Abstract
Objectives
The impact of pre-transplant hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection in patients with hepatocellular carcinoma (HCC) is not well described. This study was conducted to test the hypothesis that viral status is an independent predictor of retransplantation rates, graft survival (GS) and overall survival (OS) in patients undergoing liver transplantation for HCC.
Methods
Patients with HCC were identified from the Organ Procurement and Transplantation Network database (2005–2012), and categorized by viral status according to these categories: HBV−/HCV−; HBV+/HCV−; HBV−/HCV+, and HBV+/HCV+.
Results
Of 7742 patients transplanted for HCC, 7060 had known HBV and HCV status. Five-year GS and OS were highest in recipients who were HBV+/HCV−, at 75% and 78%, respectively, compared with patients who were HBV−/HCV− (GS = 63%, OS = 66%), HBV−/HCV+ (GS = 64%, OS = 60%) or HBV+/HCV+ (GS = 60%, OS = 62%). In multivariable analyses, HBV−/HCV+ patients were more likely than HBV+/HCV− patients to undergo repeat transplantation. Patients who were HBV−/HCV+ also had poorer GS and OS than both HBV−/HCV− and HBV+/HCV− patients. Other independent predictors of poorer OS included older age, higher Model for End-stage Liver Disease score, African-American race, and diabetes. The few HBV+/HCV+ patients (n = 138) showed trends toward fewer retransplantations, prolonged GS and prolonged OS compared with HBV−/HCV+ patients. In adjusted models, antiviral medications did not impact GS or OS.
Conclusions
In the era of modern selection criteria, viral status is an independent predictor of outcome following liver transplantation for HCC. Both HBV−/HCV− and HBV+/HCV− patients have superior GS and OS compared with HBV−/HCV+ patients.
Introduction
Hepatocellular carcinoma (HCC) is the world's most common solid-organ tumour and third most common cause of cancer-related death worldwide (600 000 deaths/year).1–4 The incidence of HCC has increased over the past several decades5 and may not plateau for the next 10 years.6 Because HCC often arises in the setting of background liver disease, liver transplantation is an ideal treatment that enables the excision of the tumour and the replacement of the underlying tumorigenic parenchyma. Early experience with transplantation in HCC was associated with relatively low 5-year survival rates of 30–40%.7 Eventually, patients with more extensive disease were noted to have increased recurrence rates and poorer survival.8 Since the adoption of modern selection criteria (the Milan Criteria) for liver transplantation in the setting of HCC,7 many centres have reported 5-year survival rates of >75%.9,10 Although more inclusive criteria have been developed and validated,11,12 the Milan Criteria are still considered to represent the benchmark for patient selection.8
Chronic hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infections account for the majority of cases of HCC worldwide. Although both are widely accepted as risk factors for HCC,13,14 their carcinogenic mechanisms differ at both the molecular and morphologic levels,14,15 and the prognostic interdependence of viral status (the presence or absence of HBV or HCV) and HCC has been controversial. Patients infected with both viruses may develop HCC through additive (or possibly synergistic) cellular mechanisms,14,16,17 and may have a worse prognosis than seronegative HCC patients.13 By contrast, several reports have found that HBV superinfection in previously HCV+ patients suppresses HCV replication.18,19 Likewise, other series have found that HCV superinfection can inhibit HBV replication.18,20 Extending this concept to transplantation, several reports suggest that graft survival (GS) and overall survival (OS) in HBV+/HCV+ recipients are greater than in HBV−/HCV+ recipients.21–23 These reports, however, encompass liver transplantation for all indications and are not specific to patients with HCC.
