Abstract
Osteoporosis in men contributes to significant morbidity and mortality. Hip fractures in men are associated with greater mortality compared with women, with a mortality rate of up to 37.5% within a year following the fracture. Its timely diagnosis and treatment are therefore essential. However, despite one-third of all hip fractures worldwide occurring in men, osteoporosis in men remains an immensely under-recognized and undertreated public health problem. Bisphosphonates are well studied first-line treatments for postmenopausal women with osteoporosis and have been shown to reduce fragility fractures at all clinically important sites (vertebral, nonvertebral, hip and wrist). However, the majority of studies of oral or intravenous bisphosphonate therapy in men with osteoporosis report effects on surrogate markers, including bone mineral density (BMD) and biochemical bone turnover markers, rather than on fragility fractures. Oral or intravenous bisphosphonate therapy increases spinal, total hip and femoral neck BMD compared with placebo in men with osteoporosis. Both bone resorption and bone formation markers are decreased following bisphosphonate therapy, with the onset of the decrease in bone formation markers being delayed. In a study of intravenous zoledronic acid given to older men and women following a hip fracture, any clinical vertebral and nonvertebral fractures were all reduced compared with placebo infusions. In addition, mortality was reduced in patients who received zoledronic acid.
Recent studies in men with osteoporosis have increasingly reported reductions in incident vertebral fractures with oral or intravenous bisphosphonate therapy, although all studies have been underpowered to detect effects on nonvertebral and hip fracture outcomes. Bisphosphonates have a role as monotherapy, as consolidative therapy after a course of teriparatide therapy, or in combination with testosterone replacement in men with hypogonadism and osteoporosis. Bisphosphonate therapy is validated and important in the treatment of osteoporosis in men.
Keywords: alendronate, bisphosphonates, hypogonadism, osteoporosis, risedronate, zoledronic acid
Introduction
Osteoporosis remains an immensely under-recognized and undertreated cause of morbidity and mortality in men, with one-third of all hip fractures worldwide occurring in men [Gullberg et al. 1997]. One in six men will sustain a hip fracture by the age of 90 [Nguyen et al. 1996], with almost half of these occurring before the age of 80 [Chang et al. 2004]. Up to 40% of hip fractures in men occur in residential care, and one-fifth of those who sustain a hip fracture will subsequently have a second hip fracture. Importantly, hip fractures in men are associated with greater mortality compared with women, with a mortality rate of up to 37.5% within a year of fracture [Cooper et al. 1992; Jones et al. 1996; O’Neill et al. 1996; Orwoll and Klein, 1995].
Vertebral fractures are also common among older men and are strongly associated with subsequent hip and nonvertebral fractures. Although the majority of vertebral fractures are painless, they can impart significant morbidity, including height loss, reduced quality of life, respiratory dysfunction and social withdrawal [Khosla et al. 1994; Orwoll and Klein, 1995]. Vertebral fractures tend to occur earlier in men than women, with a peak incidence in the fourth and fifth decades of life [Cooper et al. 1992; Jones et al. 1996; O’Neill et al. 1996], whereas above the age of 65, incidence rates of vertebral fractures in men are half those in women. The incidence of vertebral fractures at a younger age in men may be partially accounted for by traumatic fractures.
Despite the considerable health burden of fractures and osteoporosis in men, there is a paucity of reported clinical studies that explore the efficacy of osteoporosis therapy in men as opposed to postmenopausal women, particularly with regards to fracture reduction as a primary outcome [Boonen et al. 2009; Orwoll et al. 2000, 2010a, 2010b]. Increasingly, recent data support the role of bisphosphonate therapy in the treatment of osteoporosis in men.
Aetiology of bone loss in men
Bone mass and strength are determined by numerous factors, including the attainment of peak bone mass and subsequent age-related bone loss; both processes being dependent on sex steroid production and action. Notably, the majority of nonvertebral fractures occur in men without osteoporosis, implying that other factors distinct from bone mineral density (BMD) contribute to fracture risk [Seeman et al. 2006].
