Abstract
Osteoarthritis is a primary cause of disability and functional incapacity. Pharmacological treatment is currently limited to symptomatic management, and in advanced stages, surgery remains the only solution. The therapeutic armamentarium for osteoarthritis remains poor in treatments with an effect on joint structure, that is, disease-modifying osteoarthritis drugs (DMOADs). Glucosamine sulfate and chondroitin sulfate are the only medications for which some conclusive evidence for a disease-modifying effect is available. Strontium ranelate is currently indicated for the prevention of fracture in severe osteoporosis. Its efficacy and safety as a DMOAD in knee osteoarthritis has recently been explored in the SEKOIA trial, a 3-year randomized, double-blind, placebo-controlled trial. Outpatients with knee osteoarthritis, Kellgren and Lawrence grade 2 or 3, and joint space width (JSW) of 2.5–5 mm received strontium ranelate 1 g/day (n = 558) or 2 g/day (n = 566), or placebo (n = 559). This sizable population was aged 62.9 years and had a JSW of 3.50 ± 0.84 mm. Treatment with strontium ranelate led to significantly less progression of knee osteoarthritis: estimates for annual difference in joint space narrowing versus placebo were 0.14 mm [95% confidence interval (CI) 0.05–0.23 mm; p < 0.001] for 1 g/day and 0.10 mm (95% CI 0.02–0.19 mm; p = 0.018) for 2 g/day, with no difference between strontium ranelate groups. Radiological progression was less frequent with strontium ranelate (22% with 1 g/day and 26% with 2 g/day versus 33% with placebo, both p < 0.05), as was radioclinical progression (8% and 7% versus 12%, both p < 0.05). Symptoms also improved with strontium ranelate 2 g/day only in terms of total WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) score (p = 0.045), and its components for pain (p = 0.028) and physical function (p = 0.099). Responder analyses using a range of criteria for symptoms indicated that the effect of strontium ranelate 2 g/day on pain and physical function was clinically meaningful. Strontium ranelate was well tolerated. The observation of both structure and symptom modification with strontium ranelate 2 g/day makes SEKOIA a milestone in osteoarthritis research and treatment.
Keywords: joint space narrowing, osteoarthritis, strontium ranelate, symptoms, treatment
Osteoarthritis: impact, prevalence, and pathology
Osteoarthritis is a primary cause of disability and functional incapacity in adults. This progressive disease is characterized by faulty repair of joint damage and degeneration of the joint, and is thought to be triggered by atypical intra-articular biomechanical or biochemical stress [Bijlsma et al. 2011; Lane et al. 2011; van Dijk et al. 2008]. Osteoarthritis is diagnosed according to clinical and radiographic features, with pain being the predominant reason for medical consultation. Mainly affecting the hand, knee, hip, spine, and foot, osteoarthritis is largely associated with episodic pain, physical disability, and a reduction in quality of life. Recent evidence indicates an association of osteoarthritis with increased risk of mortality [Nuesch et al. 2011].
The prevalence of osteoarthritis rises markedly from the age of 50 years. A study in the United States showed that major knee radiographic changes were rare (1%) in young adults aged 25–34 years, but common (50%) in the very old, aged 75 years or more [Jordan et al. 2007]. Knee osteoarthritis occurs more frequently in women than in men. A European study reported the incidence of knee osteoarthritis in men and women as 1.2% and 2.8% [Picavet et al. 2000]. Rates are higher in older men and women, and the prevalence of knee osteoarthritis observed in Dutch people aged 55 years and over was 31% in women and 16% in men [van Saase et al. 1989]. A range of inherent risk factors are associated with the occurrence and progression of osteoarthritis, such as age, sex, and genetics, as well as a number of modifiable risk factors such as sporting activities, obesity, and smoking [Bijlsma et al. 2011]. Certain diseases, like osteoporosis and sarcopenia, and knee injury also predispose individuals to developing osteoarthritis.
The underlying defect in osteoarthritis is often considered to be cartilage degeneration. Recent work indicates that many other joint tissues are affected, such as ligaments, periarticular muscle, synovial membrane, and subchondral bone [Kwan et al. 2010]. Notably, the subchondral bone appears to play a major role in the pathogenesis of osteoarthritis and the expression of pain (e.g. painful microfractures of subchondral bone) [Karsdal et al. 2008; Kwan et al. 2010]. In this context, bone marrow lesions, alterations in magnetic resonance imaging (MRI) signal intensity in bone have been associated with pain [Felson et al. 2001, 2007; Lo et al. 2009; Neogi 2012]. Other MRI findings in bone, such as osteophytes and bone attrition, may also be linked to pain, but these associations are at best moderate or controversial [Yusuf et al. 2011]. In clinical terms, the involvement of noncartilaginous joint structures may explain why cartilage degradation and pain are not experienced simultaneously by many patients with osteoarthritis [Bijlsma et al. 2011].
