Abstract
The central hypoxic ventilatory response (HVR) comprises a reduction in ventilatory activity that follows a peripherally mediated ventilatory augmentation. Chronic early developmental exposure to nicotine or ethanol are both known to impair the peripherally mediated HVR, and nicotine impairs the central HVR, but the effect of ethanol on the central HVR has not been investigated. Additionally, chronic nicotine and ethanol exposure are known to impair ventilatory responses to hypercapnia in bullfrog tadpoles but HVRs have not been tested. Here early and late metamorphic tadpoles were exposed to either 30 µg/L nicotine or 0.15 – 0.05 g/dL ethanol for 10 wk. Tadpole brainstems were then isolated and the neurocorrelates of ventilation were monitored in vitro over 180 min of hypoxia (PO2 = 5.05 ± 1.04 kPa). Both nicotine and ethanol exposure disrupted central HVRs. Nicotine impairments were dependent on development. Central HVRs were impaired only in early metamorphic nicotine-exposed tadpoles. Both early and late metamorphic ethanol-exposed tadpoles failed to exhibit central HVRs. Thus, central HVRs are impaired following both nicotine and ethanol exposure. Such failure to decrease ventilatory activity during hypoxia indicates that central hypoxic ventilatory depression is an active suppression of neural activity in response to hypoxia rather than a metabolic consequence of O2 limitation, and that exposure to ethanol (across development) or nicotine (during early development) disrupts mechanisms that normally induce active ventilatory depression.
Keywords: control of breathing, development, alcohol
1. Introduction
Breathing is a vital behavior controlled by a brainstem neural network that must be responsive to respiratory stressors like hypercapnia and hypoxia. The ability to modulate breathing in response to hypoxia is characteristic of all vertebrate classes (McKenzie and Taylor, 1996). Prenatal exposure to the neuroteratogens, nicotine or ethanol, has been found to elicit functional impairments in the control of breathing and ventilatory responses to hypoxia (Smith et al., 1991; Simakajornboon et al., 2004; Dubois et al., 2008; Campos et al., 2009). Chronic exposure to these neuroteratogens impairs the hypercapnic response of bullfrog tadpoles (Taylor et al., 2008). Functional impairments in the breathing responses of neonates often results in infant morbidity and mortality (Hunt et al., 1981; Kotagal, 2003; Cohen and Katz-Salamon, 2005; Bavis and Mitchell, 2008). Nicotine or ethanol exposures during early development are both associated with an increased risk of Sudden Infant Death Syndrome (SIDS; Haglund and Cnattingius, 1990; Feng, 1993; Iyasu et al., 2002; Duncan et al., 2008).
In mammals the hypoxic ventilatory response (HVR) comprises a peripherally mediated augmentation to compensate for low O2 levels, followed by a ventilatory reduction that reflects a metabolic depression to adapt to low O2 levels (Vizek et al., 1987; Bisgard and Neubauer, 1995; Powell et al., 1998). Most studies investigating the consequences of prenatal nicotine exposure on the HVR have focused on the augmentation phase (Milerad et al., 1995; Bamford et al., 1996; Ueda et al., 1999). Simakajornboon et al. (2004) identified that both phases of the HVR are attenuated in 5-d rat pups following 15 ± 2 d of continuous prenatal nicotine exposure. Prenatal ethanol exposure reduces baseline ventilation in juvenile rats and attenuates the peripherally mediated augmentation phase of the HVR (Dubois et al., 2006; Dubois et al., 2008). The effect of early developmental ethanol exposure on the centrally mediated reduction phase of the HVR is currently unknown.
The developing bullfrog has been used as a model to investigate the ventilatory effects of nicotine and ethanol exposure (Taylor et al., 2008; Brundage and Taylor, 2009; 2010). Use of bullfrog tadpoles is advantageous in providing for precise control of the exposure conditions by eliminating maternal influences. Following whole-animal teratogen exposure, when the bullfrog brainstem is isolated, the preparation produces a highly robust ventilatory motor output that has been used extensively to study the control of breathing at all stages of development (Gdovin et al., 1999; Harris et al., 2002; Davies et al., 2009). Eight to 12 wk of exposure to either 30 µg/L nicotine or 0.10 % ethanol (v/v) disrupts tadpole ventilatory responses to hypercapnia, both in vivo and in isolated brainstem preparations, without affecting baseline ventilation (Taylor et al., 2008). Both the duration and timing of teratogen exposure are determining factors in nicotine-, but not ethanol-induced, hypercapnic impairments; early metamorphic tadpoles show increased vulnerability to nicotine (Brundage and Taylor, 2009). The aim of this study was to determine if the ventilatory impairments elicited by developmental nicotine and ethanol exposure extend to the bullfrog tadpole central HVR.
Early metamorphic tadpoles initially increase gill and lung activity in response to moderate hypoxia (PO2 ~100 mmHg; West and Burggren, 1982), but studies have not investigated the tadpole response to hypoxia that persists in vivo beyond the augmentation phase. The augmentation phase in lung ventilation during hypoxia becomes more dominant as metamorphosis progresses and tadpoles transition from obligate water breathers to obligate air breathers (Burggren and Doyle, 1986). In the absence of peripheral afferents the isolated brainstems of bullfrog tadpoles demonstrate only the reduction phase of the HVR (Winmill et al., 2005). Winmill and colleagues (2005) found that early metamorphic tadpoles are significantly more hypoxia tolerant than later developmental stages; reductions in lung neuroventilation are seen following 180 min of severe hypoxia (PO2 near 0 kPa). When exposed to similar conditions, brainstems from later metamorphic tadpole stages are quicker to exhibit reduction in lung neuroventilation --to the point of cessation within 30 min of severe hypoxia (Winmill et al., 2005). There is some discrepancy among studies of the tadpole central HVR. Fournier and colleagues (2007) investigated responses to 10-min hypoxia and identified an increase in lung burst frequency in early metamorphic tadpoles and reduction in late metamorphic tadpoles. It is possible that amphibians are unique in that they retain some central involvement in the augmentation phase of the HVR early in development. The studies are consistent in that neither found significant effects of hypoxia on gill neuroventilation (Winmill et al., 2005; Fournier et al., 2007). Thus, the central HVR of bullfrog tadpoles can be generally characterized as a change in lung burst frequency with persistent hypoxia.
We hypothesized that 10 wk of exposure to either nicotine or ethanol would impair the central HVR of bullfrog tadpoles. Simakajornboon and colleagues (2004) reported central HVR impairment in mammals prenatally exposed to nicotine. Ours is the first study to evaluate the consequences of ethanol exposure on central HVR, we expected that the teratogen impairment of the tadpole central hypoxic response would be similar to the impairment of the central hypercapnic response (Taylor et al., 2008). We assessed changes in neuroventilatory activity in both early and late metamorphic tadpoles to determine if developmental changes in hypoxia tolerance contributed to the degree of central HVR impairment evoked by nicotine or ethanol exposure.
2. Methods
2.1 Animals
Studies were performed on Lithobates catesbeianus (formerly Rana catesbeiana; tadpoles (n = 38) purchased from a commercial supplier (Sullivan Co. Inc., www.researchamphibians.com). Tadpoles were maintained at room temperature and were fed goldfish food daily. Tadpoles were housed for 10 wk in aquaria of either dechlorinated water only, dechlorinated water containing nicotine (30 µg/L (–)-nicotine hydrogen tartrate salt; Sigma, www.sigmaaldrich.com) or dechlorinated water containing ethanol (0.15 – 0.05 g/dL). The concentrations of nicotine and ethanol were consistent with those of a previous study using bullfrogs (Taylor et al., 2008). The nicotine concentration was based on that found in the body fluids of average smokers (Moyer et al., 2002). The ethanol concentration varied due to the volatilization from the aquarium water and was equivalent to 0.63–1.88 times the 0.08 g/dL blood alcohol content that is the legal limit of many western countries (Caldeira et al., 2004). We chose 10-wk exposure because that duration of exposure impairs ventilatory responses to hypercapnia in both early and late metamorphic tadpoles (Taylor et al., 2008; Brundage and Taylor, 2009). We sought to determine if responses to hypoxia were affected after the same 10-wk teratogen exposure.
The developmental stage of each tadpole was determined twice, once at the start of treatment and again at the time of dissection to confirm equivalent developmental of the treatment groups. At the time of dissection each tadpole was either early metamorphic (forelimbs absent, hind limbs paddle-like without joints or separated toes) or late metamorphic (forelimbs and hind limbs present, tail being resorbed), corresponding to developmental stages 5–14 or 19–25, respectively, in the classification scheme of Taylor and Köllros (1946). Animals that were not within these stage ranges after 10 wk were excluded from the datasets. Tadpoles included in this study consisted of 19 early metamorphic tadpoles (6 control, 7 nicotine-, and 6 ethanol-exposed) and 19 late metamorphic tadpoles (6 control, 6 nicotine-, and 7 ethanol-exposed). All care and experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alaska Fairbanks and complied with all state and federal ethical guidelines
2.2 Surgical preparation
Each tadpole was anesthetized by immersion for 1–2 min in cold (4 °C) 0.2 mM tricaine methanesulfonate (MS222; Sigma, www.sigmaaldrich.com) in dechlorinated water buffered to pH 7.8 with NaHCO3. The front of the head rostral to the nares and the back of the body (hind limbs and tail, if present) were removed. The dorsal cranium and forebrain rostral to the diencephalon were resected and the fourth ventricle opened by removing the choroid plexus. The remaining brainstem and spinal cord were removed en bloc and further trimmed rostrally to the optic tectum and caudally to the brachial nerve. During dissection, exposed tissues were superfused with cold artificial cerebral spinal fluid (aCSF) composed of (in mM) 104 NaCl, 4 KCl, 1.4 MgCl2, 10 D-glucose, 25 NaHCO3 and 2.4 CaCl2 equilibrated with 100 % O2. This single aCSF composition has been used in previous studies of neuroventilation in bullfrog tadpoles and juveniles (Taylor et al., 2003a; 2003b; 2008) and was employed here to ensure comparability with those studies and between experiments on animals of different metamorphic stages.
