Abstract
Objectives
Hepatectomy patients are known to be at significant risk for venous thromboembolism (VTE), but previous studies have not differentiated pre- versus post-discharge events. This study was designed to evaluate the timing, rate and predictors of pre- (‘early’) versus post-discharge (‘late’) VTE.
Methods
All patients undergoing elective hepatectomy during 2005–2010 and recorded in the American College of Surgeons National Surgical Quality Improvement Program participant use file were identified. Perioperative factors associated with 30-day rates of early and late VTE were analysed.
Results
A total of 7621 patients underwent 4553 (59.7%) partial, 802 (10.5%) left, 1494 (19.6%) right and 772 (10.1%) extended hepatectomies. Event rates were 1.9% for deep venous thrombosis, 1.2% for pulmonary embolus and 2.8% for VTE. Of instances of VTE, 28.6% occurred post-discharge. The median time of presentation of late VTE was postoperative day 14. Multivariate analysis determined that early VTE was associated with age ≥75 years [odds ratio (OR) 1.92, P = 0.007], male gender (OR 1.87, P = 0.002), intraoperative transfusion (OR 2.49, P < 0.001), operative time of >240 min (OR 2.28, P < 0.001), organ space infection (OSI) (OR 2.60, P < 0.001), and return to operating room (ROR) (OR 3.25, P < 0.001). Late VTE was associated with operative time of >240 min (OR 2.35, P = 0.008), OSI (OR 3.78, P < 0.001) and ROR (OR 2.84, P = 0.011).
Conclusions
Late VTE events occur in patients with clearly identifiable intraoperative and postoperative risk factors. This provides a rationale for the selective use of post-discharge VTE chemoprophylaxis in high-risk patients.
Introduction
Until recently, many liver surgeons have withheld postoperative venous thromboembolism (VTE) chemoprophylaxis due to a perceived increased risk for perioperative bleeding.1 This practice has continued despite increasing evidence over the past two decades demonstrating the benefits of incorporating postoperative VTE chemoprophylaxis within the standard of care in major abdominal surgery, especially for cancer patients.2–8 The historical fear of bleeding was supported by the popular belief that transient postoperative liver insufficiency, especially after major and extended hepatectomies, would make the patient's blood ‘thin’ and thus resistant to the VTE commonly seen in general surgery patients. In a previous study, the current authors disproved that paradigm by demonstrating that hepatectomy patients were at significantly increased risk for postoperative VTE, especially those who had undergone major hepatectomy or longer operations and those who suffered postoperative organ space infection (OSI) or bile leaks.1
Most guidelines for VTE prevention recommend the use of postoperative chemoprophylaxis for major abdominal surgery, especially in cancer patients, as long as the risk for bleeding does not outweigh that for VTE.5,9,10 Several studies have shown the benefit of longer-term, post-discharge chemoprophylaxis in high-risk patients.9–12 However, prolonged VTE chemoprophylaxis has significant implications, including prescription costs, patient discomfort and bleeding risk.10 The optimal strategy for the prevention of late VTE would, therefore, be to focus post-discharge chemoprophylaxis only on patients who are at high risk for late VTE. As postoperative chemoprophylaxis is still not uniformly given to all hepatectomy patients, asking liver surgeons to give even longer prescriptions beyond discharge seems impractical without proof that high-risk patients do exist. To appropriately influence practice patterns, it is first necessary to establish evidence that post-discharge VTE represents a significant proportion of all postoperative VTE. Secondly, a way to identify high-risk patients who might benefit from extended chemoprophylaxis must be established rather than prescribing it to all hepatectomy patients.
A multi-institution national database, such as that of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), facilitates the analysis of risk factors associated with major surgical complications simply because its scale is much greater than that of smaller institutional databases, especially for hepatobiliary surgery.13 The ACS-NSQIP allows surgeons to look beyond the bias and small-number limitations of single-institution databases to study surgical morbidity and mortality event rates and risk factors on a broader scale, which can more accurately describe overall trends and identify areas in need of surgical quality improvement.14
The hypothesis of this study was that a certain subset of post-hepatectomy patients are at risk for post-discharge VTE and that certain clinical factors might help surgeons identify these patients at high risk for late VTE. To test this hypothesis, this study was designed to evaluate the rates and timing of, and risk factors for, pre-discharge versus post-discharge VTE after hepatectomy.