Natural history of bone loss in men
Analogous to the process in women, both cortical and trabecular bone density increase significantly during puberty in response to the actions of sex steroid hormones [Krabbe et al. 1984]. In particular, testosterone exerts an indirect effect on bone, through the skeletal aromatization of testosterone to oestrogen [Ebeling, 1998]; mutations of the aromatase enzyme have been associated with severe osteoporosis in men [Morishima et al. 1995; Smith et al. 1994]. The finding of low bone mass in men with idiopathic hypogonadotropic hypogonadism further emphasizes the role of sex steroid hormones in attainment of optimal peak bone mass [Finkelstein et al. 1987].
Bone loss commences soon after peak bone mass is achieved [Nordstrom et al. 2007], with longitudinal studies suggesting that the rate of loss accelerates after the age of 70 in men [Jones et al. 1994; Szulc and Delmas, 2001]. In older men, bone formation markers remain stable or decline slightly, whereas bone resorption markers increase, mainly after age 70, indicating a likely role for antiresorptive therapy, such as bisphosphonates. This increased bone resorption may be related to low levels of bioavailable oestradiol and testosterone [Pietschmann et al. 2001]. In men over age 65, rapid bone loss is more common in men with testosterone or oestradiol deficiency compared with controls [Fink et al. 2006].
Bone loss in men is less rapid than postmenopausal bone loss in women, and occurs primarily by trabecular thinning secondary to reduced formation within each basic multicellular unit as demonstrated by noninvasive high-resolution peripheral quantitative computed tomography scanning. Although women lose trabeculae with an increase in trabecular spacing, men sustain trabecular thinning with no change in trabecular numbers or spacing [Khosla et al. 2006]. The preservation of trabecular numbers in men may also help explain their lower lifetime risk of fractures. For men, trabecular bone loss begins early in life, with 42% of trabecular bone being lost before age 50; changes in concentrations of insulin-like growth factor 1 being a possible factor. In contrast, 85% of cortical bone is lost after age 50, being related to subsequent decline in bioavailable sex steroids and increased bone remodelling [Riggs et al. 2008].
Secondary causes of bone loss in men
Secondary causes of osteoporosis in men are common and a significant cause of osteoporosis in men (Table 1) [Ebeling, 2008]. Secondary causes need to be excluded in men younger than 50 years when the Z score is less than −2 (2 standard deviations below the age-specific mean) on bone densitometry, and in men older than 50 years with osteoporosis. The most common secondary causes are glucocorticoid use, excessive alcohol use, tobacco use and hypogonadism.
Table 1.
Secondary causes of osteoporosis.
| Common | Cushing’s syndrome or exogenous corticosteroid use (>5 mg/day for >3 months) |
| Excessive alcohol use (more than two standard drinks per day) | |
| Primary or secondary hypogonadism | |
| Inadequate calcium intake (<600 mg/day) | |
| Vitamin D deficiency/insufficiency | |
| Tobacco | |
| Family history | |
| Less common | Low body mass index (<20) |
| Lack of exercise or excessive exercise | |
| Antiepileptic drugs (phenytoin, phenobarbitone, primidone, carbamazepine) | |
| Thyrotoxicosis | |
| Primary hyperparathyroidism | |
| Type 1 and type 2 diabetes mellitus | |
| Chronic liver or kidney disease | |
| Malabsorption, including coeliac disease | |
| Hypercalciuria | |
| Rheumatoid arthritis or ankylosing spondylosis | |
| Rare | Multiple myeloma |
| Human immunodeficiency virus infection or its treatment with protease inhibitors (tenofovir) | |
| Mastocytosis | |
| Immunosuppressive therapy (cyclosporin, tacrolimus) | |
| Osteogenesis imperfect |
Hypogonadism
Hypogonadism occurs in up to 12.3% of men [Mohr et al. 2005], and is a significant contributor to osteoporosis due to the role of sex steroids in bone loss. In older male nursing home residents with hip fractures, up to 66% were found to have hypogonadism [Abbasi et al. 1995], whereas up to 20% of men with spinal fractures had hypogonadism, with most being asymptomatic. BMD has been shown to be lower in men with hypogonadism [Finkelstein et al. 1987; Stoch et al. 2001], although bone turnover may be increased. These findings are concordant with the skeletal effects seen with hypogonadism induced by androgen deprivation therapy for prostate cancer, this being associated with decreased BMD, increased bone turnover and a higher fracture risk [Shahinian et al. 2005]. In cases of elevated bone turnover markers, testosterone replacement may reduce biochemical markers of bone resorption [Dillon et al. 2008; Wang et al. 2001]. Similarly, this would provide a justification for the use of bisphosphonate therapy given its mechanism of action and effects on bone resorption and remodelling frequency.
