Abstract
The present study has used electron microscopic techniques to rapidly detect the success or failure of bone marrow transplantation in three patients with the Chediak-Higashi Syndrome (CHS). The most rapid procedure was the whole mount technique to determine the presence or absence of dense bodies, which are inherently electron-opaque, serotonin-containing storage organelles in platelets. Dense bodies were present in normal numbers in platelets from two patients with successful transplantation and absent in thrombocytes from another patient in whom the transplant had failed.
Keywords: Chediak-Higashi Syndrome, bone marrow transplantation, ultrastructural techniques
Introduction
The Chediak-Higashi syndrome(CHS) [1–4] is a rare, autosomal recesive disorder characterized clinically by photophobia, horizontal nystagmus, hypopigmentation, marked susceptibility to infection, and hemophagocytic lymphohistiocytosis, known as the accelerated phase [5–12]. Laboratory diagnosis is based on the presence of giant organelles in nearly all leukocytes on Wright-stained peripheral blood smears. The giant granules have been found in neutrophils, eosinophils, basophils, monocytes and lymphocytes. Giant granules have also been observed in platelets, but their presence is rare [13].
A more common platelet abnormality in CHS patients is the virtual absence of the serotonin-rich [14], electron-opaque dense bodies [15] referred to as δ granules. As a result, storage pool deficiency due to near absence of δ granules is a characteristic feature of CHS.
Due to severe infections and the accelerated phase of the disease, CHS results in the death of most patients in childhood [5–12]. Treatment of infections and malignancy have not improved patient survival, but bone marrow transplantation has eliminated the risk of life-threatening infections and hemophagocytic lymphohistiocytosis; it has become the treatment of choice for CHS. It is important to determine if the transplantation has been successful or a failure in order to treat with transfusion therapy and schedule additional transplantation procedures.
The present investigation has used transmission electron microscopy to study blood from three HPS patients after bone marrow transplantation whom we had evaluated at the time of their initial diagnosis. Whole mount preparations of their platelets and thin sections of their plastic embedded white blood cells were studied. The findings indicate that ultrastructural techniques can rapidly and accurately determine the success or failure of transplantation in the CHS.
Methods
Patients
All patients were reenrolled in NIH Clinical Protocol 00-HG-0153, “Investigations into Chediak-Higashi Syndrome and Related Disorders.” They or their parents gave written informed consent.
The ultrastructure of white blood cells and platelets from the three patients had been evaluated in the electron microscope before transplantation.
Patient #1 was 3 years old at the time of transplant from an unrelated, matched donor. She is now 21.
Patient #2 was 20 months old at the time of first transplant, 24 months at the time of a rescue transplant and 25 months at the time of the last transplant. The donor was an unrelated, matched donor. She is now 5 years of age.
Patient #3 was transplanted initially at 10 months of age. He received his second transplant at 18 months of age, and is currently awaiting his third transplant.
Platelet electron microscopy
Venous blood was mixed immediately with citrate – citric acid-dextrose, pH 6.5, or 3.8% trisodium citrate in a ratio of 9 parts blood to 1 part anticoagulant and placed in a 37° C water bath for 30 min before further processing. Tubes of blood were centrifuged at 200 × g for 20 min at room temperature to obtain platelet-rich plasma (PRP) [16, 17]. A drop of PRP was placed on a formvar coated,300 mesh grid. After 10 seconds the drop of PRP was washed off the grid with distilled water. A wedge of filter paper was used to remove residual water, and the grid waved in the air to complete the drying process. The grid was introduced into the electron microscope without fixation or staining.
Leucocyte electron microscopy
To obtain leukocyte-rich buffy coats, 5 ml of whole blood was transferred to a 13 × 100-mm polystyrene tube and centrifuged at 200× g for 10 min. PRP above the buffy coat was carefully removed and replaced with 0.1% glutaraldehyde in White's saline [18]. After 15 min the fixative was replaced with 3.0% glutaraldehyde in White's saline for 45 min. At that time the solidified buffy coat was removed intact, cut into small pieces, added to fresh 3.0% glutaraldehyde in White's saline and maintained overnight at 4° C. The buffy coat fragments were then rinsed in chilled Hank's balanced salt solution (HBSS) and placed in either cold 1.0% osmic acid in distilled water containing 0.5% potassium ferrocyanide for 1 h. After fixation the samples were dehydrated in a graded series of alcohols, embedded in epon, sectioned, stained with uranyl acetate and lead citrate and examined in a Philips 301 electron microscope.