The isolated brainstem-spinal cord was transferred en bloc to a 2.5-ml, Plexiglas, flow-through recording chamber and was supported, ventral side up, between coarse nylon mesh such that all surfaces were bathed with aCSF flowing from rostral to caudal at a rate of 5 ml/min. A supply of aCSF, equilibrated with O2-CO2 mixtures, flowed through plastic tubing to the recording chamber and bathed the isolated brainstem. CO2 was monitored with a CO2 analyzer (Capstar 100; CWE, www.cwe-inc.com). The pH of the aCSF was maintained at pH 7.8 (~9 torr PCO2) by adjusting the fractional concentration of CO2 in the equilibration gas. After isolation the brainstem was allowed to stabilize for 1 h while superfused at 23 °C.
2.3 Nerve recording
Roots of the facial and hypoglossal nerves were drawn into glass suction electrodes pulled from 1-mm-diameter capillary glass to tip diameters that fit the nerve roots. Whole-nerve discharge was amplified (X100 by DAM 50 amplifiers, World Precision Instruments, www.wpiinc.com; X1000 by a four-channel model 1700 amplifier, A–M Systems, www.a-msystems.com) and filtered (100 Hz high pass to 1 kHz low pass). The amplified and filtered nerve output was sent to a data acquisition system (Powerlab, AD Instruments, www.adinstruments.com), which sampled at 1 kHz, data were archived as whole-nerve discharge, and duplicate integrated (full-wave rectified and averaged over 200 ms) neurograms were acquired simultaneously. Such recordings were made during the initial 1-h post-isolation stabilization period and recorded continuously throughout the duration of each treatment protocol.
2.4 Hypoxia treatment
After the stabilization period the aCSF brainstem perfusate remained at pH 7.8 with the equilibrated O2-CO2 mixtures for an additional 30 min to establish normoxic baseline neuroventilation. Then the O2 gas was replaced with N2 while CO2 levels remained constant. The N2/CO2 equilibrated aCSF (hypoxia) bathed the tissue at a flow rate of 5–7 ml/min for 180 min. After the hypoxia treatment the N2 was replaced with O2 for 30 min of normoxic recovery. The aCSF remained isocapnic throughout the entire treatment protocol. The hypoxia treatment and duration is similar to that employed in other bullfrog studies (Winmill et al., 2005; Fournier et al., 2007) and differs only by our use of a single aCSF reservoir rather than separate reservoirs for normoxic and hypoxic aCSF. Using an oxygen electrode (MI-730, Microelectrodes Inc., www.microelectrodes.com) the PO2 in the recording chamber of our apparatus was evaluated; within 10 min of switching to hypoxic equilibration of the aCSF reservoir, the recording chamber reached 5.05 ± 1.04 kPa of O2. There was no subsequent change in PO2 over the 180 min of hypoxia treatment.
2.5 Data and statistical analyses
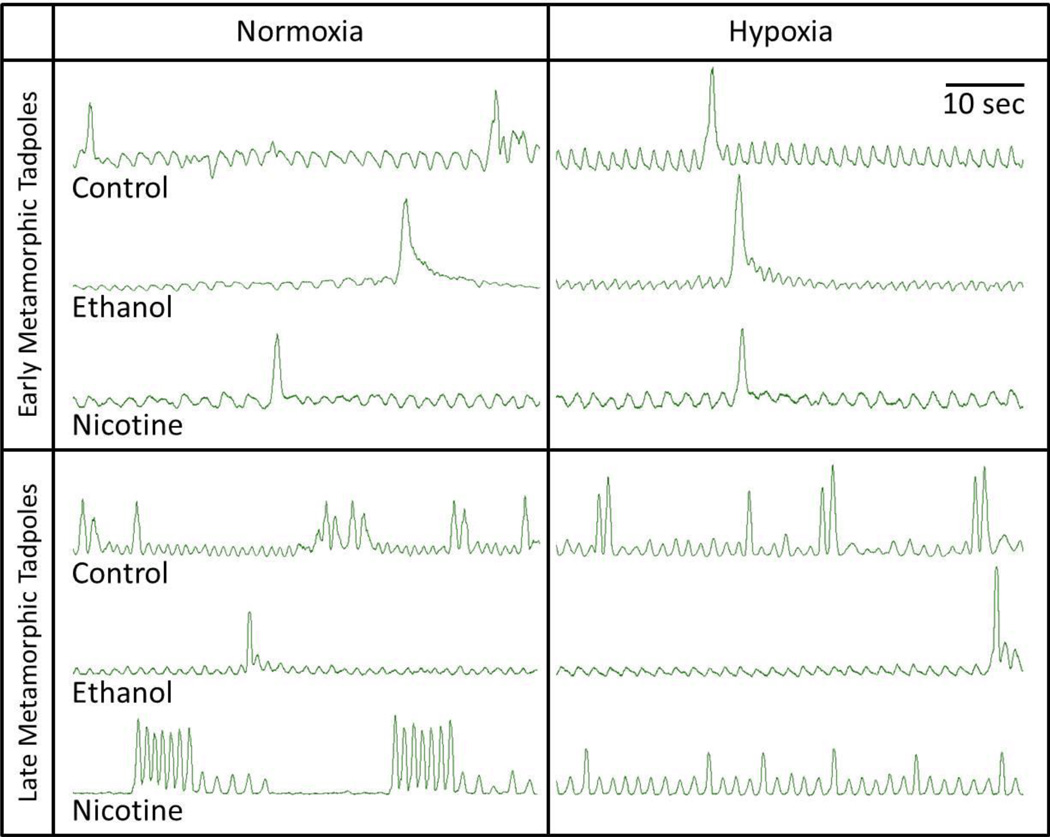
Neurograms recorded from the isolated brainstems were quantified for 30 min of normoxia, 180 min of hypoxia, and for 30 min of subsequent return to normoxia. Burst activity patterns were designated as either putative gill or putative lung breaths on the basis of the amplitude of the integrated nerve activity and the presence or absence of coincident firing in both the facial and hypoglossal nerves as previously described (Torgerson et al., 1998). Putative gill breaths had lower integrated burst amplitude on the facial nerve than putative lung breaths, and little or no coincident burst activity in the hypoglossal nerve. Putative lung breaths had higher integrated burst amplitude in the facial nerve and coincident burst activity in the hypoglossal nerve. Fig. 1 provides representative neurograms recorded from the isolated brainstems of early and late metamorphic tadpoles that had been chronically exposed to no teratogen (control) or ethanol or nicotine and were subsequently treated with normoxia and hypoxia.
Fig 1. Representative neurograms recorded from the isolated brainstems of early and late metamorphic tadpoles.
The intact animals had been chronically exposed to no teratogen (control) or ethanol or nicotine and the brainstem preparations isolated from these animals were subsequently treated with normoxia and hypoxia. Lower amplitude, higher frequency bursts represent gill ventilation and higher amplitude, lower frequency bursts represent lung ventilation. In general, gill bursting is similar in early and late metamorphic tadpoles and under all treatments, while lung bursting increases in frequency with metamorphic development of tadpoles and appears to be decreased in hypoxia.
The frequency of lung ventilation was quantified, from the neurograms of the facial nerve, as the mean number of lung bursts per minute over 10-min intervals of normoxia and hypoxia. Each animal’s hypoxic response was calculated as the 10-min mean lung burst frequency for the last 10 min of the 180-min hypoxia. The change in lung burst frequency for each 10 min of hypoxia relative to the last 10 min of baseline was used to quantify treatment group responses over time. The duration and amplitude of putative lung and gill bursts as well as the frequency of gill bursts were quantified for the last 3 consecutive minutes of normoxic baseline, the 10-min intervals ending at 10, 60, 90, 120, 150, and 180 min of hypoxia, and normoxic recovery. Within each exposure treatment (nicotine, ethanol or control), the mean values for each of the quantified neuroventilatory parameters were normally distributed and, thus, were compared using a parametric statistical test, a repeated-measures analysis of variance (RM-ANOVA; SigmaStat, www.systat.com). Comparisons within the early and late metamorphic tadpole groups and between control and treatment groups were made using a 2-way analysis of variance (SigmaStat, www.systat.com). When an RM-ANOVA or 2-way-ANOVA indicated that significant differences existed, multiple comparisons were made using the Holm-Sidak multiple comparison test. All values reported in the text are mean ± SE.
3. Results
3.1 Effect of chronic nicotine or ethanol exposure on early metamorphic tadpole lung neuroventilation
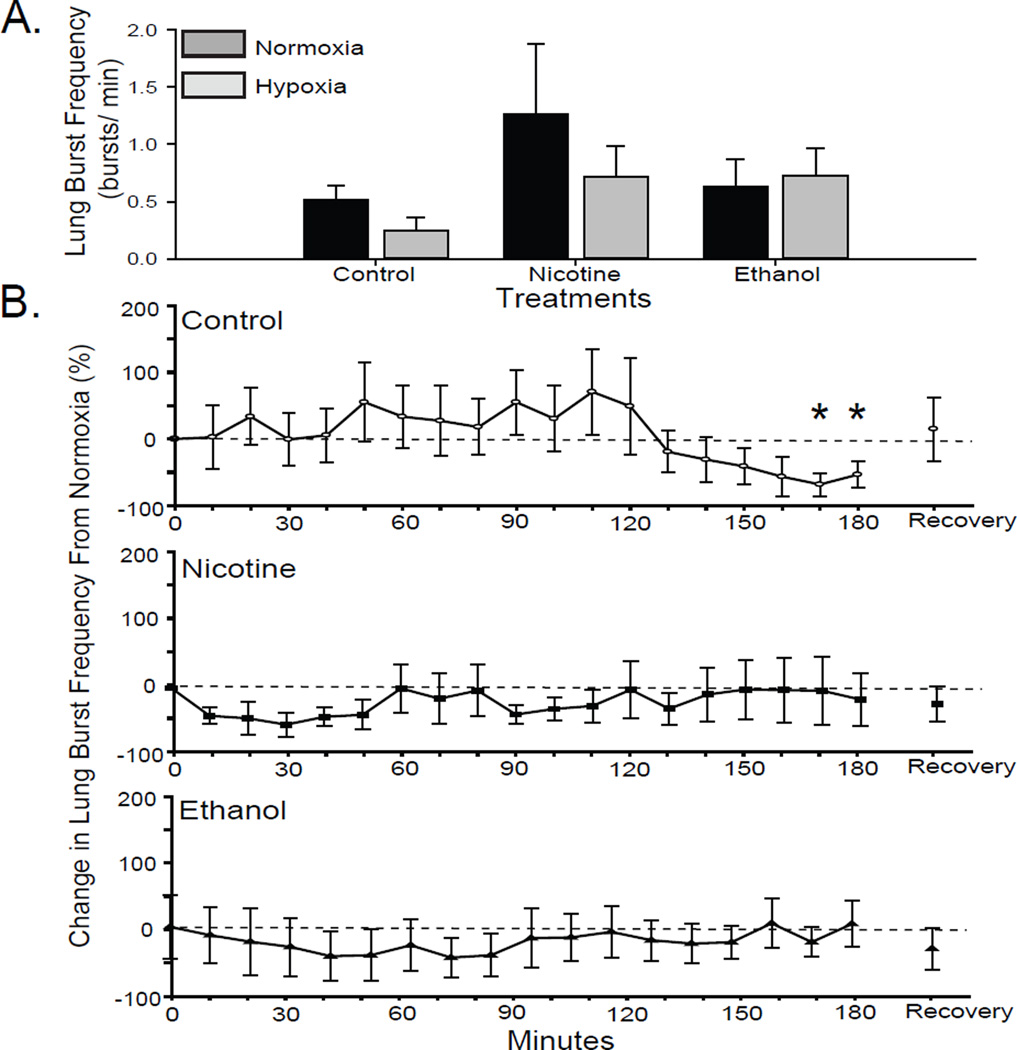
Control tadpole brainstems exhibited significantly decreased lung burst frequency in response to hypoxia in the last 20 min of the 180-min hypoxia exposure (RM-ANOVA P = 0.01; Fig 2B). In comparing among treatment groups, early metamorphic control tadpoles did not significantly reduce lung burst frequencies from 0.52 ± 0.12 to 0.25 ± 0.11 bursts/min (2-way-ANOVA P = 0.39; Fig. 2A).