Hypogonadism may be primary or secondary (Table 2). Secondary iatrogenic causes such as androgen deprivation therapy in prostate cancer management are especially important to identify since, comparable with the skeletal effects of glucocorticoids, bone loss may occur as little as 6 months after commencement of therapy [Ross and Small, 2002]. Furthermore, given that nutrient supplementation alone, with calcium and vitamin D, may be insufficient to prevent bone loss in this setting, some guidelines advocate use of bisphosphonate therapy if the BMD T score is less than or equal to −2.0 in men who have not previously sustained a minimal trauma fracture [Grossmann et al. 2011].
Table 2.
Causes of hypogonadism.
| Primary hypogonadism | Genetic/chromosomal disorders (Klinefelter’s syndrome) |
| Anorchia (congenital or postorchidectomy) | |
| Cryptorchidism | |
| Chemotherapy (alkylating agents), radiotherapy | |
| Orchitis (mumps, human immunodeficiency virus, autoimmune) | |
| Testicular trauma or torsion | |
| Medications (glucocorticoids, colchicine) | |
| Alcohol | |
| Chronic liver or kidney disease, haemochromatosis | |
| Secondary hypogonadism | Idiopathic |
| Kallmann syndrome | |
| Functional | |
| Excessive exercise, weight change | |
| Systemic or intercurrent illness | |
| Structural | |
| Pituitary or hypothalamic tumour, prolactinoma | |
| Infiltration (sarcoidosis, haemochromatosis) | |
| Cranial irradiation, surgery, head trauma | |
| Medications/iatrogenic | |
| Androgen deprivation therapy for treatment of prostate cancer | |
| Opioids | |
| Exogenous administration of androgens |
Bisphosphonate therapy
Bisphosphonates are a well studied class of antiresorptive drugs that have been approved as a first-line treatment for osteoporosis. They have a high affinity for hydroxyapatite, allowing avid attachment of the drug to bony surfaces and thus incorporation into bone. Once internalized by osteoclasts, amino-bisphosphonates inhibit the action of farnesyl pyrophosphate synthase. This reduces the activity and viability of osteoclasts and ultimately results in reduced bone resorption.
Oral bisphosphonates have been demonstrated to be effective in fracture reduction in postmenopausal women [Wells et al. 2008a, 2008b]. Alendronate is effective for the primary prevention of vertebral fractures and secondary prevention of vertebral, nonvertebral, hip and wrist fractures [Wells et al. 2008b]. Similarly, risedronate has a role in secondary prevention of vertebral, nonvertebral and hip fractures [Wells et al. 2008a].
In contrast, there are few quality studies with primary fracture outcome data in men, with many utilizing surrogate markers including bone turnover markers and BMD [Boonen et al. 2009; Orwoll et al. 2000, 2010a, 2010b]. Despite this, the similar antiresorptive effect seen in men, as supported by increases in BMD and reductions in bone resorptive markers, supports the use of bisphosphonates in men. In circumstances of higher bone turnover such as hypogonadism, bisphosphonates may play an even more substantial role.
Alendronate
Numerous studies have demonstrated the efficacy of alendronate in men with osteoporosis. The most recent randomized controlled study reaffirmed the results of numerous previous studies by demonstrating improved BMD and reduced bone turnover markers [Orwoll et al. 2010b].
In terms of antifracture efficacy, a randomized double-blind trial in 241 men with hypogonadism or eugonadism showed that alendronate at a dose equivalent to 10 mg per day increased spinal and femoral neck BMD while also reducing the incidence of quantitative, rather than clinical, vertebral fractures at 2 years (0.8% versus 7.1% in the placebo arm) [Orwoll et al. 2000]. This trial was not powered to assess reductions in other fractures, including hip fractures. A subsequent meta-analysis demonstrated a reduction in vertebral fractures (odds ratio 0.44) [Sawka et al. 2005]. However, the reduction in nonvertebral fractures was not statistically significant due to a lack of study power secondary to the small number of nonvertebral fractures in the treatment and placebo groups, and the paucity of nonvertebral fracture data in the assessed trials. Importantly, the use of alendronate in men with primary osteoporosis having a high fracture risk is supported by a cost-effectiveness analysis [Borgstrom et al. 2004].
