Abstract
Objectives
There is controversy about the roles of locoregional therapies in patients with liver metastases from breast cancer (LMBC). The aim of this study was to analyse survival after laparoscopic radiofrequency ablation (RFA) of LMBC and to compare this with survival in patients receiving systemic therapy (ST) alone.
Methods
During 1996–2011, 24 patients who had failed to respond or had shown an incomplete response to ST underwent laparoscopic RFA for LMBC. Outcomes in these patients were compared with those in 32 patients with LMBC matched by tumour size and number, but treated with ST alone. Clinical parameters and overall survival were compared using t-tests, chi-squared tests and Kaplan–Meier analysis.
Results
The groups were similar in hormone receptor status and chemotherapy exposure. In the laparoscopic RFA and ST groups, respectively, the mean ± standard deviation size of the dominant liver tumour and the number of tumours per patient were 3.7 ± 0.4 cm and 2.4 ± 0.4 cm, and 2.6 ± 0.4 tumours and 3.3 ± 0.4 tumours, respectively. These differences were not significant. At a median follow-up of 20 months in the laparoscopic RFA group, 42% of patients were found to have developed local liver recurrence, 63% had developed new liver disease and 38% had developed extrahepatic disease. Overall survival after the diagnosis of liver metastasis was 47 months in the laparoscopic RFA group and 9 months in the ST-only group (P = 0.0001). Five-year survival after the diagnosis of liver metastasis was 29% in the RFA group and 0% in the ST-only group.
Conclusions
This is the first study to compare outcomes in RFA and ST, respectively, in LMBC. The results show that survival after laparoscopic RFA plus ST is better than that after ST alone.
Introduction
In the USA, breast cancer is the leading cause of cancer-related death in women.1 More than 50% of these patients develop liver metastases, 8.1% of which are isolated to the liver.2 Although 5-year survival in patients with disease localized to the breast is 95%, this decreases to 50% in patients with metastatic disease.1
Breast cancer with liver metastases is generally considered a disseminated disease with a poor prognosis and is associated with median survival of 6 months without treatment and 14–22 months with chemotherapy.2,3 In a minority of patients in whom disease is limited to the liver, resection has been shown to result in a median survival of 32 months and 5-year survival of 37%.4
In another subset of patients with predominant liver involvement, locoregional treatment may be an important adjunct to systemic treatment and may improve survival.1 There are various reports of the use of radiofrequency ablation (RFA) for liver metastases from breast cancer (LMBC), predominantly in the radiology literature.5–7 These studies have reported encouraging results and 5-year survival of 27%.5–7 However, experience with laparoscopic RFA is limited.5–7 Previous studies by the current authors reported efficient local tumour control in hepatocellular cancer, and in patients with metastases from colorectal and neuroendocrine tumours.8,9 The aim of this study was to compare survival in patients with LMBC undergoing laparoscopic liver RFA with that in patients treated with systemic therapy (ST) alone.
Materials and methods
This study was approved by the institutional review board (IRB) of the Cleveland Clinic Foundation. The RFA patients were part of a prospective study evaluating the results of laparoscopic RFA in patients with malignant liver tumours and hence their data were collected prospectively.
Inclusion in the laparoscopic RFA group was subject to the following criteria: (i) the presence of liver metastases from breast cancer; (ii) no or minimal extrahepatic disease (EHD); (iii) <20% of liver volume involvement by tumour, and (iv) no evidence of biliary dilatation on computed tomography (CT) scans. Upon referral to the RFA programme, patients underwent extensive preoperative staging using abdominal, chest and brain CT scans, as well as bone scans to rule out EHD. Patients with EHD were treated with RFA only if the disease was limited and stable for ≥6 months. The liver was evaluated with triphasic CT scans obtained preoperatively and 1 week postoperatively. Patients were followed up quarterly for the first 2 years and then biannually with abdominal and chest CT scans.
During 1996–2011, a total of 120 patients with LMBC underwent ST alone. Data for these patients were extracted from an IRB-approved prospective oncology registry and analysed retrospectively. Thirty-two of these patients were matched with the RFA patients for liver tumour size (dominant tumour of <10 cm), number of tumours (fewer than nine), and the interval between the diagnoses of the primary disease and liver metastases (<24 months).