Materials and methods
Data acquisition, patients and definitions
All hepatectomy procedures carried out in 2005–2010 and recorded in the ACS-NSQIP participant use file (PUF) were identified. Patients who underwent emergency operations and wedge resections [current procedural terminology (CPT) code 47100] were excluded; all remaining patients were included for analysis. The extent of liver resection was classified according to the primary CPT code and included in order of increasing magnitude partial (CPT 47120), left (CPT 47125), right (CPT 47130) and extended (CPT 47122) hepatectomies. Risk factors for pre-discharge and post-discharge VTE were derived from the analysis of the full spectrum of NSQIP-collected clinical factors.
The preoperative NSQIP risk factors assessed included age, sex, race, weight/body mass index (BMI; obesity was defined as BMI of ≥30 kg/m2), performance status, albumin, haematocrit, platelets, blood urea nitrogen, creatinine, partial thrombin time, international normalized ratio (INR), alkaline phosphatase, aspartate aminotransferase (AST), bilirubin, white blood cell count, American Society of Anesthesiologists (ASA) class, smoking, chronic obstructive pulmonary disease, pneumonia, sepsis, disseminated cancer, diabetes, bleeding disorder, ascites, preoperative chemotherapy, preoperative radiation therapy, preoperative transfusion, previous operation within 30 days, and preoperative hospitalization.
Intraoperative variables included operative time, intraoperative transfusion, extent of hepatectomy, concurrent major operation, and radiofrequency ablation. Concurrent major operations included gastrointestinal resection, gastrointestinal anastomosis, biliary resection/reconstruction, thoracic operation and ventral hernia repair. Major operations were not considered to include cholecystectomy, vena caval repair, diaphragm repair, lymphadenectomy or diagnostic laparoscopy.
Postoperative metrics included postoperative transfusion, bleeding transfusion (major transfusion of >4 units of blood within 72 h after surgery), return to operating room (ROR) within the same hospitalization, renal insufficiency or failure, respiratory failure, cardiac arrest, myocardial infarction, postoperative sepsis/septic shock, surgical site infection, OSI (abscess/biloma), wound disruption (fascial dehiscence), length of stay (LoS), and mortality, as standardized and defined by the NSQIP.13 Based on the limitations intrinsic to NSQIP data, post-hepatectomy mortality was defined as death within 30 days post-surgery, or death at a later date if the patient was hospitalized continuously from surgery to the date of death.
The analysis of post-hepatectomy thrombotic events within 30 days of hepatectomy focused on deep vein thrombosis (DVT), pulmonary embolism (PE) and the combination of the two. Venous thromboembolism was defined as a clinically detected DVT or PE. Patients documented to have both DVT and PE were labelled only once for VTE. The timing of VTE was compared with the discharge date and stratified as ‘early’ (pre-discharge) or ‘late’ (post-discharge). The minimum number of patients needed to treat (NNT) to prevent a clinically apparent VTE event as defined by NSQIP criteria was estimated for various at-risk populations based on late VTE rates and hypothetical cohort sizes treated with extended VTE chemoprophylaxis on the assumption that chemoprophylaxis would prevent VTE events. This was calculated using this formula: 1/(VTE rate, %).
Statistical analysis
The associations of clinical factors with early and late VTE were analysed using the chi-squared test or Fisher's exact test for non-parametric categorical data and the Mann–Whitney U-test for non-parametric continuous data. To determine independent associations, significant univariate risk factors (i.e. P < 0.05) were entered into a multivariate logistic regression model. In subsequent analyses, the ‘high-risk’ VTE group was defined as having at least one independent risk factor from the multivariate model. Statistical analyses were performed using IBM spss Statistics 19 (IBM Corp., Armonk, NY, USA). All tests were two-sided. Statistical significance was indicated by a P-value of < 0.05.
Results
Patients and hepatectomies analysed
For the period 2005–2010, a total of 7621 patients who met the study inclusion criteria were identified. The median age of these patients was 60 years (range: 17–90 years). A total of 3931 (51.6%) were female and 5735 (75.3%) were of White European ethnicity. Liver resections included 4553 (59.7%) partial, 802 (10.5%) left, 1494 (19.6%) right and 772 (10.1%) extended hepatectomies. Indications for surgery included malignant diagnoses in 6508 (85.4%) patients. There was no difference in VTE rates between patients with malignant (3.0%) and benign (2.1%) indications (P = 0.173). Other clinically relevant preoperative, intraoperative and postoperative factors are described in Table 1.
Table 1.