Alendronate has also been shown to be efficacious in men with secondary causes of osteoporosis, such as glucocorticoid use and androgen deprivation therapy. Alendronate has been shown to increase the lumbar spine BMD in men with glucocorticoid-induced osteoporosis, with few adverse events [Lespessailles, 2013]. In one clinical trial comparing weekly alendronate 70 mg with placebo, there was a significant increase in BMD at the lumbar spine and hip after 12 months [Stoch et al. 2009]. Similarly, weekly alendronate increases lumbar spine BMD compared with placebo in the setting of androgen deprivation therapy [Greenspan et al. 2007], while also being cost effective [Ito et al. 2010].
Risedronate
Like alendronate, data support the efficacy of risedronate in men with osteoporosis. In one 12-month study, risedronate 5 mg per day given for 12 months increased spinal and proximal femur BMD in men with osteoporosis [Ringe et al. 2006]. This open-label study showed a reduction in vertebral fractures, but had the shortcoming of not being blinded.
A more recent randomized clinical trial of 284 men with osteoporosis compared weekly risedronate 35 mg with placebo, reported that risedronate increased lumbar spine BMD [Boonen et al. 2009]. Importantly, there was no reduction in incident fractures with risedronate as these occurred infrequently and the study was not powered to detect improvements in fracture endpoints. Therefore, more data are required to determine the antifracture efficacy of oral bisphosphonates, including risedronate, particularly regarding nonvertebral and hip fractures.
Intravenous bisphosphonates
Intravenous bisphosphonates, such as zoledronic acid, present an alternative option to oral bisphosphonates, avoiding gastrointestinal adverse effects and offering regimens with less frequent dosing and thus improving adherence to therapy. Potential adverse effects include an acute phase reaction with fever and myalgia after the first infusion, worsening of renal impairment and, rarely, jaw osteonecrosis [Bilezikian, 2006]. While monthly ibandronate has been shown to improve BMD and bone turnover markers [Orwoll et al. 2010a], zoledronic acid is the better studied of the intravenous bisphosphonates in terms of antifracture efficacy.
In one randomized trial, zoledronic acid at an annual dose of 5 mg reduced overall clinical fractures (35% relative risk reduction) and mortality, but not hip fractures, in older men and women over a 1.9-year follow-up period after hip fracture [Lyles et al. 2007]. Furthermore, zoledronic acid reduced mortality by 28% compared with placebo (p = 0.02).
A recent comparative study of once-yearly zoledronic acid 5 mg and once-weekly alendronate demonstrated noninferiority of zoledronic acid with regards to changes in BMD and bone turnover markers in men with osteoporosis [Orwoll et al. 2010b]. Zoledronic acid increased lumbar spine BMD at 24 months by 6.1% while bone turnover markers at 12 months were comparable in both treatment arms. Adverse event rates were similar and, although few in number, incident vertebral fracture rates were similar in both groups. Significantly, based on questionnaire responses, most men preferred the once-yearly zoledronic acid infusion (74.2%) compared with alendronate.
The most recently published multicentre double-blind, placebo-controlled trial of 1199 men reported fewer incident vertebral fractures with zoledronic acid (1.6% versus 4.9%, p = 0.002), along with significant increases in BMD and decreases in bone turnover markers (Figure 1) [Boonen et al. 2012]. Compared with men who received placebo, men who received zoledronic acid also had fewer moderate to severe vertebral fractures and less height loss. Although this study supports the antifracture efficacy for zoledronic acid in men with eugonadism and hypogonadism, overall there remains a lack of data demonstrating antifracture efficacy for nonvertebral and hip fractures in men; most studies had few such fractures and thus were underpowered to assess the effects on these clinical endpoints.
Figure 1.