Surgical technique
The procedure was performed with the patient under general anaesthesia. A diagnostic laparoscopy with biopsy of any suspicious EHD was performed. Laparoscopic ultrasonography of the liver was then performed, during which the sizes and locations of all suspected tumours were determined. Under ultrasound guidance, 18-gauge core biopsies of the representative lesions were obtained using a spring-loaded biopsy gun in order to achieve histological confirmation. The radiofrequency thermoablation catheter was placed percutaneously into the lesion, and the tines deployed under laparoscopic ultrasound guidance. The procedures were performed using various ablation catheters and generators supplied by Angiodynamic, Inc. (Latham, NY, USA). The ablation parameters were identical to those used to ablate hepatocellular cancer and colorectal and neuroendocrine liver metastases. All patients were monitored overnight and discharged to home the morning after surgery.
Statistical analysis
Data were analysed using jmp Version 9.0 (SAS Institute, Inc., Cary, NC, USA). Demographic and clinical parameters were compared using the Mann–Whitney U-test and the chi-squared test. Overall and disease-free Kaplan–Meier survival analyses were performed. Disease-free survival was calculated for the 16 patients in the RFA group without EHD at the time of diagnosis.
Results
During 1996–2011, 910 patients underwent laparoscopic RFA of liver tumours. Among these, 24 patients had LMBC. These patients had received extensive chemotherapy and/or hormonal therapy prior to their referral for RFA. All patients in both groups were female. The RFA group included 24 patients with a total of 57 liver lesions who underwent a total of 35 laparoscopic RFA sessions. The ST group included 32 patients with a total of 107 liver lesions. One patient underwent concomitant resection of primary breast disease and laparoscopic RFA of liver metastasis. Table 1 summarizes the demographic and clinical data for the study groups.
Table 1.
Demographic and clinical details of patients with breast cancer metastases to the liver
| Parameters | RFA group (n = 24) | ST group (n = 32) | P-value |
|---|---|---|---|
| Age, years, mean ± SD (range; median) | 50 ± 2 (31–65; 51) | 50 ± 1.8 (28–73; 51) | 0.968 |
| Primary tumour pathology, n | 0.728 | ||
| Infiltrating ductal adenocarcinoma | 22 | 28 | |
| Inflammatory cancer | 1 | 1 | |
| Invasive lobular carcinoma | 1 | 2 | |
| Medullary cancer | 0 | 1 | |
| Oestrogen receptor status positive, n | 6 | 14 | 0.144 |
| Progesterone receptor status positive, n | 4 | 11 | 0.132 |
| HER-2 status positive, n | 6 | 13 | 0.216 |
| Interval between diagnosis of primary tumour and liver metastasis, months, mean ± SD (range; median) | 26.5 ± 5.6 (0–80; 23) | 20.1 ± 3.3 (0–73; 15) | 0.307 |
| Liver tumour site, n | 0.131 | ||
| Bilobar | 10 | 13 | |
| Left lobe only | 2 | 9 | |
| Right lobe only | 11 | 10 | |
| Caudate lobe | 1 | 0 | |
| Liver tumours per patient, mean ± SD (range; median) | 2.4 ± 0.4 (1–9; 2) | 3.3 ± 0.4 (1–8; 3) | 0.080 |
| Dominant liver tumour size, cm, mean ± SD (range; median) | 3.7 ± 0.4 (1–10; 3.4) | 2.6 ± 0.4 (0.4–9; 1.5) | 0.064 |
| RFA sessions per patient, mean ± SD (range; median) | 1.5 ± 0.1 (1–3; 2) | _ | |
| Type of chemotherapy | 0.112 | ||
| Anthracycline | 11 | 19 | |
| Antimetabolite | 2 | 0 | |
| Taxane | 6 | 13 | |
RFA, radiofrequency ablation; ST, systemic therapy; SD, standard deviation; HER-2, human epidermal growth factor receptor 2.
The median follow-up in the RFA group was 20 months (range: 6–101 months). During this follow-up, 10 of the RFA patients developed local liver recurrence at the site of ablation, 15 developed new liver disease and nine developed EHD. Local recurrence per liver lesion treated with RFA was 19%. Repeat RFA (n = 10), chemotherapy (n = 15) and liver resection (n = 1) were used to obtain local tumour control for recurrences during follow-up. In the RFA group, median disease-free survival was 9 months and overall survival was 48 months from the diagnosis of liver metastasis. Five-year actuarial survival was 29%. By contrast, in the ST-only group, median follow-up was 27 months, overall survival was 9 months and 5-year actuarial survival was 0% (Fig. 1).
Figure 1.