Fig 2. Effect of 10-wk nicotine or ethanol exposure on lung burst frequency of early metamorphic tadpoles.
(A) Mean lung burst frequency during last 10 min of normoxia and response to hypoxia (the last 10 min of a 180-min hypoxia treatment) in control and nicotine- or ethanol-exposed early metamorphic tadpoles. (B) Percent change in lung burst frequency from normocapnia during each 10-min period of hypoxia. Control, but not nicotine- or ethanol-exposed, early metamorphic tadpoles decreased lung burst frequency in response to hypoxia (* = P ≤ 0.05). Data presented are mean ± SE for 6 – 7 tadpoles.
Early metamorphic tadpoles exposed to nicotine for 10 wk had a mean lung burst frequency of 1.26 ± 0.61 burst/min, which was not significantly different from control tadpoles (2-way-ANOVA P = 0.20; Fig. 2A). In response to hypoxia, early metamorphic nicotine-exposed tadpoles did not lower lung burst activity (0.21 ± 0.07 bursts/min; RM-ANOVA P = 0.98; Fig. 2B). The relative change in lung burst frequency from baseline showed an early drop, but did not change significantly over the course of hypoxic exposure.
Early metamorphic tadpoles exposed to ethanol for 10 wk had a mean lung burst frequency of 0.63 ± 0.24 burst/min, which was not significantly different from control tadpoles (2-way-ANOVA P = 0.20; Fig. 2A). Mean of the last 10-min period of lung neuroventilation was not depressed significantly during hypoxia. The relative change in lung burst frequency from baseline in brainstems from chronic ethanol-exposed early metamorphic tadpoles did not vary significantly over the course of hypoxic exposure (RM-ANOVA P = 0.997; Fig. 2B). Thus, 10 wk of either nicotine or ethanol exposure appears to have disrupted the early metamorphic tadpole central HVR.
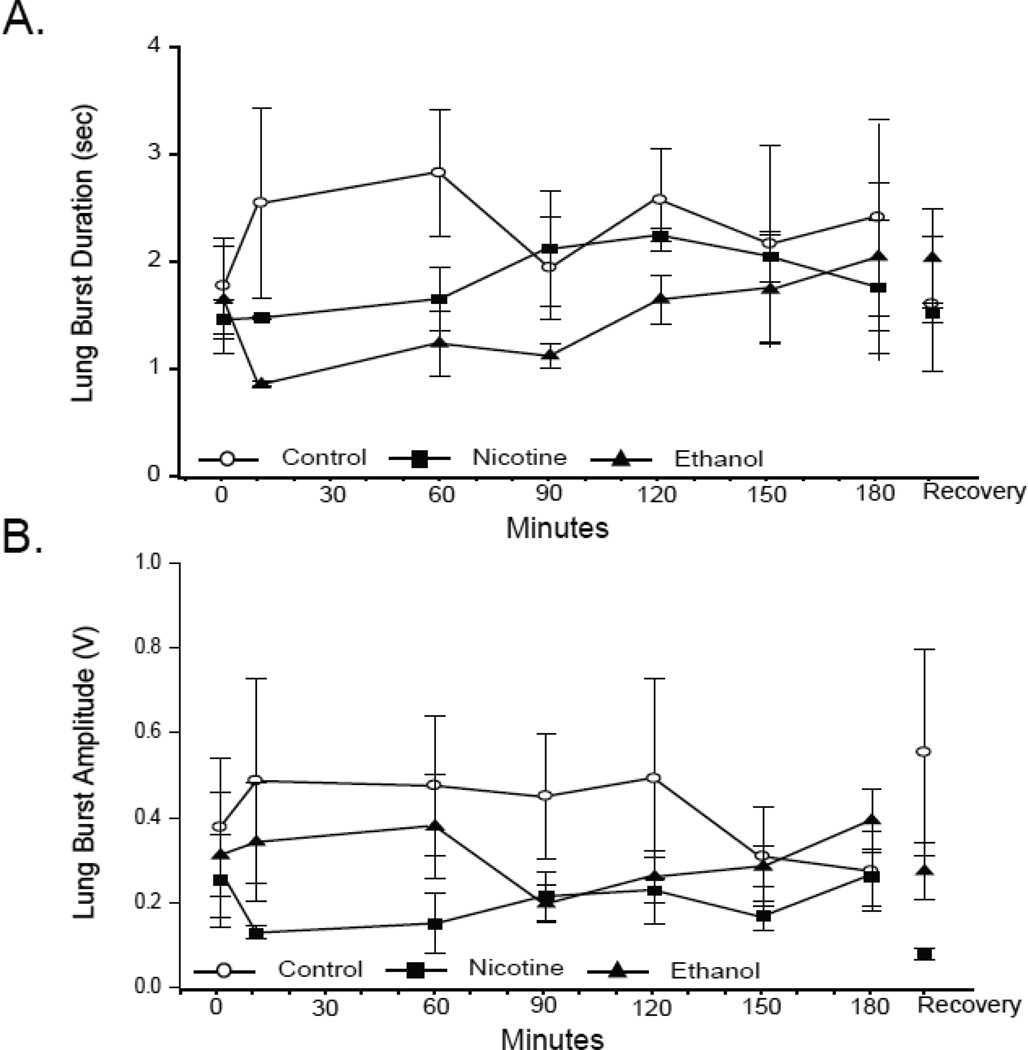
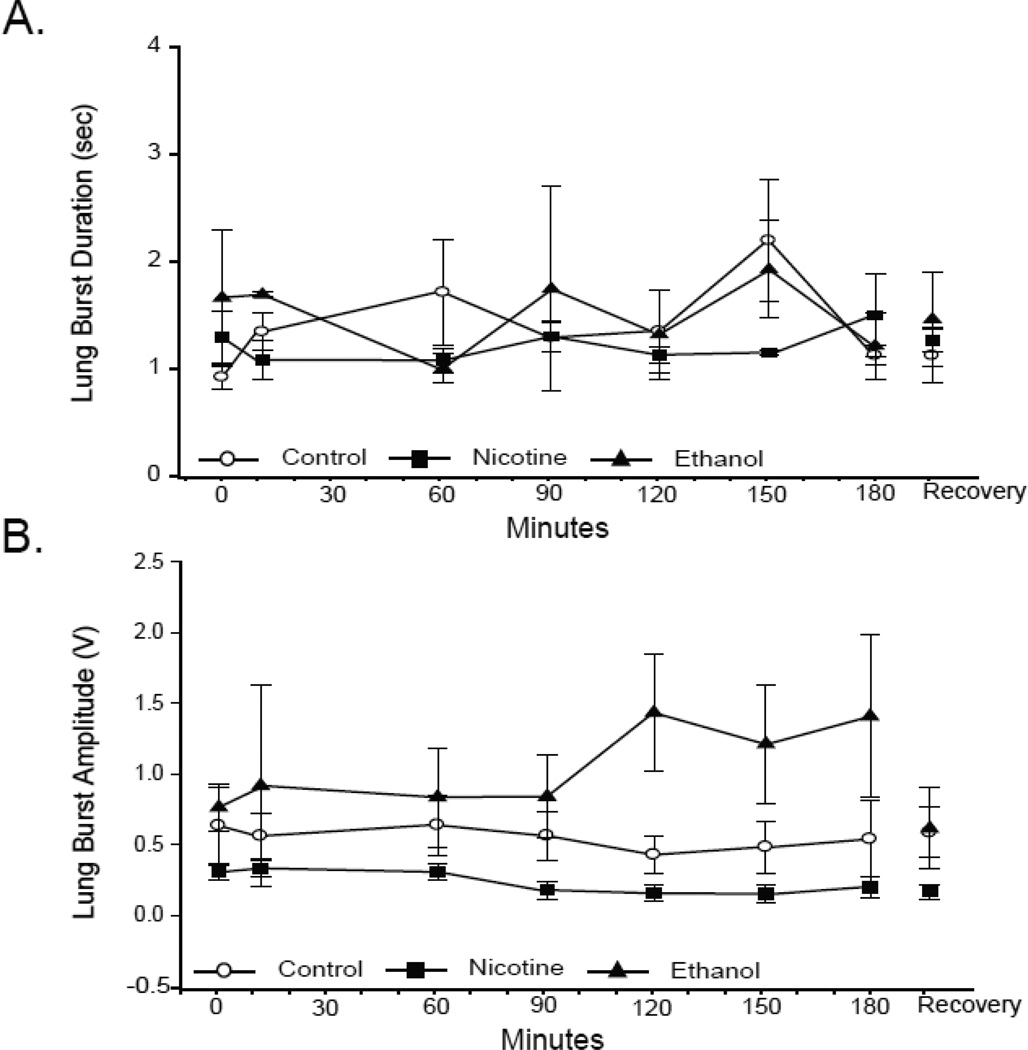
Neither the duration (Fig. 3A) nor the amplitude (Fig. 3B) of lung bursts varied significantly during hypoxia in early metamorphic tadpoles (RM-ANOVA P = 0.67 and 0.94, respectively). Neither nicotine nor ethanol exposure affected the amplitude or duration of lung bursts in early metamorphic tadpoles, nor did either teratogen alter these burst parameters significantly during hypoxia (all P values ≥ 0.35). Therefore, only lung burst frequency, the only burst parameter affected by hypoxia, was altered following 10-wk nicotine or ethanol exposure.
