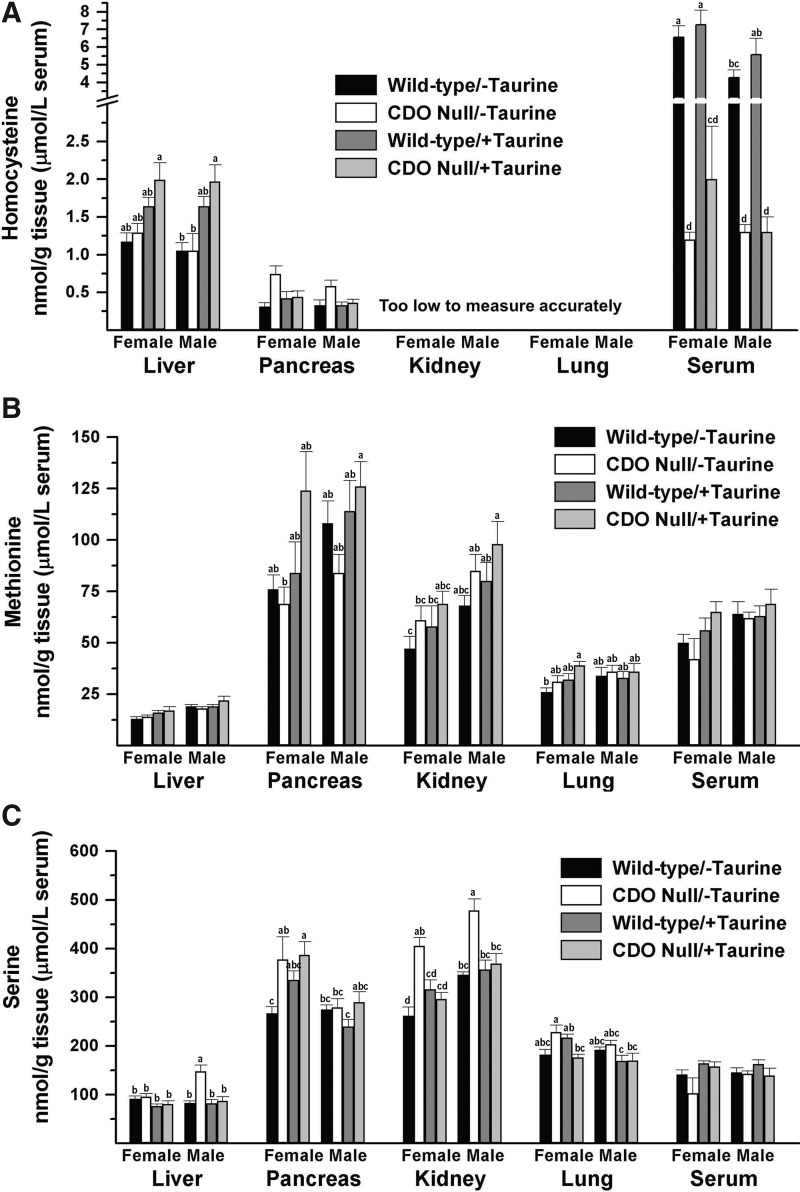
Consistent with the lack of flux through the CDO-dependent pathway for taurine synthesis, CDO−/− mice fed the taurine-free diet had low taurine levels compared with CDO+/+ mice, with liver values being less than 5%, kidney and serum values being less than 15%, and lung and pancreas values being less than 40% of the respective control values (Fig. 3A). Tissue and serum taurine levels in taurine-supplemented null mice were the same or higher than those in unsupplemented wild-type mice. Taurine levels were lower in the kidney of male wild-type mice than in the kidney of female wild-type mice, regardless of diet.
FIG. 3.
Tissue and serum taurine and hypotaurine levels and abundance of CDO, CSD, ADO, and SLC6A6 in female and male null mice and female and male wild-type mice fed a diet enriched in sulfur amino acids without or with supplemental 1% (w/w) taurine. (A, B) Taurine and hypotaurine levels. Values are means±SEM for seven mice. Values for a given tissue that are not denoted with the same letter are significantly different (p<0.05) by Tukey's test. Numerical values and details of the statistical analysis are given in Supplementary Table S1. (C) Western blots for CDO, CSD, ADO, and SLC6A6. Blots are for pooled samples (equal amounts of protein from each of seven mice in the group). Efforts to measure pancreatic CSD and SLC6A6 levels were not successful due to nonspecific antibody reactions. Western blots were quantified and normalized by actin, and the data were analyzed by a general linear model for the three categorical variables: genotype (N, null; W, wild-type); taurine supplementation (−, no taurine; +, with taurine); sex (F, female; M, male); and some two-way interactions. Differences significant at p<0.05 are indicated as the direction of difference. Relative intensities of the bands are reported in Supplementary Table S2. ADO, 2-aminoethanethioldioxygenase; CSD, cysteine sulfinate decarboxylase; SLC6A6, solute carrier 6A6 (taurine transporter).
Hypotaurine levels were low, less than 7% of wild-type levels, in all four tissues of CDO−/− mice, regardless of whether the diet contained taurine (Fig. 3B). The proportionately greater drop in hypotaurine levels than in taurine levels in tissues of CDO−/− mice compared with wild-type controls, especially in pancreas and lung, reflects the fact that hypotaurine is an intermediate in the biosynthesis of endogenous taurine. Interestingly, there was a significant (p<0.0005) overall effect of taurine supplementation on liver and kidney hypotaurine levels, with hypotaurine being lower in taurine-supplemented mice than in their nonsupplemented counterparts. For wild-type mice, the hepatic hypotaurine level was higher in male mice than in female mice, regardless of diet, and kidney and lung hypotaurine levels were higher in male mice fed the taurine-free diet than in their female counterparts.
Unexpectedly, hypotaurine levels varied markedly among tissues. Those in the pancreas were 71- and 60-times those in liver for female and male mice, respectively, whereas kidney and lung had levels that ranged from 1.9- to 5.0-times the hepatic levels. In liver, kidney, and lung of wild-type mice, the hypotaurine levels were less than 3% of the taurine levels in the same tissue, whereas hypotaurine levels in the pancreas of wild-type mice were 33% (female) and 86% (male) of the pancreatic taurine levels. The identification of the hypotaurine peaks obtained by HPLC of samples from pancreas and other tissues was substantiated by conversion to taurine when samples were treated with H2O2 and is consistent with the dramatic reduction in size of the hypotaurine peak in tissues of CDO−/− mice.
Western blots for CDO, cysteine sulfinate decarboxylase (CSD), cysteamine (2-aminoethanethiol dioxygenase [ADO]), and taurine transporter SLC6A6 are shown in Figure 3C. CSD catalyzes the second step of the CDO pathway for taurine synthesis, converting cysteine sulfinate to hypotaurine. ADO oxidizes cysteamine to hypotaurine in an alternative pathway of taurine production via coenzyme A synthesis and breakdown. SLC6A6 is responsible for taurine uptake by tissues. CDO, of course, was present in tissues from wild-type but not CDO−/− mice. Other effects of genotypes included higher CSD and SLC6A6 abundances in liver of null mice, especially in those fed the taurine-free diet, in which CSD and SLC6A6 in liver of CDO−/− mice averaged 15- and 13-fold, respectively, the levels in liver of CDO+/+ mice. Lung SLC6A6 abundance was 8% to 40% more in CDO−/− mice than in wild-type mice. Taurine supplementation affected CSD and SLC6A6 abundances in liver, with both being higher in mice fed taurine-free diets than in their taurine-supplemented counterparts; these effects were much larger for null mice (11- to 54-fold) than for wild-type mice (1- to 3-fold). Thus, the up-regulation of these enzymes paralleled decreases in hepatic taurine and hypotaurine levels in liver. Interestingly, CDO, CSD, ADO, and SLC6A6 abundances in the kidney were higher in male mice, whereas kidney taurine levels were higher in female mice. Other effects of sex included higher CSD in the liver of female mice fed the taurine-free diet and slightly higher SLC6A6 in the lung of female mice compared with their male counterparts.