Factors associated with no venous thromboembolism (VTE), early VTE and late VTE
| Clinical characteristic | All patients (n = 7621) | No VTE (DVT or PE) | Early (pre-discharge) VTE | Late (post-discharge) VTE | Early versus none | Late versus none | Early versus late | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n or median | % | n or median | % | n or median | % | n or median | % | P-value | P-value | P-value | |
| Patients | 7621 | 100% | 7411 | 97.2% | 150 | 2.1 | 60 | 0.8% | |||
| Preoperative factors | |||||||||||
| Age, years, median (range) | 60 (17–90) | 60 (17–90) | 65 (23–90) | 62 (26–83) | <0.001 | 0.616 | 0.066 | ||||
| Age ≥75 years | 894 | 11.7% | 860 | 11.6% | 29 | 19.3% | 5 | 8.3% | 0.004 | 0.546 | 0.051 |
| Sex, male | 3690 | 48.4% | 3556 | 48.0% | 98 | 65.3% | 36 | 60.0% | <0.001 | 0.175 | 0.600 |
| Race, White | 5735 | 75.3% | 5561 | 75.0% | 122 | 81.3% | 52 | 86.7% | 0.077 | 0.038 | 0.354 |
| Lack of functional independence | 148 | 1.9% | 138 | 1.9% | 7 | 4.7% | 3 | 5.0% | 0.013 | 0.075 | 1.000 |
| Albumin <4 g/dl | 2837 | 37.2% | 2738 | 36.9% | 77 | 51.3% | 22 | 36.7% | <0.001 | 0.965 | 0.054 |
| Alkaline phosphatase >93 IU/l | 3349 | 43.9% | 3240 | 43.7% | 85 | 56.7% | 24 | 40.0% | 0.002 | 0.563 | 0.029 |
| AST ≥30 IU/l | 2947 | 38.7% | 2847 | 38.4% | 73 | 48.7% | 27 | 45.0% | 0.011 | 0.296 | 0.631 |
| Haematocrit < 39% | 3435 | 45.1% | 3332 | 45.0% | 83 | 55.3% | 20 | 33.3% | 0.011 | 0.071 | 0.004 |
| BUN ≥20 mg/dl | 1205 | 15.8% | 1159 | 15.6% | 34 | 22.7% | 12 | 20.0% | 0.019 | 0.355 | 0.673 |
| ASA class ≥3 | 5074 | 66.6% | 4913 | 66.3% | 120 | 80.0% | 41 | 68.3% | <0.001 | 0.739 | 0.071 |
| Intraoperative factors | |||||||||||
| Operative time, min, median (range) | 220 (7–1029) | 218 (7–1029) | 298 (100–780) | 273 (115–734) | <0.001 | <0.001 | 0.112 | ||||
| Operative time >240 min | 3283 | 43.1% | 3133 | 42.3% | 110 | 73.3% | 40 | 66.7% | <0.001 | <0.001 | 0.398 |
| Any intraoperative transfusion | 1514 | 26.6% | 1431 | 25.9% | 68 | 57.1% | 15 | 31.3% | <0.001 | 0.397 | 0.002 |
| RBC ≥2 units | 1207 | 21.2% | 1134 | 20.5% | 61 | 51.3% | 12 | 25.0% | <0.001 | 0.443 | 0.002 |
| RBC ≥4 units | 523 | 9.2% | 486 | 8.8% | 30 | 25.2% | 7 | 14.6% | <0.001 | 0.159 | 0.135 |
| Extent of hepatectomy | <0.001 | 0.006 | 0.024 | ||||||||
| Partial | 4553 | 59.7% | 4458 | 60.2% | 64 | 42.7% | 31 | 51.7% | |||
| Left | 802 | 10.5% | 787 | 10.6% | 12 | 8.0% | 3 | 5.0% | |||
| Right | 1494 | 19.6% | 1433 | 19.3% | 39 | 26.0% | 22 | 36.7% | |||
| Extended | 772 | 10.1% | 733 | 9.9% | 35 | 23.3% | 4 | 6.7% | |||
| Partial versus left/right/extended | <0.001 | 0.181 | 0.237 | ||||||||
| Right/extended versus left/partial | <0.001 | 0.017 | 0.432 | ||||||||
| Right/extended | 2266 | 29.7% | 2166 | 29.2% | 74 | 49.3% | 26 | 43.3% | |||
| Left/partial | 5355 | 70.3% | 5245 | 70.8% | 76 | 50.7% | 34 | 56.7% | |||
| Biliary resection or anastomosis | 364 | 4.8% | 340 | 4.6% | 20 | 13.3% | 4 | 6.7% | <0.001 | 0.360 | 0.231 |
| Concurrent major GI or abdominal case | 912 | 12.0% | 867 | 11.7% | 36 | 24.0% | 9 | 15.0% | <0.001 | 0.419 | 0.151 |
| Postoperative factors | |||||||||||
