Abstract
Children born very preterm (≤32 weeks gestation) show greater internalizing (anxious/depressed) behaviors compared to term-born peers as early as 2 years corrected age (CA), however, the role of early stress in the etiology of internalizing problems in preterm children remains unknown. Therefore, we examined the relationship between neonatal pain and internalizing behavior at 18 months CA in children born very preterm, and examined whether parent behavior and stress moderated this relationship. Participants were 145 children (96 very preterm, 49 full-term) seen at 18 months CA. Neonatal data were obtained from medical and nursing chart review. Neonatal pain was defined as the number of skin-breaking procedures. Cognitive ability was measured using the Bayley Scales of Infant Development-II. Parents completed the Parenting Stress Index-III, Child Behavior Checklist 1.5–5, and participated in a videotaped play session with their child, which was coded using the Emotional Availability Scale-IV. Very preterm children displayed greater Internalizing behaviors compared to full-term controls (P =0.02). Parent Sensitivity and Nonhostility moderated the relationship between neonatal pain and Internalizing behavior (all P <0.05); higher parent education (P <0.03), lower Parenting Stress (P =0.001), and fewer children in the home (P <0.01) were associated with lower Internalizing behavior in very preterm children, after adjusting for neonatal medical confounders, gender and child cognitive ability (all P >0.05). Parent Emotional Availability and stress were not associated with Internalizing behaviors in full-term controls. Positive parent interaction and lower stress appears to ameliorate negative effects of neonatal pain on stress-sensitive behaviors in this vulnerable population.
Keywords: Pain, Infant, Parent-Child Interaction, Stress, Behavior, Internalizing, Preterm, Prematurity, Mother-Child Interaction
1. Introduction
The majority of infants born very preterm (≤32 weeks gestational age [GA]) now survive due to advances in medical care, however their long-term neurodevelopmental outcomes, including problems with behavior, have not improved [34, 35, 43, 67]. Very preterm infants are exposed to numerous skin-breaking procedures in the neonatal intensive care unit (NICU). Greater procedural pain exposure has been associated with altered stress hormone (cortisol) regulation in children born at extremely low gestational age (ELGA; 24–28 weeks) compared to very low gestational age (VLGA; 29–32 weeks) [31, 33]. Furthermore, ELGA children demonstrate higher associations between cortisol expression and internalizing (anxious/depressed) behaviors at 18 months corrected age (CA) relative to VLGA children [15]. Greater internalizing behaviors in preterm children compared to full-term controls have been reported as early 2 years CA, persist to late adolescence, and appear to be independent of cognitive ability [1, 5, 9, 35, 45, 66]. Experimental animal models have also demonstrated that early stress can permanently reorganize hormonal, physiological and behavioral systems [47, 48, 54, 58]. While rat pups exposed to neonatal pain showed increased anxiety-mediated behavior during adulthood [4], the role of neonatal pain in the etiology of internalizing problems in preterm children is unknown.
Parents play a vital role in the regulation of stress and development of their infant [36]. However, the birth of a preterm infant is a highly stressful experience for parents [49, 50, 72]. Parenting stress in families with preterm infants has been found to be high during infant hospitalization [27, 57, 68], persisting well beyond discharge from the NICU [14, 26, 38, 63]. Moreover, parenting stress was found to be a significant and independent predictor of child internalizing behavior [73], and was associated with decreased parent emotional availability at 2 years CA in preterm children [74]. Emotional Availability (EA) is a construct characterizing a supportive caregiver whose authenticity of affect, appropriate responding (sensitivity/nonhostility/nonintrusiveness), and provision of guidance (structuring), increases their child’s autonomy [10]. It is important to consider parent’s level of stress together with parental emotional availability when examining behavior in preterm children.
Parent support to promote sensitive and responsive interactions during hospitalization appears to improve white matter maturation in infants born preterm [53]. This is important given that early alterations of white matter development have been associated with deficits in social-emotional behaviors at age 5 in preterm children [61]. While more positive parent interaction was found to buffer the relationship between neonatal pain and poorer focused attention at 8 months CA [70], the extent that parent EA moderates the relationship between neonatal pain and internalizing behavior remains unknown.
Therefore, we examined whether neonatal pain (adjusted for neonatal and medical confounders) is related to parent report of internalizing behaviors at 18 months CA, and whether parent EA (adjusted for parenting stress), moderates the relationship between neonatal pain and internalizing behaviors (adjusted for child cognition) in children born very preterm. Further, we examined the relationship between parent EA and internalizing in children born full-term. We hypothesized that greater parent EA would be associated with fewer internalizing behaviors at 18 months CA in children born very preterm exposed to greater neonatal pain.
2. Methods
This study was approved by the University of British Columbia/Children’s and Women’s Health Centre of British Columbia Research Ethics Board, and parents provided written informed consent.
2.1. Participants
Ninety-six infants born very preterm (≤32 weeks gestational age) and 49 full-term controls born at the B.C. Children’s & Women’s Hospitals between February 2001 and July 2004, were recruited as part of a larger ongoing study of the effects of neonatal pain on the neurodevelopment of infants born very preterm [15, 31, 32, 70]. Infants were excluded if they were born small or large for gestational age, had a major congenital anomaly, major neurosensory impairment (legally blind, non-ambulatory cerebral palsy, sensory-neural hearing impairment), severe brain injury evident on neonatal ultrasound (periventricular leucomalacia and/or grade 3 or 4 intraventricular hemorrhage), or if the mother reported use of illicit drugs during pregnancy. All full-term infants in our study were born healthy, and none were under observation for medical complications. Ninety-four mothers and 2 fathers of children born very preterm, and 47 mothers and 2 fathers of children born full-term participated in the study at 18 months CA (see Fig. 1).
Fig. 1.
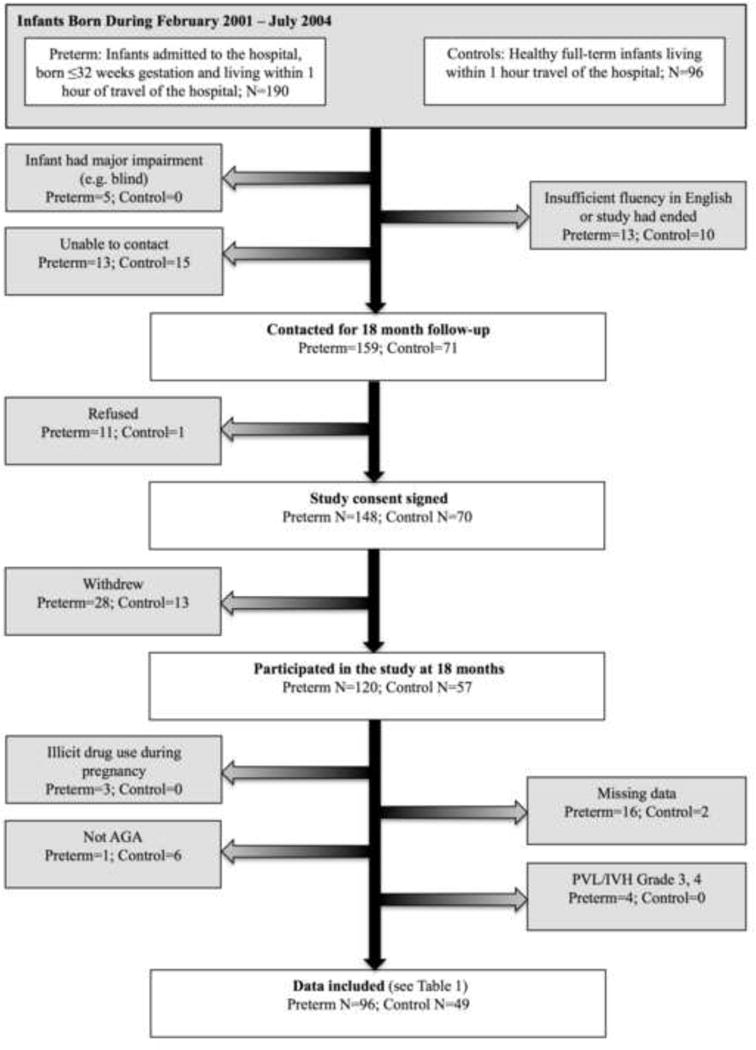
Participant flow chart
2.2. Measures
2.2.1. Demographics
Parent information was obtained by questionnaire. Since parent’s years of education is the most important socioeconomic status (SES) indicator in relation to child development [12, 59], we used parent’s years of education as the index of SES for statistical analysis.
