Abstract
Sensing others’ emotions through subtle facial expressions is a highly important social skill. We investigated the effects of intranasal oxytocin treatment on the evaluation of explicit and ‘hidden’ emotional expressions and related the results to individual differences in sensitivity to others’ subtle expressions of anger and happiness. Forty healthy volunteers participated in this double-blind, placebo-controlled crossover study, which shows that a single dose of intranasal oxytocin (40 IU) enhanced or ‘sharpened’ evaluative processing of others’ positive and negative facial expression for both explicit and hidden emotional information. Our results point to mechanisms that could underpin oxytocin’s prosocial effects in humans. Importantly, individual differences in baseline emotional sensitivity predicted oxytocin’s effects on the ability to sense differences between faces with hidden emotional information. Participants with low emotional sensitivity showed greater oxytocin-induced improvement. These participants also showed larger task-related pupil dilation, suggesting that they also allocated the most attentional resources to the task. Overall, oxytocin treatment enhanced stimulus-induced pupil dilation, consistent with oxytocin enhancement of attention towards socially relevant stimuli. Since pupil dilation can be associated with increased attractiveness and approach behaviour, this effect could also represent a mechanism by which oxytocin increases human affiliation.
Keywords: emotion, locus coeruleus, pupillometry, empathy, hormones, social
INTRODUCTION
Recent excitement over oxytocin’s putative prosocial effects in humans has been fuelled by repeated reports that intranasally administered oxytocin enhances ‘mind-reading’, or the ability to assess others’ emotions. Related studies have demonstrated effects of centrally enhanced oxytocin on social memory, behaviour in economic games, social attention and the focus of eye gaze (e.g. Kosfeld et al., 2005; Guastella et al., 2008; Unkelbach et al., 2008; Gamer et al., 2010; Ellenbogen et al., 2012). However, oxytocin’s effects on emotional processing have been variable and, in some cases, even contradictory (for a comprehensive review, see Bartz et al., 2011). This is illustrated by reports from three different research groups investigating oxytocin’s effects on emotion recognition with morphed emotional faces of varying intensity. These found (i) enhanced recognition of positive but not negative expressions (Marsh et al., 2010); (ii) enhanced recognition of fear but not other emotions (Fischer-Shofty et al., 2010) and (iii) a detrimental effect on fear perception (Di Simplicio et al., 2009).
A related set of studies have used images of eyes and more complex emotional expressions such as amusement or scepticism. Oxytocin’s enhancement of performance on this task has been reported for both more difficult (Domes et al., 2007) and ‘easy’ items (Guastella et al., 2010). Interestingly, since the study populations differed in social competence, the ‘easy’ items in the study by Guastella et al. and the ‘difficult’ items used by Domes et al. may have represented a comparable challenge to their respective study populations (high-functioning autists vs healthy volunteers). In other words, oxytocin’s prosocial effects may be most pronounced for tasks that are challenging, but not too difficult (see also Schultze et al., 2011).
Bartz et al. (2010) demonstrated that oxytocin’s effects on empathic accuracy in healthy males were proportional to their level of autistic traits, as assessed by the Autism Spectrum Quotient (AQ). Empathic accuracy was defined by how closely participants’ assessment of another’s emotion, during a videotaped speech, matched the speaker’s self-reported emotion. Since a high AQ score is associated with low sensitivity towards others’ expressions of emotion, this finding suggests that oxytocin’s prosocial effects are modulated by individual differences in the ability to correctly judge others’ emotional states. Specifically, it may be that oxytocin only improves empathic accuracy for those participants who experience difficulty in evaluating others’ emotions. If so, this provides a possible explanation of the discrepancies as well as the small effect sizes reported in the literature on oxytocin and emotion recognition in healthy volunteers.
The basis for the recent increase in human studies using intranasally administered oxytocin is an extensive body of research on oxytocin’s often dramatic effects in non-human animals (for reviews, see e.g. Insel and Young, 2001; Campbell, 2008). For instance, oxytocin enhances nurturing and reduces maternal aggression towards rat pups. Interestingly, it also enhances maternal aggression towards potential threats (Campbell, 2008). Here, we investigated the role of oxytocin for evaluation of two facial expressions related to prosocial and aggressive behaviour in humans, happiness and anger.
The dataset of this study included faces showing happiness and anger presented either explicitly or implicitly. Images containing implicitly presented, ‘hidden’, emotional information were included to allow us to investigate oxytocin’s effects of evaluative processing at a level of greater task difficulty. Implicit images containing subtle or hidden emotions were ‘hybrids’ created through the superimposition of high-spectrum visual information from neutral facial expressions over low-spectrum visual information from emotional expressions (see Figure 1). Such ‘hybrid’ faces containing hidden anger or happiness, sadness or fear have been shown to be judged as neutral, when observers are asked to choose among various emotion labels, thus indicating that the underlying, ‘hidden’, expression cannot be consciously acknowledged (Laeng et al., 2010). Despite the lack of awareness, the implicit emotional information in the images did influence how friendly the faces were rated in relation to a social trait (i.e. friendliness). These findings are consistent with the idea that only a core emotional expression (Berridge, 2003) can be processed when visual information is degraded or data-limited, as in subliminal presentations using backward masking procedures. However, in contrast to the backward masking technique, hybrid stimuli avoid the interruption of visual processing of the emotional information. The emotional stimulus (contained within a narrow band of spatial frequency information) is neither ‘interrupted’ nor ‘erased’ and forms a constituent part of the stimulus that remains available in the visual input at all times. This property may be very advantageous when studying physiological responses like changes in pupillary dilation, which typically evolve slowly over time (e.g. Laeng et al., 2011, 2012). In addition, such low-passed emotional hybrids may provide a privileged window into the functioning of the human amygdala. A seminal functional magnetic resonance imaging study by Vuilleumier et al. (2003) has revealed that this important brain structure is essentially ‘blind’ to all but the lowest visible spatial frequencies (>6 cycles/image). Therefore, ‘hiding’ low-frequency information from emotional expressions underneath high-frequency visual information from neutral expressions is not only a practical and useful method to present emotional expressions implicitly but also to preferentially stimulate part of the emotional subcortical network.