The effect of CDO knockout and taurine supplementation on cysteine and glutathione levels
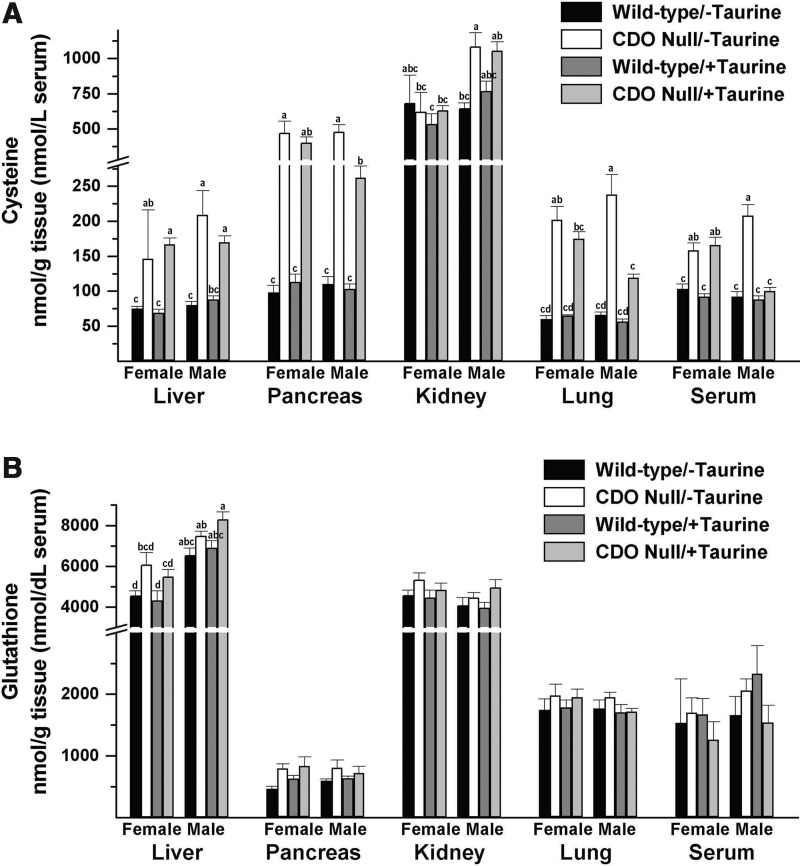
Levels of nonprotein bound cysteine in liver, pancreas, and lung and levels of total cysteine in serum of CDO−/− mice were significantly higher than those in CDO+/+ mice, with tissue levels being 2- to 4-times wild-type levels and serum levels being 1.1- to 2.3-times wild-type levels, depending on the diet-sex group (Fig. 4A). There was little effect of genotype on kidney cysteine levels, which are usually 8- to 9-times those in liver. In pancreas and lung of male CDO−/− mice, taurine supplementation significantly reduced cysteine levels (p<0.05). Male CDO−/− mice on the taurine-free diet had higher kidney cysteine levels, whereas male CDO−/− mice on the taurine-supplemented diet had lower serum cysteine levels compared with their female counterparts. Glutathione levels varied much less than cysteine levels (Fig. 4B). There was an overall effect of genotype on glutathione levels in liver, with levels being 14% to 33% higher in null mice than in wild-type mice of the same sex and diet group. Similarly, pancreas glutathione levels were 14% to 70% higher in null mice than in their wild-type counterparts, although differences did not reach significance for the individual mean comparisons. Hepatic glutathione levels were consistently higher in male mice than in their female counterparts.
FIG. 4.
Cysteine and glutathione levels in serum and tissues of female and male null mice and female and male wild-type mice fed a diet enriched in sulfur amino acids with or without supplemental 1% (w/w) taurine. (A) Total cysteine levels in serum and nonprotein bound cysteine levels in tissues. (B) Total glutathione levels in serum and nonprotein bound glutathione levels in tissues. Values are means±SEM for seven mice. Values for a given tissue that are not denoted with the same letter are significantly different (p<0.05) by Tukey's test. Numerical values and details of the statistical analyses are given in Supplementary Table S1.
The effect of CDO knockout and taurine supplementation on production of sulfide/sulfane sulfur in tissues
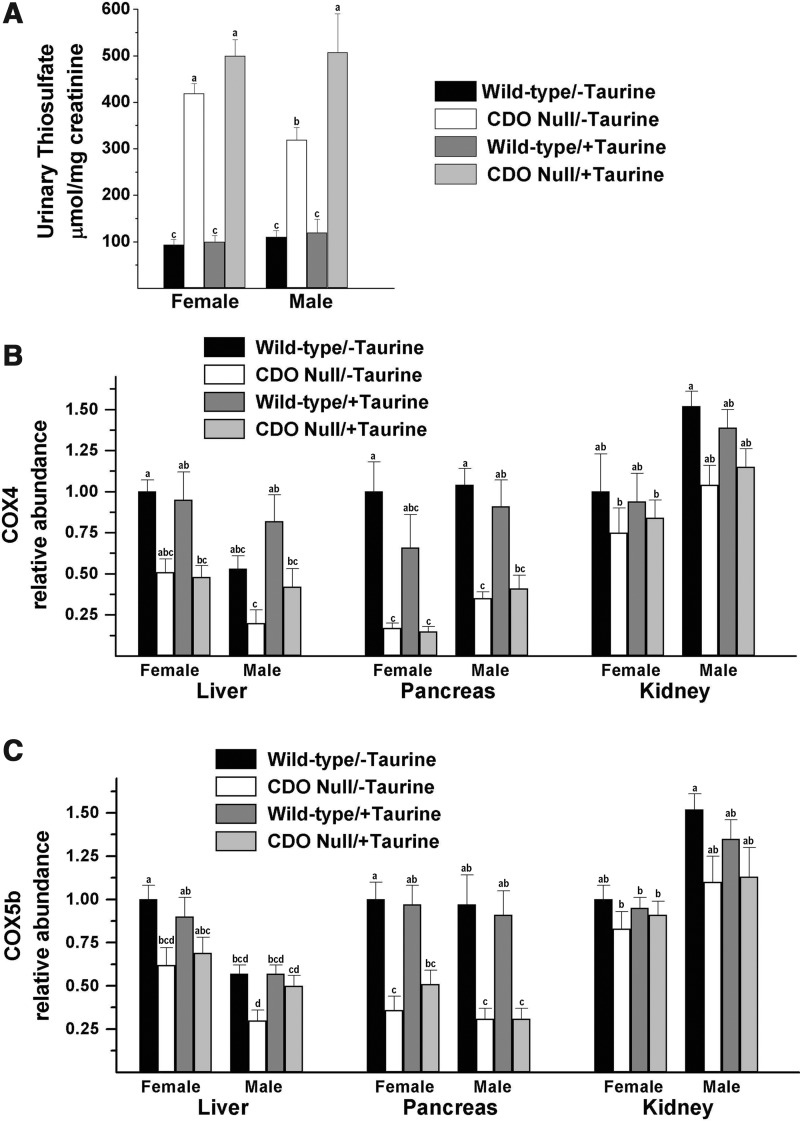
Although H2S/HS− is readily formed and measured when tissue preparations or desulfhydration enzymes are incubated with substrate in vitro, assessment of H2S/HS− levels in tissues of intact animals is difficult (18, 53). The ability of H2S to readily move through lipid membranes results in rapid loss of sulfide from the cell, with HS− shifting to H2S(aq) as H2S escapes. In addition, HS− is readily removed by oxidation to thiosulfate, sulfite, and sulfate, keeping H2S/HS− levels in the low nanomolar range. Furthermore, cysteine sulfur can also be incorporated into the sulfane sulfur pool, which includes protein-bound persulfides as well as thiosulfate. Thus, we used indirect [i.e., thiosulfate excretion, cytochrome c oxidase (COX) inhibition] rather than direct measures to assess the extent of H2S/HS− production in mouse tissues. Greater flux of cysteine through desulfhydration pathways in CDO−/− mice was indicated by elevated levels of urinary thiosulfate that were 4.5-times those in CDO+/+ mice (Fig. 5A). Since many of the COX subunits undergo rapid degradation subsequent to enzyme destabilization by H2S/HS− binding (7), we measured the abundance of COX subunits 4 and 5b to further assess the consequence of increased H2S/HS− production. The overall effect of genotype on COX4 and COX5b indicated that liver, pancreas, and kidney of CDO−/− mice had significantly lower abundances of these subunits than wild-type mice (ANOVA, p<0.05, see Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/ars), but not all individual mean comparisons were significantly different. COX4 and COX5b subunit abundances were most markedly affected in pancreas, in which levels in CDO null mice ranged from 17% to 53% of levels in CDO+/+ mice (Fig. 5B, C). Regardless of genotype or diet, COX4 and COX5b abundances were consistently lower in male liver than in female liver but higher in male kidney than in female kidney (ANOVA, p<0.05, see Supplementary Table S1).
FIG. 5.