Osteoarthritis is a significant burden in terms of cost as well as health to society and individuals [Hiligsmann and Reginster 2013]. Although there is some variation in the direct medical cost of osteoarthritis in Europe, which ranged from €534 to €1788 per patient per year, total cost including indirect costs, such as hospitalization, diagnosis, and temporary caregivers, varied much more (from €1330 to €10,452 per patient per year). In Belgium, Italy, Holland, and Spain, indirect costs accounted for 60%, 57%, 83%, and 14% of total cost respectively [Hiligsmann and Reginster 2013]. Indirect costs were especially high for active patients with osteoarthritis. Hence, any improvement in management could be expected to translate into savings in healthcare budgets.
Treatment options in osteoarthritis
Osteoarthritis can be treated nonpharmacologically, pharmacologically, or surgically [Bijlsma et al. 2011; Hochberg 2012; Zhang et al. 2010]. Nonpharmacological treatments generally involve self-management interventions such as patient information, exercise, weight reduction, joint protection, and heat/cold. Self-management may have a positive impact on compliance with other interventions in the long term, but otherwise its effects are limited. Pharmacological treatment in osteoarthritis is currently limited to symptomatic management, including analgesia with paracetamol, oral or topical nonsteroidal anti-inflammatory drugs, or weak opiates. Intra-articular glucocorticoid is used to treat acute inflammatory episodes. Hyaluronic acid is also injected intra-articularly to alleviate knee pain in osteoarthritis. Slow-acting, symptom-modifying drugs, such as glucosamine sulfate and chondroitin sulfate, can be taken alongside these other drugs. When osteoarthritis becomes too painful or function too greatly impaired, surgery remains the only solution.
The therapeutic armamentarium remains poor in treatments with an effect on joint structure, the so-called disease-modifying osteoarthritis drugs (DMOADs). Many drugs with different modes of action have been tested in cartilage or bone for a disease-modifying effect in osteoarthritis, but a DMOAD has yet to receive approval in osteoarthritis. The search for DMOADs is therefore a great challenge, and one that would meet an important medical need for patients with osteoarthritis. The ultimate goal of pharmacological treatment is to avoid, or at least delay, surgery, and DMOADs stand the best chance of achieving this target.
One of the challenges in the field has been defining a proper framework for the evaluation of DMOAD activity. The regulatory authorities currently require clinical trials of DMOADs to demonstrate an impact on radiographic joint space narrowing (JSN) over at least 2 years [European Medicines Agency, 2010; US Food and Drug Administration, 1999; Reichmann et al. 2011]. JSN is deemed to be an appropriate primary endpoint and surrogate endpoint for total joint replacement [Conaghan et al. 2011]. DMOADs also need to have evidence for an impact on symptoms (pain and function) in phase III trials of at least 2 years’ duration.
Recent research has highlighted several promising avenues for DMOADs, including regeneration of cartilage and prevention of degradation. This review aims to focus on the latter and to describe the latest evidence for the use of oral strontium ranelate as a DMOAD in knee osteoarthritis.
Strontium ranelate
Randomized clinical trials in osteoporosis have shown that strontium ranelate reduces the relative risk of fractures, both vertebral and nonvertebral (including hip), in postmenopausal osteoporotic women [Meunier et al. 2004; Reginster et al. 2005]. The effects of strontium ranelate on bone mineral density, a widely used surrogate endpoint for fracture risk in osteoporosis, are similar in men with osteoporosis and postmenopausal women with osteoporosis [Kaufman et al. 2013]. Strontium ranelate is currently indicated in severe osteoporosis in men and women at high risk of fracture. The long-term safety of strontium ranelate in osteoporosis in women over a period of 10 years has been ascertained [Reginster et al. 2011].
Rationale for strontium ranelate in osteoarthritis
A large body of evidence in preclinical, molecular, cellular, and animal models shows that strontium ranelate may have an effect in osteoarthritis [Reginster et al. 2013b]. As previously mentioned, our current understanding of osteoarthritis is that it is a disease of the whole joint, not just cartilage. As such, drugs that have an effect on any joint tissue may be promising for the treatment of osteoarthritis [Bijlsma et al. 2011; Cooper et al. 2012]. Modification of subchondral bone occurs early in osteoarthritis, and both subchondral bone structure and function are affected [Tat et al. 2010]. Pathological changes in the vasculature of subchondral bone may be responsible for disturbing normal blood flow, reducing nutrient availability for articular cartilage, or causing ischemia-induced articular damage [Findlay, 2007].
Preclinical studies have shown that strontium ranelate enhances preosteoblast replication and promotes osteoblastic differentiation, and inhibits osteoclastic activity [Marie, 2007]. Strontium ranelate’s effects on bone remodeling have been linked to activation of calcium-sensing receptors [Brennan et al. 2009; Chattopadhyay et al. 2007], which are expressed by osteoclasts, osteoblasts, and osteocytes, as well as by chondrocytes [Chang et al. 1999]. Strontium ranelate’s antiresorptive action appears to be linked to this and also to regulation of the osteoprotegerin/receptor activator of nuclear factor kappa B (RANK)/RANK ligand (OPG/RANK/RANKL) system [Atkins et al. 2009; Brennan et al. 2009]. These positive effects on bone made strontium ranelate an interesting candidate for the treatment of osteoarthritis.
