Abstract
Background
The factors associated with delayed gastric emptying (DGE) after a pancreaticoduodenectomy (PD) are not definitively known.
Methods
From November 2011 through to May 2012, data were prospectively collected on 711 patients undergoing a pancreaticoduodenectomy or total pancreatectomy as part of the American College of Surgeons-National Surgical Quality Improvement Program Pancreatectomy Demonstration Project. Bivariate and multivariate models were employed to determine the factors that predicted DGE.
Results
In the 711 patients, the overall rate of DGE was 20.1%. In a bivariate analysis, intra-operative factors such as pylorus-preservation (47.1% versus 43.7%, P = 0.40), intra-operative drain placement (85.5%, versus 85.1%, P = 0.91) and an antecolic compared with a retrocolic gastrojejunostomy (60.1% versus 65.1%, P = 0.26) were not different between the DGE and no DGE groups. Pancreatic fistula formation (31.2% versus 10.1%), post-operative sepsis (21.7% versus 7.0%), organ space surgical site infection (SSI) (23.9% versus 7.9%), need for percutaneous drainage (23.0% versus 10.6%) and reoperation (10.6% versus 3.1%) were higher in patients with DGE (P < 0.0001). In a multivariable model, only pancreatic fistula, post-operative sepsis and reoperation were independently associated with DGE.
Discussion
In this multicentre study, only post-operative complications were associated with DGE. Neither pylorus preservation nor route of enteric reconstruction (antecolic versus retrocolic) was associated with delayed gastric emptying.
Introduction
Delayed gastric emptying (DGE) is a frequent complication of a pancreatectomy, affecting 14–30% of patients post-operatively.1–4 In spite of recent advances leading to decreased mortality with pancreatic surgery, delayed gastric emptying continues to be a significant cause of post-operative morbidity. DGE has been associated with increased hospital length of stay,5 cost,6–8 hospital readmission9 and significant patient discomfort.
The exact pathogenesis of DGE is unclear. Technical approaches to a pancreatic resection and post-operative complications both have been suggested to play causative roles in the aetiology of DGE. Previous studies of operative techniques have suggested that classic Whipple versus pylorus preservation,10–20 antecolic versus retrocolic gastric/duodenal reconstruction,21–24 pancreaticogastrostomy versus pancreaticojejunostomy reconstruction,25 duodenal preservation in benign disease,26–28 and even preservation of the right gastric artery29 can influence DGE. However, these studies have been limited by a small sample size and/or retrospective analyses, and results have been mixed. As a result, no consensus exists regarding the influence of surgical technique on DGE.
Intra-abdominal complications such as a pancreatic fistula,30 pancreatic leak or abscess,13,31–33 pancreatitis,14 and pancreatic fibrosis34 also have been associated with the development of DGE post-operatively. Based on these findings, some authors have suggested that regardless of the surgical approach, avoidance of post-operative complications can reduce DGE.13,35,36 All of the above findings have been reported in single institutional studies with a limited sample size; as a result, they lack the external validity that would make them generalizable to all patients undergoing pancreatic surgery. Although much has been published on the subject, no conclusive association has been made amongst operative techniques, post-operative complications and DGE. Therefore, we used data from the American College of Surgeons-National Surgical Quality Improvement Program Pancreatectomy Demonstration Project to identify patients undergoing a pancreatic head resection. Using this multi-institutional database, we sought to analyse pre-, intra- and post-operative factors that would be associated with DGE.
Methods
This study was designated exempt from review by the Institutional Review Board at the University of Texas Medical Branch.
Data source
The American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) is a nationally validated, multicentre, prospectively-collected database that uses patient information directly abstracted from the medical record in real time to identify 30-day post-operative morbidity and mortality at participating institutions.37–39 For the ACS-NSQIP Pancreatectomy Demonstration Project, 33 institutions submitted prospectively collected data on 1384 patients undergoing a pancreatic resection (median = 28 per institution, range 3–213) from November 2011 through to May 2012.
Cohort selection
We identified our cohort from the 931 patients who underwent a pancreatic head resection (pancreaticoduodenectomy or total pancreatectomy) during the study period. A pancreatic resection was defined by the American Medical Association Common Procedure Terminology (CPT codes) 48150, 48152, 48153, 48154 and 48155. Patients in whom information on DGE or route of enteric reconstruction was missing were excluded. Our final cohort had 711 patients for analysis.
Variables
The Participant Use File (PUF) contains 240 Health Insurance Portability and Accountability Act (HIPAA) compliant variables for each case, including patient demographics, pre-operative risk factors, baseline comorbidities, intra-operative variables, and 30-day postoperative morbidity and mortality. The list and definitions of variables collected in the database can be found at the American College of Surgeons NSQIP website.21
In addition to the usual NSQIP variables, 16 additional pancreas-specific variables were included: pre-operative variables (jaundice, biliary stent, chemotherapy in the 90 days before surgery and radiation in the 90 days before surgery), operative variables (surgery type, pancreatic duct size, pancreatic gland texture, vascular resection, pancreatic reconstruction, gastrojejunostomy versus duodenojejunostomy and drain placement) and post-operative variables (drain amylase on post-operative day one, the last day of drain removal, pancreatic fistula, delayed gastric emptying and percutaneous drainage). Standard NSQIP Pancreatectomy Demonstration Project definitions of these 16 variables were used at all participating institutions and are shown in Table 1. Overall morbidity was defined as any NSQIP or pancreas-specific complication including re-operation, pancreatic fistula, delayed gastric emptying, superficial surgical site infection (SSI), deep SSI, organ space SSI, percutaneous drainage, wound dehiscence, urinary tract infection, sepsis, septic shock, renal insufficiency, acute renal failure, stroke, cardiopulmonary resuscitation, myocardial infarction, deep venous thrombosis, pneumonia, unplanned intubation or ventilator > 48 h. The primary outcome variable was DGE, defined as the need for gastric decompression for 7 days post-operatively or the absence of oral intake by post-operative day 14. We further defined isolated DGE as an additional outcome variable of DGE occurring in the absence of any associated measured complication, including reoperation, gastrointestinal complications, pulmonary complications or infectious complications.