Indirect measures of H2S/HS− production in female and male null mice and female and male wild-type mice fed a diet enriched in sulfur amino acids without or with supplemental 1% (w/w) taurine. (A) Urinary thiosulfate levels. (B) COX4 subunit levels as determined by western blotting. (C) COX5b subunit levels as determined by western blotting. Values are means±SEM for seven mice. Values for a given tissue that are not denoted with the same letter are significantly different (p<0.05) by Tukey's test. If no letters are above bars for a given tissue, there are no significant differences among any of the eight groups for that tissue. Numerical values and details of the statistical analysis are given in Supplementary Table S1. COX, cytochrome c oxidase.
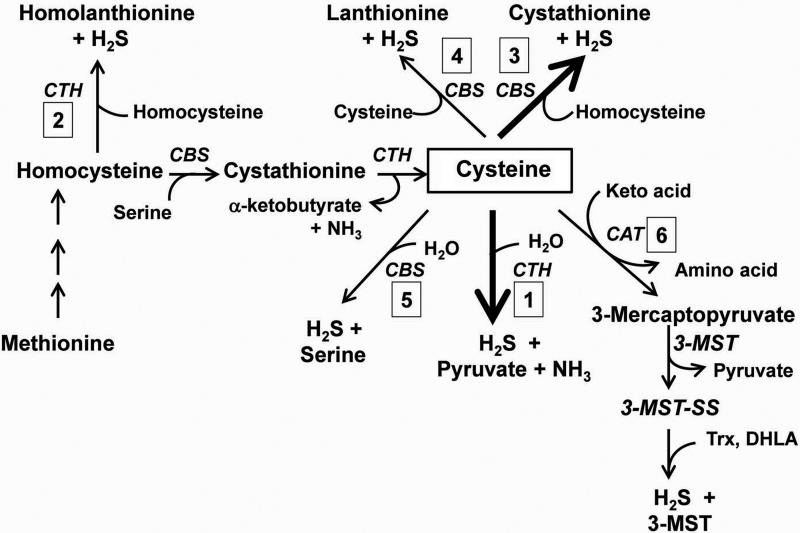
The effect of CDO knockout on tissue levels of substrates and products of desulfhydration enzymes
The major enzymes involved in cysteine desulfhydration are CTH and CBS. The major reactions, both transsulfuration and desulfhydration, catalyzed by these enzymes are shown in Figure 6. To further assess the effect of changes in substrate concentrations on cysteine desulfhydration, we measured the levels of homocysteine, which serves both as a cosubstrate for CBS-catalyzed transsulfuration and desulfhydration and as a substrate for CTH-catalyzed desulfhydration, and serine, which serves as a cosubstrate with homocysteine for CBS-catalyzed transsulfuration (Fig. 7A, B). Neither liver nor pancreas homocysteine levels were affected by genotype. Hepatic homocysteine levels tended to be higher in taurine-supplemented mice than in unsupplemented mice of the same genotype and sex (ANOVA, p<0.0001, Supplementary Table S1). Serum homocysteine levels in CDO−/− mice were only 15% to 30% of those in wild-type mice. Homocysteine levels in kidney and lung were below the limits of accurate measurement with our assay. Serine was elevated in kidney of male and female CDO−/− mice, in pancreas of female CDO−/− mice, and in liver of male CDO−/− mice fed the taurine-free diet compared with CDO+/+ mice fed the same diet, but serine levels in kidney, pancreas, and liver did not differ between genotypes for mice fed the taurine-supplemented diet. Serine levels in serum and lung were not affected by genotype regardless of diet. Thus, the major desulfhydration substrate that consistently increased in all tissues of null mice was cysteine itself. Since methionine is a precursor of homocysteine, we also measured methionine levels, and there were no differences in methionine levels in tissues of null and wild-type mice within any of the sex and diet groups (Fig. 7C). The ratio of S-adenosylmethionine to S-adenosylhomocysteine was determined for pancreas but did not differ among any of the groups (mean ratio of 2.5; see Supplementary Table S1).
FIG. 6.
Major pathways for production of hydrogen sulfide (H2S/HS−) from sulfur-containing amino acids, methionine, and cysteine. Methionine sulfur is used to synthesize cysteine via the transmethylation plus transsulfuration pathways. The reduced thiol sulfur of cysteine can be released as H2S/HS− by the action of CTH, CBS, or possibly by transamination of cysteine to mercaptopyruvate coupled with the action of MPST and reducing agents such as thioredoxin or dihydrolipoate. Due to its much higher molar abundance compared with CBS and due to the marked effects of CTH deficiency on H2S/HS− production, CTH is thought to be the major enzyme that catalyzes cysteine desulfhydration in mammalian tissues other than brain. The major desulfhydration reaction catalyzed by CTH is α,β-elimination of cysteine to form H2S+pyruvate+NH3 (reaction #1.) At equimolar concentrations of CTH and CBS and near-physiological substrate concentrations, the major cysteine desulfhydration reaction is the condensation of cysteine and homocysteine to form cystathionine (reaction #3). The quantitative importance of these two reactions is indicated by the heavy arrows. Other reactions that may contribute to H2S/HS− production include the desulfhydration of homocysteine by CTH (reaction #2), the condensation of two molecules of cysteine to form lanthionine+H2S (reaction #4), the desulfhydration of cysteine by CBS (reaction #5), or the transamination of cysteine and desulfhydration of mercaptopyruvate (pathway #6). Based on the work of Chiku et al. (6) and Singh et al. (41). CBS, cystathionine β-synthase; CTH, cystathionine γ-lyase.
FIG. 7.
Levels of potential substrates or precursors of substrates, other than cysteine, for desulfhydration enzymes in tissues and serum of female and male null mice and female and male wild-type mice fed a diet enriched in sulfur amino acids with or without supplemental 1% (w/w) taurine. (A) Homocysteine. (B) Methionine. (C) Serine. Values are means±SEM for seven mice. Values for a given tissue that are not denoted with the same letter are significantly different (p<0.05) by Tukey's test. If no letters are above bars for a given tissue, there are no significant differences among any of the eight groups for that tissue. Numerical values and details of the statistical analyses are given in Supplementary Table S1.
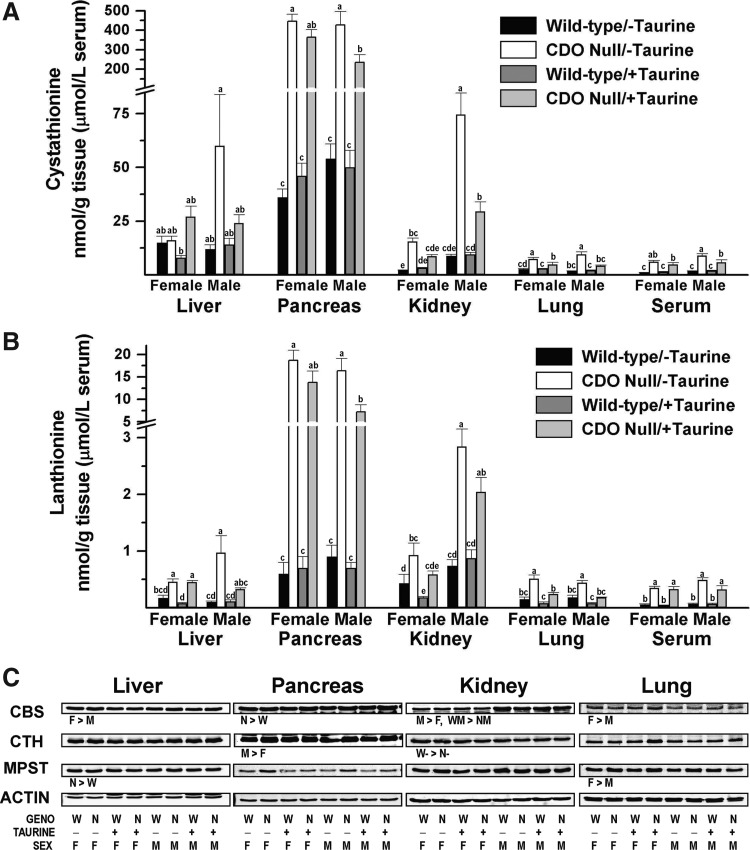
Products of desulfhydration reactions, in addition to H2S/HS−, include cystathionine, lanthionine, and serine from reactions catalyzed by CBS and α-ketobutyrate and pyruvate from reactions catalyzed by CTH and transamination/MPST. Null mice had much higher levels of cystathionine and lanthionine in tissues and serum than did wild-type mice (Fig. 8A, B). Both cystathionine and lanthionine were markedly elevated in pancreas of null mice; in the pancreas, lanthionine was 20-times and cystathionine was 8.5-times the levels present in wild-type mice. Similarly, serum levels were consistently elevated in null mice, with cystathionine levels being 3.0- to 5.5-fold levels in wild-type mice and lanthionine levels being 4.6- to 8.3-fold levels in wild-type mice. Cystathionine and lanthionine levels were consistently higher in lung of null mice compared with their wild-type controls (1.7- to 5.3-fold control for cystathionine and 2.3- to 3.4-fold for lanthionine), with the effects being greater for null mice fed the unsupplemented diet. For the kidney, cystathionine and lanthionine levels were also consistently higher for null mice than for their wild-type controls (2.7- to 8.5- fold for cystathionine and 2.1- to 3.8-fold for lanthionine), with effects being greater for male mice than for female mice. There were also significant overall trends for higher cystathionine (1.1- to 5.0-fold, ANOVA, p<0.0005, Supplementary Table S1) and lanthionine (2.7- to 9.7-fold, ANOVA, p<0.0001, Supplementary Table S1) levels in liver of CDO−/− mice. Liver lanthionine was significantly elevated in null female mice fed either diet and in null male mice fed the taurine-free diet compared with their wild-type counterparts (Fig. 8B), but differences in liver cystathionine between null and wild-type mice in a given sex and diet group did not reach significance (Fig. 8A). The lesser apparent effects of CDO knockout on cystathionine levels in liver are likely due to the very high rate of basal cystathionine production in liver due to the liver's high capacity for methionine transsulfuration (27, 31). Tissue alanine levels were also measured (not shown) and were unaffected by genotype in any tissue.