In addition, the increased synthesis of OPG by strontium ranelate can have an indirect beneficial effect in osteoarthritis [Narmazi et al. 2012], and strontium ranelate stimulated the production of insulin-like growth factor 1 (IGF-1) and boosted the stimulating effect of IGF-1 on proteoglycan synthesis in chondrocytes in a human model [Gulhan et al. 2008; Narmazi et al. 2012; Reginster et al. 2013b]. Strontium ranelate may also inhibit the resorption of subchondral bone by modulating matrix metalloproteinases (MMP-2 and MMP-9), which degrade type I collagen, fibronectin, and aggrecans [Tat et al. 2011].
There have been a number of preclinical studies that have demonstrated that strontium ranelate prevents the resorption of subchondral bone, by regulating the secretion of biochemical messengers by osteoblasts, and rebuilds cartilage, by correcting the disequilibrium between cartilage production and degradation [Henrotin et al. 2001; Tat et al. 2011]. In early osteoarthritis, resorption of subchondral bone is a dominant feature. As osteoarthritis progresses, sclerosis becomes evident [Tat et al. 2010]. A wide variety of cells, factors, and systems affect subchondral bone metabolism in osteoarthritis: mesenchymal cells; Wnt (Wingless-related integration site); IGF and transforming growth factor β1; and the OPG/RANK/RANKL and ephrin systems. Many of these subchondrally produced factors also affect cartilage catabolism [Tat et al. 2010].
Other indications that strontium ranelate may have an effect in osteoarthritis came from post hoc analyses of the phase III osteoporosis trials. The effect of strontium ranelate on cartilage was analyzed in 2617 postmenopausal women with osteoporosis participating in the TROPOS (Treatment of Peripheral Osteoporosis) trial [Alexandersen et al. 2007, 2011], who had had urinary sampling at every visit during the 3-year study. Of these, 22% (565 patients) had baseline symptoms of osteoarthritis. The patients in the strontium ranelate treatment group had 15–20% lower levels of a urinary biomarker of cartilage degradation (type II collagen C telopeptide neoepitope) than patients in the placebo group (p < 0.0001 versus placebo). The effect could be detected after 3 months and lasted over 3 years. Interestingly, it did not depend on osteoarthritis status at baseline.
An analysis of 1105 postmenopausal patients with osteoporosis and radiological spinal osteoarthritis was also made using a pool of the participants in the SOTI (Spinal Osteoporosis Therapeutic Intervention) or TROPOS trials [Bruyère et al. 2008]. All the patients in this analysis had lumbar X-rays available at baseline and over 3 years of treatment. For each intervertebral space, an overall osteoarthritis score [Lane et al. 1993] was calculated, accounting for the presence of osteophytes, disc space narrowing, and sclerosis in the lumbar intervertebral spaces. The results over 3 years showed a 42% lower overall osteoarthritis score versus placebo (p = 0.0005) and a 33% reduction in disc space narrowing score (p = 0.03) with strontium ranelate. The number of patients free of back pain also increased by 34% versus placebo (p = 0.03) [Bruyère et al. 2008].
SEKOIA
The results of the preclinical, molecular, and cellular studies combined with observations made in post hoc analyses of the osteoporosis trials provide a strong rationale for a possible disease-modifying effect of strontium ranelate in osteoarthritis. It was with this in mind that the investigators of SEKOIA (Strontium Ranelate Efficacy in Knee Osteoarthritis Trial) set up a study to explore the efficacy and safety of strontium ranelate as a DMOAD in patients with knee osteoarthritis [Cooper et al. 2012].
Study design
The rationale, design, and methods of SEKOIA have already been described in detail [Cooper et al. 2012; Reginster et al. 2013a]. Briefly, outpatients with knee osteoarthritis, most from secondary care, were recruited for this 3-year-long international, multicenter, randomized, double-blind, placebo-controlled phase III trial. White men and women aged at least 50 years with knee osteoarthritis, as defined by the American College of Rheumatology [Altman et al. 1986], who were mobile but had been in pain [> 40 mm on a visual analogue scale (VAS)] for at least half of the days in the previous month, were eligible for inclusion. Patients also had to be Kellgren and Lawrence grade 2 or 3 on radiographic examination [Kellgren and Lawrence 1963], and to have a joint space width (JSW) of 2.5–5 mm with predominant knee osteoarthritis in the medial tibiofemoral compartment. Knee prosthesis, recent intra-articular injection (especially glucocorticoids < 3 months before), clinical deformations, secondary knee osteoarthritis, previous treatments affecting cartilage or bone metabolism, and venous thromboembolism were all exclusion criteria.