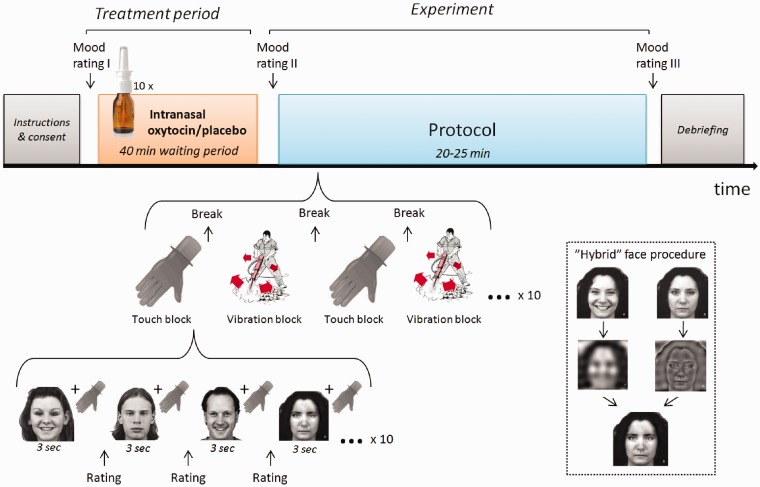
Fig. 1.
Overview of study design. After administration of nasal spray containing oxytocin or placebo, participants underwent a test protocol consisting of face stimuli presented simultaneously with either soft stroking touch or vibration. The visual stimuli included five expression types: explicit anger, implicit (hybrid) anger, neutral, implicit (hybrid) happiness and explicit happiness. Implicit expressions contained high-frequency visual information from a neutral expression and low-frequency visual information from the same person expressing either anger or happiness. These stimuli are perceived as neutral, but were previously shown to evoke a core impression such that faces containing implicit happiness are perceived as more friendly than faces containing implicit anger, fear or sadness (Laeng et al., 2010). Participants rated perceived emotion, social characteristics or tactile characteristics after each stimulus pair. Images were adapted from the Karolinska Directed Emotional Faces—KDEF (CD-ROM), by D. Lundqvist, A. Flykt, & A. Ohman, 1998, Stockholm, Sweden: Department of Clinical Neuroscience, Psychology section, Karolinska Institutet. Reprinted with permission.
This study specifically investigated oxytocin’s effect on the evaluation of happy and angry facial expressions presented both explicitly and implicitly and related the results to the participants’ sensitivity to others’ subtle emotions. Previous studies indicate that the level of autistic traits moderates the effects of oxytocin on the evaluation of others’ emotions when the task is relatively difficult (Bartz et al., 2010; Guastella et al., 2010). As autism is marked by low sensitivity to others’ emotions, we hypothesised that oxytocin’s effects on the evaluation of implicitly presented emotions would be moderated by emotional sensitivity. Since the emotional content of the hybrid images is so subtle that they cannot be consciously distinguished from neutral expressions, differentiating between the images containing hidden anger or happiness may require greater sensitivity towards others’ facial expressions of emotion. Therefore, we used each individual’s ability to detect the difference between the implicitly presented happy and angry expressions (after placebo treatment) to calculate an ‘emotional sensitivity score’ for each individual. Specifically, high scores were given to participants who perceived more anger in the ‘angry hybrids’ than in the ‘happy hybrids’ and similarly perceived more happiness in the ‘happy hybrids’ than in the ‘angry hybrids’.
We also recorded pupil diameter changes during stimulus presentation as a measure of sympathetic nervous system activity. Phasic and tonic pupil increases are tightly coupled to activity within the locus coeruleus (LC), a brainstem nucleus, which is the seat of the brain’s noradrenergic pathways (Aston-Jones and Cohen, 2005). Pupil dilation can be used as a sensitive measure of cognitive load and task difficulty (Kahneman, 1973; Laeng et al., 2012). We hypothesised that oxytocin would improve the evaluation of others’ emotions and that this prosocial effect would be mirrored by oxytocin’s effects on stimulus-related pupil dilation, reflecting altered arousal or attentional effort. Furthermore, we expected pupil dilation to reflect between-subject differences in task difficulty, as assessed by the sensitivity to differences in implicitly presented happy and angry expressions.
METHODS
Participants
Forty healthy right-handed volunteers were recruited for this study. One participant participated in one session only and was excluded, yielding a final study group size of 39 (20 females, mean age 26 years, range 20–39 years). All participants gave written informed consent to participate in the study, which was approved by the local ethics committee. Exclusion criteria were pregnancy and breastfeeding. Fourteen of the female participants used oral contraceptives. Of the remaining females, we estimated four to be in the luteal phase and two in the follicular phase of the cycle during the two test sessions, based on reported number of days since the last menses. Participants received 200 NOK (about 36 USD) per session.
Study design
Procedure
An overview of the study design is presented in Figure 1. Each individual participated in two sessions on separate days, once with 40 IU oxytocin (Syntocinon®, Novartis; 10 puffs alternating between the left and the right nostril) and once with placebo (0.9% saline, Miwana; 10 puffs alternating as above). Since previous studies of oxytocin and emotion perception using a smaller dose of 24 IU have yielded inconsistent results, we chose to administer this higher dose used in some earlier studies (e.g. Zak et al., 2007). The order of administration was counterbalanced across participants, and neither experimenters nor participants were aware of the contents of the spray (double-blind design). Behavioural ratings and pupil diameter were recorded during the experimental protocol, which was identical in the two sessions, with exception of the identity and order of presentation of stimuli. In both sessions, participants viewed black-and-white images of faces displaying a range of emotional expressions on a computer screen. Images were explicitly angry, implicitly angry, neutral, implicitly happy or explicitly happy. While viewing the images, participants received concomitant tactile stimulation on the left forearm. After each stimulus pair, participants rated qualities of the visual and tactile stimuli. Only results from visual stimuli are presented here; results relating to touch perception are presented elsewhere (manuscript in preparation). Each session lasted for about 2 h. The test phase commenced on average 40 min after administration of the nasal spray and lasted ∼25 min. Before the test phase, participants were seated alone in a room and were asked to refrain from any type of social interaction. The experimental protocol consisted of 10 blocks: 5 touch blocks and 5 vibration blocks presented in alternating order. The order of the first block type was counterbalanced across participants and conditions. Each block consisted of 10 stimulus pairs (simultaneous visual and tactile stimulation) presented for 3 s each. Before each stimulus pair, participants viewed a fixation cross for 5 s. Each stimulus pair was followed by the presentation of two rating scales; each scale was presented until the participant made a response.