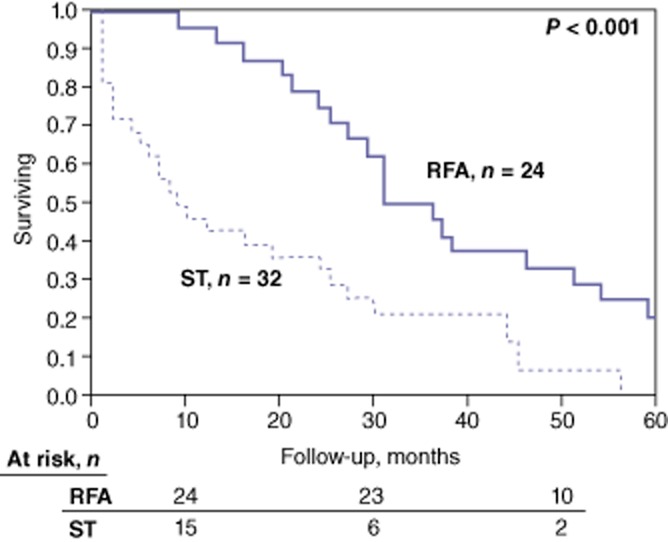
Kaplan–Meier curves for overall survival in patients in the laparoscopic radiofrequency ablation (RFA) group and the systemic therapy (ST)-alone group. Median overall survival after the diagnosis of liver metastases was 47 months in the RFA group and 9 months in ST-alone group (P < 0.001)
Discussion
This study analysed a 15-year experience with laparoscopic RFA for LMBC and compared findings in RFA patients with those in patients treated with ST alone. This is the largest laparoscopic series to be reported to date. The present group of patients with predominantly liver disease showed encouraging results, demonstrating a local tumour control rate of 81% and 5-year actuarial survival of 29% after RFA, compared with 0% after ST alone.
Overall survival in patients who have liver deposits from breast cancer at the time of the first chemotherapy cycle is 16.3 months and the median time to disease progression is 7.9 months.10 If metastasis is present at other sites, overall survival decreases from 23–27 months to 14–17 months.2 The results of salvage chemotherapy in those patients who have not responded to prior chemotherapy are worse: median overall survival is 5–13 months and time to progression is 4.4–9.1 months.11–13 These dismal results underscore the need for additional treatment options for patients with LMBC.
Surgical studies have shown that survival in LMBC can be improved with hepatic resection rather than chemotherapy alone.4,14 In the largest study of patients undergoing liver resection (n = 85), 33% had EHD. Median survival was 32 months and 5-year survival was 37% during a 38-month median follow-up. Median and 5-year disease-free survival were 20 months and 21%, respectively. Twenty-eight patients developed liver recurrence, 22 developed liver disease plus EHD and nine developed EHD only.4 In a recent study conducted in the USA in a series of 31 patients with LMBC subjected to liver resection, the median tumour size was 2.9 cm and the median number of tumours was 1.7. Median overall and disease-free survival were 25 months and 13 months, respectively. Five-year actuarial survival was 61% and disease-free survival was 31%.14
As a result of the encouraging results achieved in patients with other tumour types, RFA has also been used for the treatment of LMBC. Table 2 summarizes these studies.5,6,15–19 In general, the patient characteristics and oncologic results described in the current study are similar to those reported in these earlier studies.
Table 2.
Summary of the major studies in the literature reporting the utility of radiofrequency ablation in patients with breast cancer metastases to the liver
| Authors (year) | Patients, n | Tumour size, cm, mean | Tumours per patient, n | Follow-up, months, median | Overall survival, % |
|---|---|---|---|---|---|
| Meloni et al. (2009)5 | 52 | 2.5 | <5 | 19 | 27% (5-year) |
| Jakobs et al. (2009)6 | 43 | 2.1 | ≤5 | 37 | 38% (5-year) |
| Gunabushanam et al. (2007)15 | 14 | 1.9 | ≤3 | 19 | 64% (1-year) |
| Carrafiello et al. (2011)16 | 13 | 3.5 | ≤3 | 13a | 11 monthsa |
| Sofocleous et al. (2007)17 | 12 | – | ≤3 | 22.5 | 30% (5-year) |
| Lawes et al. (2006)18 | 19 | 3 | <7 | 15 | 42% (2.5-year) |
| Sakamoto et al. (2005)19 | 34 | 4 | – | 72 | 21% (5-year) |
Shows mean value, not median.
The interval between the diagnoses of, respectively, primary disease and liver metastasis has been found to affect survival after RFA of LMBC in some studies. In these studies, the interval found to predict prognosis in a positive manner varied between 1 year and 4 years.20–22 In the current study, the median interval was 27 months.
The management of patients with EHD and predominant liver involvement is controversial. Some studies have excluded these patients from liver ablation,14,23–25 whereas others, similarly to the current study, have offered RFA.4–6,17–20,26 Different groups have shown that limited amounts of EHD do not adversely affect survival in these patients.19,26 It is important to document the stability of these extrahepatic deposits before committing to treat the liver disease.