SEKOIA patients were randomly allocated to one of three groups (strontium ranelate 1 g/day, strontium ranelate 2 g/day, or placebo) at inclusion. The study dose was one sachet of treatment with water at bedtime at least 2 h after food. Physiotherapy, rehabilitation, alternative medicines, and pain relief were permitted. Concomitant treatment with chondroitin, glucosamine sulfate ≥1500 mg, bisphosphonates, or glucocorticoids was not.
A SynaFlexer positioning frame (Synarc Inc., San Francisco, California, USA) was used to take radiographs of both knees in a fixed semi-flexion posterioanterior view (fixed angle 10°) for the target knee at selection and once a year or upon withdrawal [Cooper et al. 2012; Kothari et al. 2004]. A standardized computer-assisted method was used to assess minimum JSW (mm) annually in the medial tibiofemoral compartment [Gensburger et al. 2009]. Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and VAS global knee pain were also assessed at inclusion and every 6 months. A total WOMAC score and pain, stiffness, and physical function WOMAC subscores indicate health and outcomes in osteoarthritis [Bellamy et al. 1988]. Adverse events and clinical and laboratory parameters were recorded.
SEKOIA evaluated radiographic change in JSW over 3 years (primary endpoint), as well as radiological progression, radioclinical progression, WOMAC scores, and global knee pain (secondary endpoints). Radiological progression was defined as JSN at least 0.5 mm over 3 years, and radioclinical progression as JSN at least 0.5 mm plus lack of improvement in WOMAC pain (≤20%) over 3 years.
Strontium ranelate affects structure and symptoms in knee osteoarthritis
SEKOIA showed that treatment with both strontium ranelate 1 g/day and 2 g/day was associated with a beneficial structure-modifying effect in patients with knee osteoarthritis, while strontium ranelate 2 g/day also had a beneficial effect on osteoarthritic symptoms [Reginster et al. 2013a].
Of the 1683 included patients (n = 558, strontium 1 g/day; n = 566, strontium 2 g/day; and n = 559, placebo), 1371 (82%) were included in the intention-to-treat set; they were treated for a mean duration of 29.8 ± 10.5 months. At baseline, patients had had knee osteoarthritis for a mean duration of 77 ± 78 months and mean age was 62.9 ± 7.5 years. The predominantly female population (70%) was generally overweight (mean body mass index 29.9 ± 5.5 kg/m2). Nearly two-thirds (62%) were Kellgren and Lawrence grade 2, and over a third (38%) were grade 3. Mean JSW of the target knee at baseline was 3.50 ± 0.84 mm. At inclusion, over three-quarters (76%) declared a musculoskeletal or connective tissue disorder, and over two-thirds (68%) were taking medication for osteoarthritis. During the study, over three-quarters (76%) of patients received a concomitant treatment for osteoarthritis, mainly anilides (39%), propionic acid-related treatments (25%), or acetic acid derivatives (18%) [Reginster et al. 2013a].
Over 3 years, treatment with strontium ranelate was associated with significantly less radiological osteoarthritis progression (JSN: −0.23 ± 0.56 mm, 1 g/day; −0.27 ± 0.63 mm, 2 g/day) than placebo (−0.37 ± 0.59 mm) (Figure 1) [Reginster et al. 2013a]. Estimates for the annual difference in JSN versus placebo were 0.14 mm (95% CI 0.05–0.23 mm; p < 0.001) for strontium ranelate 1 g/day and 0.10 mm (95% CI 0.02–0.19 mm; p = 0.018) for strontium ranelate 2 g/day, with no difference between the strontium ranelate groups. Radiological progression was less frequent with strontium ranelate (22% with 1 g/day and 26% with 2 g/day versus 33% with placebo, both p < 0.05) (Figure 2), as was radioclinical progression (8% and 7% versus 12%, both p < 0.05), and this was evident throughout the study [Reginster et al. 2013a].
Figure 1.