Table 1.
Definition of variables included in the ACS-NSQIP pancreatectomy demonstration project, November 2011 to May 2012
| Variable | Definition |
|---|---|
| Pre-operative | |
| Obstructive jaundice | Jaundice present on history and physical exam or total bilirubin < 2.0 mg/dl, at any time within 2 months of surgery |
| Biliary stent | Endoscopic or percutaneous stent placement |
| Chemotherapy | Any chemotherapy within 90 days prior to operation |
| Radiation | Any radiation within 90 days prior to operation |
| Intra-operative | |
| Operative approach | Open, laparoscopic, hand-assisted laparoscopic, laparoscopic with conversion to open, robotic, robotic with conversion to open, other hybrid approach, other |
| Pancreatic duct size | Size of pancreatic duct as indicated in preoperative imaging or operative report; <3 mm, 3–6 mm, >6 mm, or unknown |
| Pancreatic duct texture | As defined by physician in operative report; soft, intermediate, hard, or unknown |
| Vascular resection | Resection of portal vein, superior mesenteric vein, celiac artery, hepatic artery, or superior mesenteric artery |
| Pancreatic reconstruction | None, pancreaticojejunal invagination, pancreaticojejunal duct-to-mucosal, pancreaticogastrostomy, or unknown |
| Gastrojejunostomy/duodenojejunostomy | Reconstruction in antecolic or retrocolic fashion |
| Intra-operative drains | Yes or No, pancreatic anastomosis, biliary anastomosis |
| Post-operative | |
| POD no.1 drain amylase | Highest amylase level recorded from drain(s) on POD#1 |
| Last pancreatic drain removal day | Postoperative date that last pancreatic drain was removed |
| Delayed gastric emptying | Need for gastric decompression for seven days postoperatively or absence of oral intake by postoperative day 14 |
| Pancreatic fistula | Persistent drainage of amylase-rich fluid from an intraoperative drain or a clinical diagnosis; also require one of three following criteria: 1) drain in place longer than 7 days, 2) percutaneous drainage performed, or 3) reoperation performed |
| Percutaneous drainage | Any percutaneous drainage procedure performed within 30 days postoperatively. Quality of drainage recorded as amylase-rich, purulent, bilious, or other |
ACS-NSQIP, American College of Surgeons-National Surgical Quality Improvement Program; POD, post-operative day.
Statistical analysis
Summary statistics were calculated for the overall cohort. Demographic, pre-, intra-, post-operative and disease characteristics were compared in patients who did and did not develop DGE. Additional bivariate analyses were performed in patients who experienced isolated DGE compared with patients who did not experience DGE. Chi-square tests were used to identify differences in categorical variables, and t-tests were used compare continuous variables. Factors that were significantly associated with DGE in bivariate analyses were included in a multivariable logistic regression model to determine factors that were independently associated with DGE. Factors that were not significant on bivariate analysis but previously reported to be associated with DGE (diabetes, biliary stent, drain placement, pylorus preservation, deep SSI and retrocolic gastric/duodenojejunostomy) were forced into the model. An additional multivariable logistic regression model controlling for diabetes, pre-operative biliary stent, intra-operative drain placement, laparoscopic versus open approach, pylorus preservation, retrocolic versus antecolic gastrojejunostomy, vascular resection and pancreatic texture (hard versus soft) was also performed to determine factors independently associated with isolated DGE. A logistic regression model with a fixed effect for hospitals also was used to control for unobserved hospital differences and clustering of patients within hospitals. However, owing to the low number of observations within some hospitals, the model fit was poor. The findings and conclusions from the fixed effects model were similar to the logistic regression model so only the results of the logistic regression model are presented. Significance was accepted at the P < 0.05 level. Statistical analysis was carried out using SAS Version 9.2 (SAS Inc., Cary, NC, USA).
Results
From November 2011 through to May 2012, 931 patients underwent a pancreatico- duodenectomy as part of the demonstration project. After excluding patients with missing information on delayed gastric emptying or route of enteric reconstruction, the final cohort had 711 patients. Delayed gastric emptying was observed in 143 of patients (20.1%).
Pre-operative factors (Table 2)
Table 2.