FIG. 8.
Cystathionine and lanthionine levels in tissues and serum and desulfuration enzyme abundances in tissues of female and male null mice and female and male wild-type mice fed a diet enriched in sulfur amino acids without or with supplemental 1% (w/w) taurine. (A) Cystathionine. (B) Lanthionine. Values are means±SEM for seven mice. Values for a given tissue that are not denoted with the same letter are significantly different (p<0.05) by Tukey's test. Numerical values and details of the statistical analyses are given in Supplementary Table S1. (C) Western blots for CBS, CTH, and MPST. Blots are for pooled samples (equal amount of protein per mouse in each group). Western blots were quantified and normalized by actin, and the data were analyzed by a general linear model for the three categorical variables: genotype (N, null; W, wild-type); taurine supplementation (−, no taurine; +, with taurine); sex (F, female; M, male); and some two-way interactions. Differences significant at p<0.05 are indicated as the direction of difference. Relative intensities of the bands are reported in Supplementary Table S2. MPST, 3-mercaptopyruvate sulfur transferase.
Taurine supplementation had no effect on cystathionine or lanthionine levels in any tissue or serum of wild-type mice but resulted in significantly lower levels of cystathionine and lanthionine in some tissues of null mice. Significant effects of taurine were observed for pancreas, kidney, lung, and serum cystathionine levels in null male mice; pancreas and lung lanthionine levels in null male mice; lung cystathionine levels in null female mice; and kidney and lung lanthionine levels in null female mice. For CDO−/− mice, but not wild-type mice, males had higher kidney cystathionine and kidney lanthionine levels than their female counterparts.
Compared with other tissues, pancreas had much higher cystathionine and lanthionine levels, regardless of genotype, sex, or diet. Cystathionine levels in the pancreas were up to 30-fold those in other tissues of wild-type mice and up to 75-fold those in other tissues of null mice. Similarly, lanthionine levels in the pancreas were up to 9-fold those in other tissues of wild-type mice and up to 58-fold those in other tissues of null mice.
As shown in Figure 8C and Supplementary Table S2, the abundance of pancreatic CBS was 30% to 50% higher in CDO−/− mice than in CDO+/+ mice in the same sex/diet group. In male, but not in female, mice, the abundance of kidney CBS was 10% to 20% higher in CDO+/+ mice than in CDO−/− mice. CBS abundance in kidney of male mice was 100% to 140% higher than for female mice of the same sex/diet group, consistent with the higher cystathionine and lanthionine levels in kidney of male mice (Fig. 8A, B). In contrast, hepatic CBS abundance was 25% to 37% higher, and lung CBS abundance was 12% to 66% higher in female mice than in their male counterparts.
CTH abundance was affected by the genotype in kidney of mice fed the taurine-free diet, with wild-type mice having 25% to 43% higher levels. CTH abundance in the pancreas was affected by sex with male mice having 8% to 40% higher CBS than female mice in the same genotype/diet group. MPST abundance was affected by genotype in the liver, with null mice having 11% to 70% more MPST compared with wild-type mice in the same diet/sex group. MPST abundance was affected by sex in the lung, with female mice having 12% to 66% higher levels.
Since tissue levels of H2S/HS− depend on its rate of removal as well as rate of production, we screened for the abundance of several sulfide-metabolizing enzymes (Fig. 9). The relative abundances of sulfide quinone reductase (SQRD), persulfidedioxygenase (ethylmalonic encephalopathy 1 [ETHE1]), thiosulfate sulfur transferase (TST), and SUOX are shown in Figure 9 and Supplementary Table S1. Significant effects of genotype were observed in pancreas and lung, with pancreas TST being 50% higher in null females and 13% higher in null males than in wild-type mice and with both lung TST and lung SQRD being 50% higher in null mice than in wild-type mice. An effect of dietary taurine was observed for ETHE1 abundance in the pancreas, which was 25% higher for mice fed the taurine-free diet than for taurine-supplemented mice. Effects of sex were observed for ETHE1 (higher in female liver and pancreas; higher in male kidney), TST (higher in female liver; higher in male lung), and SUOX (higher in female liver; higher in male kidney).
FIG. 9.
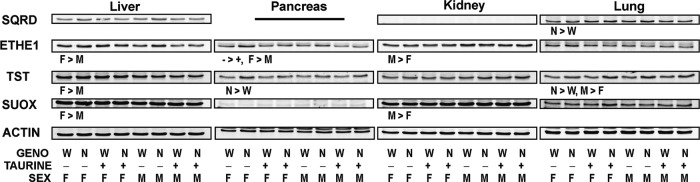
Relative abundance of sulfide oxidizing enzymes in tissues of female and male null mice and female and male wild-type mice fed a diet enriched in sulfur amino acids with or without supplemental 1% (w/w) taurine. Western blots for SQRD, ETHE1, TST, and SUOX are for pooled samples (equal amount of protein per mouse in each group). Western blots were quantified and normalized by actin, and the data were analyzed by a general linear model for the three categorical variables: genotype (N, null; W, wild-type); taurine supplementation (−, no taurine; +, with taurine); sex (F, female; M, male); and some two-way interactions. Differences significant at p<0.05 are indicated as the direction of difference. Relative intensities of the bands are reported in Supplementary Table S2. ETHE1, ethylmalonic encephalopathy 1; SUOX, sulfite oxidase; TST, thiosulfate sulfur transferase.
Discussion
The immunohistochemistry of liver CDO is consistent with previous reports that the highest expression of CDO is in the liver (15, 45, 46, 49) In addition, the greater abundance of CDO in centrilobular hepatocytes than in periportal hepatocytes is consistent with the higher levels of CDO and CSD activities and greater capacity for taurine synthesis that were previously observed in centrilobular hepatocytes (2). Lower levels of CDO protein have been reported for pancreas, kidney, and lung, and lower levels of CDO mRNA have been reported for kidney and lung (14, 45, 46, 49, 50), consistent with our identification of CDO in these tissues. It should be noted, however, that somewhat different subcellular localizations of CDO in these tissues have been reported (32, 47); the discrepancies are at least partially due to the use of biotinylated secondary antibodies in the earlier work. In the work shown here, we used a nonbiotin detection system and also used tissues of CDO−/− mice as a control for nonspecific staining.
One of the end products of cysteine metabolism by the CDO pathway is taurine. Taurine is reported to be involved in cell volume regulation, neuromodulation, antioxidant defense, protein stabilization, and immunomodulation (5, 38, 56, 57). Taurine was severely depleted in liver of CDO−/− mice fed a taurine-free diet, as previously reported (50, 51). A dramatic up-regulation of hepatic CSD and SLC6A6 in CDO−/− mice fed the taurine-free diet, but not in CDO−/− mice fed the taurine-supplemented diet, is consistent with previous observations of inverse associations of taurine with CSD in liver (37, 51) and with SLC6A6 in kidney (13). Although taurine levels in nonhepatic tissues were also depleted, the substantially lesser degree of depletion of taurine in pancreas and lung compared with liver was unexpected. Mice had access to taurine from their CDO+/− dams during gestation and suckling but had no source of exogenous taurine after weaning; so, taurine present in tissues was either synthesized by a CDO-independent pathway or transferred from the dam before weaning. Hypotaurine in the tissues of null mice was presumably formed via the alternative pathway of taurine synthesis (cysteine→coenzymeA→cysteamine→hypotaurine→taurine) or by decarboxylation of cysteine sulfinate formed by nonenzymatic oxidation of cysteine (8). Since hypotaurine levels in the pancreas and lung of CDO−/− mice were extensively depleted, similar to those in liver and kidney, it seems likely that pancreas and lung may retain their taurine pools more efficiently than liver and kidney in the face of a taurine deficiency.