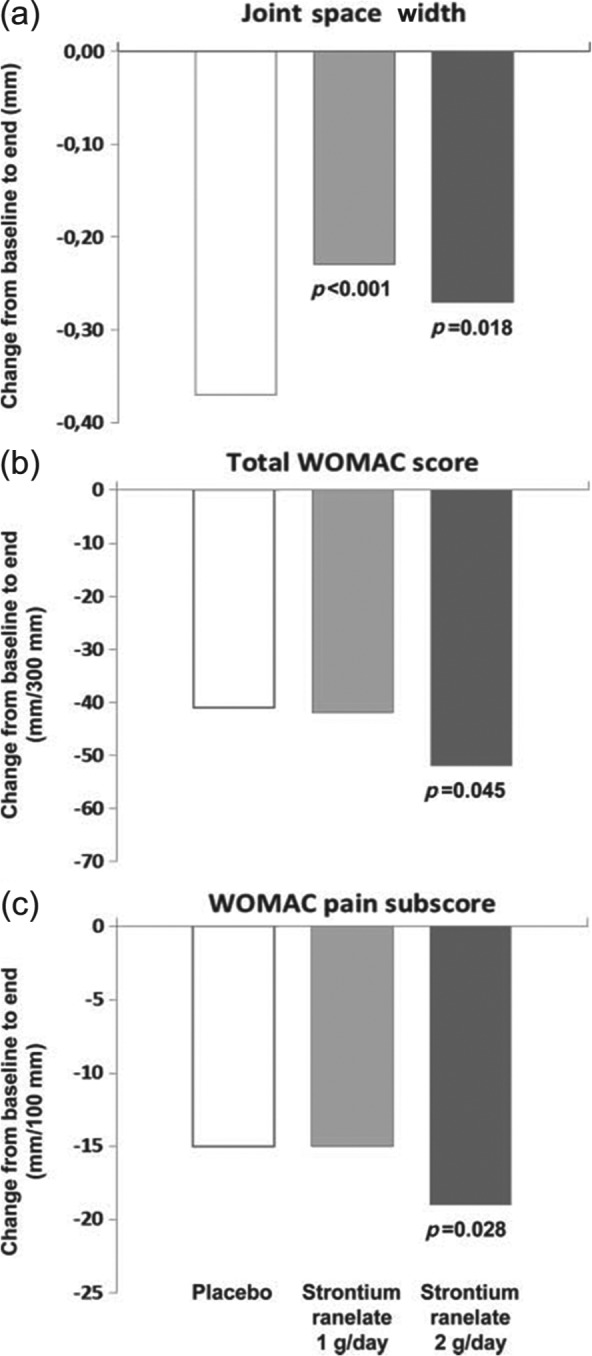
Effect of strontium ranelate on outcomes in knee osteoarthritis from baseline to end. (a) Change in joint space width (mm). (b) Change in total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score. (c) Change in WOMAC pain subscore. p Values are presented versus placebo. Reproduced from Reginster et al. [2013a].
Figure 2.
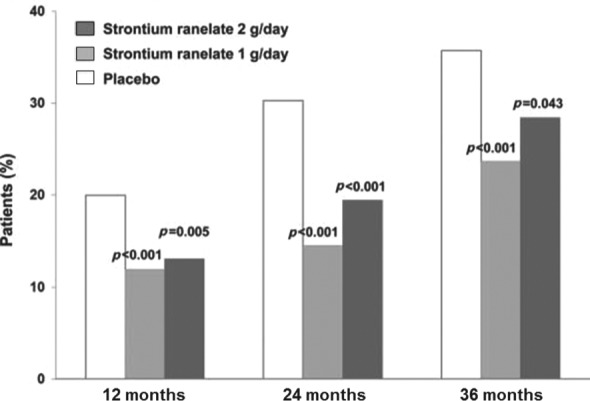
Impact of strontium ranelate on knee osteoarthritis progression: percentage of patients who were radiological progressors [joint space narrowing (JSN) ≥0.5 mm versus baseline] at 12, 24, and 36 months. p Values are presented versus placebo. Modified from Reginster et al. [2013a].
A post hoc analysis of SEKOIA has further explored the notion of progression or nonprogression [Cooper et al. 2013]. In this analysis, patients with no progression of osteoarthritis were defined as those with JSN up to 0.1 mm, up to 0.2 mm, or up to 0.3 mm (i.e. those with JSN ≤ 0.1 mm have the least progression of disease). When the JSN up to 0.3 mm definition was used, there was a significant difference in the proportion of patients in the 1 g/day and 2 g/day groups with no radiological progression compared with placebo (41% and 44% versus 33%, both p < 0.01) (Figure 3). Similar results were obtained when the thresholds of JSN up to 0.1 mm and JSN up to 0.2 mm were used, with nearly 30% of patients with no progression with strontium ranelate.
Figure 3.
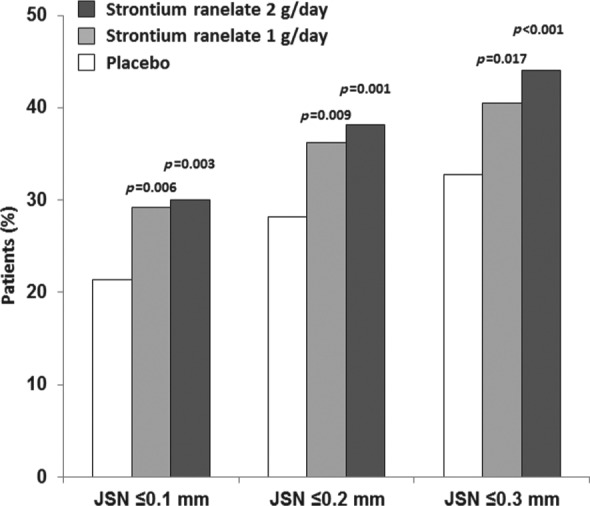
Impact of strontium ranelate on knee osteoarthritis progression: percentage of patients who were radiological nonprogressors at 36 months [joint space narrowing (JSN) ≤0.1, ≤0.2, and ≤0.3 mm versus baseline]. p Values are presented versus placebo. Modified from Cooper et al. [2013].