Bivariate analysis of pre-operative factors associated with delayed gastric emptying
| DGE (N = 143) | No DGE (N = 568) | P-value | |
|---|---|---|---|
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
| Patient characteristics | |||
| Age | 64.9 ± 11.7 | 64.0 ± 12.2 | 0.43 |
| Gender | 0.41 | ||
| Male | 80 (55.9) | 296 (52.1) | |
| Female | 63 (44.1) | 272 (47.9) | |
| Race/ethnicity | 0.49 | ||
| White | 117 (84.2) | 479 (86.9) | |
| Black | 12 (8.6) | 46 (8.4) | |
| Other | 10 (7.2) | 26 (4.7) | |
| Wound class | 0.13 | ||
| Clean | 0 (0.0) | 6 (1.1) | |
| Clean contaminated | 114 (79.7) | 483 (85.0) | |
| Contaminated | 22 (15.4) | 65 (11.4) | |
| Dirty | 7 (4.9) | 14 (2.5) | |
| Diagnosis | 0.98 | ||
| Peri-ampullary cancer | 103 (72.0) | 417 (73.4) | |
| Pancreatitis | 17 (11.9) | 62 (10.9) | |
| Neuroendocrine tumour/carcinoid | 9 (6.3) | 33 (5.8) | |
| Other | 14 (9.8) | 56 (9.9) | |
| ASA Class (N = 707) | 0.74 | ||
| 1 | 1 (0.7) | 3 (0.5) | |
| 2 | 32 (22.4) | 147 (25.9) | |
| 3 | 103 (72.0) | 391 (68.8) | |
| 4 | 7 (4.9) | 23 (4.1) | |
| BMI | 0.92 | ||
| Underweight (<18) | 2 (1.4) | 13 (2.3) | |
| Normal weight (18 − < 25) | 53 (37.1) | 211 (37.2) | |
| Overweight (25 − < 30) | 50 (35.0) | 194 (34.2) | |
| Obese (≥30) | 38 (26.6) | 150 (26.4) | |
| Pre-operative comorbidity | |||
| Diabetes | 0.86 | ||
| Insulin dependent | 18 (12.6) | 69 (12.2) | |
| Non-insulin dependent | 15 (10.5) | 69 (12.2) | |
| No | 110 (77.0) | 430 (75.7) | |
| Smoker (% Yes) | 27 (18.9) | 122 (21.5) | 0.49 |
| Hypertension | 75 (52.5) | 306 (53.9) | 0.76 |
| Disseminated cancer | 2 (1.4) | 19 (3.4) | 0.22 |
| Steroids | 6 (4.2) | 16 (2.8) | 0.40 |
| Obstructive jaundice | 81 (56.6) | 271 (47.9) | 0.06 |
| Biliary sent | 0.16 | ||
| Yes | 76 (53.2) | 265 (46.6) | |
| No | 67 (46.8) | 303 (53.4) | |
| Pre-operative chemotherapy | 13 (9.1) | 49 (8.7) | 0.87 |
| Pre-operative radiotherapy | 4 (2.8) | 24 (4.3) | 0.42 |
DGE, delayed gastric emptying; ASA, American Society of Anesthesiologists; BMI, body mass index.
Patient characteristics did not differ significantly between patients who did or did not develop DGE in the bivariate analysis. No significant differences were observed between the two groups with regards to age, gender or race. The majority of both groups had a primary pre-operative diagnosis of peri-ampullary cancer (72.0% versus 73.4%, P = 0.98), a clean-contaminated wound class (79.7% versus 85.0%, P = 0.13) and ASA class of 3 (72.0% versus 68.8%, P = 0.74). Most patients in both groups had a body mass index (BMI) that would indicate normal weight or overweight. Patient comorbidities also were similar between the groups with no difference in the rate of diabetes (12.6% versus 12.2%, P = 0.86).
Intra-operative factors (Table 3)
Table 3.
Bivariate analysis of intra-operative factors associated with delayed gastric emptying
| DGE (N = 143) | No DGE (N = 568) | P-value | |
|---|---|---|---|
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
| Operative factors | |||
| Operative time (hours) | 6.5 ± 2.0 | 6.2 ± 2.2 | 0.13 |
| Type of operation | 0.56 | ||
| Pancreaticoduodenectomy | 140 (97.9) | 551 (97.0) | |
| Total pancreatectomy | 3 (2.1) | 17 (3.0) | |
| Laparoscopic procedure | 5 (3.5) | 25 (4.4) | 0.63 |
| Pylorus preservation | 0.40 | ||
| Yes | 66 (47.1) | 238 (43.2) | |
| No | 74 (52.9) | 313 (56.8) | |
| Texture | 0.64 | ||
| Soft | 39 (27.3) | 134 (23.8) | |
| Intermediate | 13 (9.1) | 50 (8.9) | |
| Hard | 40 (28.0) | 188 (33.4) | |
| Unknown | 51 (35.7) | 191 (33.9) | |
| Vascular resection | 0.85 | ||
| Yes | 23 (16.8) | 92 (17.5) | |
| No | 114 (83.2) | 435 (82.5) | |
| Reconstruction | 0.93 | ||
| PG | 2 (1.5) | 10 (1.8) | |
| PJ | 127 (92.7) | 506 (92.8) | |
| None | 8 (5.8) | 29 (5.3) | |
| DJ/GJ | 0.26 | ||
| Antecolic | 86 (60.1) | 370 (65.1) | |
| Retrocolic | 57 (39.9) | 198 (34.9) | |
| Intra-operative drain | 0.91 | ||
| Yes | 118 (85.5) | 457 (85.1) | |
| No | 20 (14.5) | 80 (14.9) | |
DGE, delayed gastric emptying; PG, pancreaticogastrostomy; PJ, pancreaticojejunostomy; DJ. duodenojejunostomy; GJ, gastrojejunostomy.
Operative time, type of operation, operative approach, pylorus preservation, texture of the pancreas, vascular resection, pancreatic reconstruction, gastric/duodenal reconstruction (antecolic versus retrocolic) and use of drains intra-operatively did not differ between patients who did and did not have DGE. No intra-operative complications occurred in either group.
Post-operative factors (Table 4)
Table 4.