Fig 3. Effect of 10-wk nicotine or ethanol on lung burst duration and amplitude of early metamorphic tadpoles.
Mean lung burst duration (A) and amplitude (B) during last 3 min of normoxia,10, 60, 90, 120, 150, and 180 of min hypoxia, and 30 min of normoxic recovery in control and nicotine- or ethanol-exposed early metamorphic tadpoles. 10-wk nicotine or ethanol exposure had no effect on lung burst duration or amplitude. Hypoxia had no effect on lung burst duration or amplitude of any early metamorphic tadpole treatment group. Data presented are mean ± SE for 6 – 7 tadpoles (P > 0.05).
3.2 Effect of chronic nicotine or ethanol exposure on late metamorphic tadpole lung neuroventilation
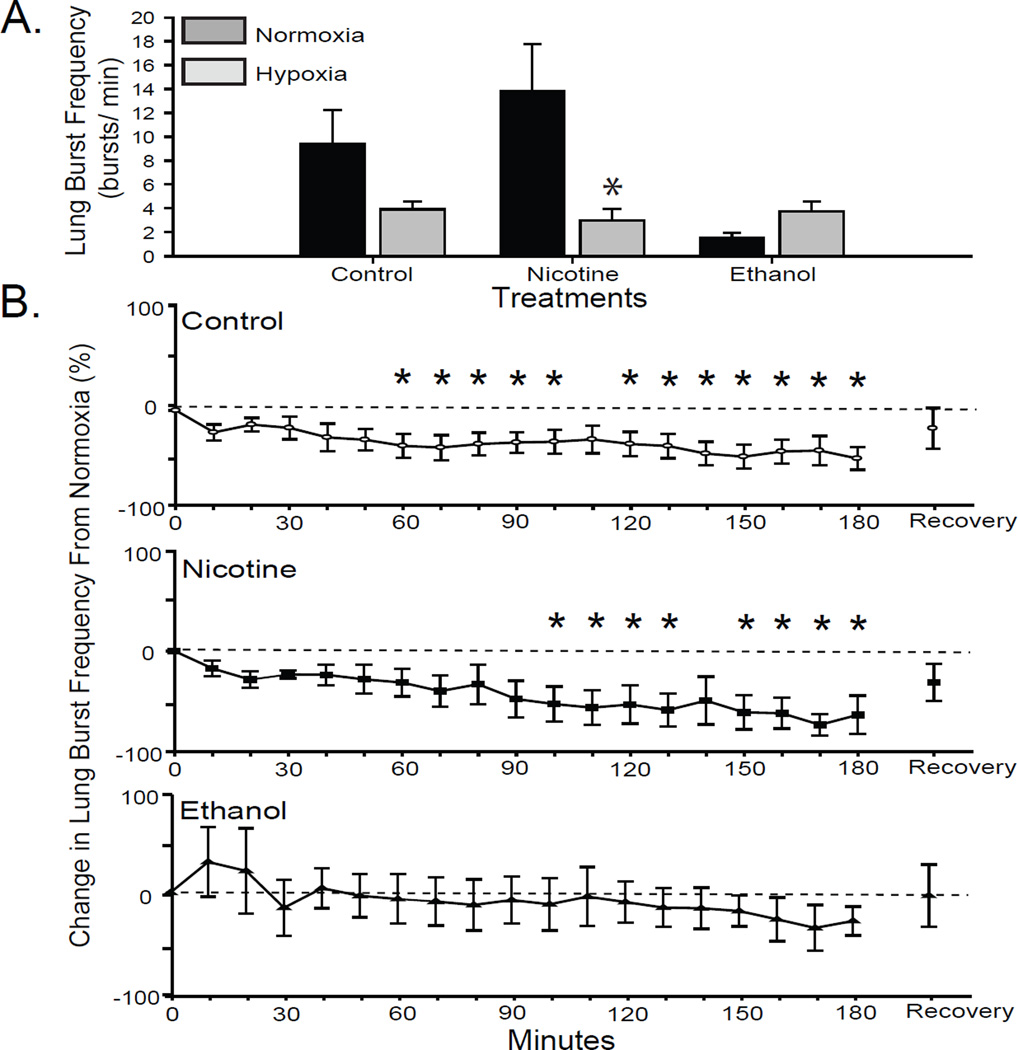
The lung burst frequency of late metamorphic tadpoles was significantly greater than early metamorphic tadpoles (9.39 ± 2.83 bursts/min; P = 0.01). In response to hypoxia, brainstems from control late metamorphic tadpoles significantly reduced lung burst frequencies to 3.93 ± 0.64 bursts/min (RM-ANOVA P = 0.03; Fig. 4B). In contrast to early metamorphic tadpoles, late metamorphic tadpoles demonstrated a more rapid reduction in lung burst frequency, a significant percent decrease was apparent after 60 min of hypoxia and extended throughout the hypoxia treatment except at 110 min (RM-ANOVA P < 0.05 for all; Fig. 4B). Lung burst frequency had recovered from the hypoxia-induced decrease by 30 min of normoxia, no longer exhibiting a change from baseline normocapnia. When comparisons are made between all treatment groups, however, the lung burst frequency exhibited by late metamorphic tadpoles is not significantly decreased during hypoxia compared to normoxia (2-way-ANOVA P = 0.08; Fig. 4A).
Fig 4. Effect of 10-wk nicotine or ethanol exposure on lung burst frequency of late metamorphic tadpoles.
(A) Mean lung burst frequency during last 10 min of normoxia and response to hypoxia (the last 10 min of a 180-min hypoxia treatment) in control and nicotine- or ethanol-exposed late metamorphic tadpoles. (B) Percent change in lung burst frequency from normocapnia during each 10-min period of hypoxia. Control and nicotine-exposed tadpoles decreased lung burst frequency in response to hypoxia (* = P ≤ 0.05). Data presented are mean ± SE for 6 – 7 tadpoles.
The lung burst frequency of late metamorphic tadpoles following 10 wk of nicotine exposure was elevated, but not significantly different from control tadpoles (13.80 ± 3.98; 2-way-ANOVA P = 0.14; Fig. 4A). In response to hypoxia, this lung burst frequency decreased significantly to 3.01 ± 0.94 bursts/min; 2-way-ANOVA P < 0.001). The significant reductions in lung burst frequency exhibited by nicotine-exposed late metamorphic tadpoles occurred at 100 – 130 min and 150 – 180 min of hypoxia (RM-ANOVA P < 0.001; Fig. 4B), which is later than the 60-min response time of control late metamorphic animals. Following 30 min of normoxic recovery, the lung burst frequency recovered to a level not significantly different from baseline.
Ten wk of ethanol exposure did not significantly alter the normoxic lung burst frequency of late metamorphic tadpoles (1.53 ± 0.45 bursts/min; 2-way-ANOVA P = 0.47; Fig. 4A) although this frequency was less than controls. The last 10-min interval of lung neuroventilation during hypoxia, though lower, was not significantly different from baseline. Hypoxia had no significant effect on the lung burst frequency of 10-wk ethanol-exposed late metamorphic tadpoles through the duration of hypoxic exposure (RM-ANOVA P = 0.85; Fig. 4B). Thus, 10 wk of ethanol, but not nicotine, exposure disrupts the central HVR of late metamorphic tadpoles.
Neither the duration (Fig. 5A) nor the amplitude (Fig. 5B) of lung bursts varied significantly during hypoxia in late metamorphic tadpoles (P = 0.65 and 0.40, respectively). Neither nicotine nor ethanol exposure affected the amplitude or duration of lung bursts in late metamorphic tadpoles, nor did either teratogen significantly alter these burst parameters during hypoxia (all P values ≥ 0.22). It is interesting to note that the lung burst amplitude of ethanol-exposed late metamorphic tadpoles does increase, although not significantly, during late hypoxia treatment. Ultimately, only those lung burst parameters associated with late metamorphic tadpole responses to hypoxia were significantly altered following 10-wk ethanol but not nicotine exposure. Therefore, like early metamorphic tadpoles, lung burst frequency was the only lung neuroventilatory response parameter altered in 10-wk ethanol-exposed late metamorphic tadpoles, and unlike the early developmental group, 10-wk nicotine exposure had no effect on lung burst parameters during normoxia or hypoxia.
Fig 5. Effect of 10-wk nicotine or ethanol on lung burst duration and amplitude of late metamorphic tadpoles.
Mean lung burst duration (A) and amplitude (B) during last 3 min of normoxia,10, 60, 90, 120, 150, and 180 min of hypoxia, and 30 min of normoxic recovery in control and nicotine- or ethanol-exposed late metamorphic tadpoles. 10-wk nicotine or ethanol exposure had no effect on lung burst duration or amplitude. Hypoxia had no effect on lung burst duration or amplitude of any late metamorphic tadpole treatment group. Data presented are mean ± SE for 6 – 7 tadpoles (P > 0.05).