2.2.2. Neonatal Medical Chart Review
A neonatal research nurse carried out medical and nursing chart review from birth to term-equivalent age, as described previously [15, 31]. Data included but were not limited to GA, gender, illness severity on day 1 (Score for Neonatal Acute Physiology II [SNAP-II]) [60], number of skin-breaking procedures, days of mechanical ventilation, and cumulative morphine exposure adjusted for weight. Neonatal pain was defined as the number of skin-breaking procedures, adjusted for illness severity on day 1, days of mechanical ventilation, and cumulative morphine exposure.
2.2.3. Cognitive Development
At 18 months CA, child development was assessed using the Bayley Scales of Infant Development-II [8]. We used the Bayley Mental Development Index (MDI) to adjust our statistical models for child cognitive function. The Bayley MDI measures language, memory and problem-solving abilities in infants and toddlers aged 1 to 42 months. The Bayley MDI is a standardized score for overall cognitive development, with a mean of 100 and standard deviation of 15.
2.2.4. Parenting Stress
Parent’s completed the Parenting Stress Index-III (PSI) [2], that includes 120-items rated on a 6-point Likert scale from 1 (strongly agree) to 6 (strongly disagree). The PSI yields a Total Score and two domain scores: Child Domain (concern about the child), Parent Domain (concern about their own parenting ability). Given that the Child Domain reflects parent’s concerns about the child’s behavior including internalizing behaviors, we only included the Parent Domain in the statistical analysis, since our focus was on how parent factors may influence child behavior.
2.2.5. Child Internalizing Behavior
Parent’s rated their child’s behavior using the Child Behavior Checklist for children ages 1½ to 5 years (CBCL) [3], a widely used questionnaire for identifying problem behaviors in children. Ninety-nine items are rated on a Likert scale ranging from 0 (not true) to 2 (very true or often true). Seven syndrome scales (Emotionally Reactive [e.g. moody, whining], Anxious/Depressed [e.g. nervous, sad], Somatic Complaints [e.g. does not eat well, stomach aches], Withdrawn [e.g. avoids eye contact, unresponsive to affection], Sleep Problems [e.g. nightmares, wakes often], Attention Problems [e.g. cannot concentrate, cannot sit still] and Aggressive Behavior [e.g. hits others, easily frustrated) are empirically derived, and form two broad domains, Internalizing and Externalizing Problems. The Internalizing scale encompasses the Emotionally Reactive, Anxious/Depressed, Somatic Complaints, and Withdrawn behaviors, whereas the Externalizing scale includes Attention Problems and Aggressive Behaviors. However, only the Internalizing domain was used, given that Internalizing, not Externalizing problems are associated with prematurity [1, 35]. Reliability for the Internalizing subscale is high (Test-Retest Pearson r = 0.90; Cronbach’s alpha 0.92) [3].
2.2.6. Emotional Availability
The primary caregiver participated in a 5-minute, videotaped, semi-structured teaching scenario with their child. This involved the caregiver trying to teach their child to perform tasks of varying difficulty. The easier and more familiar task involved stacking or nesting colored cups of varying sizes. The novel and more difficult task involved sorting plastic pigs and cows into separate containers. Parent behavior during this interaction was later scored from videotape using the Emotional Availability Scale-IV [10].
Parent-child interaction videos were coded using the Emotional Availability (EA) Scale-IV [10]. While the EA scale captures the same parameters as the scale used previously in this cohort [15, 70], it is a more holistic measure of parent behavior derived from attachment theory and emotional perspectives [10]. The EA scale captures 4 dimensions of parent behavior: Sensitivity (appropriateness/authenticity of affect), Structuring (provision of guidance), Nonintrusiveness (no overstimulation/overprotection) and Nonhostility (nonthreatening/non-frightening) [10]. Each EA dimension has 7 sub-scales, which are summed to provide a total score for the dimension. Scores range from 7–29, and higher scores denote emotionally available parenting. According to the clinical cut-offs for the EA scale, parents with scores ranging from 7–17 are considered to be Nonoptimally EA, 18–25 Inconsistently EA, and 26–29 Optimally EA [10]. An average score, i.e. in the mid-range falls within the Inconsistent category. This represents a parent that shows adequate EA, but may have moments during the interaction where they are less emotionally available to their child. Trained coders assessed EA from videotape: one primary coder and two reliability coders blinded to all other information about the participants. Inter-rater reliability assessed with intraclass correlation coefficients was 0.89, 0.86, 0.89, and 0.87 for Sensitivity, Structuring, Nonintrusiveness, and Nonhostility respectively.
2.3. Procedure
Very preterm infants were recruited from the NICU at the B.C. Children’s & Women’s Hospitals, the major tertiary neonatal unit for the province of British Columbia, Canada. Full-term infants were born at the B.C. Women’s Hospital, and were contacted through their pediatricians. Written consent was obtained from a parent. At 18 months CA, the children and their parent(s) returned to the Centre for the study visit. The Bayley Scales of Infant Development-II [8] was administered, followed by the videotaped semi-structured parent-child teaching session, later scored for parent EA [10]. The questionnaires on demographics, parenting stress and child behavior were mailed to the family approximately 4 weeks prior to the 18-month visit. Completed questionnaires were returned on the study day.
2.4. Data Analysis
Predictive Analytics SoftWare (PASW) Statistics 18.0.3 (IBM) was used. Normality plots were examined and neonatal pain (number of skin-breaking procedures from birth to term-equivalent) was log transformed. Demographic characteristics of the preterm and full-term groups, and comparisons between infants included and excluded in this study, were examined using t-tests or Chi Square, when appropriate. Univariate ANOVA was performed to examine group (preterm and full-term; ELGA and VLGA) by gender differences on EA and Internalizing scores at 18 months. Pearson correlations were used to examine associations among measures for both the preterm and full-term groups. Multivariate analyses were performed using Generalized Linear (GENLIN) modeling. For each EA dimension (Sensitivity, Structuring, Nonintrusiveness, Nonhostility) GENLIN modeling was used to examine whether parent EA adjusted for Parenting Stress, parent’s years of education, number of children in the home and parent age moderated the relationship between neonatal pain (adjusted for illness severity on day 1, days on mechanical ventilation and total morphine exposure) and Internalizing behaviors (adjusted for gender and Bayley MDI) at 18 months CA in children born very preterm. In addition, we adjusted for extent of prematurity at birth (ELGA or VLGA), given that ELGA children showed greater associations between an altered pattern of cortisol expression and internalizing behaviors at 18 months CA relative to VLGA children [15]. An interaction term between EA and neonatal pain was included in each of the models. Post hoc t-tests were used to further explore statistically significant interactions. The Univariate ANOVAs, GENLIN models and Post hoc t-tests were repeated excluding the 3 mothers in our sample that reported drinking alcohol during their pregnancy. Lastly, to better understand the etiology of the highly prevalent internalizing behaviors seen in children born prematurely relative to full-term controls, GENLIN models for each EA dimension (Sensitivity, Structuring, Nonintrusiveness, Nonhostility) were used to examine whether parent EA adjusted for parenting stress, parent’s years of education, number of children in the home and parent age, was associated with Internalizing behaviors.