For strontium ranelate 2 g/day, in addition to a positive effect on structure, there was also a significant reduction in symptoms (total WOMAC score) versus placebo (E (difference), −8.0 mm; 95% CI −15.7 to −0.2 mm; p = 0.045), with significant reductions in the WOMAC pain subscore (E (difference), −3.0 mm; 95% CI −5.6 to −0.3 mm; p = 0.028) and physical function subscore (E (difference), −2.2 mm; 95% CI −4.7 to −0.4 mm; p = 0.099) (Figure 1) [Reginster et al. 2013a].
Strontium ranelate was well tolerated. There were no cases of drug reaction with eosinophilia and systemic symptoms, and the rate of venous thromboembolic events was less than 1% in all groups. A similar incidence of cardiac events was reported in the three treatment groups (5.5%, 5.7%, and 5.8% in the 1 g/day, 2 g/day, and placebo groups respectively).
There were a number of limitations to the SEKOIA study [Reginster et al. 2013a]. The relatively high dropout rate (14% per year) was similar to other trials in osteoarthritis, and was mostly for nonmedical reasons and unrelated to treatment. This was anticipated in the sample size calculations and did not affect the power of the trial. The difficulties associated with an elevated placebo response and with recording the magnitude of changes in pain are common to all trials in osteoarthritis, or indeed any trial involving subjective changes. For this reason, the effect of strontium ranelate on symptoms was explored in more depth using responder analyses, which are described in the next section.
Clinical improvement of knee osteoarthritis with strontium ranelate
Results from clinical trials are not always simple to translate into clinically meaningful improvements for patients. This is especially the case for subjective symptoms like osteoarthritic pain and stiffness. Fortunately, there are a number of responder criteria available to assess response to treatment, including thresholds based on the WOMAC scale [Bellamy et al. 2005], thresholds developed by the Outcome Measures in Rheumatoid Arthritis Clinical Trials/Osteoarthritis Research Society International (OMERACT-OARSI) [Pham et al. 2004], and thresholds for Minimal Perceptible Clinical Improvement (MPCI) [Ehrich et al. 2000] or Minimal Clinically Important Improvement (MCII) [Tubach et al. 2005]. Clinical response to strontium ranelate has been determined using MPCI, MCII, and a set of responder criteria based on the OMERACT-OARSI criteria (which had been modified since SEKOIA did not include a global assessment). These results have been published in the form of an abstract [Bruyère et al. 2013] and demonstrated that, after 3 years, there was a significant reduction in WOMAC pain subscore of at least 20% in patients on strontium ranelate 2 g/day versus placebo (p < 0.01) and a trend toward a difference in the percentage of patients reaching a reduction in the WOMAC pain subscore of at least 50% (p = 0.083). The percentage of OARSI-OMERACT-like responders also increased significantly with strontium ranelate 2 g/day compared with placebo (p < 0.01) and a greater proportion of MPCI and MCII responders was observed.
Conclusion
The results from SEKOIA indicate that pharmacological structure modification is possible in knee osteoarthritis. Treatment with strontium ranelate 2 g/day was associated with a significant effect on structure, with a smaller degradation of JSW over 3 years (p = 0.018) compared with placebo. There was also a beneficial concomitant effect on symptoms, with a significant impact on the WOMAC total score and pain subscore (p = 0.045 and 0.028, respectively). Future results from SEKOIA substudies should also shed new light on the action of strontium ranelate in osteoarthritis. An analysis of the effect of strontium ranelate on knee osteoarthritis progression in an MRI subset of SEKOIA is ongoing using the Whole-Organ Magnetic Imaging Resonance Score (WORMS) method [Peterfy et al. 2004], and the effect of strontium ranelate on loss of cartilage volume and bone marrow lesions is also being studied in SEKOIA patients using quantitative MRI.
SEKOIA has been hailed as a milestone in osteoarthritis research [Lafeber and van Laar 2013]. It is likely to lead to further research to consolidate the potential of strontium ranelate in osteoarthritis and also to stimulate new research into other treatment options for this debilitating disease.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Jean-Yves Reginster, Department of Public Health and Health Economics, University of Liege, 4020 Liege, Belgium.
Charlotte Beaudart, Department of Public Health and Health Economics, University of Liege, Liege, Belgium.
Audrey Neuprez, Department of Public Health and Health Economics, University of Liege, Liege, Belgium.
Olivier Bruyère, Department of Public Health and Health Economics, University of Liege, Liege, Belgium.