In theory, cancer progression poses the greater threat to life, making viral status a negligible contributing variable in patient survival following liver transplantation for HCC. One study concluded that HCV+ patients transplanted for HCC have a poorer prognosis than seronegative HCC patients undergoing transplant.1 By contrast, another report showed a higher post-transplant 4-year OS in HCV+ HCC patients (72%) than in seronegative HCC patients (68%).3 To the present authors’ knowledge, no prior reports have studied the effects of HBV infection on survival in patients undergoing liver transplantation for HCC. The objective of this study was to stratify for risk patients undergoing transplantation for HCC according to their HBV and HCV viral status. The study sought to verify the hypothesis that viral status is an independent predictor of retransplantation rates, GS and OS in patients undergoing liver transplantation for HCC.
Materials and methods
The United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research Files were used to obtain records for all patients undergoing liver transplantation from September 1987 until January 2012. This study was exempt from institutional review board oversight. Liver diagnosis codes 4400 and 4401 were used to identify patients transplanted for HCC and to determine the presence of cirrhosis. Status for HBV infection was elucidated by recipient surface antigen testing; HCV status was established by recipient viral serology. Because viral status represented the primary variable of interest, all patients whose HBV or HCV viral status was coded as ‘not done’ or ‘unknown’ were excluded. Patients were stratified into four groups by viral status: HBV−/HCV−; HBV+/HCV−; HBV−/HCV+, and HBV+/HCV+. To study trends in the prevalences of HBV and HCV infection among transplanted patients, patients were divided into three groups based on their date of transplant for the periods 1987–1999, 2000–2004 and 2005–2012. Each group was duration-weighted to account for the increasing number of transplants performed in recent years. These groups were also used to analyse trends over the last several decades in viral status, retransplantation rates, GS and OS in transplanted HCC patients.
The primary endpoints were rates of retransplantation, GS and OS stratified by viral status. Graft survival was defined as the time (in months) from initial transplant to either the date of retransplantation or end of follow-up. Overall survival was defined as the time (in months) from initial transplant until either death or end of follow-up. To ensure that the present results would be representative of modern selection criteria, only the cohort transplanted during the period 2005–2012 was included for these primary endpoints. Several other known potential prognostic variables were included in this study to avoid the limitations of empirically derived models, including age, Model for End-stage Liver Disease (MELD) score, body mass index (BMI), gender, race, cirrhosis, pre-transplant diabetes status, and antiviral treatments. Donor viral status was also accounted for.
Statistical significance was set at α = 0.05; all tests were two-sided. Similarity between group characteristics was determined using the Kruskal–Wallis test (for continuous variables) and Fisher's exact test (for categorical variables). Survival curves were compared with the log-rank test for equality of survivor functions. Factors predictive of the need for retransplantation were identified with logistic regression. Variables associated with GS and OS were identified using Cox proportional hazards models. Univariate and multivariate models were generated for each regression analysis. Final adjusted models automatically included viral status (the primary variable of interest) and were otherwise limited to significant variables by backwards selection. stata Version 12 IC (StataCorp LP, College Station, TX, USA) was used for all statistical tests.
Results
Of 10 353 patients who underwent liver transplantation for HCC between September 1987 and January 2012, 8880 had known HBV and HCV status; patient characteristics are shown in Table 1. The majority of patients transplanted for HCC were HCV+ (n = 5721, 64%) and relatively few were HBV+ (n = 798, 9%).
Table 1.
Characteristics of 8880 patients transplanted for hepatocellular carcinoma (1987–2012)
| Age, years, median (IQR) | 57 (53–62) |
| MELD score, median (IQR) | 13 (9–18) |
| BMI, median (IQR) | 27.8 (24.8–31.5) |
| Gender, n (%) | |
| Female | 1931 (22%) |
| Male | 6949 (78%) |
| Race, n (%) | |
| White | 5777 (65%) |
| African-American | 765 (9%) |
| Asian | 779 (9%) |
| Hispanic | 1436 (16%) |
| Other | 123 (1%) |
| Cirrhosis, n (%) | |
| No | 2347 (26%) |
| Yes | 6533 (74%) |
| Diabetes, n (%) | |
| No | 6339 (71%) |
| Yes | 2422 (27%) |
| Unknown | 119 (0.01%) |
| Viral status, n (%) | |
| HBV−/HCV− | 2530 (28%) |
| HBV+/HCV− | 629 (7%) |
| HBV−/HCV+ | 5552 (62%) |
| HBV+/HCV+ | 169 (2%) |
| Era, n (%) | |
| Pre-2000 | 482 (5%) |
| 2000–2004 | 1338 (15%) |
| 2005–2012 | 7060 (80%) |
IQR, interquartile range; MELD, Model for End-stage Liver Disease; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus.