Results
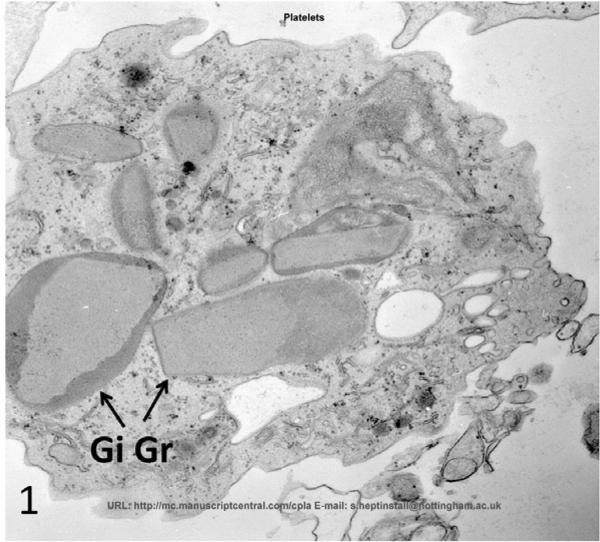
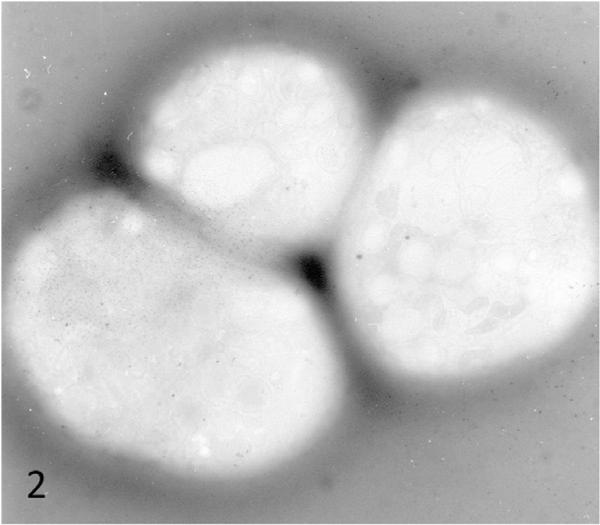
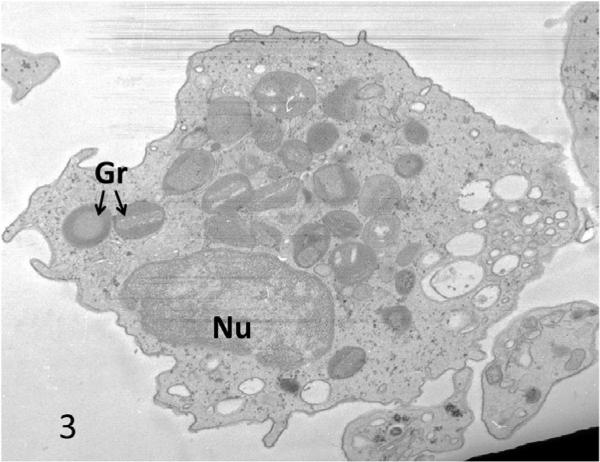
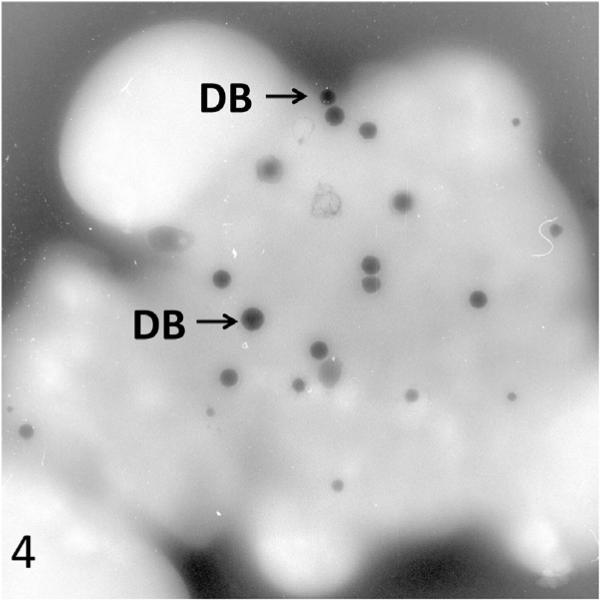
At the time of diagnosis for each patient, the ultrastructure of white blood cells (WBC) examined in thin sections and platelets by the whole mount technique revealed characteristic features of the Chediak-Higashi Syndrome (CHS) [1–4]. White cells contained giant lysosomal organelles in various stages of development (Figure 1). The 2 to 8 inherently electron opaque, serotonin rich dense bodies in normal platelets were absent, or nearly so, in CHS platelets (Figure 2). Whitecells in patients with successful bone marrow transplantation had changed completely. Giant lysosomes had disappeared from all leucocytes (Figure 3). Whole mounts of their platelets revealed normal numbers of dense bodies (Figures 4 and 5).