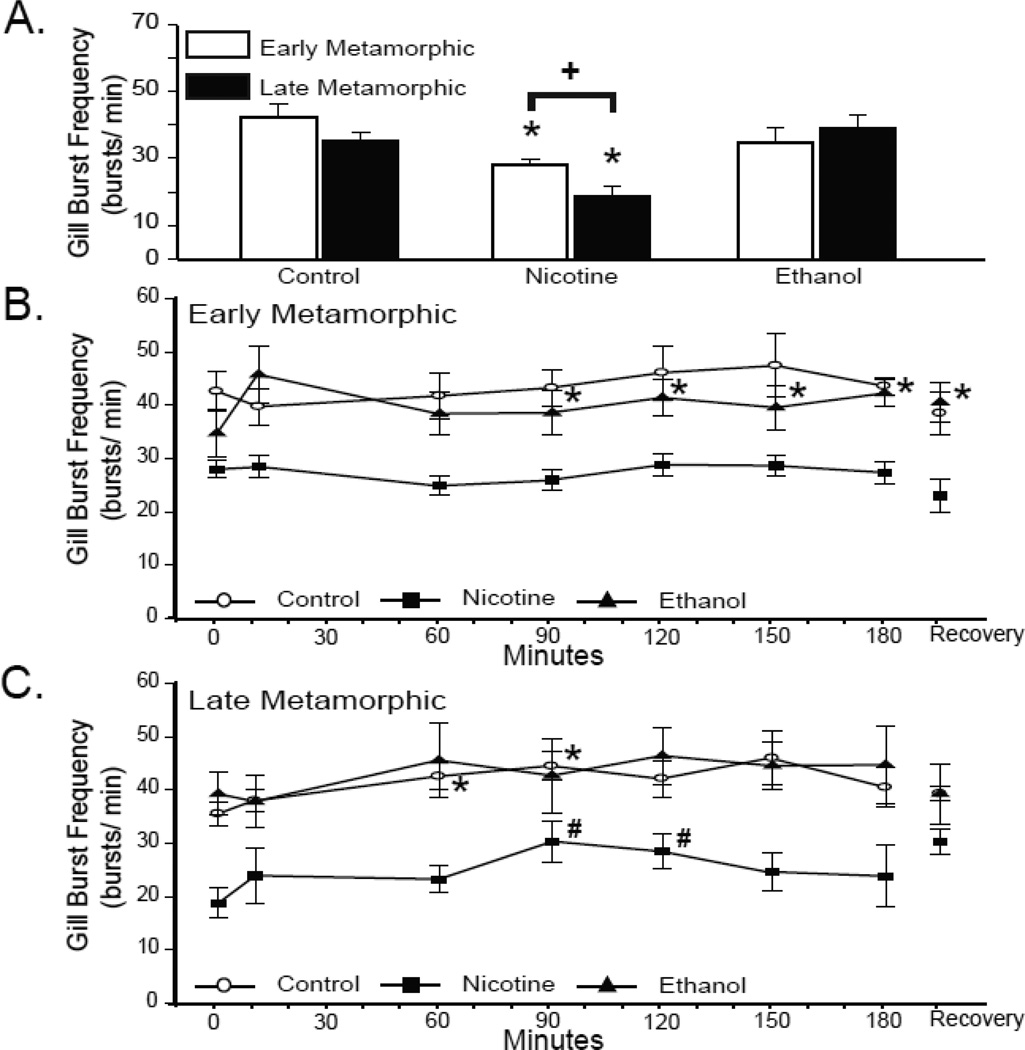
3.3 Effect of chronic nicotine or ethanol exposure on gill neuroventilation
Gill burst frequency of control early metamorphic tadpoles was not significantly different during normoxia and hypoxia (42.67 ± 3.72 bursts/min; P = 0.29; Fig. 6B). Furthermore, this gill burst frequency was not significantly different from late metamorphic tadpoles (35.57 ± 2.31 bursts/min; P = 0.12; Fig. 6A). The gill frequency of late metamorphic tadpoles did increase during hypoxia but not consistently. The increases occurred only around 60 and 90 min of hypoxic exposure (42.57 ± 2.45 bursts/min; P = 0.03 and 44.50 ± 2.72 bursts/min; P = 0.05 for 60 and 90 min of hypoxia, respectively; Fig. 6C).
Fig 6. Effect of 10-wk nicotine or ethanol exposure on tadpole gill burst frequency.
(A) Mean gill burst frequency during last 3 min of normoxia in control and nicotine- or ethanol-exposed early and late metamorphic tadpoles. The gill burst frequency of early and late metamorphic nicotine-exposed tadpoles were both less than controls (* = P ≤ 0.05). Late metamorphic nicotine-exposed tadpoles had a lower gill burst frequency than early metamorphic nicotine-exposed tadpoles (+ = P = 0.01). Mean lung burst frequency during last 3 min of normoxia,10, 60, 90, 120, 150, and 180 min of hypoxia, and 30 min of normoxic recovery in control and nicotine- or ethanol-exposed early (B) and late metamorphic tadpoles (C). Early metamorphic ethanol-exposed tadpoles increased gill burst frequency from 90 –180 min of hypoxia (* = P ≤ 0.05). Late metamorphic control tadpoles increased gill burst frequency at 60 and 90 min of hypoxia (* = P ≤ 0.05). Late metamorphic nicotine-exposed tadpoles increased gill burst frequency at 90 and 120 min of hypoxia (* = P ≤ 0.05). Data presented are mean ± SE for 6 – 7 tadpoles.
Compared to control tadpoles 10 wk of nicotine exposure had a considerable effect on gill burst frequency (Fig. 6A). The gill burst frequencies of early and late metamorphic 10-wk nicotine-exposed tadpoles were significantly different from one another (P = 0.01) and both were significantly less than that of control animals (28.10 ± 1.64 bursts/min; P = 0.003 and 18.78 ± 2.86 bursts/min; P < 0.001 for early and late 10-wk nicotine-exposed tadpoles, respectively). Similar to controls, the gill burst frequency of early metamorphic nicotine-exposed tadpoles was unchanged during hypoxia (P = 0.27; Fig. 6B & C), but the gill frequency of late metamorphic tadpoles increased briefly around 90 and 120 min of hypoxia (30.39 ± 3.87 bursts/min; P = 0.02 and 28.44 ± 3.21 bursts/min; P = 0.01 for 90 and 120 min of hypoxia, respectively).
The normoxic gill burst frequencies of early and late metamorphic 10-wk ethanol-exposed tadpoles was not significantly different from those of controls (34.83 ± 4.50 bursts/min; P = 0.21 and 39.29 ± 4.03 bursts/min; P = 0.44 for early and late metamorphic tadpoles, respectively; Fig. 6A) nor did they differ significantly from each other (P = 0.47). Unlike controls, the gill burst frequency of early metamorphic ethanol-exposed tadpoles increased by 90 min of hypoxia and remained elevated for the duration of the hypoxic exposure (P = 0.04; Fig. 6B). This gill frequency remained significantly increased above baseline levels despite 30 min of normoxic recovery (40.67 ± 3.70 bursts/min; P = 0.01; Fig. 6C). Late metamorphic 10-wk ethanol-exposed tadpoles also differed from controls in that they did not exhibit a significant change in gill burst frequency throughout the course of hypoxic exposure (P = 0.59).
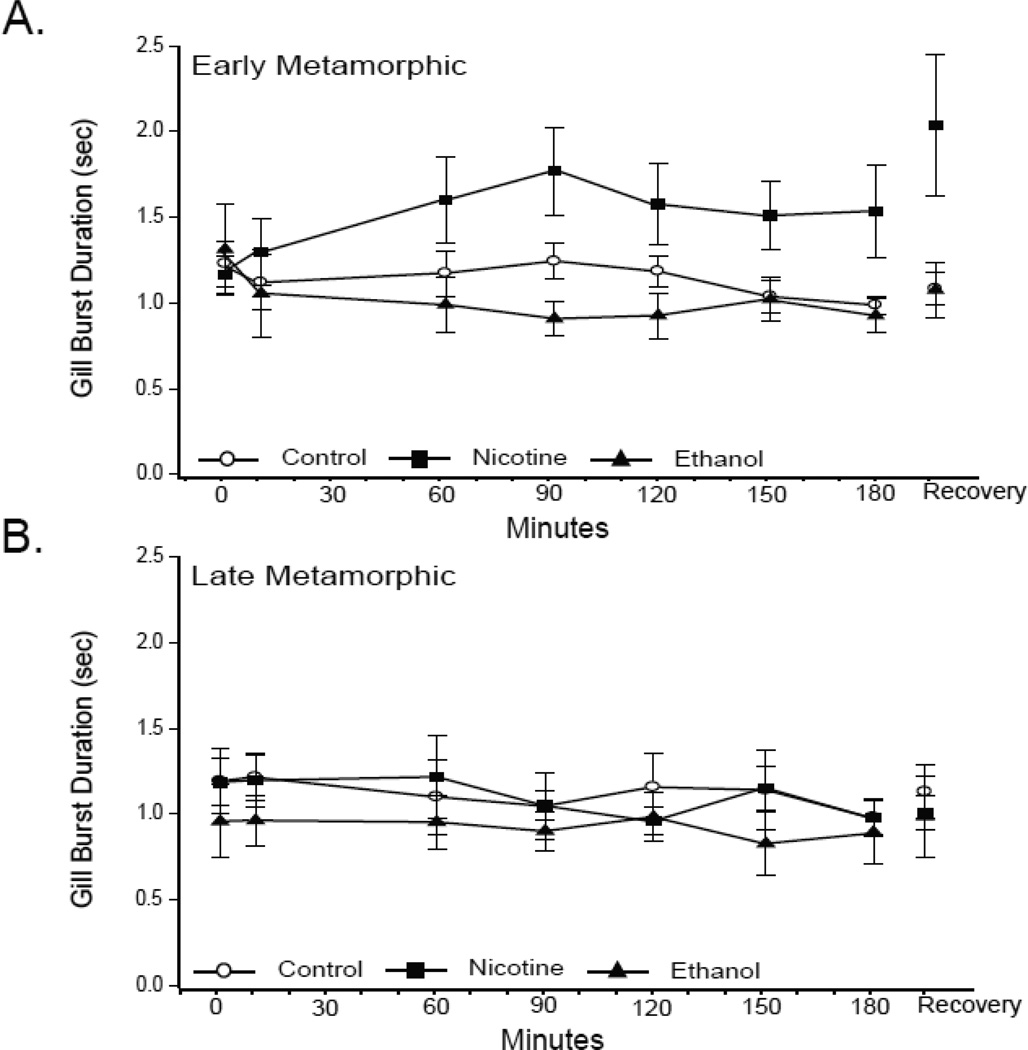
Gill burst frequency is closely linked with burst duration. Gill burst duration did not change significantly either in response to hypoxia or following exposure to nicotine or ethanol (all P values ≥ 0.20; Fig. 7). Early metamorphic nicotine-exposed tadpoles did demonstrate a transient increase in gill duration; however, this change was not significant and did not offset the lack of gill burst frequency change in that treatment group (P = 0.31; Fig. 7A). Thus, nicotine exposure alters baseline gill burst frequencies without affecting gill burst frequency responses to hypoxia, and ethanol exposure altered gill burst frequency responses to hypoxia without changing baseline gill burst activity.
Fig 7. Effect of 10-wk nicotine or ethanol exposure on tadpole gill burst duration.