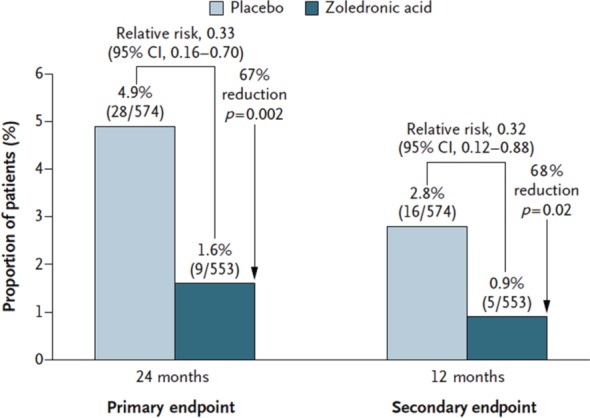
Relative risks of one or more new morphometric vertebral fractures in the modified intention-to-treat population of men with osteoporosis, for zoledronic acid versus placebo. Reproduced with permission from Boonen et al. [2012].
In the setting of androgen deprivation therapy for prostate cancer, there is also evidence supporting the use of intravenous bisphosphonates. Pamidronate has been shown to prevent the initial bone loss seen following initiation of androgen deprivation therapy [Smith et al. 2001], whereas zoledronic acid increased lumbar spine BMD by 5.6% at 1 year compared with a decrease of 2.2% with placebo [Smith et al. 2003]. However, both studies were underpowered to assess antifracture efficacy.
Alternative and adjunctive therapies
Denosumab
Denosumab, a fully human monoclonal antibody that specifically targets receptor activator of nuclear factor κB ligand, is a potential alternative antiresorptive agent to bisphosphonate therapy. Similarly, its effectiveness in the prevention of bone loss in the setting of androgen deprivation therapy for nonmetastatic prostate cancer has been demonstrated [Smith et al. 2009]. In addition to improvements in BMD, the incidence of new vertebral fractures was decreased over three years. However, there were no significant reductions in nonvertebral or hip fractures.
Teriparatide
Anabolic agents, such as teriparatide, may remedy an underlying defect in osteoblast function, which has been implicated in men with idiopathic osteoporosis [Orwoll and Klein, 1995; Rosen and Bilezikian, 2001]. Studies show teriparatide or PTH(1-34) increases spinal and proximal femur BMD in men with hypogonadism or eugonadism and osteoporosis [Orwoll et al. 2003]. In follow-up studies, prior teriparatide therapy significantly reduced moderate and severe vertebral fractures, and nonvertebral while data on nonvertebral or hip fractures in men are lacking [Kaufman et al. 2005].
Despite the purported complementary actions of bisphosphonates and teriparatide on osteoclasts and osteoblasts respectively, the addition of alendronate to teriparatide blunts the increase in BMD stimulated by teriparatide in men [Finkelstein et al. 2003]. Antecedent treatment with risedronate compared with alendronate led to greater increases in bone formation markers and BMD [Miller et al. 2008]. Notwithstanding, bisphosphonates continue to play a significant role in men who receive teriparatide for severe osteoporosis since their commencement after cessation of teriparatide is mandatory and instigates further gains in BMD [Kurland et al. 2004].
Testosterone
Studies of testosterone in men with osteoporosis are limited and none have used fractures as a primary endpoint. In men with hypogonadism, testosterone therapy has a comparable effect with bisphosphonate therapy, with improvements in bone turnover markers and BMD [Katznelson et al. 1996; van der Werff ten Bosch and Bot, 1992]. In a 2-year observational study, microscopic magnetic resonance imaging parameters of trabecular connectivity improved, suggesting testosterone may improve trabecular architecture in men with hypogonadism [Benito et al. 2005]. The effects of testosterone therapy in men with eugonadism remain uncertain.
Although there are no studies investigating the combination of testosterone and bisphosphonates and despite both having similar effects on bone turnover and density, there remains a justification for bisphosphonate use in men receiving sex steroids to restore eugonadism. As previously discussed, bisphosphonate therapy is effective in men with hypogonadism and eugonadism, with studies demonstrating antifracture efficacy. Therefore, in addition to bisphosphonate therapy, eugonadal status should be restored in men without contraindications to sex hormone replacement through adjunctive testosterone therapy in those with high fracture risk.