Bivariate analysis of post-operative factors associated with delayed gastric emptying
| Postoperative Complications | DGE (N = 143) | No DGE (N = 568) | P-value |
|---|---|---|---|
| N (%) or Mean ± SD | N (%) or Mean ± SD | ||
| General | |||
| Overall morbiditya | 95 (66.4) | 209 (36.8) | <0.0001 |
| Reoperation | 15 (10.6) | 17 (3.1) | 0.0001 |
| In hospital > 30 days | 18 (12.6) | 14 (2.5) | <0.0001 |
| 30-day mortality | 4 (2.8) | 7 (1.2) | 0.17 |
| Death after 30 days | 2 (1.5) | 2 (0.4) | 0.13 |
| Overall mortality | 6 (4.6) | 9 (1.7) | 0.05 |
| Gastrointestinal | |||
| Pancreatic fistula | 44 (31.2) | 57 (10.1) | <0.0001 |
| Percutaneous drainage | 31 (23.0) | 56 (10.6) | 0.0001 |
| Infectious | |||
| Superficial SSI | 20 (14.0) | 62 (10.9) | 0.30 |
| Deep SSI | 6 (4.2) | 15 (2.6) | 0.32 |
| Organ Space SSI | 34 (23.9) | 45 (7.9) | <0.0001 |
| Wound Disruption | 6 (4.2) | 8 (1.4) | 0.03 |
| UTI | 2 (1.4) | 28 (4.9) | 0.06 |
| Sepsis | 31 (21.7) | 40 (7.0) | <0.0001 |
| Septic Shock | 13 (9.1) | 15 (2.6) | 0.0004 |
| Renal | |||
| Progressive Renal Insufficiency | 3 (2.1) | 6 (1.1) | 0.32 |
| Acute renal failure | 1 (0.70) | 2 (0.35) | 0.57 |
| Cardiovascular | |||
| Stroke | 1 (0.7) | 4 (0.7) | 0.99 |
| CPR/Arrest | 2 (1.4) | 3 (0.5) | 0.27 |
| MI | 1 (0.7) | 4 (0.7) | 0.99 |
| DVT | 3 (2.1) | 20 (3.5) | 0.39 |
| Pulmonary | |||
| Pneumonia | 12 (8.4) | 18 (3.2) | 0.0055 |
| Unplanned Intubation | 13 (9.1) | 12 (2.1) | <0.0001 |
| Pulmonary Embolus | 2 (1.4) | 6 (1.1) | 0.73 |
| Ventilator >48 h | 14 (9.8) | 11 (1.9) | <0.0001 |
DGE, delayed gastric emptying; CPR, cardiopulmonary arrest; MI, myocardial infarction; DVT, deep venous thrombosis; UTI, urinary tract infection; SSI, surgical site infection.
Overall morbidity = reoperation, fistula, percutaneous drainage, super SSI, deep SSI, organ space SSI, wound disruption, UTI, sepsis, septic shock, renal insufficiency, ARF, stroke, CPR, MI, DVT, pneumonia, unplanned intubation, or ventilator > 48 h.
Nearly half of patients (49.5%, n = 352) experienced one or more post-operative complications. Post-operative complications were much more common in patients with DGE compared with those who did not have DGE. Overall morbidity, the need for reoperation and prolonged hospitalization (>30 days) all were more common in DGE patients (all P < 0.001). Overall mortality also was higher in the DGE patients (4.6% versus 1.7%, P = 0.05). Patients who developed DGE also were more likely to have a pancreatic fistula and to require percutaneous drainage (both P < 0.001). In addition, DGE patients were more likely to have developed an organ space infection (P < 0.0001), a wound disruption (P = 0.03), sepsis (P < 0.0001) or septic shock (P < 0.001). Furthermore, DGE patients had more pulmonary complications including pneumonia (P < 0.01), unplanned intubation (P < 0.0001) and prolonged intubation (P < 0.0001).
Multivariable analysis: factors associated with delayed gastric emptying (Table 5)
Table 5.
Multivariable logistic regression analysis of factors associated with delayed gastric emptying and isolated delayed gastric emptying
| Factors | OR (95% CI) | |
|---|---|---|
| DGE | Isolated DGEa | |
| Pre-operative | ||
| Diabetes (Yes versus No) | 1.13 (0.69–1.83) | 1.31 (0.67–2.57) |
| Biliary stent (Yes versus No) | 1.39 (0.92–2.10) | 1.30 (0.70–2.41) |
| Intra-operative | ||
| Drain placement (Yes versus No) | 0.92 (0.51–1.66) | 1.10 (0.47–2.60) |
| Pylorus preservation (Yes versus No) | 1.11 (0.73–1.69) | 0.99 (0.52–1.86) |
| Retrocolic versus antecolic gastrojejunostomy | 1.20 (0.78–1.85) | 1.67 0.90–3.13) |
| Pancreatic texture (soft versus hard) | NA | 0.50 (0.19–1.32) |
| Vascular resection (Yes versus No) | NA | 0.85 (0.37–1.92) |
| Post-operative | ||
| Deep SSI (Yes versus No) | 1.16 (0.40–3.34) | NA |
| Organ SSI (Yes versus No) | 1.79 (0.93–3.46) | NA |
| Pancreatic fistula (Yes versus No) | 3.05 (1.76–5.27) | NA |
| Sepsis (Yes versus No) | 2.06 (1.09–3.90) | NA |
| Reoperation (Yes versus No) | 2.54 (1.05–6.18) | NA |
Isolated DGE: DGE excluding any associated complication (reoperation, gastrointestinal, pulmonary, or infectious complications), n = 52. SSI, surgical site infection, NA, not applicable.
In the multivariable analysis, only post-operative complications were associated with delayed gastric emptying. In the final model, a post-operative pancreatic fistula [odds ratio (OR) 3.05, 95% confidence interval (CI) 1.76–5.27], post-operative sepsis (OR 2.06, 95% CI 1.09–3.90) and the need for reoperation (OR 2.54, 95% CI 1.05–6.18) were independently associated with delayed gastric emptying (Fig. 1). Diabetes, a pre-operative biliary stent, pylorus preservation, intra-operative drain placement, position of the gastro/duodenojejunostomy (antecolic versus retrocolic) and surgical site infections were not associated with delayed gastric emptying.
Figure 1.