Changes in viral status over time
An examination of the entire dataset (1987–2012) revealed an increase in the proportion of HBV−/HCV+ patients who underwent transplantation for HCC until 2000 (from 51% to 62%), whereas the distribution of viral status among the study cohort remained steady over the subsequent 12 years (Fig. 1a). Patients who were HBV+/HCV− demonstrated general trends toward fewer retransplantations (Fig. 1b) and the highest rates of 5-year GS and OS (Fig. 1c, d). Although a cursory examination of retransplantation rates over time might suggest that modern rates are lower (Fig. 1b), this certainly represents lead-time bias and should not be considered as a measure of improved quality.
Figure 1.
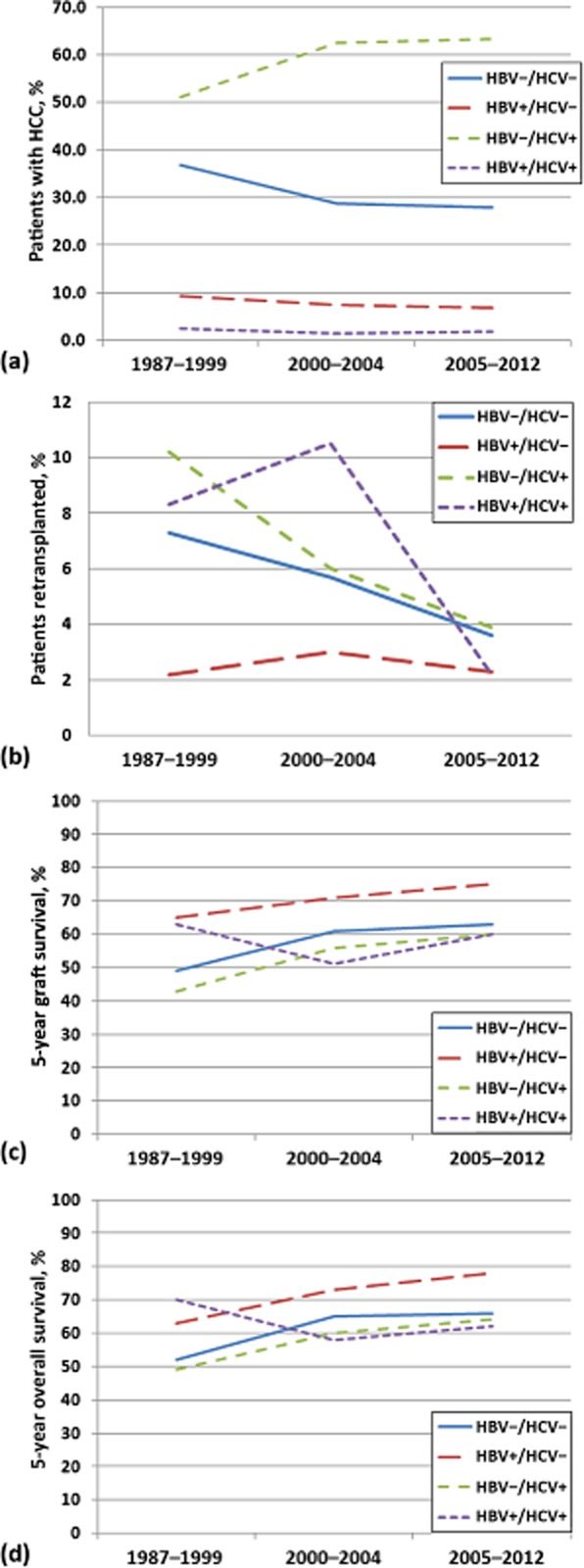
(a) Distribution of viral status in patients undergoing liver transplantation for hepatocellular carcinoma (HCC), and its effect on (b) retransplantation rates, (c) graft survival and (d) overall survival over the 25 years from September 1987 to January 2012
Impact of viral status on primary endpoints
To ensure that the present results would reflect modern selection criteria, this analysis of the primary endpoints was limited to the 7060 patients transplanted between 2005 and 2012. Cold and warm ischaemia times were similar between groups (P = 0.545 and P = 0.556, respectively) (Table 2). In comparison with patients in the other viral status groups, HBV+/HCV− patients had lower MELD scores and were predominantly of Asian descent. Retransplantation was performed in 71 HBV−/HCV− patients (4%), 11 HBV+/HCV− patients (2%), 173 HBV−/HCV+ patients (4%), and three HBV+/HCV+ patients (2%) (P = 0.250). On univariate analysis, retransplantation rates were found to correlate only with patient age and gender (Table 3): younger and male patients were more likely to undergo retransplantation.