Stimulus presentation
In all, 120 images of faces (20 males and 20 females) displaying angry, neutral and happy facial expressions were chosen from the Karolinska Directed Emotional Faces (Lundqvist et al., 1998; Calvo and Lundqvist, 2008). Two implicitly emotional images of each face (happy–neutral and angry–neutral) were created as described by Laeng et al. (2010). In brief, images of happy and angry facial expressions were passed through a spatial low-pass filter, keeping only frequencies of 1–6 cycles/image. Onto these low-frequency images were overlaid high-frequency images of the same individual displaying a neutral expression (high-pass filtered to exclude spatial information below seven cycles/image).
A total of 200 images were used, depicting 20 males and 20 females with the following five facial expressions: explicitly angry, implicitly angry, neutral, implicitly happy and explicitly happy. One hundred unique images were presented in each session. The order of presentation was pseudo-randomised according to the following rules: no more than two consecutive images of the same expression; no more than two consecutive images of the same person; all expressions were presented within each 10-stimulus block; at least two images of each gender within each block and finally, the same proportion of expressions were displayed during the five touch blocks and during the five vibration blocks within each session. Images of all 40 individuals within the stimulus set were presented in each session, and no images were repeated across sessions.
As pupil size is affected by ambient luminance, the background section of each image was altered to obtain the same net luminance for all images using Matlab (The Mathworks Inc., Natick, MA, USA). The section of the images containing face or hair was unaltered. Each image (11 × 11 cm) was presented on a computer monitor situated 104 cm in front of the participant, yielding a visual angle of 6°, as used by Laeng et al. (2010). Participants were tested in a windowless room with constant artificial lighting. All visual stimuli and rating scales were presented using e-Prime 2.0 (Psychology Software Tools, Inc).
Two types of innocuous tactile stimulation were presented during viewing of facial expressions, stroking touch to the dorsal aspect of the participant’s left forearm (5 cm/s) and 70 Hz vibration to the back of the hand. The stimuli were matched for intensity.
Behavioural measures
Ratings of two aspects of the face stimuli were recorded during the experimental protocol: (i) perceived mood/facial expression and (ii) perception of social characteristics. Each aspect was measured via two visual analogue scales (VAS); one such rating scale pair was displayed after each combined stimulus in pseudo-randomised order. The rating scales were as follows—1A: How happy was the person? (anchors: Not Happy–Happy); 1B: How angry was the person? (anchors: Not angry–Angry); 2A: How attractive was the person? (anchors: Unattractive–Attractive) and 2B: How friendly was the person? (anchors: Not Friendly–Friendly). The intensity and pleasantness of touch perception were also rated; these data are presented elsewhere (Ellingsen, Wessberg, Olausson, Laeng, Chelnokova and Leknes, manuscript in preparation). The order of presentation of the rating scale pairs was pseudo-randomised within each session and within each rating scale pair (with the rules: no more than two consecutive rating scale pairs; at least two of each pair type within each block). Therefore, the participants were unable to predict the occurrence of the rating scales. Participants were informed of this before the experiment onset and were instructed to pay attention to all aspects of the visual and the tactile stimuli in every trial since the subsequent rating scales could revolve around either.
Mood was measured at three time points during each session: (i) before the nasal spray administration; (ii) immediately before the experimental protocol and (iii) immediately after the experimental protocol. Participants rated their current level of fear, sadness, irritability, happiness, calmness and anxiety using VAS scales with anchors Not at all–Very much so.
Pupillometry
The pupil diameter of the participant’s left eye was measured by using a non-invasive infrared eye tracker (remote eye-tracking device, SMI-SensoMotoric Instruments®, Teltow, Germany) at a rate of 240 Hz for the duration of each stimulus pair (3000 ms).
Data analysis
Behavioural data
Repeated-measures analyses of variance (ANOVAs) were conducted using PASW Statistics version 18 (SPSS Inc.) for each of the rating scales using the following within-subjects factors: treatment (oxytocin or placebo); tactile stimulation (touch or vibration); facial expression (explicit anger, implicit anger, neutral, implicit happiness and explicit happiness) and face gender (male or female). We also investigated the following between-subjects factors: participant gender (male or female) and session order (oxytocin or placebo at session 1). A separate repeated-measures ANOVA was conducted on the mood ratings treatment, session and mood scale as factors. Additional ANOVAs assessing oxytocin’s putative ‘sharpening’ effects on evaluative processing of emotion were run for ratings of happiness or anger using the within-subjects factors treatment (oxytocin or placebo) and facial expression (mean anger and mean happiness). For statistically significant effects, contrasts were performed to establish the exact nature of the differences. Pearson’s correlation test and linear regression analyses were used to assess the relationship between two or more normally distributed measures.
Assessing sensitivity to others’ subtle emotions
We defined high emotional sensitivity as the successful differentiation of implicitly presented anger and happiness in the placebo session. To calculate an emotional sensitivity score for each participant, we computed the average of (i) the difference in ratings of perceived anger between the faces containing hidden anger and happiness and (ii) the difference in ratings of perceived happiness between the faces containing hidden anger and happiness. Those participants who rated the implicitly angry expressions as angrier and less happy than the implicitly happy expressions (and thus reported more happiness and less anger in response to the implicitly happy expressions compared with the implicitly angry images) during this baseline session received a high emotional sensitivity score. In contrast, ratings of perceived emotion in participants with low emotional sensitivity either did not reliably differ between the two implicitly presented expressions or they differed in the opposite direction. We entered this normally distributed score in a linear regression analysis, where we also modelled treatment order (oxytocin or placebo) as a covariate. This analysis assessed whether emotional sensitivity at baseline predicted oxytocin-induced improvement of emotion evaluation. To illustrate the relationship between this baseline measure and the effects of oxytocin, we also used this baseline emotional sensitivity score to divide the participants into two groups of high and low sensitivity (median split). Furthermore, a related analysis was run where we assessed the relationship of task-induced pupil dilation to baseline emotional sensitivity, as well as to oxytocin-induced improvement of emotion evaluation.