Conclusion
Despite the relative paucity of studies investigating the use of bisphosphonates in men with osteoporosis, particularly with respect to antifracture efficacy, both oral and intravenous bisphosphonates have positive effects on surrogate markers, including bone turnover markers and bone density. Furthermore, despite the lack of data on efficacy on hip and nonvertebral fractures recent studies confirm the benefit of both oral and intravenous bisphosphonate therapy in reducing vertebral fractures in men with osteoporosis. Bisphosphonates have a role as monotherapy, as consolidative therapy after a course of teriparatide therapy or in combination with testosterone replacement in men at high risk of fracture with hypogonadism.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: Ie-Wen Sim declares no conflicts of interest in preparing this article. Peter R Ebeling has received research support from Merck, Novartis, Amgen and Eli-Lilly and an honorarium from Merck.
Contributor Information
Ie-Wen Sim, NorthWest Academic Centre, Department of Endocrinology, University of Melbourne, Western Health & Southern Health, St Albans, Victoria, Australia.
Peter R. Ebeling, NorthWest Academic Centre, Department of Endocrinology, University of Melbourne, Western Health, 176 Furlong Road, St Albans 3021, Victoria, Australia
References
- Abbasi A., Rudman D., Wilson C., Drinka P., Basu S., Mattson D., et al. (1995) Observations on nursing home residents with a history of hip fracture. Am J Med Sci 310: 229–234 [PubMed] [Google Scholar]
- Benito M., Vasilic B., Wehrli F., Bunker B., Wald M., Gomberg B., et al. (2005) Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res 20: 1785–1791 [DOI] [PubMed] [Google Scholar]
- Bilezikian J. (2006) Osteonecrosis of the jaw – do bisphosphonates pose a risk? N Engl J Med 355: 2278–2281 [DOI] [PubMed] [Google Scholar]
- Boonen S., Orwoll E., Wenderoth D., Stoner K., Eusebio R., Delmas P. (2009) Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 24: 719–725 [DOI] [PubMed] [Google Scholar]
- Boonen S., Reginster J., Kaufman J., Lippuner K., Zanchetta J., Langdahl B., et al. (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367: 1714–1723 [DOI] [PubMed] [Google Scholar]
- Borgstrom F., Johnell O., Jonsson B., Zethraeus N., Sen S. (2004) Cost effectiveness of alendronate for the treatment of male osteoporosis in Sweden. Bone 34: 1064–1071 [DOI] [PubMed] [Google Scholar]
- Chang K., Center J., Nguyen T., Eisman J. (2004) Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 19: 532–536 [DOI] [PubMed] [Google Scholar]
- Cooper C., Atkinson E., O’Fallon W., Melton L., 3rd (1992) Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7: 221–227 [DOI] [PubMed] [Google Scholar]
- Dillon E., Urban R., Angel J., Casperson S., Paddon-Jones D., Sheffield-Moore M. (2008) Continuous testosterone administration for 5 months reduces markers of bone turnover in older men. FASEB J 22: 1188 [Google Scholar]
- Ebeling P. (1998) Osteoporosis in men. New insights into aetiology, pathogenesis, prevention and management. Drugs Aging 13: 421–434 [DOI] [PubMed] [Google Scholar]
- Ebeling P.R. (2012) Clinical practice. Osteoporosis in men. New Engl J Med 358(14): 1474–82 [DOI] [PubMed] [Google Scholar]
- Fink H., Ewing S., Ensrud K., Barrett-Connor E., Taylor B., Cauley J., et al. (2006) Association of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older men. J Clin Endocrinol Metab 91: 3908–3915 [DOI] [PubMed] [Google Scholar]
- Finkelstein J., Hayes A., Hunzelman J., Wyland J., Lee H., Neer R. (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349: 1216–1226 [DOI] [PubMed] [Google Scholar]
- Finkelstein J., Klibanski A., Neer R., Greenspan S., Rosenthal D., Crowley W., Jr (1987) Osteoporosis in men with idiopathic hypogonadotropic hypogonadism. Ann Intern Med 106: 354–361 [DOI] [PubMed] [Google Scholar]
- Greenspan S., Nelson J., Trump D., Resnick N. (2007) Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med 146: 416–424 [DOI] [PubMed] [Google Scholar]