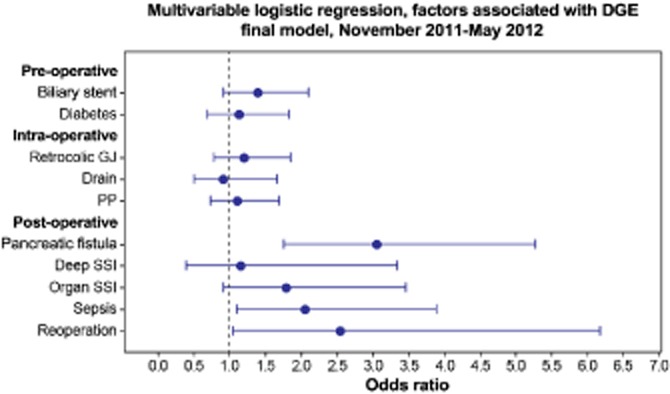
Forest plot, multivariable logistic regression analysis of factors associated with delayed gastric emptying. This figure demonstrates the observed odds ratio (OR) for 10 pre-, intra- and post-operative variables on the development of delayed gastric emptying (DGE) in a multivariable model. The variables of interest are plotted on the y-axis and OR with 95% confidence interval (CI) on the x-axis. In this adjusted analysis, only sepsis (OR 2.06, 95% CI 1.09–3.90), post-operative pancreatic fistula (OR 3.05, 95% CI 1.76-5.27) and reoperation (OR 2.54, 95% CI 1.05–6.18) were independently associated with DGE. PP, pylorus preservation; SSI, surgical site infection; fistula, pancreatic fistula; Retrocolic GJ, retrocolic gastrojejunostomy
Isolated DGE
After excluding patients with DGE who also underwent reoperation or experienced pulmonary, gastrointestinal and infectious complications (n = 91), 52 patients (8.4%) experienced isolated DGE. In a bivariate analysis, patients who experienced isolated DGE did not differ significantly from patients without DGE with regards to operative factors, including pylorus preservation, pancreatic reconstruction and intra-operative drain placement. The rate of DGE did not differ significantly in patients who underwent an antecolic enteric reconstruction versus retrocolic reconstruction (7.3% versus 10.4%, P = 0.18). In the multivariable model, we did not identify any factors that were independently associated with isolated DGE, but there was a trend towards increased DGE with retrocolic reconstruction (OR 1.67, 95% CI 0.90–3.13, Table 5).
Discussion
This analysis is the largest multi-institutional study of delayed gastric emptying after a pancreatic resection to date. By including both high- and low-volume centres, with the number of cases per hospital ranging from 3 to 213, we were able to evaluate factors associated with delayed gastric emptying in a ‘real world’ setting. In spite of previous evidence suggesting that operative factors such as pylorus-preservation and the position of the gastro/duodenojejunostomy influence DGE, these potential factors were not significant in our multivariable analysis. In addition, diabetes, which is known to be associated with gastroparesis, and other patient characteristics had no effect on DGE in our analysis. It was demonstrated that only the development of post-operative complications, specifically a pancreatic fistula, sepsis and the need for reoperation was strongly associated with delayed gastric emptying. Patients with DGE had significantly higher operative mortality and overall morbidity, were more likely to require hospitalization longer than 30 days and were more likely to have associated infections and pulmonary complications compared with those who did not have DGE.
Our findings are consistent with previous retrospective studies demonstrating that intra-abdominal complications are strongly associated with DGE. In a review of 260 patients, Malleo et al.30 demonstrated that post-operative pancreatic and biliary fistulae were independently associated with DGE, whereas operative factors such as duration of operation and retrocolic/antecolic gastric/duodenal reconstruction were not. Another similar study by Lermite and associates of 131 patients found that DGE was more common when other intra-abdominal complications occurred.35 These studies and the present are limited by a retrospective design, and as a result it cannot be determined if DGE occurred secondarily to these complications or if these complications were consequences of DGE.
Analyses evaluating the effect of operative factors on DGE have demonstrated mixed results. Some previous studies have found that antecolic compared with retrocolic gastric/duodenal reconstruction is associated with decreased DGE,21,40–42 whereas others have found no difference with retrocolic reconstruction. 13,22,23 Pylorus preservation was once considered to be a risk factor for DGE, but more recent prospective randomized trials have found no difference between pylorus preservation and the classic Whipple operation.14,16,20 In addition, two studies of pylorus preservation compared with the classic Whipple resection and DGE have found that DGE occurred exclusively in the presence of other complications, regardless of the choice of operation.13,15 Similar to the findings from these prospective data, we did not find any intra-operative variable that played a significant role in the development of DGE. We also did not observe any effect of operative factors on isolated DGE for the small sample of patients who experienced DGE in the absence of other complications.
None of the 16 pre-operative factors that were measured were associated with post-operative delayed gastric emptying. There is no mechanism to explain an association between DGE and many of these characteristics, such as age, gender and race/ethnicity. However, other factors, such as diabetes, diagnosis, wound class and ASA class have been suggested to play a role in delayed gastric emptying. Diabetes is a known risk factor for gastroparesis, but its role in patients undergoing a pancreatic resection and subsequent DGE is not well known, and it has not been confirmed to be a risk factor for DGE.43 Obstructing duodenal tumours may have also played a role in DGE, but we are limited by our sample size to draw any definitive conclusions in this regard.
The association between delayed gastric emptying and other post-operative complications observed in this analysis is understandable. First, as others have observed,30 pancreatic fistulae and organ space infections are the underlying cause of delayed gastric emptying in the majority of patients. These post-operative complications also lead to sepsis, unplanned intubation, wound disruptions, re-operations, prolonged hospitalizations and increased mortality. As a result, these other post-operative complications, while associated with DGE, do not necessarily represent direct consequences of DGE. We did not observe an association between delayed gastric emptying and cardiovascular or renal complications.