| Any transfusion | 494 | 6.5% | 469 | 6.3% | 22 | 14.7% | 3 | 5.0% | <0.001 | 1.000 | 0.060 |
| Bleeding transfusion only | 46 | 0.6% | 39 | 0.5% | 6 | 4.0% | 1 | 1.7% | <0.001 | 0.276 | 0.676 |
| Return to operating room | 335 | 4.4% | 293 | 4.0% | 30 | 20.0% | 12 | 20.0% | <0.001 | <0.001 | 1.000 |
| Renal insufficiency/failure | 169 | 2.2% | 150 | 2.0% | 16 | 10.7% | 3 | 5.0% | <0.001 | 0.124 | 0.288 |
| Any SSI, wound disruption | 895 | 11.7% | 828 | 11.2% | 47 | 31.3% | 20 | 33.3% | <0.001 | <0.001 | 0.779 |
| Organ space infection | 478 | 6.3% | 430 | 5.8% | 33 | 22.0% | 15 | 25.0% | <0.001 | <0.001 | 0.640 |
| Postoperative LoS, days, median (range) | 6 (1–138) | 6 (1–138) | 15 (1–117) | 7 (3–24) | <0.001 | 0.004 | <0.001 | ||||
| Postoperative LoS ≥7 days | 3062 | 40.2% | 2888 | 39.0% | 139 | 92.7% | 35 | 58.3% | <0.001 | 0.002 | <0.001 |
| Death within 30 days | 177 | 2.3% | 162 | 2.2% | 14 | 9.3% | 1 | 1.7% | <0.001 | 1.000 | 0.072 |
P-values in bold indicate significance at P < 0.05.
Non-significant factors: year of operation; diabetes; body mass index; smoking; alcohol use; dyspnoea; stroke; pulmonary disease; sodium; white blood cell count; platelet count; creatinine; partial thrombin time; international normalized ratio; total bilirubin; ascites; varices; pneumonia; steroids; open wound; disseminated cancer; preoperative transfusion; bleeding disorder; operation in preceding 30 days; chief resident involvement; preoperative chemotherapy within 30 days; radiation therapy within 90 days; preoperative weight loss of >10%; admission before operation; postoperative re-intubation/ventilator >48 h; cardiac arrest; myocardial infarction; sepsis, and septic shock.
DVT, deep venous thrombosis; PE, pulmonary embolism; AST, aspartate aminotransferase; BUN, blood urea nitrogen; ASA, American Society of Anesthesiologists; RBC, red blood cells; GI, gastrointestinal; SSI, surgical site infection; LoS, length of stay.
Rates and timing of post-hepatectomy thrombotic events
Overall thrombotic events included 91 (1.9%) DVTs, 142 (1.2%) PEs, and 210 (2.8%) VTEs. Incidences of VTE amounted to 4.4% in patients undergoing right and extended hepatectomies, and 2.1% in those undergoing left and partial hepatectomies (P < 0.001). Operative time was stratified as >240 min and ≤240 min; incidences of VTE amounted to 4.6% in the cohort with longer operative time and 1.4% in that with shorter operative time. Late events accounted for 27.5%, 33.0% and 28.6% of all occurrences of DVT, PE and VTE, respectively (Fig. 1). The median date of any VTE was postoperative day (PoD) 9 and the median discharge date was PoD 6 (Fig. 2). Among patients with early VTE, the median date of VTE was PoD 6 and the median discharge date was PoD 15. Among patients with late VTE, the median date of VTE was PoD 14 and the median discharge date was PoD 7.
Figure 1.

Post-discharge instances of venous thromboembolism (VTE) represent 28.6% of all post-hepatectomy occurrences of VTE. Columns are labelled with the number and percentage of patients in whom each event occurred. PE, pulmonary embolism; DVT, deep venous thrombosis
Figure 2.