3. Results
3.1. Characteristics of the Sample
Of the families we contacted 120/159 (75%) of the parents of children born very preterm, and 57/71 (80%) of the parents of children born full-term returned for the 18 month follow-up. After exclusions (see Fig. 1), we included 96 very preterm and 49 full-term children in this study. Importantly the 96 children born very preterm that were included in the present study did not differ in GA, birthweight or sex from the original sample of infants recruited from the NICU at the B.C. Children’s & Women’s Hospitals between February 2001 and July 2004 (all P > 0.05). Similarly, the 49 full-term controls did not differ in GA or sex from the original sample of full-term infants, recruited at birth (all P > 0.05). The full-term controls included in this study had a lower mean birth weight than the full-term infants in the original sample (t [96[ = −2.26, P = 0.03). This difference, however, was no longer significant after the 6 children born large for their gestational age were removed from the analysis (t [90[ = −1.35, P = 0.18). As expected, GA and birth weight differed between infants born very preterm versus full-term. The only significant difference between parents of preterm versus full-term infants was parent’s years of education, such that parents of infants born very preterm had fewer years of education than parents of infants born full-term. Children born very preterm had lower Bayley MDI cognitive scores and demonstrated more Internalizing behaviors at 18 months CA than children born full-term. Characteristics of the sample are shown in Table 1.
Table 1.
Characteristics of the cohort
| Preterm | Full-term | ||
|---|---|---|---|
| Neonatal characteristics | N = 96 | N = 49 | P |
| Gestational age (weeks), median (IQR) | 29.4 (26.6–31.3)* | 40.0 (39.4–40.6) | 0.001 |
| Birth weight (grams), median (IQR) | 1222 (813–1641) | 3475 (3240–3678) | 0.001 |
| Gender (male), number (%) | 47 (49) | 21 (43) | 0.49 |
| Illness severity on day 1 (score), median (IQR) | 9 (5–19) | – | – |
| Total skin breaks (number), median (IQR) | 87 (49–176) | – | – |
| Mechanical ventilation (days), median (IQR) | 3 (0–18) | – | – |
| Total morphine exposure (grams/kg), median (IQR) | 0.10 (0.00–1.28) | – | – |
|
| |||
| Parent characteristics at 18 month visit | |||
|
| |||
| Age (years), median (IQR) | 36 (31–39) | 37 (33–40) | 0.07 |
| Marital status (married), number (%) | 93 (97) | 46 (94) | 0.50 |
| Ethnicity (Caucasian), number (%) | 73 (76) | 37 (76) | 1.00 |
| Parent education (years), median (IQR) | 15 (13–17) | 18 (15–19) | 0.001 |
| Children at home (number), median (IQR) | 2 (1–3) | 2 (1–2) | 0.18 |
| PSI Parenting stress (score), median (IQR) | 109 (97–128) | 115 (92–122) | 0.24 |
| EA Sensitivity (score), median (IQR) | 19 (16–22) | 21 (15–23) | 0.72 |
| EA Structuring (score), median (IQR) | 20 (17–22) | 19 (16–24) | 0.37 |
| EA Nonintrusiveness (score), median (IQR) | 19 (16–22) | 20 (16–22) | 0.62 |
| EA Nonhostility (score), median (IQR) | 21 (19–24) | 22 (19–24) | 0.66 |
|
| |||
| Child Characteristics | |||
|
| |||
| Bayley MDI (score), median (IQR) | 91 (78–101) | 97 (87–107) | 0.01 |
| CBCL Internalizing (t-score), median (IQR) | 45 (41–51) | 41 (33–49) | 0.02 |
44 children (46%) were born extremely low gestational age (24–28 weeks gestational age), 52 children (54%) were born very low gestational age (29–32 weeks gestational age)
3.2. Parent and Child Behavior: Group by Gender
Assumptions were met for the ANOVAs: Levene’s Test for Equality of Variances was nonsignificant, and skewness was between −1 and 1 for the variables Internalizing, Sensitivity, Structuring, Nonintrusiveness for the preterm, full-term, ELGA and VLGA groups. Children born preterm demonstrated significantly more Internalizing behaviors at 18 months CA than children born full-term (F [1, 141[ = 5.88, P = 0.02); gender was not significant, and there was no group X gender interaction (P = 0.76). Internalizing behaviors, however, did not differ between ELGA and VLGA children (P = 0.39). Internalizing behavior was not correlated with the Bayley MDI for the preterm (r = −0.19, P = 0.07) or full-term children (r = 0.06, P = 0.67). Parent EA (Sensitivity, Structuring, Nonintrusiveness, Nonhostility) did not differ significantly by group (preterm, full-term) or by gender (all P > 0.36). However, parents of children born ELGA children provided less Structure than parents of VLGA children (F [1, 92[ = 4.68, P = 0.03).
3.3. Correlations Among Neonatal and Parent Characteristics
3.3.1. Correlations Among Neonatal Variables
Among the infants born very preterm, lower GA at birth was correlated with higher illness severity on day 1, greater number of skin breaking procedures, more days of mechanical ventilation and more total morphine exposure (see Table 2). Given that all correlations were r < 0.80, multicollinearity among the neonatal predictors was not considered to be problematic [41].
Table 2.
Pearson correlations among the neonatal variables of the preterm infants
| Illness severity on day 1 | Neonatal pain (Number of skin breaks) | Days on mechanical ventilation | Total morphine exposure | |
|---|---|---|---|---|
| Gestational age group | −0.59*** | −0.77*** | −0.64*** | −0.39*** |
| Illness severity on day 1 | – | 0.58*** | 0.51*** | 0.28*** |
| Neonatal pain (Number of skin breaks) | – | – | 0.80*** | 0.52*** |
| Days on mechanical ventilation | – | – | – | 0.77*** |
P < 0.001
Gestational age group (24–28 weeks gestational age or 29–32 weeks gestational age)
3.3.2. Correlations Among Parent Variables
The correlations among the four EA dimensions for parents of preterm children ranged from r = 0.47 to r = 0.80, and for parents of full-term children ranged from r = 0.62 to r = 0.88 (see Table 3). Each EA dimension was entered in a separate multivariate model.
Table 3.