The relatively high levels of hypotaurine in pancreas, kidney, and lung of wild-type mice are consistent with these nonhepatic tissues playing important roles in taurine biosynthesis in the animal. These observations challenge the view that the liver is the only major site of taurine biosynthesis, as does our previous observation that liver-specific knockout of CDO does not result in taurine depletion (50). The very high levels of hypotaurine in pancreas of wild-type mice could indicate that hypotaurine per se carries out an important function in the pancreas, possibly as an antioxidant (12). Very high levels of hypotaurine, as well as relatively high ratios of hypotaurine to taurine, have been observed in epididymal fluid, seminal plasma, and sperm; in these cases, hypotaurine has been suggested to play important roles in sperm maturation and capacitation, antioxidation and volume regulation (16, 20). Clearly, tissue levels of hypotaurine are measureable with current HPLC methods, and they serve as a better indicator of tissue capacity for taurine synthesis than do taurine levels, because hypotaurine, unlike taurine, is not obtained from the diet or present in the more oxidized plasma compartment.
CDO knockout resulted in elevated cysteine levels in serum and tissues. Cysteine levels in CDO−/− mice were, on average, 1.7-times control in the serum, 2-times control in the liver, 3-times control in the lung, and 4-times control in the pancreas. Kidney cysteine levels, which were already high relative to those in other tissues, were about 50% higher in null males than in wild-type males but were not different between null and wild-type females. Although cysteine levels were markedly elevated in CDO−/− mice, glutathione levels were only 14% to 70% higher in liver and pancreas of null mice and not different in lung, kidney, or serum of null mice compared with wild-type controls. The limited effect of genotype on glutathione levels in tissues was somewhat unexpected given the close correlation that is frequently observed between cysteine and glutathione concentrations in tissues, particularly under conditions of sulfur amino-acid deficiency. However, mice in this study received a diet with a slight excess of sulfur amino acids; so, even wild-type mice had relatively high cysteine and glutathione levels. In addition, glutamate-cysteine ligase activity is down-regulated when cysteine levels are high, and this limits the ability of the cell to remove excess cysteine by glutathione synthesis (27, 28). Thus, the capacity for glutathione synthesis was close to saturated in tissues of all mice in this study so that CDO−/− mice could not dispose of much additional cysteine by converting it to glutathione.
In our initial characterization of the CDO−/− mouse (51), we found a slight elevation in plasma sulfate levels and evidence for excess H2S/HS− production. Thus, we wanted to evaluate the hypothesis that a lack of CDO results in elevated cysteine levels, which, in turn, result in excess flux of cysteine through desulfhydration pathways, leading to increased exposure of tissues to sulfide and sulfane sulfur compounds. The desulfhydration enzymes function physiologically at substrate concentrations far below their Km values and, thus, are very sensitive to an increase in cysteine concentration (3, 4, 6, 40, 41, 59). H2S/HS− has been shown to play a regulatory role in a number of physiological processes, including vasorelaxation, inflammation and cell survival, with the major mode of signaling currently believed to involve modification of oxidized cysteine residues of target proteins to form cysteine persulfide residues (35). Although exogenous H2S/HS− has long been recognized to have lethal and sublethal effects on humans and animals, consideration of the physiological and pathophysiological effects of endogenous H2S/HS− production has been minimal (53).
An increase in H2S/HS− production or tissue levels in CDO−/− mice was confirmed by a marked increase in urinary excretion of thiosulfate and by loss of COX4 and COX5b subunit abundance in liver, kidney, and pancreas. Thiosulfate is a metabolite formed in the mitochondria by the transfer of enzyme-bound persulfide sulfur (formed from H2S/HS− by SQRD) to sulfite (30, 48); when thiosulfate production exceeds its further oxidation to sulfate, thiosulfate accumulates in plasma and is excreted in the urine. A well-known feature of H2S/HS− toxicity is the inhibition of COX activity due to rapid heme a inhibition, and this has, more recently, been shown to result in destabilization of the enzyme with accelerated long-term degradation of COX subunits (7). Both of these indirect measures of H2S/HS− toxicity have been validated in Ethe1−/− mice and in humans with ethylmalonic encephalopathy, a rare autosomal recessive disorder caused by mutations in the ETHE1 gene, which encodes the mitochondrial persulfide (sulfur) dioxygenase (7, 11, 48). Presumably, sulfite produced by the CDO-mediated pathway can serve as the acceptor of the sulfane sulfur generated from sulfide in the SQRD-catalyzed reaction when mitochondrial sulfite production is prevented by disruption of the ETHE1 gene.
Supplementation of the diet with taurine did not significantly affect any of the parameters used to assess sulfide production. Overall, the phenotype of the CDO−/− mouse seems largely unrelated to taurine status (51). Clearly, taurine supplementation does little to abate H2S/HS− toxicity. However, the possibility that lack of hypotaurine impairs some unique function that is not served by taurine itself, and which hence would not be rescued by taurine supplementation, has not yet been tested.
It seems likely that the elevated flux through desulfhydration reactions in null mice is due mainly to elevated cysteine levels, although the increase in CBS abundance in pancreas of null mice likely also contributed to the increase in H2S/HS− production in pancreas. It seems unlikely that elevated homocysteine levels were driving increased flux through desulfhydration pathways, because tissue homocysteine levels were not significantly different between wild-type and CDO−/− mice and as serum homocysteine levels in CDO−/− mice were consistently less than 30% of levels in wild-type mice.
Although it is commonly stated that H2S/HS− production in liver and most other peripheral tissues is catalyzed by CTH (21, 29, 58), our observations of elevated cystathionine and lanthionine levels indicate substantial cysteine desulfhydration by CBS in pancreas, kidney, liver, and lung. As shown in Figure 6, the thioethers cystathionine and lanthionine are formed when cysteine and either homocysteine or another molecule of cysteine, respectively, are condensed with release of H2S. Lanthionine levels were much lower than cystathionine levels, consistent with kinetic studies showing that the condensation of two molecules of cysteine is much less favorable than the condensation of cysteine with homocysteine (41). Lanthionine and cystathionine levels in tissues followed similar patterns, with both being higher in CDO null mice than in wild-type mice and with the molar ratio of lanthionine to cystathionine being in the range of 0.01 to 0.1 for all tissue and serum samples. In pancreas and liver, the ratio of lanthionine to cystathionine averaged 0.026 and 0.014, respectively, which is very close to the 0.017 predicted by kinetic simulations for CBS-catalyzed H2S/HS− production (560 μM serine, 100 μM cysteine, 10 μM homocysteine, and pH 7.4) (41). Although cystathionine and lanthionine can potentially be produced from cysteine by CTH-catalyzed reactions, reactions leading to these products are unfavorable under physiological conditions and predicted to account for less than 0.2% of the total CTH-catalyzed desulfhydration reactions (6).
A role of CBS in H2S/HS− production is also consistent with the expression of high levels of CBS in pancreas, liver, and kidney (46). In particular, CBS appears to play a large role in H2S/HS− production from cysteine in pancreas as evidenced by the very large fold-elevations of cystathionine and lanthionine in pancreas of CDO−/− mice compared with wild-type mice and by this being the only tissue in which CBS abundance was higher in CDO−/− mice than in wild-type mice. Cystathionine levels exceeding 200 nmol/g were reached in pancreas of CDO−/− mice compared with levels of ∼50 nmol/g for pancreas of wild-type mice or 12 nmol/g for liver of wild-type mice. Likewise, lanthionine levels exceeding 7 nmol/g were reached in pancreas of CDO−/− mice compared with levels of ∼1 nmol/g for pancreas of wild-type mice or 0.2 nmol/g for liver of wild-type mice (Fig. 8). Striking sex differences in both cystathionine and lanthionine levels in kidney, with male mice having three- to five-times the levels in their female counterparts, along with the higher abundance of CBS in kidney of male mice compared with female mice, further supports the conclusion that CBS is an important contributor to H2S/HS− production and that cystathionine and lanthionine accumulate in vivo under conditions of enhanced CBS-catalyzed cysteine desulfhydration (Fig. 8). Our results suggest that serum, as well as tissue lanthionine levels, could be useful specific biomarkers for H2S/HS− production by CBS. Serum lanthionine levels in CDO−/− mice, although low, were about 6-times those in wild-type mice, and the observed range of values for individual mice did not overlap, being 0.05 to 0.11 μM for wild-type compared with 0.20 to 0.60 μM for CDO−/− mice.