References
- Alexandersen P., Karsdal M., Byrjalsen I., Christiansen C. (2011) Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric 14: 236–243 [DOI] [PubMed] [Google Scholar]
- Alexandersen P., Karsdal M., Qvist P., Reginster J., Christiansen C. (2007) Strontium ranelate reduces the urinary level of cartilage degradation biomarker CTX-II in postmenopausal women. Bone 40: 218–222 [DOI] [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., et al. (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29: 1039–1049 [DOI] [PubMed] [Google Scholar]
- Atkins G., Welldon K., Halbout P., Findlay D. (2009) Strontium ranelate treatment of human primary osteoblasts promotes an osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporos Int 20: 653–664 [DOI] [PubMed] [Google Scholar]
- Bellamy N., Bell M., Goldsmith C., Pericak D., Walker V., Raynauld J., et al. (2005) Evaluation of WOMAC 20, 50, 70 response criteria in patients treated with hylan G-F 20 for knee osteoarthritis. Ann Rheum Dis 64: 881–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N., Buchanan W., Goldsmith C., Campbell J., Stitt L. (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15: 1833–1840 [PubMed] [Google Scholar]
- Bijlsma J., Berenbaum F., Lafeber F. (2011) Osteoarthritis: an update with relevance for clinical practice. Lancet 377: 2115–2126 [DOI] [PubMed] [Google Scholar]
- Brennan T., Rybchyn M., Green W., Atwa S., Conigrave A., Mason R. (2009) Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol 157: 1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyère O., Delferriere D., Roux C., Wark J., Spector T., Devogelaer J., et al. (2008) Effects of strontium ranelate on spinal osteoarthritis progression. Ann Rheum Dis 67: 335–339 [DOI] [PubMed] [Google Scholar]
- Bruyère O., Richette P., Bellamy N., Brown J., Chapurlat R., Chevalier X., et al. (2013) Strontium ranelate improves osteoarthritis symptoms compared to placebo in patients with knee osteoarthritis – the SEKOIA study. Osteoporos Int 24(Suppl. 1): OC27 [Google Scholar]
- Chang W., Tu C., Chen T., Komuves L., Oda Y., Pratt S., et al. (1999) Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140: 5883–5893 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N., Quinn S., Kifor O., Ye C., Brown E. (2007) The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem Pharmacol 74: 438–447 [DOI] [PubMed] [Google Scholar]
- Conaghan P., Hunter D., Maillefert J., Reichmann W., Losina E. (2011) Summary and recommendations of the OARSI FDA osteoarthritis Assessment of Structural Change Working Group. Osteoarthritis Cartilage 19: 606–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C., Berenbaum F., Nash P., Zamani O., Cohen-Solal M., Bianchi G., et al. (2013) Strontium ranelate prevents radiological progression in patients with primary knee osteoarthritis. Osteoporos Int 24(Suppl. 1): P581 [Google Scholar]
- Cooper C., Reginster J., Chapurlat R., Christiansen C., Genant H., Bellamy N., et al. (2012) Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of a randomised double-blind, placebo-controlled trial. Curr Med Res Opin 28: 231–239 [DOI] [PubMed] [Google Scholar]
- Ehrich E., Davies G., Watson D., Bolognese J., Seidenberg B., Bellamy N. (2000) Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 27: 2635–2641 [PubMed] [Google Scholar]
- European Medicines Agency (2010) Guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis. Available at: http://www.ema.europa.eu (accessed 25 May 2013).
- Felson D., Chaisson C., Hill C., Totterman S., Gale M., Skinner K., et al. (2001) The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med 134: 541–549 [DOI] [PubMed] [Google Scholar]
- Felson D., Niu J., Guermazi A., Roemer F., Aliabadi P., Clancy M., et al. (2007) Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 56: 2986–2992 [DOI] [PubMed] [Google Scholar]
- Findlay D. (2007) Vascular pathology and osteoarthritis. Rheumatology (Oxford) 46: 1763–1768 [DOI] [PubMed] [Google Scholar]
- Gensburger D., Arlot M., Sornay-Rendu E., Roux J., Delmas P. (2009) Radiologic assessment of age-related knee joint space changes in women: a 4-year longitudinal study. Arthritis Rheum 61: 336–343 [DOI] [PubMed] [Google Scholar]
- Gulhan I., Bilgili S., Gunaydin R., Gulhan S., Posaci C. (2008) The effect of strontium ranelate on serum insulin like growth factor-1 and leptin levels in osteoporotic post-menopausal women: a prospective study. Arch Gynecol Obstet 278: 437–441 [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Labasse A., Zheng S., Galais P., Tsouderos Y., Crielaard J., et al. (2001) Strontium ranelate increases cartilage matrix formation. J Bone Miner Res 16: 299–308 [DOI] [PubMed] [Google Scholar]
- Hiligsmann M., Reginster J. (2013) The economic weight of osteoarthritis in Europe. Medicographia 35: 197–202 [Google Scholar]
- Hochberg M. (2012) Osteoarthritis year 2012 in review: clinical. Osteoarthritis Cartilage 20: 1465–1469 [DOI] [PubMed] [Google Scholar]
- Jordan J., Helmick C., Renner J., Luta G., Dragomir A., Woodard J., et al. (2007) Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 34: 172–180 [PubMed] [Google Scholar]
- Karsdal M., Leeming D., Dam E., Henriksen K., Alexandersen P., Pastoureau P., et al. (2008) Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage 16: 638–646 [DOI] [PubMed] [Google Scholar]
- Kaufman J., Audran M., Bianchi G., Braga V., Diaz-Curiel M., Francis R., et al. (2013) Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab 98: 592–601 [DOI] [PubMed] [Google Scholar]