Despite the wider experience with percutaneous RFA reported in the literature, the laparoscopic technique has a role in the management of these patients because it provides the advantages of abdominal exploration for more accurate staging, more precise targeting of the lesions as a result of the immobilization of the liver with pneumoperitoneum, and increased safety afforded by the ability to retract the surrounding organs to prevent inadvertent injuries. Four of the current patients underwent concomitant cholecystectomy for lesions close to the gallbladder in order to prevent a postoperative bile leak. The current study found no complications associated with the laparoscopic technique, which compares favourably with morbidity of 2.2–5.0% and mortality of 0.3% associated with percutaneous RFA.27
Because the current study did not include a comparison group of resection patients, it is difficult to comment on whether RFA may represent an alternative to resection in patients with resectable disease. Recently, Bergenfeldt et al. reviewed 25 surgical and seven local ablation studies on LMBC and showed that median survival after RFA (30–60 months) was similar to that reported after resection (20–67 months).28 The low rate of complications and high local tumour control rate suggest that laparoscopic RFA should be considered among the liver-directed treatment options for LMBC in appropriate patients. Patients with bulky tumours that are not amenable to ablation should be directed to resection. The overall survival of 47 months established in the current study is comparable with rates reported in surgical series. The complication rate and the length of recovery time after hepatic resection should not be underestimated. Patients in the current study required only an overnight hospital stay.
In conclusion, this study reports that survival after laparoscopic RFA plus ST was better than that after ST alone. Hence, these findings support the proposal that patients with metastatic breast cancer with predominantly liver involvement should be considered for locoregional treatment options, including RFA.
Conflicts of interest
None declared.
References
- 1.Covey AM, Sofocleous CT. Radiofrequency ablation as a treatment strategy for liver metastases from breast cancer. Semin Intervent Radiol. 2008;25:406–412. doi: 10.1055/s-0028-1102996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atalay G, Biganzoli L, Renard F, Paridaens R, Cufer T, Coleman R, et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomized metastatic breast cancer trials. Eur J Cancer. 2003;39:2439–2449. doi: 10.1016/s0959-8049(03)00601-4. [DOI] [PubMed] [Google Scholar]
- 3.Elias D, Maisonnette F, Druet-Cabanac M, Ouellet JF, Guinebretiere JM, Spielmann M, et al. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am J Surg. 2003;185:158–164. doi: 10.1016/s0002-9610(02)01204-7. [DOI] [PubMed] [Google Scholar]
- 4.Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg. 2006;244:897–907. doi: 10.1097/01.sla.0000246847.02058.1b. discussion 907–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meloni MF, Andreano A, Laeseke PF, Livraghi T, Sironi S, Lee FT., Jr Breast cancer liver metastases: US-guided percutaneous radiofrequency ablation – intermediate and longterm survival rates. Radiology. 2009;253:861–869. doi: 10.1148/radiol.2533081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobs TF, Hoffmann RT, Schrader A, Stemmler HJ, Trumm C, Lubienski A, et al. CT-guided radiofrequency ablation in patients with hepatic metastases from breast cancer. Cardiovasc Intervent Radiol. 2009;32:38–46. doi: 10.1007/s00270-008-9384-7. [DOI] [PubMed] [Google Scholar]
- 7.Livraghi T, Goldberg SN, Solbiati L, Meloni F, Ierace T, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology. 2001;220:145–149. doi: 10.1148/radiology.220.1.r01jl01145. [DOI] [PubMed] [Google Scholar]
- 8.Akyildiz HY, Mitchell J, Milas M, Siperstein A, Berber E. Laparoscopic radiofrequency thermal ablation of neuroendocrine hepatic metastases: longterm follow-up. Surgery. 2010;148:1288–1293. doi: 10.1016/j.surg.2010.09.014. discussion 1293. [DOI] [PubMed] [Google Scholar]
- 9.Ballem N, Berber E, Pitt T, Siperstein A. Laparoscopic radiofrequency ablation of unresectable hepatocellular carcinoma: longterm follow-up. HPB. 2008;10:315–320. doi: 10.1080/13651820802247102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pentheroudakis G, Fountzilas G, Bafaloukos D, Koutsoukou V, Pectasides D, Skarlos D, et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat. 2006;97:237–244. doi: 10.1007/s10549-005-9117-4. [DOI] [PubMed] [Google Scholar]