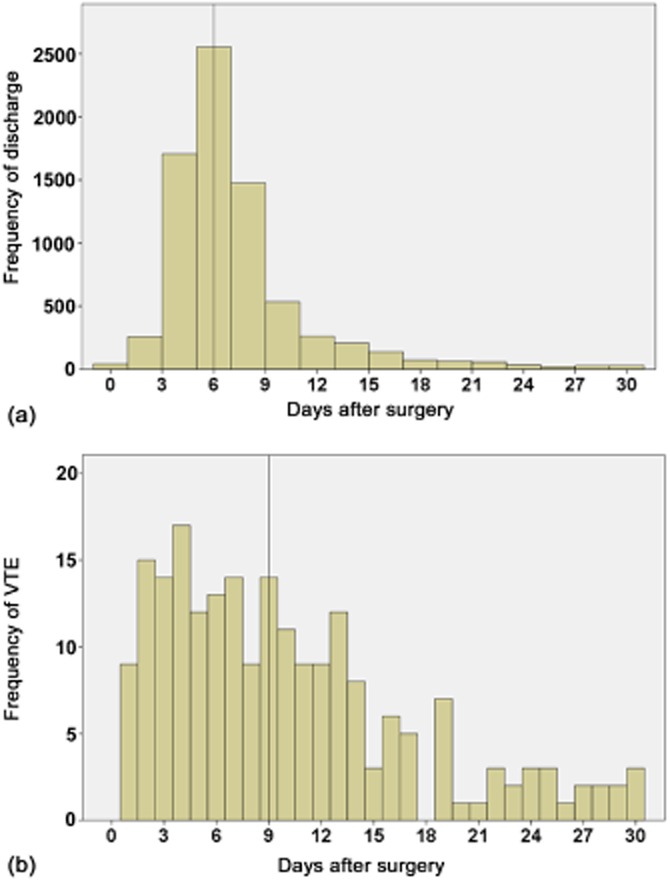
Distribution of dates of (a) discharge after hepatectomy and (b) venous thromboembolism (VTE). Vertical lines mark the median dates for each
Factors associated with pre-discharge VTE
Univariate factors associated with early VTE are detailed in Table 1. Multivariate analysis of these risk factors determined that preoperative, intraoperative and postoperative risk factors were independently associated with pre-discharge VTE (Table 2). These risk factors included age ≥75 years, male sex, intraoperative transfusion, operative time >240 min, postoperative OSI and ROR (all P < 0.01). Longer operative time was used as a surrogate for the magnitude of the operation because of the variability in operative time for each type of hepatectomy. Organ space infection occurred in 6.3% of all patients, 9.4% of those undergoing right or extended hepatectomy, and 5.0% of those receiving partial or left hepatectomy (P < 0.001). Although the overall rate of early VTE was 2.0%, patients with OSI or ROR had pre-discharge VTE rates of 6.9% and 9.8%, respectively (P < 0.001). Patients with early VTE suffered a 30-day mortality rate of 9.3%, whereas the overall baseline mortality rate was 2.3% (P < 0.001).
Table 2.
Rates of and independent factors associated with early versus late venous thromboembolism (VTE)
| Risk factor | Any VTE | Early VTE | Early VTE | Late VTE | Late VTE | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||||
| Preoperative factors | |||||||||
| Age ≥75 years | 3.8% | 3.2% | 1.92 | 1.19–3.10 | 0.007 | ||||
| Male | 3.6% | 2.7% | 1.87 | 1.26–2.76 | 0.002 | ||||
| Intraoperative factors | |||||||||
| Intraoperative transfusion | 5.5% | 4.5% | 2.49 | 1.69–3.69 | <0.001 | ||||
| Operative time >240 min | 4.6% | 3.4% | 2.28 | 1.47–3.52 | <0.001 | 1.2% | 2.35 | 1.26–4.40 | 0.008 |
| Postoperative factors | |||||||||
| Organ space infection | 10.0% | 6.9% | 2.60 | 1.61–4.19 | <0.001 | 3.1% | 3.78 | 1.87–7.66 | <0.001 |
| Return to operating room | 12.5% | 9.8% | 3.25 | 1.97–5.37 | <0.001 | 2.7% | 2.84 | 1.27–6.33 | 0.011 |
P-values in bold indicate significance at P < 0.05.
Early, pre-discharge; late, post-discharge; OR, odds ratio; 95% CI, 95% confidence interval.
Factors associated with post-discharge VTE and the number of high-risk patients needed to treat analysis
Univariate factors associated with late VTE are detailed in Table 1. Multivariate analysis identified independent associations with post-discharge VTE; these indicated intraoperative and postoperative risk factors, but not preoperative variables (Table 2). These independent risk factors included an operative time of >240 min, ROR and OSI (all P < 0.02). In patients at high risk for late VTE, incidences of all VTE, early VTE and late VTE were, respectively, 4.6%, 3.4% and 1.2% in patients in whom surgery lasted >240 min, 10.0%, 6.9% and 3.1% in patients who experienced an OSI, and 12.5%, 9.8% and 2.7% in ROR patients. Although the overall rate of late VTE was 0.8%, patients with an OSI and ROR patients had post-discharge VTE rates of 3.1% and 2.7%, respectively (P < 0.001).
On the assumption that chemoprophylaxis prevents VTE, if extended chemoprophylaxis to PoD 30 were to be administered to all patients with the risk factors of operative time >240 min, OSI and/or ROR, this would cover 78.3% of patients who experienced late events and thus imply that 75 patients is the minimum NNT to prevent one late VTE. Expanding these criteria to include patients who underwent right or extended hepatectomy might avoid 88.3% of late events with a minimum NNT of 81 patients. Tightening the selection criteria to include only patients with postoperative OSI and ROR improves the minimum NNT to 32 patients, but would theoretically reduce the coverage of patients with late events to only 36.7%.