Pearson correlations among the parent variables for preterm and full-term groups
|
Preterm |
EA Structuring |
EA Nonintrusiveness |
EA Nonhostility |
Parenting Stress |
Parent’s years of education |
Number of children in the home |
Parent age |
|---|---|---|---|---|---|---|---|
| EA Sensitivity | 0.70*** | 0.70*** | 0.80*** | −0.05 | 0.21* | 0.41 | −0.02 |
| EA Structuring | – | 0.37*** | 0.47*** | 0.30 | 0.07 | 0.05 | −0.02 |
| EA Nonintrusiveness | – | – | 0.52*** | −0.17 | 0.11 | 0.05 | 0.12 |
| EA Nonhostility | – | – | – | 0.04 | 0.25* | −0.05 | 0.003 |
| Parenting Stress | – | – | – | – | −0.10 | 0.08 | 0.04 |
| Parent’s years of education | – | – | – | – | – | −0.14 | 0.29** |
| Number of children in the home | – | – | – | – | – | – | 0.03 |
|
| |||||||
| Full-term | |||||||
|
| |||||||
| EA Sensitivity | 0.78*** | 0.82*** | 0.88*** | −0.21 | 0.04 | 0.05 | 0.31* |
| EA Structuring | – | 0.62*** | 0.72*** | −0.19 | −0.01 | 0.14 | 0.27 |
| EA Nonintrusiveness | – | – | 0.78*** | −0.21 | 0.09 | 0.13 | 0.30* |
| EA Nonhostility | – | – | – | −0.27 | 0.09 | 0.07 | 0.31* |
| Parenting Stress | – | – | – | – | −0.30* | 0.21 | −0.18 |
| Parent’s years of education | – | – | – | – | – | −0.05 | 0.34* |
| Number of children in the home | – | – | – | – | – | – | 0.18 |
In the preterm group, more years of education was associated with higher parent age, EA Sensitivity and Nonhostility. In the full-term group, more years of education was associated with higher parent age and lower Parenting Stress. Unlike the preterm group, higher parent age among parents of full-term children was associated with greater parent Sensitivity, Nonintrusiveness and Nonhostility (see Table 3).
3.4. EA Moderates the Relationship Between Neonatal Pain-Related Stress and Internalizing Behavior in Children Born Very Preterm
3.4.1. Neonatal Pain, EA Sensitivity, and Internalizing Behavior
In the GENLIN models, there was a significant interaction between parent Sensitivity and neonatal pain in relation to Internalizing behavior at 18 months CA in children born very preterm (B = 1.30, P = 0.05; see Table 4), after adjusting for neonatal medical confounders (GA group [ELGA or VLGA], illness severity on day 1, days of mechanical ventilation, cumulative morphine exposure), concurrent environmental stressors (Parenting Stress, parent’s years of education, number of children in the home), parent age, gender and Bayley MDI. In order to understand this two-way interaction, Internalizing scores were plotted by number of skin-breaking procedures, separately for subgroups of parent EA behavior: Non-optimal Sensitivity (n = 37), Inconsistent Sensitivity (n = 54), Optimal Sensitivity (n = 5) (see Fig. 2.). Post hoc t-tests revealed significant differences in Internalizing behavior between Nonoptimal and Inconsistently Sensitive parents, such that among preterm children exposed to a higher number of skin-breaking procedures, greater parent sensitivity was associated with lower internalizing behaviors at 18 months CA (t [44] = 2.32, P = 0.03, see Fig. 2.). Higher Parenting Stress (B = 0.15, P = 0.001), fewer years of education (B = −0.87, P = 0.008) and fewer children in the home (B = −2.46, P = 0.004) were independently associated with more Internalizing behaviors at 18 months CA in children born very preterm.
Table 4.
Greater parent Sensitivity and Nonhostility were associated with fewer Internalizing behaviors at 18 months corrected age among preterm children exposed to a higher number of skin-breaking procedures
| Child Internalizing | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Sensitivity† Model |
Structuring† Model |
Nonintrusiveness† Model |
Nonhostility† Model |
|||||
|
|
||||||||
| Predictors | B | P | B | P | B | P | B | P |
| EA † | −2.32 | 0.07 | −1.31 | 0.40 | 0.22 | 0.85 | −4.01 | 0.02 |
| Neonatal pain (Number of skin breaks) | −19.08 | 0.129 | −9.69 | 0.50 | 8.15 | 0.50 | −45.76 | 0.02 |
| EA† x neonatal pain | 1.30 | 0.05 | 0.74 | 0.32 | −0.19 | 0.75 | 2.32 | 0.006 |
| Days on mechanical ventilation | −0.06 | 0.62 | −0.05 | 0.64 | −0.10 | 0.33 | −0.05 | 0.59 |
| Total morphine exposure | 0.08 | 0.67 | 0.05 | 0.80 | 0.07 | 0.71 | 0.18 | 0.33 |
| Illness severity on day 1 | −0.004 | 0.96 | −0.02 | 0.85 | −0.01 | 0.89 | −0.01 | 0.95 |
| Gestational age group | 0.12 | 0.97 | 0.39 | 0.90 | 0.78 | 0.79 | 0.22 | 0.94 |
| Gender | −2.85 | 0.14 | −3.15 | 0.12 | −3.93 | 0.05 | −2.43 | 0.20 |
| Parenting stress | 0.15 | 0.001 | 0.15 | 0.001 | 0.14 | 0.001 | 0.16 | 0.001 |
| Parent’s years of education | −0.87 | 0.008 | −0.81 | 0.01 | −0.74 | 0.03 | −0.82 | 0.01 |
| Number of children in the home | −2.46 | 0.004 | −2.29 | 0.009 | −2.17 | 0.01 | −2.25 | 0.007 |
| Parent age | −0.13 | 0.49 | −0.17 | 0.37 | −0.23 | 0.21 | −0.16 | 0.35 |
| Bayley MDI | −0.05 | 0.56 | −0.05 | 0.44 | −0.06 | 0.35 | −0.07 | 0.22 |
|
| ||||||||
| Adjusted R2 | 0.26* | 0.23* | 0.22* | 0.31* | ||||
The Generalized Linear Model was repeated four times: each time a different EA variable (Sensitivity, Structuring, Nonintrusiveness, Nonhostility) was entered into the model.
Computed using linear regression.
Parent Sensitivity and Nonhostility moderated the relationship between neonatal pain and Internalizing in children born very preterm at 18 months corrected age after adjusting for neonatal medical confounders (gestational age group [24–28 weeks gestational age or 29–32 weeks gestational age]), illness severity on day 1, days of mechanical ventilation, cumulative morphine exposure), concurrent environmental stressors (Parenting Stress, parent’s years of education, number of children in the home), parent age, child gender and cognition (Bayley MDI). Higher Parenting Stress, fewer maternal years of education and fewer children in the home were independently associated with greater Internalizing at 18 months corrected age in children born very preterm.
Fig. 2.

Predicted values of Internalizing behaviors (t-score) in relation to number of skin-breaking procedures from birth to term-equivalent age adjusted for gestational age group (24–28 weeks gestational age or 29–32 weeks gestational age), illness severity on day 1, days of mechanical ventilation, cumulative morphine exposure, Parenting Stress, parent’s years of education, number of children in the home, parent age, child gender and cognition. Differences in Internalizing scores were between non-optimal and inconsistently sensitive parents, whose infants received a high number of skin-breaking procedures. Among preterm children exposed to a higher number of skin-breaking procedures, greater parent sensitivity was associated with lower internalizing behaviors at 18 months corrected age.