- Grossmann M., Hamilton E., Gilfillan C., Bolton D., Joon D., Zajac J. (2011) Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust 194: 301–306 [DOI] [PubMed] [Google Scholar]
- Gullberg B., Johnell O., Kanis J. (1997) World-wide projections for hip fracture. Osteoporos Int 7: 407–413 [DOI] [PubMed] [Google Scholar]
- Ito K., Elkin E., Girotra M., Morris M. (2010) Cost-effectiveness of fracture prevention in men who receive androgen deprivation therapy for localized prostate cancer. Ann Intern Med 152: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Nguyen T., Sambrook P., Kelly P., Eisman J. (1994) Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo osteoporosis epidemiology study. BMJ 309: 691–695 [PMC free article] [PubMed] [Google Scholar]
- Jones G., White C., Nguyen T., Sambrook P., Kelly P., Eisman J. (1996) Prevalent vertebral deformities: relationship to bone mineral density and spinal osteophytosis in elderly men and women. Osteoporos Int 6: 233–239 [DOI] [PubMed] [Google Scholar]
- Katznelson L., Finkelstein J., Schoenfeld D., Rosenthal D., Anderson E., Klibanski A. (1996) Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81: 4358–4365 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Orwoll E., Goemaere S., San Martin J., Hossain A., Dalsky G., et al. (2005) Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int 16: 510–516 [DOI] [PubMed] [Google Scholar]
- Khosla S., Lufkin E., Hodgson S., Fitzpatrick L., Melton L., 3rd (1994) Epidemiology and clinical features of osteoporosis in young individuals. Bone 15: 551–555 [DOI] [PubMed] [Google Scholar]
- Khosla S., Riggs B., Atkinson E., Oberg A., McDaniel L., Holets M., et al. (2006) Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe S., Hummer L., Christiansen C. (1984) Longitudinal study of calcium metabolism in male puberty. II. Relationship between mineralization and serum testosterone. Acta Paediatr Scand 73: 750–755 [DOI] [PubMed] [Google Scholar]
- Kurland E., Heller S., Diamond B., McMahon D., Cosman F., Bilezikian J. (2004) The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1-34)]. Osteoporos Int 15: 992–997 [DOI] [PubMed] [Google Scholar]
- Lespessailles E. (2013) Bisphosphonates and glucocorticoid-induced osteoporosis: efficacy and tolerability. Joint Bone Spine 80: 258–264 [DOI] [PubMed] [Google Scholar]
- Lyles K., Colon-Emeric C., Magaziner J., Adachi J., Pieper C., Mautalen C., et al. (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357: 1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P., Delmas P., Lindsay R., Watts N., Luckey M., Adachi J., et al. (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93: 3785–3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr B., Guay A., O’Donnell A., McKinlay J. (2005) Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf) 62: 64–73 [DOI] [PubMed] [Google Scholar]
- Morishima A., Grumbach M., Simpson E., Fisher C., Qin K. (1995) Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80: 3689–3698 [DOI] [PubMed] [Google Scholar]
- Nguyen T., Eisman J., Kelly P., Sambrook P. (1996) Risk factors for osteoporotic fractures in elderly men. Am J Epidemiol 144: 255–263 [DOI] [PubMed] [Google Scholar]
- Nordstrom P., Neovius M., Nordstrom A. (2007) Early and rapid bone mineral density loss of the proximal femur in men. J Clin Endocrinol Metab 92: 1902–1908 [DOI] [PubMed] [Google Scholar]
- O’Neill T., Felsenberg D., Varlow J., Cooper C., Kanis J., Silman A. (1996) The prevalence of vertebral deformity in European men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 11: 1010–1018 [DOI] [PubMed] [Google Scholar]
- Orwoll E., Binkley N., Lewiecki E., Gruntmanis U., Fries M., Dasic G. (2010a) Efficacy and safety of monthly ibandronate in men with low bone density. Bone 46: 970–976 [DOI] [PubMed] [Google Scholar]
- Orwoll E., Ettinger M., Weiss S., Miller P., Kendler D., Graham J., et al. (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343: 604–610 [DOI] [PubMed] [Google Scholar]