Pupillometry data
Pupil diameter data for each participant and each session were pre-processed in Matlab. Some datasets were lost due to technical constraints (malfunction of software or hardware). Good-quality recordings from both sessions existed for 25 participants; only these data were analysed (50 sessions). Eye blinks and artifacts were excluded, leaving pupil sizes of 1–9 mm. Average time series were created for each stimulus type; these time series were smoothed using a 10 Hz cutoff low-pass filter (a five-pole Chebyshev Type II filter). The time series were normalised to reflect the total dilation of the pupil for each stimulus type by subtracting the average pupil size during the first 200 ms from all points in the time series. For statistical analysis, the trimmed mean pupil dilation was computed for the 10 250 ms- ‘bins’ between 500 and 3000 ms for each stimulus type, session and participant. The trimmed means were entered into a linear mixed models analysis using PASW with the following variables: drug treatment (oxytocin or placebo); tactile stimulation (stroking touch or vibration) and visual facial expression (explicit anger, implicit anger, neutral, implicit happiness and explicit happiness). A subsequent analysis further included participant gender and order of treatment presentation. In a third mixed models analysis, we also included the between-subjects variable of emotional sensitivity score, as defined from behavioural ratings.
RESULTS
Ratings of explicitly and implicitly presented angry and happy facial expressions
Analysis of ratings of perceived anger, happiness, friendliness and attractiveness confirmed the expected effects of the explicit and implicit facial expressions. Specifically, we found a significant linear effect of expression in ratings of anger [F(1,38) = 358, P < 0.001], happiness [F(1,38) = 441, P < 0.001], friendliness [F(1,38) = 385, P < 0.001] and attractiveness (F(1,38) = 126, P < 0.001), with explicitly angry faces rated as the most angry, least happy, friendly and attractive and explicitly happy faces perceived as the least angry and most happy, friendly and attractive. Planned contrasts confirmed that the implicitly angry images were rated as significantly more angry (P < 0.001), less happy (P < 0.001), less friendly (P < 0.001) and less attractive (P = 0.043) than the implicitly happy images.
Oxytocin enhanced evaluation of explicitly and implicitly presented angry and happy facial expressions
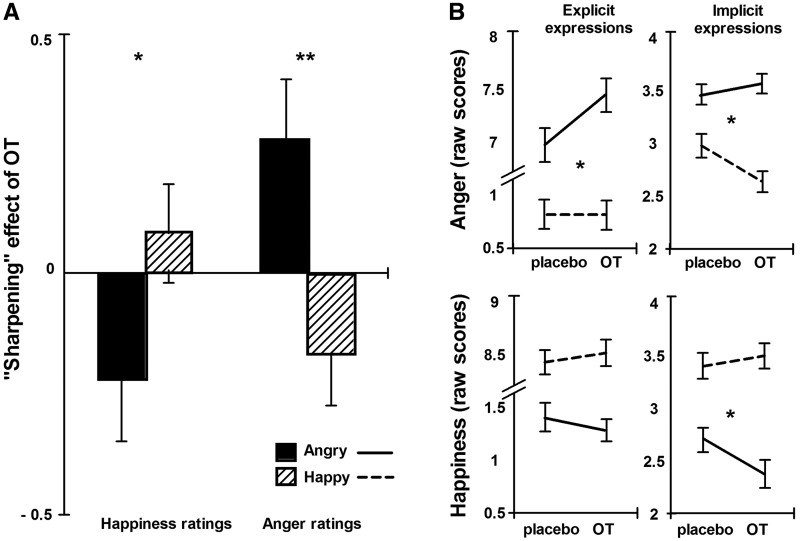
We found a significant interaction between oxytocin treatment and facial expression for ratings of perceived emotion [anger ratings, F(3.4,128) = 2.7, P = 0.042; happiness ratings F(3.3,127) = 3.3, P = 0.021]. As illustrated in Figure 2, this interaction was driven by a stimulus-congruent ‘sharpening’ effect of oxytocin on perceived emotion. Specifically, planned contrasts revealed that oxytocin increased anger ratings for faces containing anger, but decreased anger ratings of faces containing happy emotional information (explicit expressions, P = 0.028, implicit expressions, P = 0.029). Similarly, oxytocin enhanced the perception of happiness of happy expressions and decreased happiness ratings of angry expressions (explicit expressions, P = 0.24 and implicit expressions, P = 0.028). These effects of oxytocin treatment were relatively small; however, the pattern of stimulus-congruent rating changes was consistent across rating scales (perceived anger and happiness) and across expression presentation (explicit and implicit). Furthermore, separate ANOVAs set up to specifically address the hypothesised ‘sharpening’ effect of oxytocin on emotional evaluation, confirmed that oxytocin treatment increased ratings of congruent and decreased ratings of incongruent emotional expressions [see Figure 2A; facial expression × treatment interaction, anger ratings: F(1,38) = 10.24, P = 0.003; happiness ratings: F(1,38) = 5.75, P = 0.021].
Fig. 2.
(A) Oxytocin treatment caused a stimulus-congruent ‘sharpening’ of perceived anger and happiness, such that angry expressions were perceived as more angry and less happy, whereas happy expressions were perceived as more happy and less angry. Oxytocin-induced ‘sharpening’ (i.e. oxytocin-induced changes in perceived mood, calculated as the between-session difference in mean ratings for each participant and facial expression) is represented on the y axis. (B) This ‘sharpening’ effect of intranasal oxytocin treatment was evident across explicitly and implicitly presented expressions, as illustrated here with a depiction of the raw scores. Error bars represent the standard error of the mean. **P < 0.01, *P < 0.05.
Sensitivity to differences in subtle expressions at baseline predicted oxytocin-enhanced emotional sensitivity
Sensitivity to differences in emotional expression between faces containing hidden anger or hidden happiness varied substantially between participants. We computed a score for emotional sensitivity for each participant, such that those who perceived implicitly angry faces as angrier and less happy than implicitly happy faces received a high emotional sensitivity score. In contrast, a low emotional sensitivity score indicated a lack of sensitivity towards differences between the implicitly presented happy and angry facial expressions. The emotional sensitivity score significantly correlated with differences in perceived friendliness of the implicitly angry and happy expressions, such that those with high emotional sensitivity also reported the greatest differences in friendliness (r = 0.46, P = 0.003).