Mean gill burst duration during last 3 min of normoxia,10, 60, 90, 120, 150, and 180 min of hypoxia, and 30 min of normoxic recovery in control and nicotine- or ethanol-exposed early (A) and late (B) metamorphic tadpoles. 10-wk nicotine or ethanol exposure had no effect on lung burst duration. Hypoxia had no effect on gill burst duration of any early or late metamorphic tadpole treatment group. Data presented are mean ± SE for 6 –7 tadpoles (P > 0.05).
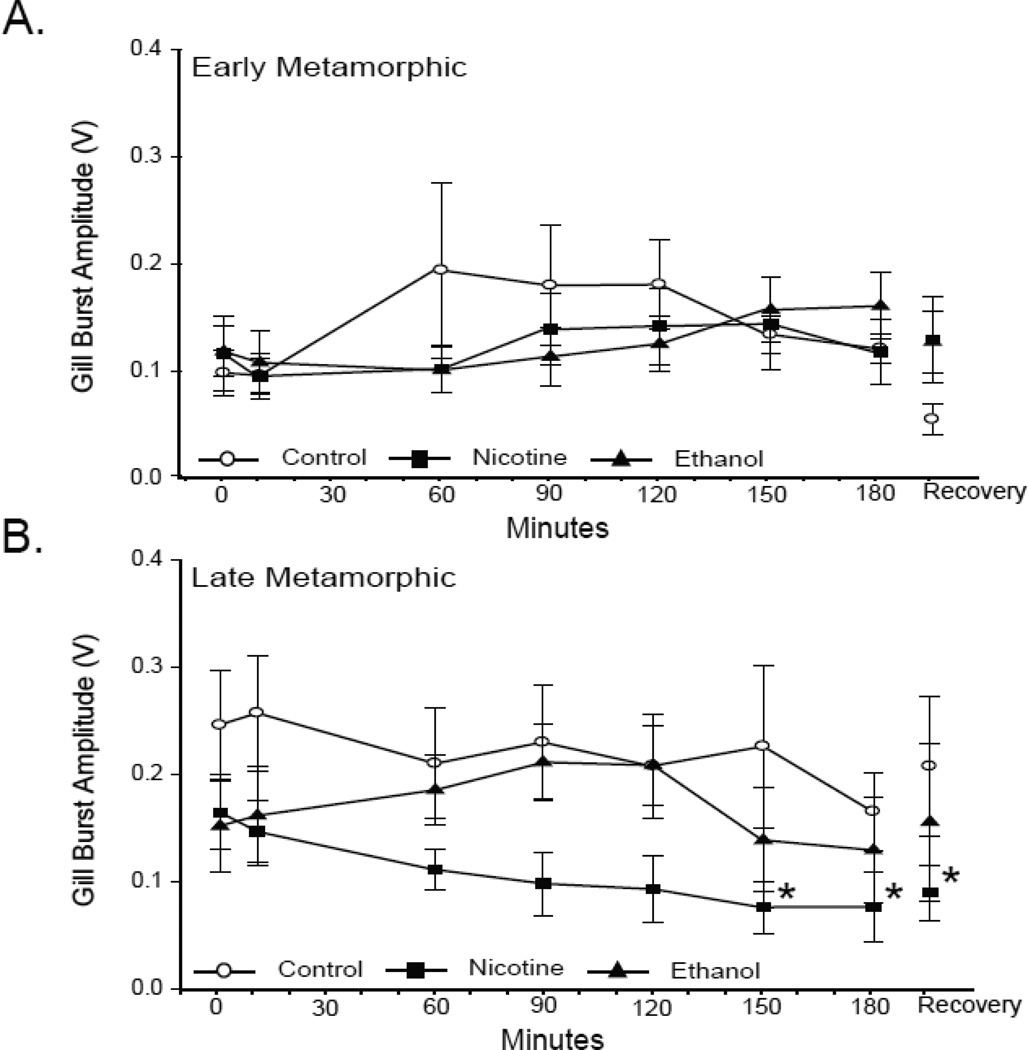
In control tadpoles the amplitude of gill bursts increased significantly with development from 0.10 ± 0.02 V in early metamorphic tadpoles to 0.25 ± 0.05 V in late metamorphic tadpoles (P = 0.04). Hypoxia, however, did not have a significant effect on gill burst amplitude in either developmental group (P = 0.78 and P = 0.40 for early and late metamorphic tadpoles, respectively; Fig. 8). Ten wk of nicotine exposure did not significantly alter the normoxic gill burst amplitude of either developmental group (0.11 ± 0.03 V; P = 0.70 and 0.17 ± 0.04 V; P = 0.23 for early and late metamorphic 10-wk nicotine-exposed tadpoles, respectively), and the gill burst amplitudes of early and late metamorphic nicotine-exposed tadpoles were not significantly different from each other (P = 0.31). In response to hypoxia, the gill burst amplitude of early metamorphic tadpoles did not change significantly during the course of hypoxia (P = 0.57; Fig. 8A). Late metamorphic tadpoles, however, demonstrated a significant reduction in gill amplitude at 150 and 180 min of hypoxia (0.08 ± 0.02 V; P = 0.04 and 0.08 ± 0.03; P = 0.03, respectively; Fig. 8B). Within this developmental group, gill burst amplitude remained significantly reduced from baseline levels following 30 min of normoxic recovery (0.09 ± 0.03 V; P = 0.046).
Fig 8. Effect of 10-wk nicotine or ethanol exposure on tadpole gill burst amplitude.
Mean gill burst amplitude during last 3 min of normoxia,10, 60, 90, 120, 150, and 180 min of hypoxia, and 30 min of normoxic recovery in control, and nicotine- or ethanol-exposed early (A) and late (B) metamorphic tadpoles. 10-wk nicotine or ethanol exposure had no effect on normoxic gill burst amplitude. Hypoxia decreased gill burst amplitude of late metamorphic nicotine-exposed tadpoles at 150 and 180 min of hypoxia, and after 30 min of normoxic recovery (* = P ≤ 0.05). Data presented are mean ± SE for 6 – 7 tadpoles
Ten wk of ethanol exposure did not significantly alter the normoxic gill burst amplitude of either early or late metamorphic tadpoles relative to that of control tadpoles (0.12 ± 0.24 V; P = 0.55 and 0.15 ± 0.04 V; P = 0.18, respectively; Fig. 8), and the gill burst amplitudes of early and late metamorphic ethanol-exposed tadpoles were not significantly different from each other (P = 0.48). Similar to controls the gill burst amplitude of both early and late metamorphic ethanol-exposed tadpoles did not vary significantly over the 180 min of hypoxia (P = 0.20 and P = 0.89 for early and late metamorphic tadpoles, respectively).
In summary, early and late metamorphic normoxic gill burst frequency and the late metamorphic hypoxic gill burst amplitude were affected by 10 wk of nicotine exposure. Ten-wk ethanol exposure did not affect normoxic gill neuroventilation; however, during hypoxia the gill burst frequency of ethanol-exposed animals was markedly changed.
4. Discussion
4.1 The central hypoxic ventilatory response of bullfrog tadpoles
The central HVR of bullfrog tadpoles differed slightly in our study from those previously conducted. The central HVR we found in early metamorphic tadpoles was similar to Winmill and colleagues (2005) who identified a reduction in lung burst frequency late in hypoxia treatment. Fournier and colleagues (2007) described an increase in early metamorphic lung burst frequency at 10 min of hypoxia exposure suggesting some central involvement in the augmentation phase of the HVR exhibited by intact tadpoles. We saw no such increase; however, lung activity of early metamorphic tadpoles fluctuated prior to the reduction in lung burst frequency. It is possible that individual variation precluded our ability to identify a significant increase. Here and in the previous studies (Winmill et al., 2005; Fournier et al., 2007) the central HVR of later metamorphic stages was a reduction in lung burst frequency. Fournier and colleagues (2007) reported a significant reduction at 10 min hypoxia, Winmill (2005) reported a cessation of lung activity by 30 min of hypoxia, and in our hands lung burst frequency was significantly reduced after 60 min of hypoxia. Variation in the time-course and degree of reduction in lung burst frequency may be due to the onset speed and degree of hypoxic insult employed in each study. In our study the onset of the hypoxic challenge was slower (10 min vs. 5 min) and the degree of hypoxic challenge less (5.05 ± 1.04 kPa vs. near 0 kPa; Winmill et al., 2005). Despite differences in technique, we recorded a central HVR in bullfrog tadpoles that was similar to previously reported responses. Furthermore, we demonstrated that the central HVR was impaired in10-wk ethanol-exposed tadpoles of either developmental group and early metamorphic 10-wk nicotine-exposed tadpoles. The nicotine- and ethanol-induced impairment of the central HVR -- failure to decrease neuroventilatory activity during hypoxia --indicates that central hypoxic ventilatory depression is an active suppression of neural activity in response to hypoxia rather than a metabolic consequence of O2 starvation.
The ability to respond to hypoxia is a fundamental feature of vertebrate ventilation (McKenzie and Taylor, 1996). 10 wk of nicotine exposure induced a development-dependent difference in gill burst frequency during normoxia and impaired the central HVR of early but not late metamorphic tadpoles. 10 wk of ethanol exposure impaired the central HVR of both early and late metamorphic tadpoles without affecting normoxic neuroventilation, gill or lung. Evidence presented here and by others suggests that developmental exposure to either nicotine or ethanol impairs both peripherally and centrally mediated responses to hypoxia in amphibians and mammals.
Ten-wk exposure to either nicotine or ethanol impaired tadpole central HVRs. The tadpole central HVR is a reduction in lung burst frequency as seen in control tadpoles. Late metamorphic tadpoles exhibited this reduction earlier in the hypoxic treatment than early metamorphic tadpoles, and in both metamorphic stages lung burst frequency recovered to baseline levels following 30 min of normoxia. Unlike lung ventilation, which increases with tadpole development, gill ventilation remains consistent. Hypoxia had little effect on gill burst frequency. Only late metamorphic tadpoles demonstrated hypoxia-induced changes in gill burst frequency, transient increases around 60 and 90 min of hypoxia. Therefore, the tadpole central HVR manifests as a decrease in lung burst frequency and an ephemeral increase in late metamorphic tadpole gill burst frequency, the amplitude and duration of lung bursts, as well as the amplitude, and duration of gill bursts, were not altered in response to hypoxia.