Although we were not able to assess the contribution of CTH to H2S/HS− production with the approach followed in this study, the fact that cystathionine levels were elevated in pancreas, kidney, lung, and serum of CDO−/− mice indicates that cystathionine production in these tissues exceeded the capacity of CTH to cleave cystathionine to cysteine plus α-ketobutyrate and ammonia. Although CTH-catalyzed formation of homolanthionine from two molecules of homocysteine is an unfavorable reaction, representing less than 0.5% of CTH-mediated desulfhydration (6), it might be possible in future work to use homolanthionine as a biomarker for H2S/HS− production by CTH. Thiosulfate excretion in the urine may also be a reasonable indicator of overall H2S/HS− exposure. A 3.5-fold excess of thiosulfate was observed in the urine of the CDO−/− mice, and excess excretion of thiosulfate has also been observed in Ethe1−/− mice (48); in children with ethylmalonic encephalopathy, caused by loss-of-function mutations of the ETHE1 gene encoding mitochondrial persulfide dioxygenase (9); and in individuals with Down syndrome (trisomy 21), who have an extra copy of the CBS allele (22).
A clear conclusion from this work with the CDO−/− mouse is that the animal's capacity to produce H2S/HS− exceeds its capacity to oxidize sulfide to sulfate. Excess levels of H2S/HS− also occur in the individuals with loss-of-function mutations of ETHE1 and in the Ethe1 knockout mouse (48). The similarities in the phenotypes of the CDO knockout mouse and the Ethe1 knockout mouse support the conclusion that excess H2S/HS− rather than deficient taurine is responsible for much of the clinical phenotype of the CDO−/− mouse. The CDO−/− mouse model also provides new insights into tissue specificity for H2S/HS− production and H2S/HS− toxicity, especially in the context of chronic postnatal exposure of tissues to high cysteine levels. The capacity for H2S/HS− production appears to be high in pancreas, kidney, and liver but low in lung, whereas the abundance of the ETHE1 persulfide dioxygenase was high in liver and kidney, moderate in pancreas, and low in lung. The high capacity of liver and kidney to oxidize sulfide to sulfite may protect the liver and kidney from H2S/HS− toxicity, whereas the moderate capacity of pancreas to oxidize sulfide along with its high capacity for H2S/HS− production and the very low capacity of lung to oxidize sulfide may contribute to greater vulnerability of pancreas and lung to H2S/HS− toxicity. Sex differences in the relative abundance of enzymes involved in cysteine and H2S/HS− metabolism were noted in some cases, most notably higher levels of enzymes involved in cysteine catabolism in kidney of male mice but higher levels of enzymes involved in sulfide oxidation in liver of female mice.
A critical function of CDO appears to be to remove cysteine by a pathway in which the sulfur atom is oxidized in the first step. Control of cysteine levels by regulation of CDO is necessary to maintain low sulfide/sulfane sulfur levels, and low basal sulfide levels may facilitate the ability of tissues to use H2S/HS− as a signaling molecule. H2S/HS− regulates a broad spectrum of physiological events (34, 36, 54, 55). There is much potential for using pharmacological agents or phytochemicals to deliver H2S/HS− in the treatment of a variety of disorders, including hypertension, cardiovascular disease, obesity, diabetes, inflammatory bowel disease, and cancer. The inhibition of COX by H2S/HS− is well known, and excess H2S/HS− may additionally interfere with oxidation of carbon-based substrates by the electron transport chain because of precedence of SQRD over Complex I (26). Knowledge of potential benefits of H2S/HS− needs to be tempered by an understanding of potential adverse effects and genotype considerations, especially if long-term use of sulfide-delivering drugs is to be considered. This work with the CDO knockout mouse is an important contribution to the elucidation of effects of endogenous H2S/HS− production.
Materials and Methods
Animals
All experimental procedures involving live animals were conducted with the approval of the Cornell University Institutional Animal Care and Use Committee (No. 2009-0138). Mice were housed in a pathogen-free barrier facility maintained at 23°C and 45%–50% humidity, with a 10-h dark period (from 20:00 h to 6:00 h). CDO+/− mice (51) were crossed to generate CDO−/− and CDO+/+ mice; 14 mice of each sex and each genotype (total of 56 mice; 7 mice per group) were used for this experiment. Genotyping and breeding were performed as previously described (51).
Mice (or their dams) had unrestricted access to a basal taurine-free or taurine-supplemented diet from birth to PND42 and to a sulfur amino acid-enriched taurine-free or taurine-supplemented diet from PND42 to PND56 (Table 2). From PND42 to PND56, food intake and body weight were measured over 2-day increments.
Table 2.
Composition of Mouse Diets
| |
Fed to lactating dams and to pups from PND1 to PND42 |
Fed to pups from PND42 to PND56 |
||
|---|---|---|---|---|
| |
Basal/−taurine |
Basal/+taurine |
High sulfur amino acids/−taurine |
High sulfur amino acids/+taurine |
| g/kg diet | ||||
| Sterile vitamin-free casein | 200 | 200 | 200 | 200 |
| l-Cystine | 1.5 | 1.5 | 4.2 | 4.2 |
| l-Methionine | 0 | 0 | 1.5 | 1.5 |
| Taurine | 0 | 10 | 0 | 10 |
| Cornstarch | 399 | 389 | 395 | 385 |
| Dyetrose | 132 | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 |
| Soybean oil | 70 | 70 | 70 | 70 |
| AIN-93G-MX mineral mix | 35 | 35 | 35 | 35 |
| AIN-93G-VX vitamin mix | 10 | 10 | 10 | 10 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.014 | 0.014 | 0.014 | 0.014 |
| Total Met equivalentsa | 7.4 | 7.4 | 12.3 | 12.3 |
For reference, the standard AIN93G diet formulation for rodents provides 9.3 methionine equivalents per kg, provided by 200 g casein and 3.0 g cystine. 1 g cystine=1.24 g methionine equivalents. The National Research Council estimates of the growth requirement and the maintenance requirement for rodents are 9.8 g and 2.3 g sulfur amino acids per kg diet, respectively.
PND, postnatal day.
Sample collection
Spot urine collection (25) was performed during the second week that mice were on the high-sulfur amino-acid diet. Urine was frozen immediately on collection. Between 10:00 and 14:00 h on PND56, mice were euthanized with isoflurane, blood was drawn via cardiac puncture, and liver, kidneys, pancreas, and lungs were removed and immediately frozen in liquid nitrogen. Blood was centrifuged at 18,000 g, and serum was removed and frozen in liquid nitrogen. Tissues and serum were stored in liquid nitrogen until analysis.
Measurement of amino acids and metabolites
Serum total aminothiols and tissue nonprotein bound aminothiols (thiols plus disulfides, after reduction) were measured by HPLC as previously described (50). Briefly, acid supernatants or serum were treated with tris(2-carboxyethyl)phosphine to reduce disulfides, and thiols were derivatized with 7-fluorobenzo-2-oxa-1,3-diazole-4-sulphonate. Chromatography was carried out on a C18 reversed-phase column with a mobile phase of 100 mM potassium phosphate buffer (pH 2.1) with 5% (vol/vol) acetonitrile. Fluorescence of derivatives in the eluate was detected using an excitation wavelength of 385 nm and an emission wavelength of 515 nm.
Taurine and hypotaurine were measured by HPLC as previously described (50). Samples were derivatized with o-phthaldialdehyde (OPA) and separated on a C18 column by gradient elution using 50 mM potassium phosphate buffer (pH 7.0) with 3.5% (vol/vol) tetrahydrofuran with or without 40% (vol/vol) acetonitrile. Detection of OPA-derivatized compounds was performed using excitation and emission peaks at 360 and 455 nm, respectively.
Cystathionine, serine, alanine, methionine, and lanthionine were determined by LC-MS/MS using a commercially available kit for amino-acid analysis (EZ:faast; Phenomenex) as previously described (24). Sample preparation according to the manufacturer's instructions involved a solid-phase extraction step, derivatization with propyl chloroformate, and extraction into an organic solvent before LC-MS/MS analysis. S-adenosylmethionine and S-adenosylhomocysteine levels in pancreas were measured according to the LC-MS/MS method previously published (23) based on chromatographic separation on a Hypercarb column filled with porous graphitic carbon stationary phase.