We investigated the association between participants’ emotional sensitivity score and oxytocin’s effects on task performance using linear regression analyses. The results showed that the participants who perceived implicitly angry faces as angrier and less happy than implicitly happy faces without oxytocin pre-treatment showed little benefit of intranasal oxytocin. In contrast, participants who were not sensitive to the differences between the implicitly presented angry and happy expressions at baseline showed greater improvement after oxytocin treatment. Specifically, the emotional sensitivity score covaried with how much oxytocin improved the sensitivity towards differences between the implicitly presented expressions of anger and happiness (perceived anger, t = –2.3, P = 0.028; perceived happiness t = –3.3, P = 0.002). This relationship was not affected by the order of oxytocin or placebo treatment (ts < 0.7).
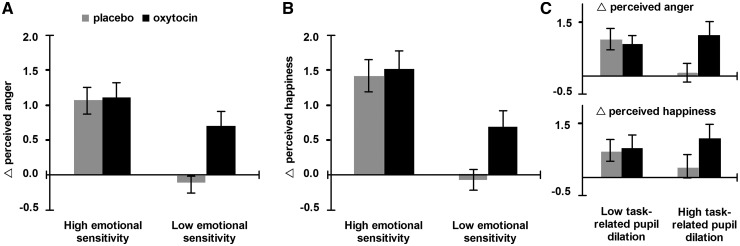
The moderating effect of baseline emotional sensitivity on oxytocin’s effects on task performance is illustrated in Figure 3, which shows the pattern of improvement when participants were divided into two groups of high and low emotional sensitivity using a median split based on the emotional sensitivity score. Paired two-tailed t-tests for each group showed significant improvement with oxytocin only for the low sensitivity group (anger ratings: high sensitivity P = 0.84, low sensitivity P = 0.002; happiness ratings: high sensitivity P = 0.73, low sensitivity P = 0.005). The median-split analysis is presented solely as an illustration of the relationship between emotional sensitivity and oxytocin’s effects and not as an independent analysis step.
Fig. 3.
(A and B) A median-split analysis based on baseline emotional sensitivity scores illustrates the relationship between sensitivity to subtle differences in emotional expressions and oxytocin enhancement of performance on this task. A high score on this measure indicates high sensitivity to differences between the two ‘hybrid’ images containing hidden anger or happiness in terms of perceived anger and perceived happiness. Participants with a high emotional sensitivity score performed only marginally better in this task after oxytocin treatment (Ps > 0.73). In contrast, oxytocin significantly improved the task performance of those who did not reliably report more anger for the implicitly angry faces (relative to implicitly happy faces, chart A) or more happiness for the implicitly happy expressions (relative to implicitly angry expressions, chart B) in the placebo condition (both Ps < 0.01). (C) A similar relationship was found when task-induced pupil dilation was used as an independent moderator, such that those with greater task-induced pupil dilation showed the largest improvement in emotional sensitivity after oxytocin treatment. Error bars represent the standard error of the mean.
Oxytocin enhances stimulus-induced pupil dilation
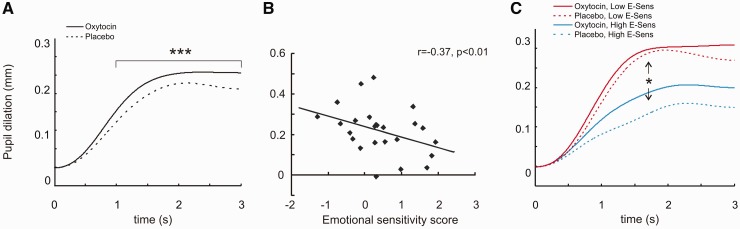
Average pupil size was 3.7 mm, and the mean stimulus-induced pupil dilation during the 3 s stimulus presentation period was 0.3 mm (8%). The effects of drug administration, facial expression and other factors on pupil dilation at 500–3000 ms were assessed using a linear mixed models approach. P values from type III F-test for fixed effects are reported. For non-significant effects, example P values are reported from 1700 ms after stimulus onset. We found a significant main effect of oxytocin on pupil dilation, such that stimulus-induced pupil dilation was larger after oxytocin pre-treatment (see Figure 4, P < 0.001 in the interval 1000–3000 ms). There was no significant main effect of facial expression on pupil dilation at any time during stimulus presentation (e.g. P = 0.45 at 1700 ms), nor did we find evidence for a treatment-by-expression interaction (P = 0.50 at 1700 ms). There were no significant effects of participants’ gender on pupil dilation (P = 0.46 at 1700 ms), or the order of treatment presentation, oxytocin or placebo first (P = 0.58 at 1700 ms).
Fig. 4.
Oxytocin pre-treatment caused significantly larger stimulus-induced pupil dilation (A). Pupil responses in the oxytocin session were significantly larger than in the placebo session during the 1000–3000 ms interval after stimulus onset. Task-induced pupil dilation correlated with individual variability in emotional sensitivity towards differences in subtle expressions (r = –0.376, P < 0.01). Those with the lowest sensitivity showed larger pupil dilation during stimulus presentation, consistent with higher task difficulty for these participants. As illustrated by the median split analysis (C), oxytocin’s beneficial effects on emotional sensitivity in the low sensitivity group were not underpinned by large increases in pupil dilation. In contrast, we found a trend towards a group-by-treatment interaction driven by a larger oxytocin-induced increase in pupil dilation in the high emotional sensitivity group.
A correlation analysis of pupil dilation around peak (2000 ms) revealed a significant negative association between stimulus-induced pupil dilation and emotional sensitivity score (r = –0.37, P < 0.05). In other words, the greatest pupil dilation was found in the participants who showed low sensitivity towards differences between the implicit angry and the implicit happy facial expressions after placebo treatment. Conversely, those who successfully distinguished between the implicitly presented expressions of anger and happiness at baseline showed smaller pupil dilation during stimulus presentation. A median-split analysis illustrates this finding. The low emotional sensitivity group (pupillometry data from 13 participants) showed a significantly greater dilation of the pupil during stimulation than did the 12 participants with high emotional sensitivity scores that were included in the pupillometry analysis (P < 0.01 in the interval 1400–3000 ms). As before, the median-split analysis is provided for illustrative purposes only. This finding is consistent with greater attention allocation to stimuli in those who showed difficulty in evaluating the implicitly presented emotional expressions at baseline. However, there was no evidence that oxytocin’s beneficial effects on emotional sensitivity in this subgroup were due to additional attention to the socially relevant stimuli. Instead we found a trend towards the opposite effect, by which the high emotional sensitivity group showed a greater oxytocin enhancement of pupil dilation than did the low sensitivity group, with the effect being greatest in the interval 1500–1800 ms (P = 0.039 at 1700 ms).