4.2 Effect of nicotine on bullfrog tadpole neuroventilation
Early metamorphic tadpoles did not exhibit a central HVR following 10-wk nicotine exposure. There was an early attenuation in lung activity, however, neither the time-course nor the degree of ventilatory depression were similar to control animals. Late metamorphic nicotine-exposed tadpoles did exhibit decreased lung burst frequency, a central HVR, and an increase in gill burst frequency at 90 and 120 min of hypoxia. Both the lung burst frequency reduction and gill burst frequency augmentation in late metamorphic nicotine-exposed tadpoles occurred later in the hypoxic treatment than similar changes in controls. This delayed hypoxic response suggests increased hypoxia tolerance, decreased hypoxia sensitivity, or some hindrance of the hypoxic response. An increase in hypoxia tolerance is unlikely as nicotine also caused a reduction in gill burst amplitude late in hypoxia that did not recover during normoxia. These findings suggest that chronic nicotine exposure delays the central HVR by impairing the breathing control network’s mechanisms for sensing and/or responding to hypoxia.
Loss of the central HVR following 10-wk nicotine exposure was dependent on the timing of that exposure coinciding with early development, which suggests that early metamorphic tadpoles are more vulnerable to the deleterious neural effects of chronic nicotine exposure. This was similar to studies looking at the effect of chronic nicotine exposure on hypercapnic ventilation, where early metamorphic tadpoles exhibited an impaired hypercapnic response after 3-wk nicotine exposure, whereas late metamorphic tadpoles required 10-wk exposure to exhibit the same impairment (Brundage and Taylor, 2009). The effects of acute nicotine exposure, however, are opposite in that they increase with bullfrog development (Brundage et al., 2010). Maturation of the cholinergic system in the bullfrog brainstem and ontogeny of ventilatory chemoreflexes may play key roles in defining the period of nicotine vulnerability. Numerous studies have determined that prenatal nicotine exposure impairs the postnatal hypoxic response of rats and lambs (Slotkin et al., 1995; Fewell and Smith, 1998; Fewell et al., 2001b, a; Hafstrom et al., 2002), but the effects of varying timing and duration of this developmental exposure have not been specifically investigated. In rat pups, prenatal nicotine exposure impairs the central HVR in an age-dependent manner in that impairments are exhibited in the early postnatal days but not later than postnatal day 10 (Simakajornboon et al., 2004). It remains to be seen if early metamorphic tadpoles can recover from nicotine-induced impairments of central ventilatory responses.
We have previously shown that 10 wk of nicotine exposure has no effect on normoxic/normocapnic gill neuroventilation nor does it affect the integrity of the isolated bullfrog brainstem lung activity over the duration of the experiment (Taylor et al., 2008; Brundage and Taylor, 2009). Here we see that 10-wk nicotine exposure did have a significant effect on gill burst activity. Nicotine-exposed tadpoles had significantly fewer gill bursts per min than control tadpoles of the same developmental group, and late metamorphic nicotine-exposed tadpoles had significantly fewer gill bursts than similarly exposed early metamorphic tadpoles. Gill amplitude was also reduced in late metamorphic nicotine-exposed tadpoles at the end of the hypoxia and did not recover, which suggests that chronic nicotine exposure may have disrupted hypoxia tolerance in these tadpoles rather than their central HVR. Early metamorphic tadpoles appear to be more vulnerable to a nicotine-induced impairment of the central HVR, but late metamorphic nicotine-exposed tadpoles appear to be more vulnerable to hypoxia given they were the only group to exhibit a hypoxia-induced neuroventilatory change that was not reversed during 30 min of normoxic recovery.
4.3 Effect of ethanol on bullfrog tadpole neuroventilation
This is the first study to investigate the effect of ethanol exposure on the central HVR. Ethanol exposure, regardless of developmental stage, disrupted the tadpole central HVR. Neither early nor late metamorphic tadpoles that were chronically exposed to ethanol demonstrated a significant change in lung burst frequency over the 180-min hypoxia treatment. There was a slight depression in the lung burst frequency of late metamorphic ethanol-exposed tadpoles at 180 min, and it is feasible that a significant reduction in lung burst frequency would occur if hypoxia was sustained longer. Late metamorphic ethanol-exposed tadpoles failed to increase gill burst frequency at any time during hypoxia; however, early metamorphic ethanol-exposed tadpoles exhibited an increased gill burst frequency during the latter half of hypoxia treatment.
All neuroventilatory parameters were consistent between ethanol-exposed and control tadpoles during normoxic conditions, this is similar to previous studies demonstrating that the assessed functionality of ethanol-exposed isolated brainstem preparations is unaltered over the duration of our experiment (Taylor et al., 2008). This would suggest that the central HVR was specifically impaired by 10-wk ethanol exposure. These results are different than those of semi-intact preparations derived from neonatal rats that had been prenatally exposed to ethanol, where baseline ventilation and the HVR were reduced (Dubois et al., 2008). It is possible that these differing results are due to chronic ethanol altering peripheral chemoreception and/or the presence of afferent signally mechanisms, which were present and functional in the semi-intact rat preparations. Additional studies with either semi-intact tadpole preparations or isolated rat brainstem preparations would be required to clarify this point.
4.4 Consequences of nicotine and ethanol exposure on neural signaling
Signaling pathways that underlie the HVR in bullfrogs and mammals and pathways that are affected by chronic nicotine or ethanol exposure, to date, have been investigated separately. We can, however, gain insight into possible mechanisms of these teratogenic effects on the central HVR by jointly considering several independent studies. Fournier and colleagues (2007) determined that the bullfrog central HVR is modulated by noradrenergic neurons via an indirect GABAergic/glycinergic pathway. The α1-antagonist prazosine blocked the central lung burst HVR in both early and late metamorphic tadpoles. Application of a bicuculine/strychine mixture blocked both the HVR and the effect of the α1-agonist phenylephrine in isolated brainstem preparations. Hehre et al. (2008) reported that the larger depression in ventilator response to hypoxia seen in younger piglets is mediated by a predominance of inhibitory neurotransmitters (GABA, glycine, taurine) in the nucleus tractus solitarius. A role for GABA in mediating the HVR has been reported by many investigators, but other neurotransmitters have been implicated as well (see review by Teppema and Dahan, 2010). In rats, prenatal nicotine exposure alters both GABAergic and glycinergic signaling in the Pre-Bötzinger complex (Luo et al., 2007), a putative respiratory rhythm generator. Nicotinic acetylcholine receptors are up-regulated on GABAergic and glycinergic neurons, and chronic stimulation of these neurons results in desensitization and a reduction in GABA and glycine release (Covernton and Lester, 2002; Neff et al., 2003; Fregosi and Pilarski, 2008). In bullfrog tadpoles, a reduction in inhibitory signaling may contribute to the loss of the central HVR in early metamorphic tadpoles. Similarly, ethanol is a known GABAmimetic and imidazobenzodiazepine GABAA receptor agonist (Breese et al., 2006; Wallner and Olsen, 2008). Prenatal ethanol exposure has also been shown to alter GABAergic transmission and GABAergic neuron activity (Janiri et al., 1994; Allan et al., 1998). An increase in central glycinergic tonic inhibition is also seen following prenatal ethanol exposure (Dubois et al., 2008). Binding characteristics and ligand specificity of GABAA receptors is similar in bullfrogs and mammals (Hollis and Boyd, 2003). Chronic ethanol may, therefore, generate similar disruptions in GABAergic and glycinergic activity in both early and late metamorphic tadpoles contributing to a loss of the central HVR. Thus, existing evidence suggests that the central HVR and the effects of chronic nicotine and ethanol exposure overlap in their links to GABAergic and glycinergic signaling pathways but not to the exclusion of other possibilities.
If chronic nicotine and ethanol exposure exert their deleterious effects on control of breathing via a common mechanism, GABAergic signaling is a potential candidate. The mechanism by which nicotine and ethanol impair the central HVR, however, need not be a common pathway. Prenatal nicotine has been found to influence serotonergic, glutamatergic, and adrenergic signaling (Campos et al., 2009). Ethanol has both direct and indirect affects on neural tissue including the desensitization of multiple ionotropic receptor subtypes (Aguayo et al., 2002; Dopico and Lovinger, 2009). Identifying the multifarious consequences of developmental nicotine and ethanol exposure remains the subject of on-going research as do the multiplicity of sites and heterogeneity of neural mechanisms involved in the control of breathing.
Highlights.
The control of breathing network within the bullfrog brainstem is sensitive to hypoxia.
The central response to hypoxia is an active suppression of activity.
Chronic exposure of tadpoles to ethanol or nicotine impairs the central response to hypoxia.