Figure 1.
Eosinophil from patient #1 with CHS prior to bone marrow transplantation. GiGr are a characteristic finding in all white blood cells. Mag× 16,800
Figure 2.
Whole mount of blood platelets from CHS patient #1 prior to transplantation. Dense bodies are essentially absent. Mag× 10,000
Figure 3.
Eosinophil from CHS patient #2 after successful bone marrow transplantation the cytoplasm is filled with normal sized granules. Mag× 16,800
Figure 4.
Platelets from a CHS patient #2 viewed in whole mount after successful transplantation. The cells are filled with normal dense bodies. Mag× 13,000
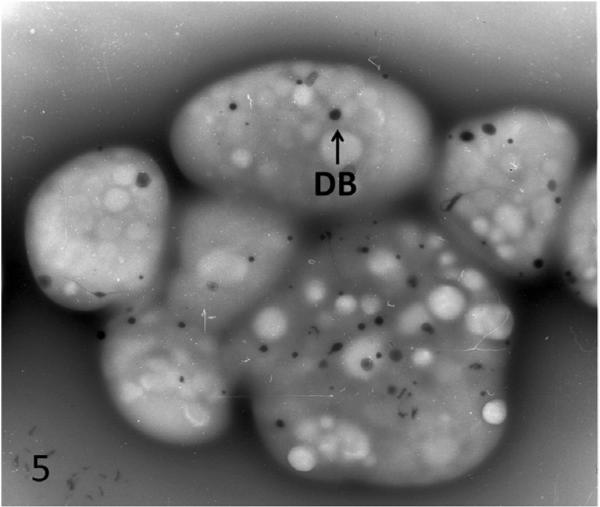
Figure 5.
Platelets from normal individual viewed in whole mount. Platelets contain numerous dense bodies like patient in Figure 4.
The leucocytes from the patient whose transplant had been unsuccessful contained the giant lysosomes seen in his diagnostic work up, and his platelets were devoid of dense bodies (data not shown).
Discussion
The present study has shown that electron microscopy can be used to detect the success or failure of bone marrow transplantation in the treatment of patients with the Chediak-Higashi Syndrome (CHS). Absence of the giant lysosomal organelles in circulating white blood cells from transplanted patients was one of the diagnostic features of success. However, the lack of giant organelles in white blood cells could have been observed by light microscopy on Wright stained blood smears several days earlier than thin sections could be cut from plastic embedded buffy coats for EM evaluation.
The same cannot be said for the reappearance of dense bodies in platelets from successfully transplanted CHS patients. A small drop of platelet-rich plasma placed on a plastic coated EM grid, dried and inserted into the electron microscope will give an immediate answer as to whether dense bodies have returned to circulating thrombocytes after transplantation. Dense bodies are beyond the resolution of the light microscope. Thus EM is a rapid technique for determining the success or failure of bone marrow transplantation in patients with CHS within minutes after arrival of the patient blood sample.
The return of a normal population of dense bodies to platelets raises an important scientific question. Dense bodies form in megakaryocytes and leave the bone marrow on delivery of platelets to the circulation. They are rich in serotonin, adenine nucleotides and calcium, but do not contain lysosomal enzymes, so they are not lysosomes. Why do platelets containing dense bodies fail to be delivered from megakaryocytes of patients with CHS? Perhaps they are formed, but platelets containing them do not reach the circulation. Further work will be required to answer this question.