We also used the pupillometry data in a linear regression analysis to support the above findings of how baseline emotional sensitivity moderated the beneficial effects of oxytocin with task-induced pupil dilation as an independent moderator. Since pupil dilation reflects task demands and we expected those with low emotional sensitivity to find the rating tasks more demanding than those with high emotional sensitivity, we expected and found a trend towards a significant association between task-induced pupil dilation and oxytocin-induced improvement of evaluation of implicit expressions (t = 2.1, P = 0.053). As before, a median-split analysis illustrates this relationship. The participants with high task-induced pupil dilation (consistent with high perceived task difficulty) show the greatest oxytocin-induced improvement in task performance for ratings of perceived anger (high sensitivity P = 0.70, low sensitivity P = 0.014; happiness ratings: high sensitivity P = 0.79, low sensitivity P = 0.021).
No effect of oxytocin on measures of mood
As expected, we found no main effect of oxytocin treatment on ratings of fear, sadness, irritability, happiness, calmness or anxiety (F(1,26) = 1.4, P = 0.246). There were also no significant interactions between oxytocin treatment and session order (oxytocin or placebo first) or time of rating (pre-treatment, pre-testing and post-testing) on mood scores (all Ps > 0.39).
DISCUSSION
The results from this study demonstrate that oxytocin consistently enhances the perception of others’ emotional facial expressions, ‘sharpening’ the impression such that happy faces appear more happy and less angry, whereas angry expressions appear more angry and less happy. We also found that oxytocin significantly increased stimulus-induced pupil dilation, consistent with oxytocin enhancement of attention towards socially relevant stimuli. These effects of treatment were present for both explicitly and implicitly presented emotional facial expressions, across negative (anger) and positive (happiness) emotions. Importantly, the degree to which oxytocin improved the discrimination between faces showing implicitly presented anger or happiness depended on each participant’s ability to perform this task at baseline. Participants with low emotional sensitivity scores showed significant improvement in task performance after oxytocin treatment. In contrast, when emotional sensitivity was already high, oxytocin afforded little or no improvement. Baseline emotional sensitivity also covaried with stimulus-induced pupil dilation. ‘Poor’ performers showed significantly greater pupil dilation, likely reflecting increased attentional demands of the task in these participants.
Our results highlight a neural mechanism potentially underpinning oxytocin’s prosocial effects. Oxytocin significantly enhanced the pupil dilation response for all facial expressions presented. Pupil dilation has been successfully used as an index of interest, attention allocation or cognitive load (Hess and Polt, 1960; Kahneman and Beatty, 1966; Laeng et al., 2012). This measure has shown remarkable covariation with the firing of neurons in the LC, the ‘hub’ of the noradrenergic system in the brain (Aston-Jones and Cohen, 2005). LC signalling is thought to be particularly important for event detection and closely related to the ‘ventral attention network’ (Corbetta et al., 2008). The LC also contains oxytocin receptors (Petersson et al., 1998). Thus, it is possible that intranasal or endogenous increase of central oxytocin levels causes pupil dilation via direct oxytocinergic actions on neurons in the LC. Given the crucial role of oxytocin in pair bonding in non-human mammals, this finding is highly interesting. Dilated pupils are associated with increased attractiveness and they can influence approach behaviour (e.g. see Wiseman and Watt, 2010). Seeing others’ dilated pupils also causes significantly higher amygdala activation than viewing undilated pupils, an effect that nonetheless appears to be unrelated to subjective reports of attractiveness (Demos et al., 2008; Amemiya and Ohtomo, 2012). A number of studies have also shown that pupil dilation closely mirrors sexual interest or reward value (Hess and Polt, 1960; Whipple et al., 1992; Laeng and Falkenberg, 2007; Bijleveld et al., 2009).
It is unclear whether the oxytocin-induced increase in pupil dilation demonstrated here similarly reflects enhanced attractiveness or interest towards the face stimuli. Oxytocin enhancement of perceived attractiveness was reported in a previous study (Theodoridou et al., 2009). However, ratings of face attractiveness were not significantly altered by oxytocin treatment in this study. Nevertheless, we cannot exclude the possibility that the pupil represents a more sensitive measure of increased attraction or interest towards others than ratings based on introspection or conscious acknowledgements. Since all stimuli in this investigation were socially relevant, it is as yet unclear whether oxytocin’s effects on stimulus-induced pupil dilation are specific for social stimuli. Future pupillometry studies including both socially relevant and non-social stimuli could clarify this question. Collecting larger datasets would enable us to exploit this sensitive physiological signal to a larger extent. This could for instance allow for expansion of the study material to other emotions that are closer to each other in their moderating effects, such as fear and sadness. Larger studies would also enable the investigation of interactions between oxytocin and sex hormones on pupil dilation and emotion perception. Oxytocin is known to interact with sex hormones such as oestrogen, but few studies in humans have so far had the necessary scope to address such questions (Campbell, 2010). These results demonstrating oxytocin-induced pupil dilation during the viewing of others’ faces nevertheless point to mechanisms by which oxytocin facilitates human affiliation, since oxytocin is thought to be released in situations of trust and warm touch (Morhenn et al., 2008).
The findings reported here on perception of others’ emotions replicate and extend upon previous reports on the prosocial effects of oxytocin. Several avenues of research, employing a range of stimuli and tasks, have reported that intranasal oxytocin treatment enhances the recognition accuracy of one or more emotions displayed in others’ faces (Domes et al., 2007; Di Simplicio et al., 2009; Bartz et al., 2010; Fischer-Shofty et al., 2010; Guastella et al., 2010; Marsh et al., 2010; Schulze et al., 2011). However, the nature of the reported improvement has varied considerably. Some have reported enhanced empathic accuracy towards only positive (Di Simplicio et al., 2009; Marsh et al., 2010) or negative emotions (Fischer-Shofty et al., 2010), some have found that the effect is strongest for difficult items (Domes et al., 2007) and others the opposite (Guastella et al., 2010; Schulze et al., 2011).