Other assays
Urine was deproteinated using an Amicon Ultra 0.5 ml centrifugal filter with a 3 kDa cutoff (Millipore Corp.) and assayed for thiosulfate (39) and for creatinine (urinary Creatinine Assay Kit; Cayman Chemical).
Western blotting
Tissue samples were homogenized in four volumes of TNES buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40] supplemented with Complete protease inhibitor and PhosSTOP phosphatase inhibitor “cocktails” (Roche). Homogenates were centrifuged at 18,000 g for 20 min at 40°C, and the total protein concentration of the supernatant fraction was determined using the BCA Protein Assay Kit (Thermo Scientific Pierce). Fifty micrograms of protein per lane was resolved by SDS-PAGE and transferred onto a 0.45-μm Immobilon PVDF membrane (Millipore Corp.). Membranes were blocked using blocking buffer for near-infrared fluorescent westerns (LI-COR Biosciences) and blotted for immunoreactive proteins. Individual samples were analyzed for COX4 and COX5b, whereas pooled samples (n=7, equal amounts of total protein) were screened for proteins involved in taurine synthesis and uptake, cysteine desulfhydration, and sulfide oxidation. The CDO and ADO antibodies were generated as previously described (8, 44), and the CSD antibody was a gift from Dr. Marcel Tappaz (Institut National de la Santé et de la RechercheMédicale). Other primary antibodies were obtained from commercial sources: COX5b and COX4 (Abcam), CBS, SQRDL, SUOX and TST (Proteintech Group), CTH (Norvus Biologicals), MPST (Santa Cruz Biotechnology), actin (Cell Signaling), ETHE1 (Thermo Scientific), and SLC6A6 (TAU11-A; Alpha Diagnostic International). An infrared fluorescent dye-labeled secondary antibody (IRDye; LI-COR Biosciences) and the Odyssey direct infrared imaging system and software (LI-COR Biosciences) were used to visualize and quantify the relative abundance of each protein.
Immunohistochemistry
Mice were anesthetized with isoflurane and perfused by cardiac puncture for 15 min with 4% (v/v) paraformaldehyde. Tissues were excised, embedded, and sectioned. After epitope retrieval via exposure to 0.025% (w/v) trypsin and blocking of endogenous peroxidase by exposure to 3% (v/v) H2O2, sections were exposed to affinity-purified polyclonal anti-CDO (1:500) and then to ImmPRESS anti-rabbit IgG (Vector Labs). After application of ImmPACT NovaRED (Vector Labs), sections were counterstained with hematoxalin. Images were obtained using an Olympus BX50 microscope with a Moticam 2300 cMOS camera (Motic North America).
Statistical analysis
Results of measurements on individual mice are expressed as means±SEM (n=7). Results for measurements on individual tissues samples or mice were analyzed as a full factorial model by three-way ANOVA using JMP version 10 (SAS, Cary, NC). Post hoc comparisons among means were made by Tukey's test. Differences were considered significant at p≤0.05 for main effects (genotype, sex, and diet) and at p<0.1 for interactions. Numerical values and statistical details are reported in Supplementary Table S1. For western blots of pooled samples, statistical analysis was run as a general linear model for the three categorical variables (genotype, diet, and sex) and some two-way interactions. Effect tests with F values significant at p<0.05 are noted in the figures; relative band intensities and all F values significant at p<0.1 are reported in Supplementary Table S2. Data for cystathionine, lanthionine, taurine, and hypotaurine levels and data for the hepatic abundances of CSD and SLC6A6 were transformed to square roots before statistical analyses.
Supplementary Material
Abbreviations Used
- ADO
2-aminoethanethiol dioxygenase
- CBS
cystathionine β-synthase
- CDO
cysteine dioxygenase
- COX
cytochrome c oxidase
- CSD
cysteine sulfinate decarboxylase
- CTH
cystathionine γ-lyase
- ETHE1
ethylmalonic encephalopathy 1
- H2S/HS−
hydrogen sulfide and its conjugate base
- MPST
3-mercaptopyruvate sulfur transferase
- OPA
o-phthaldialdehyde
- PND
postnatal day
- SLC6A6
solute carrier 6A6 (taurine transporter)
- SQRD
sulfide quinone reductase
- SUOX
sulfite oxidase
- TST
thiosulfate sulfurtransferase
Acknowledgments
This project was supported by Grant DK-056649 from National Institute of Diabetes and Digestive and Kidney Diseases and by Charles University programs PRVOUK P24/LF1/3 and UNCE204011/2012. The content is solely the responsibility of the authors and does not necessarily represent the official views either of National Institutes of Health or of Charles University.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bella DL. Hahn C. Stipanuk MH. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Physiol. 1999;277:E144–E153. doi: 10.1152/ajpendo.1999.277.1.E144. [DOI] [PubMed] [Google Scholar]
- 2.Bella DL. Hirschberger LL. Kwon YH. Stipanuk MH. Cysteine metabolism in periportal and perivenous hepatocytes: perivenous cells have greater capacity for glutathione production and taurine synthesis but not for cysteine catabolism. Amino Acids. 2002;23:453–458. doi: 10.1007/s00726-002-0213-z. [DOI] [PubMed] [Google Scholar]
- 3.Braunstein AE. Goryachenkova EV. Tolosa EA. Willhardt IH. Yefremova LL. Specificity and some other properties of liver serine sulphhydrase: evidence for its identity with cystathionineβ-synthase. Biochim Biophys Acta. 1971;242:247–260. doi: 10.1016/0005-2744(71)90105-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen X. Jhee KH. Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 5.Chesney RW. Han X. Patters AB. Taurine and the renal system. J Biomed Sci. 2010;17((Suppl 1)):S4. doi: 10.1186/1423-0127-17-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiku T. Padovani D. Zhu W. Singh S. Vitvitsky V. Banerjee R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem. 2009;284:11601–11612. doi: 10.1074/jbc.M808026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Meo I. Fagiolari G. Prelle A. Viscomi C. Zeviani M. Tiranti V. Chronic exposure to sulfide causes accelerated degradation of cytochrome c oxidase in ethylmalonic encephalopathy. Antioxid Redox Signal. 2011;15:353–362. doi: 10.1089/ars.2010.3520. [DOI] [PubMed] [Google Scholar]
- 8.Dominy JE., Jr. Simmons CR. Hirschberger LL. Hwang J. Coloso RM. Stipanuk MH. Discovery and characterization of a second mammalian thiol dioxygenase, cysteamine dioxygenase. J Biol Chem. 2007;282:25189–25198. doi: 10.1074/jbc.M703089200. [DOI] [PubMed] [Google Scholar]
- 9.Drousiotou A. DiMeo I. Mineri R. Georgiou T. Stylianidou G. Tiranti V. Ethylmalonic encephalopathy: application of improved biochemical and molecular diagnostic approaches. Clin Genet. 2011;79:385–390. doi: 10.1111/j.1399-0004.2010.01457.x. [DOI] [PubMed] [Google Scholar]
- 10.Feng C. Tollin G. Enemark JH. Sulfite oxidizing enzymes. Biochim Biophys Acta. 2007;1774:527–539. doi: 10.1016/j.bbapap.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano C. Viscomi C. Orlandi M. Papoff P. Spalice A. Burlina A. Di Meo I. Tiranti V. Leuzzi V. d'Amati G. Zeviani M. Morphologic evidence of diffuse vascular damage in human and in the experimental model of ethylmalonic encephalopathy. J Inherit Metab Dis. 2012;35:451–458. doi: 10.1007/s10545-011-9408-3. [DOI] [PubMed] [Google Scholar]
- 12.Gossai D. Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–368. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- 13.Han X. Patters AB. Jones DP. Zelikovic I. Chesney RW. The taurine transporter: mechanisms of regulation. Acta Physiol (Oxf) 2006;187:61–73. doi: 10.1111/j.1748-1716.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 14.Hildebrandt TM. Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008;275:3352–3361. doi: 10.1111/j.1742-4658.2008.06482.x. [DOI] [PubMed] [Google Scholar]
- 15.Hirschberger LL. Daval S. Stover PJ. Stipanuk MH. Murine cysteine dioxygenase gene: structural organization, tissue-specific expression and promoter identification. Gene. 2001;277:153–161. doi: 10.1016/s0378-1119(01)00691-6. [DOI] [PubMed] [Google Scholar]
- 16.Holmes RP. Goodman HO. Shihabi ZK. Jarow JP. The taurine and hypotaurine content of human semen. J Androl. 1992;13:289–292. [PubMed] [Google Scholar]