In summary the present study has shown that ultrastructural methods can be used to rapidly detect the success or failure of bone marrow transplantation in patients with the CHS.
Footnotes
Declaration of interest: The authors are report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Bequez CA. Netropeniacronicamaligna familiar con granulacionesatipicas de los leucocitos. Bol Soc Cubana Pediat. 1943;15:900–922. [Google Scholar]
- 2.Steinbring W. Ubereineneue granulationsanomolie der leukocyten. Deutsch Arch Klin Med. 1948;193:577–581. [Google Scholar]
- 3.Higashi O. Congenital gigantism of peroxidase granules. Tohoku J Exp Med. 1954;59:315–332. doi: 10.1620/tjem.59.315. [DOI] [PubMed] [Google Scholar]
- 4.Chediak M. Nouvelle anomalieleucoctaire de caractereconstitutionnelet familial. Rev Hemat. 1962;7:362–367. [Google Scholar]
- 5.Erfrati P, Jonas W. Chediak's anomaly of leukocytes in malignant lymphoma associated with leukemic manifestations: Case report with necropsy. Blood. 1958;13:1063–1073. [PubMed] [Google Scholar]
- 6.Gilloon JR, Pease GL, Mills SD. Chediak-Higashi anomaly of the leukocytes: Report of a case. Proc Mayo Clin. 1960;35:635–640. [PubMed] [Google Scholar]
- 7.Kritzler RA, Terner JY, Lindenbaum J, Magidsan J, Williams R, Presig R, Philips GB. Chediak-Higashi syndrome. Cytologic and serum lipid observations in a case and family. Amer J Med. 1964;36:583–594. doi: 10.1016/0002-9343(64)90106-8. [DOI] [PubMed] [Google Scholar]
- 8.Bernard J, Bessis M, Seligman M, Chassigneux J, Chome J. Un cas de maladie de Chediak Steinbrink-Higashi. Etude Clinique et Cytologique Presse Med. 1960;68:563–566. [PubMed] [Google Scholar]
- 9.Wolff SM, Dale DC, Clark RA, Root RK, Kimball HR. The Chediak Higashi syndrome: Studies of host defenses. Ann Intern Med. 1972;76:293–306. doi: 10.7326/0003-4819-76-2-293. [DOI] [PubMed] [Google Scholar]
- 10.Blume RS, Wolff SM. The Chediak-Higashi syndrome: Studies in four patients and a review of the literature. Medicine. 1972;51:247–280. [PubMed] [Google Scholar]
- 11.Klebanoff SJ, Clark RA. Function and clinical disorders. North-Holland Publishing; New York: 1978. [Google Scholar]
- 12.Clawson CC, Repine JE, White JG. Chediak-Higashi syndrome: Quantitative defect in bactericidal capacity. Blood. 1971;38:814. [Google Scholar]
- 13.White JG. Disorders affecting megakaryocytes and platelets: Inherited conditions. In: Farwit A, McCullough J, Erver WN, editors. Blood and Bone Marrow Pathology. 2nd edn. Churchill Livingstone; Edinburgh: 2011. pp. 491–521. [Google Scholar]
- 14.Page AR, Berendes H, Warner J, Good RA. The Chediak-Higashi syndrome. Blood. 1962;20:330–343. [PubMed] [Google Scholar]
- 15.White JG. The dense bodies of human platelets: Inherent electron density of serotonin storage particles. Blood. 1969;33:598–606. [PubMed] [Google Scholar]
- 16.White JG. The morphology of platelet function. In: Harker LA, Zimmerman TS, editors. Methods in hematology, series 8L: Measurements of platelets function. Churchill-Livingstone; New York: 1983. pp. 1–25. [Google Scholar]
- 17.White JG. Interaction of membrane systems in blood platelets. Am J Pathol. 1972;66:295–312. [PMC free article] [PubMed] [Google Scholar]
- 18.White JG. A simple method for preservation of fine structure of blood cells. Thromb Diath Haemorrh. 1967;18:745–53. [PubMed] [Google Scholar]