3.4.2. Neonatal Pain, EA Structuring, and Internalizing Behavior
In the GENLIN models, the interaction between parent Structuring and neonatal pain in relation to Internalizing behavior at 18 months CA in children born very preterm was not significant (B = 0.74, P = 0.32; see Table 4), after adjusting for neonatal medical confounders (GA group, illness severity on day 1, days of mechanical ventilation, cumulative morphine exposure), concurrent environmental stressors (Parenting Stress, parent’s years of education and number of children in the home), parent age, gender and Bayley MDI. However, higher Parenting Stress (B = 0.15, P = 0.001), fewer years of education (B = −0.81, P = 0.01) and fewer children in the home (B = −2.29, P = 0.009) were independently associated with more Internalizing behaviors at 18 months CA in children born very preterm.
3.4.3. Neonatal Pain, EA Nonintrusiveness, and Internalizing Behavior
In the GENLIN models, the interaction between parent Nonintrusiveness and neonatal pain in relation to Internalizing at 18 months CA in children born very preterm was not significant (B = −0.19, P = 0.75; see Table 4), after adjusting for neonatal medical confounders (GA group, illness severity on day 1, days of mechanical ventilation, cumulative morphine exposure), concurrent environmental stressors (Parenting Stress, parent’s years of education, number of children in the home), parent age, gender and Bayley MDI. However, higher Parenting Stress (B = 0.14, P = 0.001), fewer years of education (B = −0.74, P = 0.03) and fewer children in the home (B = −2.17, P = 0.01) were independently associated with more Internalizing behaviors at 18 months CA in children born very preterm.
3.4.4. Neonatal Pain, EA Nonhostility, and Internalizing Behavior
In the GENLIN models, there was a significant interaction between parent Nonhostility and neonatal pain in relation to Internalizing behavior at 18 months CA in children born very preterm (B = 2.32, P = 0.006; see Table 4), after adjusting for neonatal medical confounders (GA group, illness severity on day 1, days of mechanical ventilation, cumulative morphine exposure), concurrent environmental stressors (Parenting Stress, parent’s years of education, number of children in the home), parent age, gender and Bayley MDI. In order to understand this two-way interaction, Internalizing scores were plotted by number of skin-breaking procedures, separately for subgroups of parent EA behavior: Non-optimal Nonhostile (n = 16), Inconsistent Nonhostile (n = 75), Optimal Nonhostile (n = 5) (see Fig. 3.). While the relationship between Nonoptimal and Inconsistently Nonhostile parenting and neonatal pain (see Fig. 3.) was similar to the Nonoptimal and Inconsistently Sensitive parents whose infants received a high number of skin breaking procedures (see Fig. 2.), the group size was limited (t [45] = 0.57, P = 0.57). Consistent with the analyses above, higher Parenting Stress (B = 0.16, P = 0.001), fewer years of education (B = −0.82, P = 0.01) and fewer children in the home (B = −2.25, P = 0.007) were independently associated with more Internalizing behaviors at 18 months CA in children born very preterm.
Fig. 3.
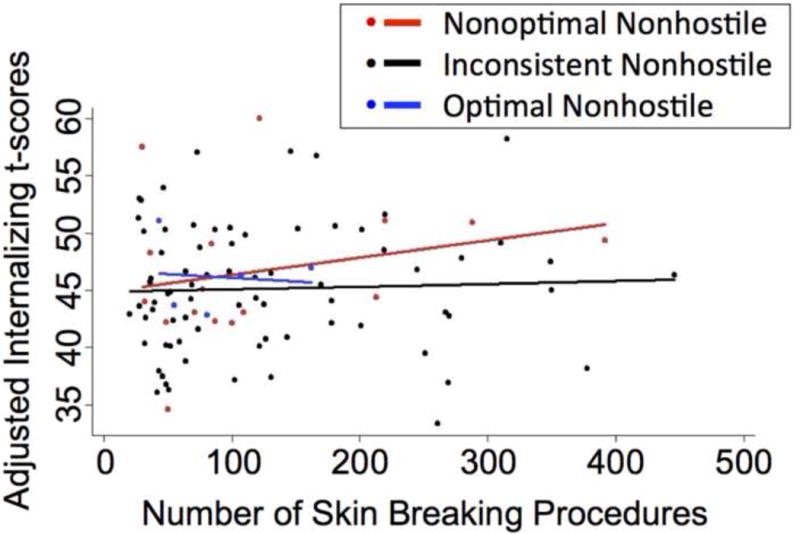
Predicted values of Internalizing behaviors (t-score) in relation to number of skin-breaking procedures from birth to term-equivalent age adjusted for gestational age group (24–28 weeks gestational age or 29–32 weeks gestational age), illness severity on day 1, days of mechanical ventilation, total morphine exposure, Parenting Stress, parent’s years of education, number of children in the home, parent age, child gender and cognition. Differences in Internalizing scores were between non-optimal and inconsistently nonhostile parents, whose infants received a high number of skin-breaking procedures. Among preterm children exposed to a higher number of skin-breaking procedures, greater parent nonhostility appears to lower internalizing behaviors at 18 months corrected age.
3.5. Exclusion of Mothers that Reported Drinking Alcohol during their Pregnancy
The Univariate ANOVAs, GENLIN models and Post hoc t-tests were repeated excluding the 3 mothers in our sample that reported to drinking alcohol during their pregnancy, and the results of our models remained unchanged.
3.6. Parent Behavior and Stress in Relation to Internalizing Behavior in Children Born Full-Term
There were no significant associations between parent EA (Sensitivity, Structuring, Nonintrusiveness, Nonhostility) or stress (Parenting Stress, parent’s years of education and number of children in the home), and Internalizing behavior (adjusted for GA parent age, gender and Bayley MDI) at 18 months in children born full-term.
4. Discussion
This is the first study, to our knowledge, to examine whether neonatal pain is related to later internalizing behaviors, and whether parent emotional availability moderates the relationship between neonatal pain and internalizing behavior in children born very preterm. We found that neonatal pain was associated with internalizing (anxious/depressed) behaviors at 18 months CA in children born very preterm. Importantly, among preterm children exposed to a higher number of skin-breaking procedures (adjusted for confounding neonatal medical factors), greater parent sensitivity and nonhostility was associated with lower internalizing behaviors at 18 months CA. In addition, environmental factors including lower parenting stress, more years of education and more children in the home were also associated with fewer internalizing behaviors in children born very preterm at 18 months CA. In contrast, none of the parent factors were significant predictors of internalizing behavior in children born full-term.
EA is a holistic measure of parental behavior, integrating both attachment theory and emotional perspectives [10]. Consistent with the attachment literature [42] we found no significant differences in the EA of parents of infants born very preterm compared to full-term. There were also no significant differences in parenting stress in this sample of children all born appropriate-for-gestational age. Consistent with our findings, Gray et al. (2012) and Treyvaud et al. (2011) also reported that parenting stress in mothers of very preterm infants assessed at 4 months and 2 years of age (respectively) did not differ from mothers of term-born infants [30, 69]. However, in a previous sample that included infants born small for gestational age (<10th percentile), we found parenting stress was higher in the parents of children born very preterm compared to full-term, when their child was 18 months CA [15]. Despite the similarities in parenting stress and parent behavior, children born very preterm were more influenced by their interactions with their parents than their term-born peers, consistent with previous findings from our group and others that preterm children are more vulnerable to environmental context [15, 18, 70].