- Orwoll E., Klein R. (1995) Osteoporosis in men. Endocr Rev 16: 87–116 [DOI] [PubMed] [Google Scholar]
- Orwoll E., Miller P., Adachi J., Brown J., Adler R., Kendler D., et al. (2010b) Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 25: 2239–2250 [DOI] [PubMed] [Google Scholar]
- Orwoll E., Scheele W., Paul S., Adami S., Syversen U., Diez-Perez A., et al. (2003) The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18: 9–17 [DOI] [PubMed] [Google Scholar]
- Pietschmann P., Kudlacek S., Grisar J., Spitzauer S., Woloszczuk W., Willvonseder R., et al. (2001) Bone turnover markers and sex hormones in men with idiopathic osteoporosis. Eur J Clin Invest 31: 444–451 [DOI] [PubMed] [Google Scholar]
- Riggs B., Melton L., Robb R., Camp J., Atkinson E., McDaniel L., et al. (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23: 205–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe J., Faber H., Farahmand P., Dorst A. (2006) Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int 26: 427–431 [DOI] [PubMed] [Google Scholar]
- Rosen C., Bilezikian J. (2001) Clinical review 123: Anabolic therapy for osteoporosis. J Clin Endocrinol Metab 86: 957–964 [DOI] [PubMed] [Google Scholar]
- Ross R., Small E. (2002) Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol 167: 1952–1956 [PubMed] [Google Scholar]
- Sawka A., Papaioannou A., Adachi J., Gafni A., Hanley D., Thabane L. (2005) Does alendronate reduce the risk of fracture in men? A meta-analysis incorporating prior knowledge of anti-fracture efficacy in women. BMC Musculoskelet Disord 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E., Bianchi G., Khosla S., Kanis J., Orwoll E. (2006) Bone fragility in men – where are we? Osteoporos Int 17: 1577–1583 [DOI] [PubMed] [Google Scholar]
- Shahinian V., Kuo Y., Freeman J., Goodwin J. (2005) Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 352: 154–164 [DOI] [PubMed] [Google Scholar]
- Smith E., Boyd J., Frank G., Takahashi H., Cohen R., Specker B., et al. (1994) Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331: 1056–1061 [DOI] [PubMed] [Google Scholar]
- Smith M., Eastham J., Gleason D., Shasha D., Tchekmedyian S., Zinner N. (2003) Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 169: 2008–2012 [DOI] [PubMed] [Google Scholar]
- Smith M., Egerdie B., Hernandez Toriz N., Feldman R., Tammela T., Saad F., et al. (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361: 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., McGovern F., Zietman A., Fallon M., Hayden D., Schoenfeld D., et al. (2001) Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med 345: 948–955 [DOI] [PubMed] [Google Scholar]
- Stoch S., Parker R., Chen L., Bubley G., Ko Y., Vincelette A., et al. (2001) Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab 86: 2787–2791 [DOI] [PubMed] [Google Scholar]
- Stoch S., Saag K., Greenwald M., Sebba A., Cohen S., Verbruggen N., et al. (2009) Once-weekly oral alendronate 70 mg in patients with glucocorticoid-induced bone loss: a 12-month randomized, placebo-controlled clinical trial. J Rheumatol 36: 1705–1714 [DOI] [PubMed] [Google Scholar]
- Szulc P., Delmas P. (2001) Biochemical markers of bone turnover in men. Calcif Tissue Int 69: 229–234 [DOI] [PubMed] [Google Scholar]
- van der Werff ten Bosch J., Bot A. (1992) Some skeletal dimensions of males with isolated gonadotrophin deficiency. Neth J Med 41: 259–263 [PubMed] [Google Scholar]
- Wang C., Swerdloff R., Iranmanesh A., Dobs A., Snyder P., Cunningham G., et al. (2001) Effects of transdermal testosterone gel on bone turnover markers and bone mineral density in hypogonadal men. Clin Endocrinol (Oxf) 54: 739–750 [DOI] [PubMed] [Google Scholar]
- Wells G., Cranney A., Peterson J., Boucher M., Shea B., Robinson V., et al. (2008a) Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev (1): CD004523. [DOI] [PubMed] [Google Scholar]
- Wells G., Cranney A., Peterson J., Boucher M., Shea B., Robinson V., et al. (2008b) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev (1): CD001155. [DOI] [PubMed] [Google Scholar]