The present study has several limitations. The most significant limitation is in qualifying the association between DGE and post-operative complications. In using the ACS-NSQIP method for gathering data we are unable to determine if post-operative complications preceded DGE or vice versa. Therefore, we are not able to draw any conclusions regarding the causative roles of post-operative complications in the development of delayed gastric emptying. For instance, it would be reasonable to conclude that DGE is a consequence of reoperation and not the cause, but identifying this temporal relationship is difficult using retrospective data. Nonetheless, our results, along with those of others32,36 implicate intra-abdominal complications as factors strongly associated with DGE.
We defined delayed gastric emptying as the need for gastric decompression for 7 days post-operatively or an inability to tolerate oral intake by post-operative day 14. This definition is consistent with grades B and C DGE as defined by the International Study Group of Pancreatic Surgery definition for delayed gastric emptying and was chosen to represent clinically significant delayed gastric emptying.30,44 However, this definition is not specific to patients who experienced DGE, and the need for gastric decompression may occur with other disease processes. For example, patients who required prolonged intubation for 14 days post-operatively owing to a post-operative pneumonia will be defined as having DGE, while this is not their primary disease process. This is evidenced by the finding of an association between pulmonary complications and DGE in our bivariate analysis. However, the incidence of pulmonary complications was low in our cohort (<10%), and this is unlikely to affect our analyses. In addition, the incidence of DGE in our cohort is comparable to findings of other previous studies,1–4 making an overestimation of DGE unlikely.
We also performed an additional multivariable analysis of the 52 patients who experienced isolated DGE and determined no effect of operative factors. There was a trend towards increased DGE in patients with retrocolic reconstruction, but this was not statistically significant. However, this analysis is limited by a small sample size and as a result is subject to a Type II error.
This study is also limited by the other variables available in our dataset. The ACS-NSQIP Pancreatectomy Demonstration Project does not include information on operative-specific variables such as length of the duodenal cuff, gastric/duodenal devascularization, or preservation of the right gastric artery. Post-procedure practice measures such as the use of prokinetic agents including erythromycin12 or metoclopramide were also not recorded. The NSQIP database also does not include information on the severity of post-operative complications that required a specific pharmacological or procedural intervention, as has been suggested by the Dindo–Clavien system.45
In addition, we excluded 220 patients in whom information was missing (190 on enteric reconstruction, 14 on DGE, 16 missing both), or 24% of the total cohort. As a result, the analysis is subject to potential sampling bias, and the population in our study may not be entirely representative of the population at large. As a result we performed an additional analysis on these patients with missing information and did not identify a systematic pattern in missing data over the study period or by hospital volume. We are blinded to institutional data so we were not able to identify specific patient or hospital characteristics, only the number of cases submitted. In addition, we believe the characteristics of our patient population and proportion that experienced post-operative complications is consistent with those established in the literature.1 Finally, the sample size was relatively small, and our analysis may have lacked sufficient power to detect significant associations between operative factors and DGE. In the adjusted analysis, antecolic reconstruction and pylorus preservation were weakly associated with increased DGE, but these associations were not statistically significant. Nevertheless, the present study is the largest multi-institutional analysis of delayed gastric emptying after a pancreatic resection to date.
In conclusion, post-operative complications were identified to be strongly associated with delayed gastric emptying, whereas operative factors such as pylorus preservation and antecolic reconstruction were not associated with DGE. In considering delayed gastric emptying, surgeons should perform the operative procedure of their own choosing. These findings were prospectively collected at both academic medical centres and community hospitals, providing more clinically relevant circumstances in which to study pancreatic surgery. Single institution studies lack the power or external validity to adequately study complications of pancreatic surgery. In comparison, the Pancreatectomy Demonstration Project within ACS-NSQIP is a multi-institutional collaborative, and a potent resource to improve evidence-based practice.
Acknowledgments
Supported by grants from the UTMB Clinical and Translational Science Award #UL1TR000071 and NIH T-32 Grant # 5T32DK007639.
This study would not have been possible without the collaboration of the following institutions: Albany Medical Center, Baptist Memorial Healthcare – Memphis, Baystate Medical Center, Boston Medical Center, Brigham & Women's Hospital, California Pacific Medical Center, Emory University Hospital, Hospital of the University of Pennsylvania, Indiana University Health – Methodist Hospital, Indiana University Hospital, Intermountain Medical Center, Johns Hopkins Hospital, Kaiser Permanente – San Francisco, Lehigh Valley Hospital, Massachusetts General Hospital, Oregon Health and Science University, Penn State Milton S. Hershey Medical Center, Providence Portland Medical Center, Sacred Heart Medical Center, Stanford Hospital and Clinics, Tampa General Hospital, The Ohio State University Medical Center, Thomas Jefferson University Hospital, University of California Irvine, University of California San Diego Medical Center, University of Iowa Hospital and Clinics, University of Kentucky Chandler Medical Center, University of Texas Medical Branch, University of Wisconsin Hospital and Clinics, Vidant Medical Center, Wake Forest University Baptist Medical Center, Washington University/Barnes Jewish Hospital and Winthrop University.
Conflicts of interest
Dr. Bruce L. Hall is a paid consultant to the American College of Surgeons National Surgical Quality Improvement Program.