Discussion
The aim of the present study was to evaluate the rates and timing of, and risk factors for, pre- versus post-discharge VTE after hepatectomy and to identify high-risk patients who might benefit from chemoprophylaxis against late VTE. This is the first study to identify distinct risk factors for pre-discharge versus post-discharge VTE after hepatectomy. Its standardized quality measures and national scale make the ACS-NSQIP database very suitable for further study of post-hepatectomy VTE.1 This analysis showed that late VTE does indeed represent a problem that has impact after liver surgery as 28.6% of all instances of VTE occurred after discharge. Although pre-discharge VTE risk factors included preoperative, intraoperative and postoperative factors, post-discharge VTE was associated with only intraoperative and postoperative risk factors. Patients with longer operative time, OSI and/or ROR status were at high risk for late VTE.
Post-discharge VTE is a clinical problem that has been previously described in studies of patients undergoing abdominal surgery, although, historically, hepatobiliary operations (the vast majority of which are carried out for malignant indications) have been under-represented in these analyses and not specifically studied.5,9,11 In one prospective observational study of 1238 general surgery patients, 40% of VTE occurred after PoD 21, and VTE was responsible for almost half of the 30-day deaths in the study cohort.15 Although postoperative VTE chemoprophylaxis in the form of low-dose unfractionated heparin or low molecular weight heparin is a Grade 1B recommendation within the most recent American College of Chest Physicians (ACCP) guidelines, issued in 2012, for all moderate- to high-risk (defined by a VTE rate of 3–6%) general surgery patients undergoing major surgery, there remains no uniform protocol regarding the length of therapy.10
Past studies comparing extended-duration 30-day VTE chemoprophylaxis with pre-discharge-only chemoprophylaxis generally showed lower VTE rates (5.9% versus 13.6%, with a relative risk of 0.44 in a recent meta-analysis) in the extended chemoprophylaxis arms.11,12,16 This meta-analysis indicated that the number of patients needed to treat to prevent a single VTE was 13, and thus the issue of cost-effectiveness raises its head as pharmacologic costs must be balanced against the morbidity and cost of each VTE. Currently, high-risk patients undergoing abdominal cancer surgery are recommended by the ACCP (Grade 1B) to undergo 4 weeks of extended-duration chemoprophylaxis if there is not a high risk for bleeding.10 Although most surgeons would consider universal prescription of extended VTE chemoprophylaxis to be safe (with no increase in bleeding complications in comparison with in-hospital chemoprophylaxis only),16 the cost of a 1-month supply of whichever drug is chosen may be quite high, especially for patients without adequate health insurance coverage. Therefore, there is a clause in the guidelines which states that patients who have to bear the financial burden of the extended-duration chemoprophylaxis may instead choose limited-duration chemoprophylaxis.10 However, as postoperative VTE is considered a preventable nosocomial complication that has both hospital cost and reimbursement implications, clinicians and administrators should be encouraged to facilitate the systematic identification and treatment of high-risk patients.7
The primary analysis of the present study determined that 28.6% of VTE events after liver surgery occurred after discharge. In current practice, most hepatectomy patients are discharged relatively quickly, as the median LoS of only 6 days in the overall national cohort reflects. The median day of any VTE was PoD 9. These data suggest that it is naïve to assume that all discharged liver surgery patients are fully ambulatory and free of thrombotic post-surgical inflammatory issues. This is especially relevant to patients who have undergone larger resections, in whom physiologic demands to regenerate the liver are higher, leaving them with some element of persistent fatigue. Hepatectomies of larger magnitude are also associated with a higher risk for bile leak, some instances of which present post-discharge.17 Those patients who have suffered major bile leaks or have required reoperation for bleeding or bile leaks would certainly be at high risk for late VTE, even after meeting hospital discharge criteria.
Another remarkable finding of this analysis that relates to the association of postoperative complications and VTE events was the temporal relationship between early or late VTE and discharge date. Early VTE, which occurred at a median of 6 days postoperatively, was associated with discharge at a median of 15 days, reflecting the impact of VTE and other complications on LoS. By contrast, late VTE occurred at a median of 14 postoperative days and was associated with discharge at a median of 7 days post-surgery. These data indicate that despite meeting discharge criteria, certain hepatectomy patients harbour significant thrombotic risks. These findings have important implications for extended VTE chemoprophylaxis, particularly with the more frequent utilization of enhanced recovery after surgery (ERAS) or fast-track protocols that reduce the LoS after hepatectomy. This will potentially increase the proportion of patients experiencing VTE in the post-discharge period and will place a greater premium on the accurate identification of high-risk patients who may benefit from post-discharge chemoprophylaxis.