Early stress, including neonatal pain related stress has been associated with altered brain microstructure in infants born very preterm [13, 65]. Additionally, at 8 years of age, children born very preterm demonstrate altered engagement of neural networks [28], altered inter-regional functional connectivity and cortical activation [19] compared to full-term controls. Furthermore, early alterations of cerebral white matter development have been associated with deficits in social-emotional behaviors at age 5 years in children born very preterm [61]. Thus, neonatal pain related stress may contribute to altered cerebral microstructure in infants born very preterm, contributing to altered processing of environmental information during childhood, and thereby eliciting greater anxiety in children born very preterm relative to full-term controls.
While preterm birth has been associated with abnormalities in brain development, particularly in infants with lower GA [6, 11, 39], we have found in another cohort of preterm infants that after accounting for GA, illness severity on day 1, days of mechanical ventilation and cumulative morphine exposure, neonatal pain was related to delayed white and subcortical gray matter maturation [13]. Thus, a higher exposure to neonatal pain from birth to NICU discharge, independent of the degree of prematurity and systemic illness, is related to altered brain microstructure, and may underlie altered cortical processing and thereby greater internalizing problems in children born very preterm at 18 months CA.
Importantly, our results suggest that positive parent interaction may ameliorate effects of early adversity. Our finding that parent sensitivity and nonhostility moderate the relationship between neonatal pain and internalizing behavior at 18 months CA in children born very preterm, is supported by animal models that have demonstrated mother-infant interactive behaviors can protect against effects of early stress on later behavior [16, 22]. It should be noted, however, that unlike animal models of early stress and behavior [7, 40, 62, 64], in the present study we did not find gender differences in behavior of children born very preterm or full-term. Offspring of low licking and grooming dams are fearful in response to novelty in adulthood [16]. Cross-fostering the biological offspring of high and low licking and grooming mothers reverses this phenotype, emphasizing the potential for high quality maternal care to ameliorate the adverse impact of early stress on behavioral reactivity [22]. A recent study by Milgrom et al. (2010) demonstrated that positive parent interaction in the NICU leads to more mature cerebral white matter micro-structural development of infants born very preterm [53].
In addition to neonatal pain, concurrent parental factors were also associated with internalizing behaviors in children born very preterm at 18 months CA. Specifically, fewer years of education, an indicator of lower SES, was associated with less parent emotional availability and greater internalizing behavior in children born very preterm. Importantly, the relationship between parent’s years of education and internalizing behavior remained significant after accounting for child cognition, which is associated with both parent’s years of education and child interactive behavior [46]. Previous studies have demonstrated that the relationship between socioeconomic risk and behavior in preterm infants in children was mediated by maternal behavior [17, 44]. NICU-based programs designed to enhance the ability of low SES mothers to provide appropriate interaction and environmental stimulation for their preterm infant may lead to improved home environments and infant temperament at 4 and 8 months CA [56]. However, it is noteworthy that the level of parent’s education was relatively high in our cohort, with parents of preterm infants having a median of 15 years education, indicating some postsecondary college attendance.
One of the most stressful experiences reported by parents of infants in the NICU is seeing their infant in pain [25, 50, 51]. NICU-based interventions that increase parental involvement in infant pain management have been found to improve parents’ efficacy in supporting their infant post-discharge from the NICU [23], and thereby may lead to improved behavioral outcomes in this population. Parental concerns regarding their inability to reduce pain in the NICU may continue to be a source of stress long after their infant’s discharge from the NICU [71]. We found that greater concern regarding parenting ability, as indexed by the level of parenting stress at 18 months CA, was associated with higher internalizing behavior in children born very preterm, consistent with a previous study examining effects of parenting stress in the NICU [73].
In the present study, we found that fewer children in the home was associated with greater internalizing behavior in children born very preterm. First-time parents may underestimate the personal impact of the birth of the infant [21]; a life altering change in combination with an unexpected preterm birth appears to increase the risk for a negative transition to parenthood [37]. Realistic expectations of parenthood may improve parent attachment/responsiveness and lessen parent psychological symptoms [21], factors that are in turn associated with fewer internalizing behaviors [29, 55]. Moreover, siblings can also play an important role in behavioral development; support from siblings can be a buffer to feelings of loneliness and depression in contexts of decreased parental and or peer support [20, 52]. Adult siblings of children born preterm have retrospectively described their relationships with their brother or sister as both positive and protective [24].
Given the correlational nature of this study, and the bi-directional nature of parent-child interaction, it is important to note that greater child internalizing behavior may contribute to lower parent emotional availability. Future research is needed to determine whether early or concurrent emotional availability training can effectively prevent or reduce the long-term effects of neonatal pain on internalizing behavior in children born very preterm.
The results of this study have important clinical implications. After accounting for GA, illness severity on day 1, days of mechanical ventilation and morphine exposure, more neonatal pain in the NICU was associated with greater internalizing behavior at 18 months CA. Therefore, the necessity and frequency of skin-breaking procedures performed in the NICU needs to be evaluated, with the goal of reducing unnecessary pain exposure. Importantly, parent sensitivity and nonhostility appears to moderate the relationship between neonatal pain and internalizing behavior in children born very preterm, who are more sensitive to their environment than full-term controls. NICU-based or follow-up programs designed to facilitate parent emotional availability may be able to effectively prevent or reduce internalizing behavior in children born very preterm. However, with limited resources for training, results from this study suggest that parents who have fewer years of education, are highly stressed, or are first-time parents may be a priority for support. Helping parents to appropriately regulate pain-related stress in their infant may also help to improve infant interactions, thereby reducing and or preventing the development of internalizing behaviors in their preterm child.
Our findings suggests that neonatal procedural pain, which is inherent to lifesaving procedures in the NICU, may be one aspect of the etiology of internalizing behaviors in children born very preterm, consistent with the animal literature on early stress. However, positive parent interaction and lower parenting stress may help to ameliorate the negative long-term effects of neonatal pain on child behavior, suggesting opportunities for intervention.