Further discrepancies can be found in the literature on oxytocin’s effects on evaluative processing. Petrovic et al. (2008) found that oxytocin reduced the negative evaluation of a face predicting unpleasant electric shocks. In contrast, Shamay-Tsoory et al. (2009) reported increases in evaluation of both negative and positive feelings induced by a monetary task. A third study on evaluative processing assessed positivity and negativity ratings of images on separate scales and reported increased vector angle (i.e. a ‘sharpening’ of ratings through opposite, stimulus-congruent effects on negativity and positivity ratings) for pleasant, socially relevant images after oxytocin treatment (Norman et al., 2010). This investigation replicated this evaluative ‘sharpening’ effect. Specifically, images of happiness were rated as happier and less angry, and the opposite effect was found for images of anger. Importantly, our findings extend upon the findings by Norman et al. to include images of negative emotional value and also implicitly presented emotional information. This type of evaluative ‘sharpening’ effect could represent one mechanism by which oxytocin enhances sensitivity to simple as well as more complex emotional expressions.
A recent study by Bartz et al. (2010) reported a compelling interaction between oxytocin treatment and social competence, as measured with the Autism Spectrum Quotient (AQ). The authors suggested that oxytocin increases the salience of social cues and that treatment with oxytocin should benefit individuals who are generally less tuned to social information, but not socially adept individuals. Our results are consistent with this notion. Here, we calculated an emotional sensitivity score based on each individual’s ability to discriminate between faces containing hidden anger or happiness. Low scores indicated participants who did not reliably rate the implicitly angry faces as expressing more anger and less happiness than the implicitly happy faces. The emotional sensitivity score significantly predicted the prosocial effect of oxytocin on this task. A median-split analysis based on this score illustrates this relationship clearly. Participants with high emotional sensitivity were able to differentiate between the hidden expressions of anger and happiness both with and without oxytocin treatment. In contrast, the performance of those with low baseline sensitivity to others’ subtle emotions was dramatically improved after oxytocin pre-treatment. This finding was corroborated by a related analysis demonstrating that the same pattern of results emerges when task-induced pupil dilation, an independent moderator, is used to index emotional sensitivity. Participants whose pupils dilated the most during the evaluative task at baseline, suggesting greater attentional allocation or cognitive load due to higher perceived task difficulty, also showed the greatest improvement of oxytocin on emotional sensitivity.
These findings support the suggestion by Bartz et al. that oxytocin’s prosocial effects depend on how attuned an individual is towards social information. Importantly, we replicate and extend their findings to include static images and hidden emotional expressions. Our data demonstrate that oxytocin’s effects can be predicted by baseline ability to evaluate other’s emotions, a more basic measure of social skills than the AQ in the sense that the present measure is behavioural and does not rely on self-report about one’s own behavioural patterns. These findings are consistent with the notion that oxytocin may prove a useful treatment for other psychiatric populations than autism spectrum disorder. Impaired emotion recognition is a symptom of several mental disorders, including schizophrenia and drug addiction (Penn et al., 2008; Fernandez-Serrano et al., 2010). Interestingly, early investigations provide some encouragement that oxytocin could prove a useful supplement to current treatment of both schizophrenia (Keri et al., 2008; Feifel et al., 2010) and drug addiction (You et al., 2001; Qi et al., 2009; Carson et al., 2010). Future studies should address whether the effects reported here for static facial expressions of happiness and anger presented in a laboratory setting would generalise to other emotions and more ecological interactions. Moreover, this study used a single dose of intranasal oxytocin and repeated administration, as used in some recent studies in clinical populations, could perhaps enhance these effects (Feifel et al., 2010; Feifel, 2011).
The ability to ‘read’ the hidden expressions, as measured by the emotional sensitivity score, also covaried with stimulus-induced pupil dilation. The validity of the emotional sensitivity measure used here is therefore supported by this physiological measure. The highest task-related dilation was shown by participants with low emotional sensitivity. We interpret this between-subjects finding as an indication of task difficulty, whereby the task of rating aspects of the presented stimuli demands higher attention allocation and represents increased cognitive load in those with low sensitivity. This finding is consistent with previous suggestions that oxytocin enhances performance on subjectively difficult tasks (Domes et al., 2007; Guastella et al., 2010). However, since oxytocin did not cause the largest pupil increases in this subset of participants, our results do not provide direct evidence that oxytocin’s prosocial effects are mediated by increased attention allocation to social stimuli. Instead, oxytocin enhanced pupil dilation relatively more in those participants whose emotional sensitivity was already high. We speculate that this finding could reflect higher sensitivity to oxytocin within brain structures related to arousal and/or emotion perception in those with high emotional sensitivity. Oxytocin treatment was recently shown to affect the autonomic nervous system in proportion to self-reported loneliness (Norman et al., 2011), a measure that has been related to reduced reactivity to pictures of other people (Cacioppo et al., 2009). An alternative explanation would be that pupil dilation during the placebo session was already at ceiling level in the low emotional sensitivity group. We find this unlikely, since maximum pupil dilation in this group after placebo treatment was only 8.3% during the 3000 ms of stimulus presentation.
In conclusion, we have shown that a single dose of intranasal oxytocin (40 IU) ‘sharpens’ evaluative processing of others’ positive and negative facial expression for both explicit and hidden emotional information. Our results point to mechanisms which could underpin oxytocin’s prosocial effects in humans. Using a performance-based measure of social sensitivity, we replicated and extended earlier findings, showing that oxytocin specifically enhances the sensitivity of others’ explicit and hidden emotions in individuals whose baseline performance was poor. We contend that a putative explanation for the numerous discrepancies in the literature on oxytocin’s prosocial effects is the high emotional sensitivity in most healthy volunteer populations, rendering effect sizes small or non-significant. Our findings support early indications that oxytocin treatment holds promise for not only for autism spectrum disorder but also for other psychiatric populations including schizophrenia and drug addiction. Finally, we report a significant increase in stimulus-related pupil dilation after oxytocin treatment, consistent with a role for oxytocin in enhancing the salience of socially relevant stimuli. Intriguingly, since large pupil sizes are associated with increased attractiveness and approach behaviour in humans (Laeng and Falkenberg, 2007; Wiseman and Watt, 2010), this physiological effect could also enhance others’ affiliative behaviours towards an individual with high central oxytocin levels.