The second focus of the study was to determine if there was a difference between risk factors for pre-discharge and post-discharge VTE. A practical aim was to identify high-risk patients who might benefit from post-discharge chemoprophylaxis. Interestingly, with regard to late VTE, variables related to the extent of and complications from hepatectomy outweighed all preoperative variables. This potentially allows for the selection of patients for extended chemoprophylaxis based on the conduct of the operation and the course of inpatient recovery. As in-hospital postoperative VTE chemoprophylaxis is now within the standard of care in all general surgery operations including hepatectomies, identifying the risk factors for early VTE is important, but not as clinically useful as selecting patients who would benefit from chemoprophylaxis beyond discharge. Although it is true that the overall absolute risk for late VTE among all hepatectomy patients is low (around 0.8%), in high-risk patients, this risk is three to four times greater. Thus the NNT of high-risk patients to prevent a VTE is lower, improving the cost-effectiveness of extended chemoprophylaxis.
According to the present analysis, the minimum NNT of high-risk patients to prevent a single late VTE ranges between 33 and 81 depending on the stringency of selection criteria and the desired yield of late VTE prevention. The validity of these NNT calculations is dependent on several assumptions, including that postoperative chemoprophylaxis from PoD 1 to the date of discharge is routine and that post-discharge chemoprophylaxis is effective.11,12,18 An additional point regarding these NNT calculations is that they are based on symptomatic VTE as captured by the NSQIP, which explains why these NNT estimates are higher than those reported in prospective studies in which patients were screened at 4 weeks to detect asymptomatic VTE.11,12,15,18
As in a previous study conducted by the present group on postoperative VTE rates and bleeding complications,1 the relationship between extent of hepatectomy or its surrogate, longer operative time, and the development of major complications should be emphasized. In general, these patients are at greatest risk for any post-hepatectomy complication, not just VTE. For example, patients undergoing right or extended hepatectomies are almost twice as likely to experience bile leak and more than twice as likely to have a VTE event in comparison with those undergoing partial or left hepatectomies. They happen to also be most likely to require reoperation. Combined, these associated risk factors are likely to create a biological prothrombotic state which explains the observed increase in VTE risk, the underlying mechanisms of which are beyond the scope of this clinical database-driven study. These patients would be predicted to benefit the most from routine postoperative VTE chemoprophylaxis. The next question to resolve concerns whether they would benefit from extended chemoprophylaxis beyond discharge, as demonstrated in past clinical trials.11,12,18 The present study suggests that patients who have undergone longer operations or have suffered major complications, including OSI and ROR (both of which are high-grade complications requiring invasive interventions), would benefit from extended chemoprophylaxis. Given that VTE was diagnosed at a median of 14 days post-surgery (7 days after discharge) in the late VTE cohort, the administration of at least 14 additional days of chemoprophylaxis from the date of discharge or until PoD 30 (whichever is later) may represent a rational recommendation with which to start. The greatest limitation of this recommendation would concern the cost to the patient and to society, but this should be weighed against the potential cost and prolonged morbidity associated with each VTE event, which may range from being asymptomatic to being completely debilitating or life-threatening.
The major potential limitation of this study is its inability to assess the utilization of VTE chemoprophylaxis in this NSQIP cohort because this was not a recorded variable during the period under study.1,19,20 Thus, it is imperative that this study be interpreted according to the disclosure that event rates and subsequent analyses are based on current national practice patterns, the granular details of which are not measured by NSQIP. Based on historical practice patterns and published cross-sectional studies, it would be reasonable to assume that the routine and timely use of postoperative VTE chemoprophylaxis was not universal and that the use of extended chemoprophylaxis was infrequent.21–23 The true, or ‘natural’, event rates for early and late VTE may not be exactly as reported by NSQIP. However, the scale of the national cohort and the standardization of the definitions used allowed for a detailed analysis of these thrombotic events, which may occur too rarely to support evaluation with adequate power in single-institution databases. As this is the first study to identify hepatectomy patients who might benefit from post-discharge VTE chemoprophylaxis, further prospective studies and cost-effectiveness analyses are required to elucidate the true benefit of extended VTE chemoprophylaxis after hepatectomy.