Acknowledgments
We thank the children and their parents who generously participated in this study and Dr. Rollin Brant for his statistical consultation. This study was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development (R01 HD39783 to REG), and Canadian Institutes of Health Research ([CIHR] MOP42469 to REG). REG is supported by a Senior Scientist award from the Child and Family Research Institute (CFRI). SPM (currently Bloorview Children’s Hospital Chair in Paediatric Neuroscience) was supported by a Tier 2 Canadian Research Chair in Neonatal Neuroscience, and Michael Smith Foundation for Health Research Scholar Award. JV holds a CIHR Frederick Banting and Charles Best Doctoral Award, and is a trainee in Pain in Child Health (CIHR Strategic Training Initiative in Health Research).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Metaanalysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 2.Abidin RR. Parenting stress index. Odessa, FL: Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- 3.Achenbach T, Rescorla L. Manual for the ASEBA Preschool Forms and Profiles. Burlington VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- 4.Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson P, Doyle LW, Victorian Infant Collaborative Study Group Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 6.Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 2012;22:1016–1024. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barna I, Balint E, Baranyi J, Bakos N, Makara GB, Haller J. Gender-specific effect of maternal deprivation on anxiety and corticotropin-releasing hormone mRNA expression in rats. Brain Res Bull. 2003;62:85–91. doi: 10.1016/s0361-9230(03)00216-8. [DOI] [PubMed] [Google Scholar]
- 8.Bayley N. Bayley scales of infant development. San Antonio, TX: TX-Psychological Corporation; 1993. [Google Scholar]
- 9.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 10.Biringen Z. Emotional availability: conceptualization and research findings. Am J Orthopsychiatry. 2000;70:104–114. doi: 10.1037/h0087711. [DOI] [PubMed] [Google Scholar]
- 11.Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, Hajnal J, Allsop JM, Rutherford MA, Edwards AD. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage. 2006;32:70–78. doi: 10.1016/j.neuroimage.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Bohm B, Katz-Salamon M, Institute K, Smedler AC, Lagercrantz H, Forssberg H. Developmental risks and protective factors for influencing cognitive outcome at 5 1/2 years of age in very-low-birthweight children. Dev Med Child Neurol. 2002;44:508–516. doi: 10.1017/s001216220100247x. [DOI] [PubMed] [Google Scholar]
- 13.Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, Gover A, Synnes AR, Miller SP. Procedural pain and brain development in premature newborns. Ann Neurol. 2012;71:385–396. doi: 10.1002/ana.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brummelte S, Grunau RE, Synnes AR, Whitfield MF, Petrie-Thomas J. Declining cognitive development from 8 to 18 months in preterm children predicts persisting higher parenting stress. Early Hum Dev. 2011;87:273–280. doi: 10.1016/j.earlhumdev.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 15.Brummelte S, Grunau RE, Zaidman-Zait A, Weinberg J, Nordstokke D, Cepeda IL. Cortisol levels in relation to maternal interaction and child internalizing behavior in preterm and full-term children at 18 months corrected age. Dev Psychobiol. 2011;53:184–195. doi: 10.1002/dev.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candelaria M, Teti DM, Black MM. Multi-risk infants: predicting attachment security from sociodemographic, psychosocial, and health risk among African-American preterm infants. J Child Psychol Psychiatry. 2011;52:870–877. doi: 10.1111/j.1469-7610.2011.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crnic KA, Greenberg MT. Transactional relationships between perceived family style, risk status, and mother-child interactions in two-year-olds. J Pediatr Psychol. 1987;12:343–362. doi: 10.1093/jpepsy/12.3.343. [DOI] [PubMed] [Google Scholar]
- 19.Doesburg SM, Ribary U, Herdman AT, Cheung T, Moiseev A, Weinberg H, Whitfield MF, Synnes A, Liotti M, Weeks D, Grunau RE. Altered Long-Range Phase Synchronization and Cortical Activation in Children Born Very Preterm. IFMBE Proc. 2010;29:250–253. doi: 10.1007/978-3-642-12197-5_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.East PL, Rook KS. Compensatory patterns of support among children’s peer relationships: A test using school friends, nonschool friends, and siblings. Dev Psychol. 1992;28:163–172. doi: 10.1037/0012-1649.28.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans T, Whittingham K, Boyd R. What helps the mother of a preterm infant become securely attached, responsive and well-adjusted? Infant Behav Dev. 2012;35:1–11. doi: 10.1016/j.infbeh.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 23.Franck LS, Oulton K, Nderitu S, Lim M, Fang S, Kaiser A. Parent involvement in pain management for NICU infants: a randomized controlled trial. Pediatrics. 2011;128:510–518. doi: 10.1542/peds.2011-0272. [DOI] [PubMed] [Google Scholar]
- 24.Gaal BJ, Pinelli J, Crooks D, Saigal S, Streiner DL, Boyle M. Outside looking in: the lived experience of adults with prematurely born siblings. Qual Health Res. 2010;20:1532–1545. doi: 10.1177/1049732310375248. [DOI] [PubMed] [Google Scholar]
- 25.Gale G, Franck LS, Kools S, Lynch M. Parents’ perceptions of their infant’s pain experience in the NICU. Int J Nurs Stud. 2004;41:51–58. doi: 10.1016/s0020-7489(03)00096-8. [DOI] [PubMed] [Google Scholar]
- 26.Garel M, Dardennes M, Blondel B. Mothers’ psychological distress 1 year after very preterm childbirth. Results of the EPIPAGE qualitative study. Child Care Health Dev. 2007;33:137–143. doi: 10.1111/j.1365-2214.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 27.Glazebrook C, Marlow N, Israel C, Croudace T, Johnson S, White IR, Whitelaw A. Randomised trial of a parenting intervention during neonatal intensive care. Arch Dis Child Fetal Neonatal Ed. 2007;92:F438–43. doi: 10.1136/adc.2006.103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gozzo Y, Vohr B, Lacadie C, Hampson M, Katz KH, Maller-Kesselman J, Schneider KC, Peterson BS, Rajeevan N, Makuch RW, Constable RT, Ment LR. Alterations in neural connectivity in preterm children at school age. Neuroimage. 2009;48:458–463. doi: 10.1016/j.neuroimage.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravener JA, Rogosch FA, Oshri A, Narayan AJ, Cicchetti D, Toth SL. The relations among maternal depressive disorder, maternal expressed emotion, and toddler behavior problems and attachment. J Abnorm Child Psychol. 2012;40:803–813. doi: 10.1007/s10802-011-9598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray PH, Edwards DM, O’Callaghan MJ, Cuskelly M. Parenting stress in mothers of preterm infants during early infancy. Early Hum Dev. 2012;88:45–49. doi: 10.1016/j.earlhumdev.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born at extremely low gestational age. J Pediatr. 2007;150:151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunau RE, Holsti L, Haley DW, Oberlander T, Weinberg J, Solimano A, Whitfield MF, Fitzgerald C, Yu W. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114:e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunau RE, Whitfield MF, Davis C. Pattern of learning disabilities in children with extremely low birth weight and broadly average intelligence. Arch Pediatr Adolesc Med. 2002;156:615–620. doi: 10.1001/archpedi.156.6.615. [DOI] [PubMed] [Google Scholar]
- 35.Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (< or =800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004;114:e725–32. doi: 10.1542/peds.2004-0932. [DOI] [PubMed] [Google Scholar]
- 36.Gunnar MR. Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev Med. 1998;27:208–211. doi: 10.1006/pmed.1998.0276. [DOI] [PubMed] [Google Scholar]