Table 2.
Characteristics of 7060 patients transplanted for hepatocellular carcinoma by viral status (2005–2012)
| HBV−/HCV− (n = 1967) | HBV+/HCV− (n = 485) | HBV−/HCV+ (n = 4470) | HBV+/HCV+ (n = 138) | P-value | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 61 (56–65) | 56 (50–62) | 57 (53–60) | 57 (52–60) | 0.0001 |
| MELD score, median (IQR) | 14 (10–19) | 9 (7–14) | 13 (9–18) | 12 (8–18) | 0.0001 |
| BMI, median (IQR) | 29.0 (25.5–33.1) | 25.2 (22.9–28.1) | 28.1 (25.1–31.4) | 26.5 (23.8–30.8) | 0.0001 |
| Gender, n (%) | <0.001 | ||||
| Female | 514 (26%) | 82 (17%) | 917 (21%) | 23 (17%) | |
| Male | 1453 (74%) | 403 (83%) | 3553 (79%) | 115 (83%) | |
| Race, n (%) | <0.001 | ||||
| White | 1392 (71%) | 127 (26%) | 2977 (67%) | 83 (60%) | |
| African-American | 78 (4%) | 48 (10%) | 478 (11%) | 18 (13%) | |
| Asian | 112 (6%) | 283 (58%) | 193 (4%) | 21 (15%) | |
| Hispanic | 353 (18%) | 21 (4%) | 758 (17%) | 15 (11%) | |
| Other | 32 (2%) | 6 (1%) | 64 (1%) | 1 (1%) | |
| Cirrhosis, n (%) | <0.001 | ||||
| No | 621 (32%) | 147 (30%) | 976 (22%) | 41 (30%) | |
| Yes | 1346 (68%) | 338 (70%) | 3494 (78%) | 97 (70%) | |
| Diabetes, n (%) | <0.001 | ||||
| No | 1154 (59%) | 388 (80%) | 3374 (75%) | 104 (75%) | |
| Yes | 797 (41%) | 94 (19%) | 1053 (24%) | 33 (24%) | |
| Unknown | 16 (1%) | 3 (1%) | 43 (1%) | 1 (1%) | |
| Cold ischaemia time, h, median | 6.8 | 6.5 | 6.8 | 6.1 | 0.545 |
| Warm ischaemia time, min, median | 10 | 14 | 10 | 12 | 0.556 |
HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; MELD, Model for End-stage Liver Disease; BMI, body mass index.
Table 3.