References
- 1.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–257. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büchler MW, Friess H, Wagner M, Kulli C, Wagener V, Z'Graggen K. Pancreatic fistula after pancreatic head resection. Br J Surg. 2000;87:883–889. doi: 10.1046/j.1365-2168.2000.01465.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsao JI, Rossi RL, Lowell JA. Pylorus-preserving pancreatoduodenectomy. Is it an adequate cancer operation. Arch Surg. 1994;129:405–412. doi: 10.1001/archsurg.1994.01420280081010. [DOI] [PubMed] [Google Scholar]
- 4.Gouma DJ, Nieveen van Dijkum EJ, Obertop H. The standard diagnostic work-up and surgical treatment of pancreatic head tumours. Eur J Surg Oncol. 1999;25:113–123. doi: 10.1053/ejso.1998.0612. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M. Gastroparesis after a pylorus-preserving pancreatoduodenectomy. Surg Today. 2005;35:345–350. doi: 10.1007/s00595-004-2961-8. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer CJ. Cost and outcome of the Whipple procedure. Ann Surg. 1995;222:211–212. [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995;221:43–49. doi: 10.1097/00000658-199501000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edge SB, Schmieg RE, Rosenlof LK, Wilhelm MC. Pancreas cancer resection outcome in American University centers in 1989–1990. Cancer. 1993;71:3502–3508. doi: 10.1002/1097-0142(19930601)71:11<3502::aid-cncr2820711107>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad SA, Edwards MJ, Sutton JM, Grewal SS, Hanseman DJ, Maithel SK, et al. Factors influencing readmission after pancreaticoduodenectomy: a multi-institutional study of 1302 patients. Ann Surg. 2012;256:529–537. doi: 10.1097/SLA.0b013e318265ef0b. [DOI] [PubMed] [Google Scholar]
- 10.Roder JD, Stein HJ, Hüttl W, Siewert JR. Pylorus-preserving versus standard pancreatico-duodenectomy: an analysis of 110 pancreatic and periampullary carcinomas. Br J Surg. 1992;79:152–155. doi: 10.1002/bjs.1800790219. [DOI] [PubMed] [Google Scholar]
- 11.Klinkenbijl JH, van der Schelling GP, Hop WC, van Pel R, Bruining HA, Jeekel J. The advantages of pylorus-preserving pancreatoduodenectomy in malignant disease of the pancreas and periampullary region. Ann Surg. 1992;216:142–145. doi: 10.1097/00000658-199208000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeo CJ, Barry MK, Sauter PK, Sostre S, Lillemoe KD, Pitt HA, et al. Erythromycin accelerates gastric emptying after pancreaticoduodenectomy. A prospective, randomized, placebo-controlled trial. Ann Surg. 1993;218:229–237. doi: 10.1097/00000658-199309000-00002. discussion 37–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Berge Henegouwen MI, van Gulik TM, DeWit LT, Allema JH, Rauws EA, Obertop H, et al. Delayed gastric emptying after standard pancreaticoduodenectomy versus pylorus-preserving pancreaticoduodenectomy: an analysis of 200 consecutive patients. J Am Coll Surg. 1997;185:373–379. doi: 10.1016/s1072-7515(97)00078-1. [DOI] [PubMed] [Google Scholar]
- 14.Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br J Surg. 1999;86:603–607. doi: 10.1046/j.1365-2168.1999.01074.x. [DOI] [PubMed] [Google Scholar]
- 15.Horstmann O, Markus PM, Ghadimi MB, Becker H. Pylorus preservation has no impact on delayed gastric emptying after pancreatic head resection. Pancreas. 2004;28:69–74. doi: 10.1097/00006676-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Tran KT, Smeenk HG, van Eijck CH, Kazemier G, Hop WC, Greve JW, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738–745. doi: 10.1097/01.sla.0000143248.71964.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosca F, Giulianotti PC, Balestracci T, Di Candio G, Pietrabissa A, Sbrana F, et al. Long-term survival in pancreatic cancer: pylorus-preserving versus Whipple pancreatoduodenectomy. Surgery. 1997;122:553–566. doi: 10.1016/s0039-6060(97)90128-8. [DOI] [PubMed] [Google Scholar]
- 18.Di Carlo V, Zerbi A, Balzano G, Corso V. Pylorus-preserving pancreaticoduodenectomy versus conventional whipple operation. World J Surg. 1999;23:920–925. doi: 10.1007/s002689900600. [DOI] [PubMed] [Google Scholar]
- 19.Seiler CA, Wagner M, Sadowski C, Kulli C, Büchler MW. Randomized prospective trial of pylorus-preserving vs. Classic duodenopancreatectomy (Whipple procedure): initial clinical results. J Gastrointest Surg. 2000;4:443–452. doi: 10.1016/s1091-255x(00)80084-0. [DOI] [PubMed] [Google Scholar]
- 20.Seiler CA, Wagner M, Bachmann T, Redaelli CA, Schmied B, Uhl W, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92:547–556. doi: 10.1002/bjs.4881. [DOI] [PubMed] [Google Scholar]
- 21.Hartel M, Wente MN, Hinz U, Kleeff J, Wagner M, Müller MW, et al. Effect of antecolic reconstruction on delayed gastric emptying after the pylorus-preserving Whipple procedure. Arch Surg. 2005;140:1094–1099. doi: 10.1001/archsurg.140.11.1094. [DOI] [PubMed] [Google Scholar]
- 22.Eshuis WJ, van Dalen JW, Busch OR, van Gulik TM, Gouma DJ. Route of gastroenteric reconstruction in pancreatoduodenectomy and delayed gastric emptying. HPB. 2012;14:54–59. doi: 10.1111/j.1477-2574.2011.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oida T, Mimatsu K, Kano H, Kawasaki A, Fukino N, Kida K, et al. Antecolic and retrocolic route on delayed gastric emptying after MSSPPD. Hepatogastroenterology. 2012;59:1274–1276. doi: 10.5754/hge10113. [DOI] [PubMed] [Google Scholar]