- 37.Harwood K, McLean N, Durkin K. First-time mothers’ expectations of parenthood: What happens when optimistic expectations are not matched by later experiences? Dev Psychol. 2007;43:1–12. doi: 10.1037/0012-1649.43.1.1. [DOI] [PubMed] [Google Scholar]
- 38.Holditch-Davis D, Miles MS, Weaver MA, Black B, Beeber L, Thoyre S, Engelke S. Patterns of distress in African-American mothers of preterm infants. J Dev Behav Pediatr. 2009;30:193–205. doi: 10.1097/DBP.0b013e3181a7ee53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 40.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 41.Katz MH. Multivariable analysis a practical guide for clinical and public health researchers third edition. New York, USA: Cambridge University Press; 2011. [Google Scholar]
- 42.Korja R, Latva R, Lehtonen L. The effects of preterm birth on mother-infant interaction and attachment during the infant’s first two years. Acta Obstet Gynecol Scand. 2012;91:164–173. doi: 10.1111/j.1600-0412.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- 43.Lind A, Korkman M, Lehtonen L, Lapinleimu H, Parkkola R, Matomaki J, Haataja L, PIPARI Study Group Cognitive and neuropsychological outcomes at 5 years of age in preterm children born in the 2000s. Dev Med Child Neurol. 2011;53:256–262. doi: 10.1111/j.1469-8749.2010.03828.x. [DOI] [PubMed] [Google Scholar]
- 44.Linver MR, Brooks-Gunn J, Kohen DE. Family processes as pathways from income to young children’s development. Dev Psychol. 2002;38:719–734. [PubMed] [Google Scholar]
- 45.Loe IM, Lee ES, Luna B, Feldman HM. Behavior problems of 9–16 year old preterm children: biological, sociodemographic, and intellectual contributions. Early Hum Dev. 2011;87:247–252. doi: 10.1016/j.earlhumdev.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowe J, Erickson SJ, MacLean P. Cognitive correlates in toddlers born very low birth weight and full-term. Infant Behav Dev. 2010;33:629–634. doi: 10.1016/j.infbeh.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–380. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 48.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Meyer EC, Garcia Coll CT, Seifer R, Ramos A, Kilis E, Oh W. Psychological distress in mothers of preterm infants. J Dev Behav Pediatr. 1995;16:412–417. [PubMed] [Google Scholar]
- 50.Miles MS, Funk SG, Carlson J. Parental Stressor Scale: neonatal intensive care unit. Nurs Res. 1993;42:148–152. [PubMed] [Google Scholar]
- 51.Miles MS, Holditch-Davis D. Parenting the prematurely born child: pathways of influence. Semin Perinatol. 1997;21:254–266. doi: 10.1016/s0146-0005(97)80067-5. [DOI] [PubMed] [Google Scholar]
- 52.Milevsky A, Levitt MJ. Sibling support in early adolescence: Buffering and compensation across relationships. Eur J Dev Psychol. 2005;2:299–320. [Google Scholar]
- 53.Milgrom J, Newnham C, Anderson PJ, Doyle LW, Gemmill AW, Lee K, Hunt RW, Bear M, Inder T. Early sensitivity training for parents of preterm infants: impact on the developing brain. Pediatr Res. 2010;67:330–335. doi: 10.1203/PDR.0b013e3181cb8e2f. [DOI] [PubMed] [Google Scholar]
- 54.Murgatroyd C, Spengler D. Epigenetic programming of the HPA axis: early life decides. Stress. 2011;14:581–589. doi: 10.3109/10253890.2011.602146. [DOI] [PubMed] [Google Scholar]
- 55.O’Connor E, Bureau JF, McCartney K, Lyons-Ruth K. Risks and Outcomes Associated with Disorganized/Controlling Patterns of Attachment at Age Three in the NICHD Study of Early Child Care and Youth Development. Infant Ment Health J. 2011;32:450–472. doi: 10.1002/imhj.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker SJ, Zahr LK, Cole JG, Brecht ML. Outcome after developmental intervention in the neonatal intensive care unit for mothers of preterm infants with low socioeconomic status. J Pediatr. 1992;120:780–785. doi: 10.1016/s0022-3476(05)80248-3. [DOI] [PubMed] [Google Scholar]
- 57.Poehlmann J, Fiese BH. The interaction of maternal and infant vulnerabilities on developing attachment relationships. Dev Psychopathol. 2001;13:1–11. doi: 10.1017/s0954579401001018. [DOI] [PubMed] [Google Scholar]
- 58.Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 59.Resnick MB, Stralka K, Carter RL, Ariet M, Bucciarelli RL, Furlough RR, Evans JH, Curran JS, Ausbon WW. Effects of birth weight and sociodemographic variables on mental development of neonatal intensive care unit survivors. Am J Obstet Gynecol. 1990;162:374–378. doi: 10.1016/0002-9378(90)90389-o. [DOI] [PubMed] [Google Scholar]
- 60.Richardson DK, Corcoran JD, Escobar GJ, Lee SK. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J Pediatr. 2001;138:92–100. doi: 10.1067/mpd.2001.109608. [DOI] [PubMed] [Google Scholar]
- 61.Rogers CE, Anderson PJ, Thompson DK, Kidokoro H, Wallendorf M, Treyvaud K, Roberts G, Doyle LW, Neil JJ, Inder TE. Regional cerebral development at term relates to school-age social-emotional development in very preterm children. J Am Acad Child Adolesc Psychiatry. 2012;51:181–191. doi: 10.1016/j.jaac.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 63.Singer LT, Fulton S, Davillier M, Koshy D, Salvator A, Baley JE. Effects of infant risk status and maternal psychological distress on maternal-infant interactions during the first year of life. J Dev Behav Pediatr. 2003;24:233–241. doi: 10.1097/00004703-200308000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skripuletz T, Kruschinski C, Pabst R, von Horsten S, Stephan M. Postnatal experiences influence the behavior in adult male and female Fischer and Lewis rats. Int J Dev Neurosci. 2010;28:561–571. doi: 10.1016/j.ijdevneu.2010.07.235. [DOI] [PubMed] [Google Scholar]
- 65.Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, Vavasseur C, Wallendorf M, Neil J, Inder T. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70:541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spittle AJ, Treyvaud K, Doyle LW, Roberts G, Lee KJ, Inder TE, Cheong JL, Hunt RW, Newnham CA, Anderson PJ. Early emergence of behavior and social-emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry. 2009;48:909–918. doi: 10.1097/CHI.0b013e3181af8235. [DOI] [PubMed] [Google Scholar]
- 67.Synnes AR, Anson S, Arkesteijn A, Butt A, Grunau RE, Rogers M, Whitfield MF. School entry age outcomes for infants with birth weight </= 800 grams. J Pediatr. 2010;157:989–994. doi: 10.1016/j.jpeds.2010.06.016. e1. [DOI] [PubMed] [Google Scholar]
- 68.Thomas KA, Renaud MT, Depaul D. Use of the parenting stress index in mothers of preterm infants. Adv Neonatal Care. 2004;4:33–41. doi: 10.1016/j.adnc.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 69.Treyvaud K, Doyle LW, Lee KJ, Roberts G, Cheong JL, Inder TE, Anderson PJ. Family functioning, burden and parenting stress 2 years after very preterm birth. Early Hum Dev. 2011;87:427–431. doi: 10.1016/j.earlhumdev.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Tu MT, Grunau RE, Petrie-Thomas J, Haley DW, Weinberg J, Whitfield MF. Maternal stress and behavior modulate relationships between neonatal stress, attention, and basal cortisol at 8 months in preterm infants. Dev Psychobiol. 2007;49:150–164. doi: 10.1002/dev.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wereszczak J, Miles MS, Holditch-Davis D. Maternal recall of the neonatal intensive care unit. Neonatal Netw. 1997;16:33–40. [PubMed] [Google Scholar]
- 72.Younger JB, Kendell MJ, Pickler RH. Mastery of stress in mothers of preterm infants. J Soc Pediatr Nurs. 1997;2:29–35. doi: 10.1111/j.1744-6155.1997.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 73.Zelkowitz P, Na S, Wang T, Bardin C, Papageorgiou A. Early maternal anxiety predicts cognitive and behavioural outcomes of VLBW children at 24 months corrected age. Acta Paediatr. 2011;100:700–704. doi: 10.1111/j.1651-2227.2010.02128.x. [DOI] [PubMed] [Google Scholar]
- 74.Zelkowitz P, Papageorgiou A, Bardin C, Wang T. Persistent maternal anxiety affects the interaction between mothers and their very low birthweight children at 24 months. Early Hum Dev. 2009;85:51–58. doi: 10.1016/j.earlhumdev.2008.06.010. [DOI] [PubMed] [Google Scholar]