Acknowledgments
We would like to thank Mr. Justin Buehner for his contribution to the dataset used in this study and Ms. Carla Nelson for her assistance analyzing nicotine treatment data. This work was supported by NIH-NINDS grant # U54 NS041069-06A1 and NSF-IOS 1022442.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- Bamford OS, Schuen JN, Carroll JL. Effect of nicotine exposure on postnatal ventilatory responses to hypoxia and hypercapnia. Respir Physiol. 1996;106:1–11. doi: 10.1016/0034-5687(96)00051-5. [DOI] [PubMed] [Google Scholar]
- Bavis RW, Mitchell GS. Long-term effects of the perinatal environment on respiratory control. J Appl Physiol. 2008;104:1220–1229. doi: 10.1152/japplphysiol.01086.2007. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Neubauer JA. Peripheral and central effects of hypoxia. In: Dempsey JA, Pack AI, editors. Lung Biology in Health and Disease; Regulation of breathing. Second edition. Marcel Dekker, Inc; 1995. pp. 617–668. [Google Scholar]
- Breese GR, Criswell HE, Carta M, Dodson PD, Hanchar HJ, Khisti RT, Mameli M, Ming Z, Morrow AL, Olsen RW, Otis TS, Parsons LH, Penland SN, Roberto M, Siggins GR, Valenzuela CF, Wallner M. Basis of the gabamimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage CM, Cartagena CM, Potter EA, Taylor BE. Nicotine elicits a developmentally dependent depression in bullfrog neuroventilatory response to CO2 . Respir Physiol Neurobiol. 2010;170:226–235. doi: 10.1016/j.resp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Brundage CM, Taylor BE. Timing and duration of developmental nicotine exposure contribute to attenuation of the tadpole hypercapnic neuroventilatory response. Dev Neurobiol. 2009;69:451–461. doi: 10.1002/dneu.20720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren W, Doyle M. Ontogeny of regulation of gill and lung ventilation in the bullfrog Rana catesbeiana. Resp Physiol. 1986;66:279–292. doi: 10.1016/0034-5687(86)90080-0. [DOI] [PubMed] [Google Scholar]
- Caldeira JC, Wu Y, Mameli M, Purdy RH, Li PK, Akwa Y, Savage DD, Engen JR, Valenzuela CF. Fetal alcohol exposure alters neurosteroid levels in the developing rat brain. J Neurochem. 2004;90:1530–1539. doi: 10.1111/j.1471-4159.2004.02686.x. [DOI] [PubMed] [Google Scholar]
- Campos M, Bravo E, Eugenin J. Respiratory dysfunctions induced by prenatal nicotine exposure. Clin Exp Pharmacol Physiol. 2009;36:1205–1217. doi: 10.1111/j.1440-1681.2009.05214.x. [DOI] [PubMed] [Google Scholar]
- Cohen G, Katz-Salamon M. Development of chemoreceptor responses in infants. Respir Physiol Neurobiol. 2005;149:233–242. doi: 10.1016/j.resp.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Covernton POJ, Lester RAJ. Prolonged stimulation of presynaptic nicotinic acetylcholine receptors in the rat interpeduncular nucleus has differential effects on transmitter release. Intl J Dev Neurosci. 2002;20:247–258. doi: 10.1016/s0736-5748(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Davies BL, Brundage CM, Harris MB, Taylor BE. Lung respiratory rhythm and pattern generation in the bullfrog: role of neurokinin-1 and mu-opioid receptors. J Comp Physiol B. 2009;179:579–592. doi: 10.1007/s00360-009-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C, Houchi H, Naassila M, Daoust M, Pierrefiche O. Blunted response to low oxygen of rat respiratory network after perinatal ethanol exposure: involvement of inhibitory control. J Physiol. 2008;586:1413–1427. doi: 10.1113/jphysiol.2007.147165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois C, Naassila M, Daoust M, Pierrefiche O. Early chronic ethanol exposure in rats disturbs respiratory network activity and increases sensitivity to ethanol. J Physiol. 2006;576:297–307. doi: 10.1113/jphysiol.2006.111138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Randall LL, Belliveaum RA, Trachtenberg FL, Randall B, Habbe D, Mandell F, Welty TK, Iyasu S, Kinney HC. The effect of maternal smoking and drinking during pregnancy upon 3H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain Pathol. 2008;18:21–31. doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T. Substance abuse in pregnancy. Curr Opin Obstet Gynecol. 1993;5:16–23. [PubMed] [Google Scholar]
- Fewell JE, Smith FG. Perinatal nicotine exposure impairs ability of newborn rats to autoresuscitate from apnea during hypoxia. J Appl Physiol. 1998;85:2066–2074. doi: 10.1152/jappl.1998.85.6.2066. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK. Prenatal exposure to nicotine impairs protective responses of rat pups to hypoxia in an age-dependent manner. Respir Physiol. 2001a;127:61–73. doi: 10.1016/s0034-5687(01)00232-8. [DOI] [PubMed] [Google Scholar]
- Fewell JE, Smith FG, Ng VK. Threshold levels of maternal nicotine impairing protective responses of newborn rats to intermittent hypoxia. J Appl Physiol. 2001b;90:1968–1976. doi: 10.1152/jappl.2001.90.5.1968. [DOI] [PubMed] [Google Scholar]
- Fournier S, Allard M, Roussin S, Kinkead R. Developmental changes in central O2 chemoreflex in Rana catesbeiana: the role of noradrenergic modulation. J Exp Biol. 2007;210:3015–3026. doi: 10.1242/jeb.005983. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respir Physiol Neurobiol. 2008;164:80–86. doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gdovin MJ, Torgerson CS, Remmers JE. The fictively breathing tadpole brainstem preparation as a model for the development of respiratory pattern generation and central chemoreception. Comp Biochem Physiol A Mol Integr Physiol. 1999;124:275–286. doi: 10.1016/s1095-6433(99)00116-6. [DOI] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am J Respir Crit Care Med. 2002;166:1544–1549. doi: 10.1164/rccm.200204-289OC. [DOI] [PubMed] [Google Scholar]
- Haglund B, Cnattingius S. Cigarette smoking as a risk factor for sudden infant death syndrome: a population-based study. Am J Public Health. 1990;80:29–32. doi: 10.2105/ajph.80.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MB, Wilson RJ, Vasilakos K, Taylor BE, Remmers JE. Central respiratory activity of the tadpole in vitro brain stem is modulated diversely by nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2002;283:R417–R428. doi: 10.1152/ajpregu.00513.2001. [DOI] [PubMed] [Google Scholar]
- Hehre DA, Devia CJ, Bancalari E, Sugihara C. Brainstem amnio acid neurotransmitters and ventilatory respons to hypoxia in piglets. Pediatr Res. 2008;63:46–50. doi: 10.1203/PDR.0b013e31815b4421. [DOI] [PubMed] [Google Scholar]
- Hollis DM, Boyd SK. Characterization of the GABAA receptor in the brain of the adult male bullfrog, Rana catesbeiana. Brain Res. 2003;992:69–75. doi: 10.1016/j.brainres.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Hunt CE, McCulloch K, Brouillette RT. Diminished hypoxic ventilatory responses in near-miss sudden infant death syndrome. J Appl Physiol. 1981;50:1313–1317. doi: 10.1152/jappl.1981.50.6.1313. [DOI] [PubMed] [Google Scholar]
- Iyasu S, Randall LL, Welty TK, Hsia J, Kinney HC, Mandell F, McClain M, Randall B, Habbe D, Wilson H, Willinger M. Risk factors for sudden infant death syndrome among northern plains Indians. J Am Med Assoc. 2002;288:2717–2723. doi: 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- Janiri L, Gobbi G, Persico AM, Santarelli M, Minciacchi D, Tempesta E. Alterations of neocortical neuronal responses to acetylcholine and GABA in rats born to alcohol-dependent mothers. Alcohol. 1994;29:611–619. [PubMed] [Google Scholar]
- Kotagal S. Sleep and breathing disturbances in infancy and early childhood. Semin Pediatr Neurol. 2003;10:281–288. doi: 10.1016/s1071-9091(03)00074-3. [DOI] [PubMed] [Google Scholar]
- Luo Z, McMullen NT, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem-spinal cord preparation. Respir Physiol Neurobiol. 2007;157:226–234. doi: 10.1016/j.resp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- McKenzie DJ, Taylor EW. Cardioventilatory responses to hypoxia and NaCN in the neotenous axolotl. Respir Physiol. 1996;106:255–262. doi: 10.1016/s0034-5687(96)00080-1. [DOI] [PubMed] [Google Scholar]
- Milerad J, Larsson H, Lin J, Sundell HW. Nicotine attenuates the ventilatory response to hypoxia in the developing lamb. Pediatr Res. 1995;37:652–660. doi: 10.1203/00006450-199505000-00017. [DOI] [PubMed] [Google Scholar]
- Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin Chem. 2002;48:1460–1471. [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Simakajornboon N, Vlasic V, Li H, Sawnani H. Effect of prenatal nicotine exposure on biphasic hypoxic ventilatory response and protein kinase C expression in caudal brain stem of developing rats. J Appl Physiol. 2004;96:2213–2219. doi: 10.1152/japplphysiol.00935.2003. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for sudden infant death syndrome. Brain Res Bull. 1995;38:69–75. doi: 10.1016/0361-9230(95)00073-n. [DOI] [PubMed] [Google Scholar]
- Smith GN, Patrick J, Sinervo KR, Brien JF. Effects of ethanol exposure on the embryo-fetus: experimental considerations, mechanisms, and the role of prostaglandins. Can J Physiol Pharmacol. 1991;69:550–569. doi: 10.1139/y91-082. [DOI] [PubMed] [Google Scholar]
- Taylor AC, Kollros JJ. Stages in the normal development of Rana pipiens larvae. Anat Rec. 1946;94:7–24. doi: 10.1002/ar.1090940103. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Croll AE, Drucker ML, Wilson AL. Developmental exposure to ethanol or nicotine inhibits the hypercapnic ventilatory response in tadpoles. Respir Physiol Neurobiol. 2008;160:83–90. doi: 10.1016/j.resp.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Coates EL, Gdovin MJ, Leiter JC. Central CO2 chemoreception in developing bullfrogs: anomalous response to acetazolamide. J Appl Physiol. 2003a;94:1204–1212. doi: 10.1152/japplphysiol.00558.2002. [DOI] [PubMed] [Google Scholar]
- Taylor BE, Harris MB, Leiter JC, Gdovin MJ. Ontogeny of central CO2 chemoreception: chemosensitivity in the ventral medulla of developing bullfrogs. Am J Physiol Regul Integr Comp Physiol. 2003b;285:R1461–R1472. doi: 10.1152/ajpregu.00256.2003. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Danah A. The ventilatory response to hypoxia in mammals: Mechaanisms, Measurement and Analysis. Physiol Rev. 2010;90:674–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Torgerson CS, Gdovin MJ, Remmers JE. Fictive gill and lung ventilation in the pre- and postmetamorphic tadpole brain stem. J Neurophysiol. 1998;80:2015–2022. doi: 10.1152/jn.1998.80.4.2015. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Stick SM, Hall G, Sly PD. Control of breathing in infants born to smoking mothers. J Pediatr. 1999;135:226–232. doi: 10.1016/s0022-3476(99)70026-0. [DOI] [PubMed] [Google Scholar]
- Vizek M, Pickett CK, Weil JV. Biphasic ventilatory response of adult cats to sustained hypoxia has central origin. J Appl Physiol. 1987;63:1658–1664. doi: 10.1152/jappl.1987.63.4.1658. [DOI] [PubMed] [Google Scholar]
- Wallner M, Olsen RW. Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABAA receptors as an opportunity for drug development? Br J Pharmacol. 2008;154:288–298. doi: 10.1038/bjp.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NH, Burggren WW. Gill and lung ventilatory responses to steady-state aquatic hypoxia and hyperoxia in the bull frog Rana catesbeiana tadpole. Resp Physiol. 1982;47:165–176. doi: 10.1016/0034-5687(82)90109-8. [DOI] [PubMed] [Google Scholar]
- Winmill RE, Chen AK, Hedrick MS. Development of the respiratory response to hypoxia in the isolated brainstem of the bullfrog Rana catesbeiana. J Exp Biol. 2005;208:213–222. doi: 10.1242/jeb.01399. [DOI] [PubMed] [Google Scholar]