- 24.Paraskevas KI, Avgerinos C, Manes C, Lytras D, Dervenis C. Delayed gastric emptying is associated with pylorus-preserving but not classical Whipple pancreaticoduodenectomy: a review of the literature and critical reappraisal of the implicated pathomechanism. World J Gastroenterol. 2006;12:5951–5958. doi: 10.3748/wjg.v12.i37.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellner UF, Sick O, Olschewski M, Adam U, Hopt UT, Keck T. Randomized controlled single-center trial comparing pancreatogastrostomy versus pancreaticojejunostomy after partial pancreatoduodenectomy. J Gastrointest Surg. 2012;16:1686–1695. doi: 10.1007/s11605-012-1940-4. [DOI] [PubMed] [Google Scholar]
- 26.Müller MW, Friess H, Beger HG, Kleeff J, Lauterburg B, Glasbrenner B, et al. Gastric emptying following pylorus-preserving Whipple and duodenum-preserving pancreatic head resection in patients with chronic pancreatitis. Am J Surg. 1997;173:257–263. doi: 10.1016/S0002-9610(96)00402-3. [DOI] [PubMed] [Google Scholar]
- 27.Miller LJ, Clain JE, Malagelada JR, Go VL. Control of human postprandial pancreatic exocrine secretion: a function of the gastroduodenal region. Dig Dis Sci. 1979;24:150–154. doi: 10.1007/BF01324743. [DOI] [PubMed] [Google Scholar]
- 28.Grace PA, Pitt HA, Longmire WP. Pancreatoduodenectomy with pylorus preservation for adenocarcinoma of the head of the pancreas. Br J Surg. 1986;73:647–650. doi: 10.1002/bjs.1800730824. [DOI] [PubMed] [Google Scholar]
- 29.Itani KM, Coleman RE, Meyers WC, Akwari OE. Pylorus-preserving pancreatoduodenectomy. A clinical and physiologic appraisal. Ann Surg. 1986;204:655–664. doi: 10.1097/00000658-198612000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malleo G, Crippa S, Butturini G, Salvia R, Partelli S, Rossini R, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB. 2010;12:610–618. doi: 10.1111/j.1477-2574.2010.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt DR, McLean R. Pylorus-preserving pancreatectomy: functional results. Br J Surg. 1989;76:173–176. doi: 10.1002/bjs.1800760223. [DOI] [PubMed] [Google Scholar]
- 32.Park YC, Kim SW, Jang JY, Ahn YJ, Park YH. Factors influencing delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Am Coll Surg. 2003;196:859–865. doi: 10.1016/S1072-7515(03)00127-3. [DOI] [PubMed] [Google Scholar]
- 33.Miedema BW, Sarr MG, van Heerden JA, Nagorney DM, McIlrath DC, Ilstrup D. Complications following pancreaticoduodenectomy. Current management. Arch Surg. 1992;127:945–949. doi: 10.1001/archsurg.1992.01420080079012. discussion 9–50. [DOI] [PubMed] [Google Scholar]
- 34.Murakami H, Suzuki H, Nakamura T. Pancreatic fibrosis correlates with delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy with pancreaticogastrostomy. Ann Surg. 2002;235:240–245. doi: 10.1097/00000658-200202000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lermite E, Pessaux P, Brehant O, Teyssedou C, Pelletier I, Etienne S, et al. Risk factors of pancreatic fistula and delayed gastric emptying after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg. 2007;204:588–596. doi: 10.1016/j.jamcollsurg.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 36.You D, Jung K, Lee H, Heo J, Choi S, Choi D. Comparison of different pancreatic anastomosis techniques using the definitions of the International Study Group of Pancreatic Surgery: a single surgeon's experience. Pancreas. 2009;38:896–902. doi: 10.1097/MPA.0b013e3181b365f7. [DOI] [PubMed] [Google Scholar]
- 37.American College of Surgeons-National Surgical Quality Improvement Program: American College of Surgeons. 2013. Available at http://site.acsnsqip.org/downloads/ [last accessed January 2013]
- 38.Pitt HA, Kilbane M, Strasberg SM, Pawlik TM, Dixon E, Zyromski NJ, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB. 2009;11:405–413. doi: 10.1111/j.1477-2574.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh P, Shiloach M, Cohen ME, Bilimoria KY, Ko CY, Hall BL, et al. Pancreatectomy risk calculator: an ACS-NSQIP resource. HPB. 2010;12:488–497. doi: 10.1111/j.1477-2574.2010.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakagawa N, et al. An antecolic Roux-en Y type reconstruction decreased delayed gastric emptying after pylorus-preserving pancreatoduodenectomy. J Gastrointest Surg. 2008;12:1081–1086. doi: 10.1007/s11605-008-0483-1. [DOI] [PubMed] [Google Scholar]
- 41.Nikfarjam M, Kimchi ET, Gusani NJ, Shah SM, Sehmbey M, Shereef S, et al. A reduction in delayed gastric emptying by classic pancreaticoduodenectomy with an antecolic gastrojejunal anastomosis and a retrogastric omental patch. J Gastrointest Surg. 2009;13:1674–1682. doi: 10.1007/s11605-009-0944-1. [DOI] [PubMed] [Google Scholar]
- 42.Tani M, Terasawa H, Kawai M, Ina S, Hirono S, Uchiyama K, et al. Improvement of delayed gastric emptying in pylorus-preserving pancreaticoduodenectomy: results of a prospective, randomized, controlled trial. Ann Surg. 2006;243:316–320. doi: 10.1097/01.sla.0000201479.84934.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu CK, Mazo AE, Sarmiento JM, Staley CA, Adsay NV, Umpierrez GE, et al. Impact of diabetes mellitus on perioperative outcomes after resection for pancreatic adenocarcinoma. J Am Coll Surg. 2010;210:463–473. doi: 10.1016/j.jamcollsurg.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 44.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–768. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]