- Kellgren J., Lawrence J. (1963) The Epidemiology of Chronic Rheumatism: Atlas of Standard Radiographs, 2nd edition. Oxford, UK: Blackwell Scientific [Google Scholar]
- Kothari M., Guermazi A., von Ingersleben G., Miaux Y., Sieffert M., Block J., et al. (2004) Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol 14: 1568–1573 [DOI] [PubMed] [Google Scholar]
- Kwan T., Lajeunesse D., Pelletier J., Martel-Pelletier J. (2010) Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol 24: 51–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeber F., van Laar J. (2013) Strontium ranelate: ready for clinical use as disease-modifying osteoarthritis drug? Ann Rheum Dis 72: 157–161 [DOI] [PubMed] [Google Scholar]
- Lane N., Brandt K., Hawker G., Peeva E., Schreyer E., Tsuji W., et al. (2011) OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 19: 478–482 [DOI] [PubMed] [Google Scholar]
- Lane N., Nevitt M., Genant H., Hochberg M. (1993) Reliability of new indices of radiographic osteoarthritis of the hand and hip and lumbar disc degeneration. J Rheumatol 20: 1911–1918 [PubMed] [Google Scholar]
- Lo G., McAlindon T., Niu J., Zhang Y., Beals C., Dabrowski C., et al. (2009) Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 17: 1562–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie P. (2007) Strontium ranelate: New insights into its dual mode of action. Bone 40: S5-S8 [Google Scholar]
- Meunier P., Roux C., Seeman E., Ortolani S., Badurski J., Spector T., et al. (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468 [DOI] [PubMed] [Google Scholar]
- Narmazi H., Cooper C., Reginster J. (2012) Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of randomised, double-blind, placebo-controlled trial: Letter to the Editor and Author’s response. Curr Med Res Opin 28: 609–610 [DOI] [PubMed] [Google Scholar]
- Neogi T. (2012) Clinical significance of bone changes in osteoarthritis. Ther Adv Musculoskelet Dis 4: 259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuesch E., Dieppe P., Reichenbach S., Williams S., Iff S., Juni P. (2011) All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 342: d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterfy C., Guermazi A., Zaim S., Tirman P., Miaux Y., White D., et al. (2004) Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 12: 177–190 [DOI] [PubMed] [Google Scholar]
- Pham T., Van der Heijde D., Altman R., Anderson J., Bellamy N., Hochberg M., et al. (2004) OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage 12: 389–399 [DOI] [PubMed] [Google Scholar]
- Picavet H., van Gils H.W.V., Schouten J. (2000) Musculoskeletal complaints in the Dutch population: prevalences, consequences, and risk groups [in Dutch]. Bilthoven, The Netherlands: CBS/RIVM; RIVM report number: 266807002. [Google Scholar]
- Reginster J., Badurski J., Bellamy N., Bensen W., Chapurlat R., Chevalier X., et al. (2013a) Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised, placebo-controlled trial. Ann Rheum Dis 72: 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J., Kaufman J., Goemaere S., Devogelaer J., Benhamou C., Felsenberg D., et al. (2011) Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos Int 23: 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J., Pelousse F., Bruyère O. (2013b) Is there potential for strontium ranelate in the management of osteoarthritis? Clin Pract 10: 201–207 [Google Scholar]
- Reginster J., Seeman E., De Vernejoul M., Adami S., Compston J., Phenekos C., et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90: 2816–2822 [DOI] [PubMed] [Google Scholar]
- Reichmann W., Maillefert J., Hunter D., Katz J., Conaghan P., Losina E. (2011) Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: a systematic review. Osteoarthritis Cartilage 19: 550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tat S., Lajeunesse D., Pelletier J., Martel-Pelletier J. (2010) Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol 24: 51–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tat S., Pelletier J., Mineau F., Caron J., Martel-Pelletier J. (2011) Strontium ranelate inhibits key factors affecting bone remodelling in human osteoarthritic subchondral bone osteoblasts. Bone 49: 559–567 [DOI] [PubMed] [Google Scholar]
- Tubach F., Ravaud P., Baron G., Falissard B., Logeart I., Bellamy N., et al. (2005) Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 64: 29–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration (1999) Guidance for industry. Clinical development programs for drugs, devices, and biological products intended for the treatment of osteoarthritis. http://www.fda.gov (accessed 27 May 2013).
- van Dijk G., Veenhof C., Schellevis F., Hulsmans H., Bakker J., Arwert H., et al. (2008) Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee. BMC Musculoskelet Disord 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Saase J., van Romunde L., Cats A., Vandenbroucke J., Valkenburg H. (1989) Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann Rheum Dis 48: 271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf E., Kortekaas M., Watt I., Huizinga T., Kloppenburg M. (2011) Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 70: 60–67 [DOI] [PubMed] [Google Scholar]
- Zhang W., Nuki G., Moskowitz R., Abramson S., Altman R., Arden N., et al. (2010) OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 18: 476–499 [DOI] [PubMed] [Google Scholar]