- 11.Onyenadum A, Gogas H, Markopoulos C, Bafaloukos D, Aravantinos G, Mantzourani M, et al. Mitoxantrone plus vinorelbine in pretreated patients with metastatic breast cancer. J Chemother. 2007;19:582–589. doi: 10.1179/joc.2007.19.5.582. [DOI] [PubMed] [Google Scholar]
- 12.Blum JL, Savin MA, Edelman G, Pippen JE, Robert NJ, Geister BV, et al. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7:850–856. doi: 10.3816/CBC.2007.n.049. [DOI] [PubMed] [Google Scholar]
- 13.Onyenadum A, Gogas H, Kosmidis P, Aravantinos G, Bafaloukos D, Bacoyiannis H, et al. Mitoxantrone plus gemcitabine in pretreated patients with metastatic breast cancer. J Chemother. 2006;18:192–198. doi: 10.1179/joc.2006.18.2.192. [DOI] [PubMed] [Google Scholar]
- 14.Vlastos G, Smith DL, Singletary SE, Mirza NQ, Tuttle TM, Popat RJ, et al. Longterm survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol. 2004;11:869–874. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Gunabushanam G, Sharma S, Thulkar S, Srivastava DN, Rath GK, Julka PK, et al. Radiofrequency ablation of liver metastases from breast cancer: results in 14 patients. J Vasc Interv Radiol. 2007;18:67–72. doi: 10.1016/j.jvir.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Carrafiello G, Fontana F, Cotta E, Petulla M, Brunese L, Mangini M, et al. Ultrasound-guided thermal radiofrequency ablation (RFA) as an adjunct to systemic chemotherapy for breast cancer liver metastases. Radiol Med. 2011;116:1059–1066. doi: 10.1007/s11547-011-0697-2. [DOI] [PubMed] [Google Scholar]
- 17.Sofocleous CT, Nascimento RG, Gonen M, Theodoulou M, Covey AM, Brody LA, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol. 2007;189:883–889. doi: 10.2214/AJR.07.2198. [DOI] [PubMed] [Google Scholar]
- 18.Lawes D, Chopada A, Gillams A, Lees W, Taylor I. Radiofrequency ablation (RFA) as a cytoreductive strategy for hepatic metastasis from breast cancer. Ann R Coll Surg Engl. 2006;88:639–642. doi: 10.1308/003588406X149129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakamoto Y, Yamamoto J, Yoshimoto M, Kasumi F, Kosuge T, Kokudo N, et al. Hepatic resection for metastatic breast cancer: prognostic analysis of 34 patients. World J Surg. 2005;29:524–527. doi: 10.1007/s00268-004-7688-6. [DOI] [PubMed] [Google Scholar]
- 20.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: longterm survival after curative resection. Surgery. 2000;127:383–389. doi: 10.1067/msy.2000.103883. [DOI] [PubMed] [Google Scholar]
- 21.Caralt M, Bilbao I, Cortes J, Escartin A, Lazaro JL, Dopazo C, et al. Hepatic resection for liver metastases as part of the ‘oncosurgical’ treatment of metastatic breast cancer. Ann Surg Oncol. 2008;15:2804–2810. doi: 10.1245/s10434-008-0072-2. [DOI] [PubMed] [Google Scholar]
- 22.Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ. Hepatic resection for breast cancer metastases: results and prognosis (65 cases) Ann Chir. 2001;126:413–420. doi: 10.1016/s0003-3944(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 23.Carlini M, Lonardo MT, Carboni F, Petric M, Vitucci C, Santoro R, et al. Liver metastases from breast cancer. results of surgical resection. Hepatogastroenterology. 2002;49:1597–1601. [PubMed] [Google Scholar]
- 24.Arena E, Ferrero S. Surgical treatment of liver metastases from breast cancer. Minerva Chir. 2004;59:7–15. [PubMed] [Google Scholar]
- 25.Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotte M. Liver resection for breast cancer metastasis: does it improve survival? Surg Today. 2008;38:293–299. doi: 10.1007/s00595-007-3617-2. [DOI] [PubMed] [Google Scholar]
- 26.Thelen A, Benckert C, Jonas S, Lopez-Hanninen E, Sehouli J, Neumann U, et al. Liver resection for metastases from breast cancer. J Surg Oncol. 2008;97:25–29. doi: 10.1002/jso.20911. [DOI] [PubMed] [Google Scholar]
- 27.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumours with percutaneous radiofrequency ablation: complications encountered in a multicentre study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 28.Bergenfeldt M, Jensen BV, Skjoldbye B, Nielsen D. Liver resection and local ablation of breast cancer liver metastases – a systematic review. Eur J Surg Oncol. 2011;37:549–557. doi: 10.1016/j.ejso.2011.04.013. [DOI] [PubMed] [Google Scholar]