- 17.Hou C. Wykes LJ. Hoffer LJ. Urinary sulfur excretion and the nitrogen/sulfur balance ratio reveal nonprotein sulfur amino acid retention in piglets. J Nutr. 2003;133:766–772. doi: 10.1093/jn/133.3.766. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MN. Centelles MN. Moore KP. Making and working with hydrogen sulfide: The chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Jackson MR. Melideo SL. Jorns MS. Human sulfide: quinone oxidoreductase catalyzes the first step in hydrogen sulfide metabolism and produces a sulfane sulfur metabolite. Biochemistry. Biochemistry. 2012;51:6804–6815. doi: 10.1021/bi300778t. [DOI] [PubMed] [Google Scholar]
- 20.Johnson LA. Pursel VG. Gerrits RJ. Thomas CH. Free amino acid composition of porcine seminal, epididymal and seminal vesicle fluids. J Anim Sci. 1972;34:430–434. doi: 10.2527/jas1972.343430x. [DOI] [PubMed] [Google Scholar]
- 21.Kabil O. Vitvitsky V. Xie P. Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal. 2011;15:363–372. doi: 10.1089/ars.2010.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamoun P. Belardinelli MC. Chabli A. Lallouchi K. Chadefaux-Vekemans B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am J Med Genet A. 2003;116A:310–311. doi: 10.1002/ajmg.a.10847. [DOI] [PubMed] [Google Scholar]
- 23.Krijt J. Dutá A. Kožich V. Determination of S-adenosylmethionine and S-adenosylhomocysteine by LC-MS/MS and evaluation of their stability in mice tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2061–2066. doi: 10.1016/j.jchromb.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krijt J. Kopecká J. Hnízda A. Moat S. Kluijtmans LA. Mayne P. Kožich V. Determination of cystathionine beta-synthase activity in human plasma by LC-MS/MS: potential use in diagnosis of CBS deficiency. J Inherit Metab Dis. 2011;34:49–55. doi: 10.1007/s10545-010-9178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurien BT. Everds NE. Scofield RH. Experimental animal urine collection: a review. Lab Anim. 2004;38:333–361. doi: 10.1258/0023677041958945. [DOI] [PubMed] [Google Scholar]
- 26.Lagoutte E. Mimoun S. Andriamihaja M. Chaumontet C. Blachier F. Bouillaud F. Oxidation of hydrogen sulfide remains a priority in mammalian cells and causes reverse electron transfer in colonocytes. Biochim Biophys Acta. 2010;1797:1500–1511. doi: 10.1016/j.bbabio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee JI. Kang J. Stipanuk MH. Differential regulation of glutamate-cysteine ligase subunit expression and increased holoenzyme formation in response to cysteine deprivation. Biochem J. 2006;393:181–190. doi: 10.1042/BJ20051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JI. Londono M. Hirschberger LL. Stipanuk MH. Regulation of cysteine dioxygenase and gamma-glutamylcysteinesynthetase is associated with hepatic cysteine level. J Nutr Biochem. 2004;15:112–122. doi: 10.1016/j.jnutbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Li L. Rose P. Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 30.Linden DR. Furne J. Stoltz GJ. Abdel-Rehim MS. Levitt MD. Szurszewski JH. Sulphide quinonereductase contributes to hydrogen sulphide metabolism in murine peripheral tissues but not in the CNS. Br J Pharmacol. 2012;165:2178–2190. doi: 10.1111/j.1476-5381.2011.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu SC. Huang HY. Comparison of sulfur amino acid utilization for GSH synthesis between HepG2 cells and cultured rat hepatocytes. Biochem Pharmacol. 1994;47:859–869. doi: 10.1016/0006-2952(94)90486-3. [DOI] [PubMed] [Google Scholar]
- 32.Luo L. Chen S. Jin H. Tang C. Du J. Endogenous generation of sulfur dioxide in rat tissues. Biochem Biophys Res Commun. 2011;415:61–67. doi: 10.1016/j.bbrc.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Mikami Y. Shibuya N. Kimura Y. Nagahara N. Ogasawara Y. Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 34.Olson KR. A practical look at the chemistry and biology of hydrogen sulfide. Antioxid Redox Signal. 2012;17:32–44. doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paul BD. Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 36.Predmore BL. Lefer DJ. Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rentschler LA. Hirschberger LL. Stipanuk MH. Response of the kitten to dietary taurine depletion: effects on renal reabsorption, bile acid conjugation and activities of enzymes involved in taurine synthesis. Comp Biochem Physiol B. 1986;84:319–325. doi: 10.1016/0305-0491(86)90084-2. [DOI] [PubMed] [Google Scholar]
- 38.Schaffer SW. Jong CJ. Ramila KC. Azuma J. Physiological roles of taurine in heart and muscle. J Biomed Sci. 2010;17((Suppl 1)):S2. doi: 10.1186/1423-0127-17-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shih VE. Carney MM. Mandell R. A simple screening test for sulfite oxidase deficiency: detection of urinary thiosulfate by a modification of Sörbo's method. Clin Chim Acta. 1979;95:143–145. doi: 10.1016/0009-8981(79)90348-6. [DOI] [PubMed] [Google Scholar]
- 40.Singh S. Banerjee R. PLP-dependent H(2)S biogenesis. Biochim Biophys Acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh S. Padovani D. Leslie RA. Chiku T. Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative transsulfuration reactions. J Biol Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 43.Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu Rev Nutr. 1986;6:179–209. doi: 10.1146/annurev.nu.06.070186.001143. [DOI] [PubMed] [Google Scholar]
- 44.Stipanuk MH. Dominy JE. Ueki I. Hirschberger LL. Measurement of cysteine dioxygenase activity and protein abundance. Curr Protoc Toxicol. 2008;38:6.15.1–6.15.25. doi: 10.1002/0471140856.tx0615s38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stipanuk MH. Londono M. Lee JI. Hu M. Yu AF. Enzymes and metabolites of cysteine metabolism in nonhepatic tissues of rats show little response to changes in dietary protein or sulfur amino acid levels. J Nutr. 2002;132:3369–3378. doi: 10.1093/jn/132.11.3369. [DOI] [PubMed] [Google Scholar]
- 46.Stipanuk MH. Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis. 2011;34:17–32. doi: 10.1007/s10545-009-9006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stipanuk MH. Ueki I. Dominy JE., Jr. Simmons CR. Hirschberger LL. Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids. 2009;37:55–63. doi: 10.1007/s00726-008-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiranti V. Viscomi C. Hildebrandt T. Di Meo I. Mineri R. Tiveron C. Levitt MD. Prelle A. Fagiolari G. Rimoldi M. Zeviani M. Loss of ETHE1, a mitochondrial dioxygenase, causes fatal sulfide toxicity in ethylmalonic encephalopathy. Nat Med. 2009;15:200–205. doi: 10.1038/nm.1907. [DOI] [PubMed] [Google Scholar]
- 49.Tsuboyama N. Hosokawa Y. Totani M. Oka J. Matsumoto A. Koide T. Kodama H. Structural organization and tissue-specific expression of the gene encoding rat cysteine dioxygenase. Gene. 1996;181:161–165. doi: 10.1016/s0378-1119(96)00496-9. [DOI] [PubMed] [Google Scholar]
- 50.Ueki I. Roman HB. Hirschberger LL. Junior CC. Stipanuk MH. Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase. Am J Physiol Endocrinol Metab. 2012;302:E1292–1299. doi: 10.1152/ajpendo.00589.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueki I. Roman HB. Valli A. Fieselmann K. Lam J. Peters R. Hirschberger LL. Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab. 2011;301:E668–E684. doi: 10.1152/ajpendo.00151.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uhteg LC. Westley J. Purification and steady-state kinetic analysis of yeast thiosulfate reductase. Arch Biochem Biophys. 1979;195:211–222. doi: 10.1016/0003-9861(79)90343-6. [DOI] [PubMed] [Google Scholar]
- 53.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 54.Whiteman M. Le Trionnaire S. Chopra M. Fox B. Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci (Lond) 2011;121:459–488. doi: 10.1042/CS20110267. [DOI] [PubMed] [Google Scholar]
- 55.Whiteman M. Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 56.Wu JY. Prentice H. Role of taurine in the central nervous system. J Biomed Sci. 2010;17((Suppl 1)):S1. doi: 10.1186/1423-0127-17-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamori Y. Taguchi T. Hamada A. Kunimasa K. Mori H. Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17((Suppl 1)):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang G. Wu L. Jiang B. Yang W. Qi J. Cao K. Meng Q. Mustafa AK. Mu W. Zhang S. Snyder SH. Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao K. Kinuta M. Akagi R. Cat liver cystathionase. Physiol Chem Phys. 1979;11:257–260. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.