In conclusion, post-hepatectomy late VTE is a clinically distinct entity accounting for 28.6% of all postoperative VTE. Longer operative time, OSI (bile leaks) and ROR are independent risk factors for late VTE. Because late VTE events occur in patients with clearly identifiable intraoperative and postoperative risk factors, these results provide a rationale for the selective use of post-discharge VTE chemoprophylaxis after hepatectomy in high-risk patients.
Conflicts of interest
None declared.
References
- 1.Tzeng CW, Katz MH, Fleming JB, Pisters PW, Lee JE, Abdalla EK, et al. Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5651 National Surgical Quality Improvement Program patients. HPB. 2012;14:506–513. doi: 10.1111/j.1477-2574.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 3.Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66 329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4:529–535. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 4.Collins R, Scrimgeour A, Yusuf S, Peto R. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopaedic, and urologic surgery. N Engl J Med. 1988;318:1162–1173. doi: 10.1056/NEJM198805053181805. [DOI] [PubMed] [Google Scholar]
- 5.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edn) Chest. 2008;133(6 Suppl.):381–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 6.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in non-orthopaedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th edn. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl.):e227–e277. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol. 2007;14:929–936. doi: 10.1245/s10434-006-9183-9. [DOI] [PubMed] [Google Scholar]
- 8.Mismetti P, Laporte S, Darmon JY, Buchmuller A, Decousus H. Meta-analysis of low molecular weight heparin in the prevention of venous thromboembolism in general surgery. Br J Surg. 2001;88:913–930. doi: 10.1046/j.0007-1323.2001.01800.x. [DOI] [PubMed] [Google Scholar]
- 9.Farge D, Debourdeau P, Beckers M, Baglin C, Bauersachs RM, Brenner B, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11:56–70. doi: 10.1111/jth.12070. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th edn. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl.):7–47. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975–980. doi: 10.1056/NEJMoa012385. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen MS, Jorgensen LN, Wille-Jorgensen P, Nielsen JD, Horn A, Mohn AC, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicentre randomized open-label study. J Thromb Haemost. 2006;4:2384–2390. doi: 10.1111/j.1538-7836.2006.02153.x. [DOI] [PubMed] [Google Scholar]
- 13.Aloia TA, Fahy BN, Fischer CP, Jones SL, Duchini A, Galati J, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB. 2009;11:510–515. doi: 10.1111/j.1477-2574.2009.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strasberg SM, Hall BL. Postoperative morbidity index: a quantitative measure of severity of postoperative complications. J Am Coll Surg. 2011;213:616–626. doi: 10.1016/j.jamcollsurg.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Agnelli G, Bolis G, Capussotti L, Scarpa RM, Tonelli F, Bonizzoni E, et al. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottaro FJ, Elizondo MC, Doti C, Bruetman JE, Perez Moreno PD, Bullorsky EO, et al. Efficacy of extended thrombo-prophylaxis in major abdominal surgery: what does the evidence show? A meta-analysis. Thromb Haemost. 2008;99:1104–1111. doi: 10.1160/TH07-12-0759. [DOI] [PubMed] [Google Scholar]
- 17.Zimmitti G, Roses RE, Andreou A, Shindoh J, Curley SA, Aloia TA, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2628 consecutive resections. J Gastrointest Surg. 2013;17:57–64. doi: 10.1007/s11605-012-2000-9. discussion 64–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakkar VV, Balibrea JL, Martinez-Gonzalez J, Prandoni P. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223–1229. doi: 10.1111/j.1538-7836.2010.03892.x. [DOI] [PubMed] [Google Scholar]
- 19.Pitt HA, Kilbane M, Strasberg SM, Pawlik TM, Dixon E, Zyromski NJ, et al. ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB. 2009;11:405–413. doi: 10.1111/j.1477-2574.2009.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy SK, Turley RS, Barbas AS, Steel JL, Tsung A, Marsh JW, et al. Postoperative pharmacologic thromboprophylaxis after major hepatectomy: does peripheral venous thromboembolism prevention outweigh bleeding risks? J Gastrointest Surg. 2011;15:1602–1610. doi: 10.1007/s11605-011-1591-x. [DOI] [PubMed] [Google Scholar]
- 21.Tooher R, Middleton P, Pham C, Fitridge R, Rowe S, Babidge W, et al. A systematic review of strategies to improve prophylaxis for venous thromboembolism in hospitals. Ann Surg. 2005;241:397–415. doi: 10.1097/01.sla.0000154120.96169.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottaro FJ, Ceresetto JM, Emery J, Bruetman J, Emery N, Pellegrini D, et al. Cross-sectional study of adherence to venous thromboembolism prophylaxis guidelines in hospitalized patients. The Trombo-Brit study. Thromb J. 2012;10:7. doi: 10.1186/1477-9560-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–394. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]