Acknowledgments
The authors are grateful to Karin Göthner and Steven van der Pavert for technical assistance, to Martin Larsson, Hannah Ellingsen, Gunnlaug Sæter Gitlestad, Gunn-Asbjørg Valen-Sendstad and Esther Wu for help with data collection and to Guido Biele and Markus Handal Sneve for discussions of content and analyses. This work was supported by the Swedish Research Council [grant number 2007-2912 to H.O.], the Marianne and Marcus Wallenberg Foundation [grant number 2009.0080 to H.O.] and the Research Council of Norway [grant number ES455867 to S.L.]
REFERENCES
- Amemiya S, Ohtomo K. Effect of the observed pupil size on the amygdala of the beholders. Social Cognitive and Affective Neuroscience. 2012;7(3):332–41. doi: 10.1093/scan/nsr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. Journal of Comparative Neurology. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, et al. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;21(10):1426–8. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain and Cognition. 2003;52:106–28. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Bijleveld E, Custers R, Aarts H. The unconscious eye opener: pupil dilation reveals strategic recruitment of resources upon presentation of subliminal reward cues. Psychological Science. 2009;20:1313–5. doi: 10.1111/j.1467-9280.2009.02443.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience. 2009;21: 83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Lundqvist D. Facial expressions of emotion (kdef): identification under different display-duration conditions. Behavior Research Methods. 2008;40:109–15. doi: 10.3758/brm.40.1.109. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological Psychology. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Pers Soc Psychol Rev. 2010;14:281–95. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, et al. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction Biology. 2010;15:448–63. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos KE, Kelley WM, Ryan SL, Davis FC, Whalen PJ. Human amygdala sensitivity to the pupil size of others. Cerebral Cortex. 2008;18:2729–34. doi: 10.1093/cercor/bhn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Simplicio M, Massey-Chase R, Cowen P, Harmer C. Oxytocin enhances processing of positive versus negative emotional information in healthy male volunteers. Journal of Psychopharmacology. 2009;23:241–8. doi: 10.1177/0269881108095705. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–3. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Linnen AM, Grumet R, Cardoso C, Joober R. The acute effects of intranasal oxytocin on automatic and effortful attentional shifting to emotional faces. Psychophysiology. 2012;49:128–37. doi: 10.1111/j.1469-8986.2011.01278.x. [DOI] [PubMed] [Google Scholar]
- Feifel D. Is oxytocin a promising treatment for schizophrenia? Expert Review of Neurotherapeutics. 2011;11: 157–9. doi: 10.1586/ern.10.199. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, et al. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biological Psychiatry. 2010;68:678–80. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Lozano O, Perez-Garcia M, Verdejo-Garcia A. Impact of severity of drug use on discrete emotions recognition in polysubstance abusers. Drug and Alcohol Dependence. 2010;109:57–64. doi: 10.1016/j.drugalcdep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48:179–84. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences. 2010;107:9400–5. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2010;67:692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–50. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154:1583–5. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Keri S, Kiss I, Kelemen O. Sharing secrets: oxytocin and trust in schizophrenia. Social Neuroscience. 2008;4:287–93. doi: 10.1080/17470910802319710. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–6. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Laeng B, Falkenberg L. Women's pupillary responses to sexually significant others during the hormonal cycle. Hormones and Behavior. 2007;52:520–30. doi: 10.1016/j.yhbeh.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Laeng B, Orbo M, Holmlund T, Miozzo M. Pupillary stroop effects. Cogn Process. 2011;12:13–21. doi: 10.1007/s10339-010-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng B, Profeti I, Sæther L, et al. Invisible expressions evoke core impressions. Emotion. 2010;10:573–86. doi: 10.1037/a0018689. [DOI] [PubMed] [Google Scholar]
- Laeng B, Sirois S, Gredebäck G. Pupillometry: a window to the preconscious? Perspectives on Psychological Science. 2012;7: 18–27. doi: 10.1177/1745691611427305. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. Karolinska directed emotional faces [database of standardized facial images] Karolinska Hospital: Department of Clinical Neuroscience; 1998. S-171 76, Psychology Section, Stockholm, Sweden) [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology. 2010;209:225–32. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Morhenn VB, Park JW, Piper E, Zak PJ. Monetary sacrifice among strangers is mediated by endogenous oxytocin release after physical contact. Evolution and Human Behavior. 2008;29:375–83. [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, et al. Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology. 2010;25:1313–9. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biological Psychology. 2011;86:174–80. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Penn DL, Sanna LJ, Roberts DL. Social cognition in schizophrenia: an overview. Schizophrenia Bulletin. 2008;34:408–11. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson M, Uvnäs-Moberg K, Erhardt S, Engberg G. Oxytocin increases locus coeruleus alpha 2-adrenoreceptor responsiveness in rats. Neuroscience Letters. 1998;255:115–18. doi: 10.1016/s0304-3940(98)00729-0. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. Journal of Neuroscience. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF. Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement. Neuropharmacology. 2009;56:856–65. doi: 10.1016/j.neuropharm.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Schulze L, Lischke A, Greif J, Herpertz SC, Heinrichs M, Domes G. Oxytocin increases recognition of masked emotional faces. Psychoneuroendocrinology. 2011;36:1378–82. doi: 10.1016/j.psyneuen.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biological Psychiatry. 2009;66:864–70. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behavior. 2009;56:128–32. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Unkelbach C, Guastella AJ, Forgas JP. Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychological Science. 2008;19:1092–4. doi: 10.1111/j.1467-9280.2008.02206.x. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience. 2003;6:624–31. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Whipple B, Ogden G, Komisaruk BR. Physiological correlates of imagery-induced orgasm in women. Archives of Sexual Behavior. 1992;21:121–33. doi: 10.1007/BF01542589. [DOI] [PubMed] [Google Scholar]
- Wiseman R, Watt C. Judging a book by its cover: the unconscious influence of pupil size on consumer choice. Perception. 2010;39:1417–9. doi: 10.1068/p6834. [DOI] [PubMed] [Google Scholar]
- You ZD, Li JH, Song CY, Lu CL, He C. Oxytocin mediates the inhibitory action of acute lithium on the morphine dependence in rats. Neuroscience Research. 2001;41:143–50. doi: 10.1016/s0168-0102(01)00272-3. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]