Univariate logistic regression analysis of factors associated with retransplantation in patients with hepatocellular carcinoma (HCC)
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Age | 0.98 (0.97–0.99) | 0.003 |
| MELD score | 0.99 (0.98–1.01) | 0.221 |
| BMI | 1.01 (1.00–1.03) | 0.085 |
| Gender | 0.041 | |
| Female | 1.00 | |
| Male | 1.39 (1.01–1.89) | |
| Race | 0.372 | |
| White | 1.00 | |
| African-American | 1.08 (0.72–1.64) | 0.701 |
| Asian | 0.78 (0.48–1.26) | 0.314 |
| Hispanic | 1.23 (0.91–1.68) | 0.182 |
| Other | 1.57 (0.68–3.63) | 0.289 |
| Cirrhosis | 0.500 | |
| No | 1.00 | |
| Yes | 0.91 (0.70–1.19) | |
| Diabetes | 0.863 | |
| No | 1.00 | |
| Yes | 0.98 (0.75–1.27) | |
| Viral status | 0.200 | |
| HBV−/HCV− | 1.00 | |
| HBV+/HCV− | 0.62 (0.33–1.18) | 0.145 |
| HBV−/HCV+ | 1.08 (0.81–1.42) | 0.614 |
| HBV+/HCV+ | 0.59 (0.18–1.91) | 0.381 |
95% CI, 95% confidence interval; MELD, Model for End-stage Liver Disease; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus.
Five-year GS was highest in HBV+/HCV− patients (75%) compared with HBV−/HCV− (63%), HBV−/HCV+ (60%) and HBV+/HCV+ (60%) patients (P < 0.0001) (Fig. 2). Similarly, 5-year OS was highest in HBV+/HCV− patients (78%) compared with HBV−/HCV− (66%), HBV−/HCV+ (64%) and HBV+/HCV+ (62%) patients (P = 0.0003) (Fig. 3). In unadjusted models, GS (Table 4) and OS (Table 5) were both associated with age, MELD score, race, diabetes status and viral status. Few patients had been treated with either lamivudine (n = 162) or hepatitis B immunoglobulin (n = 383). Although lamivudine therapy was not associated with either GS or OS, univariate analysis showed that hepatitis B immunoglobulin was associated with prolonged OS in HBV-infected patients.
Figure 2.
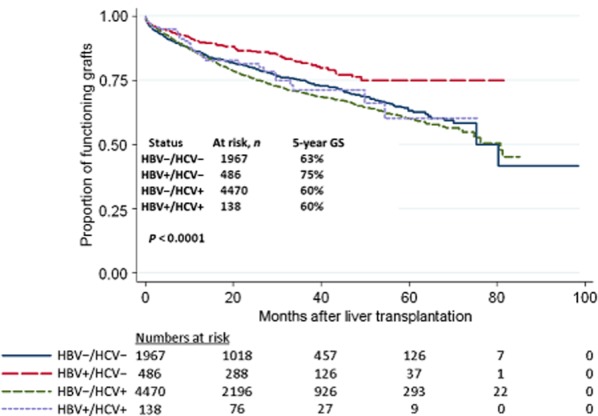
Graft survival in patients transplanted for hepatocellular carcinoma (HCC) during 2005–2012, by viral status
Figure 3.
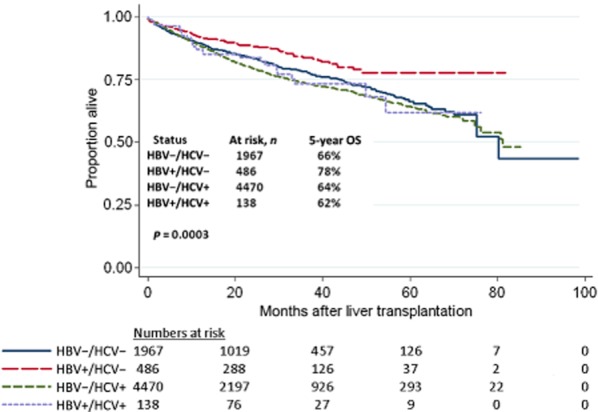
Overall survival in patients transplanted for hepatocellular carcinoma (HCC) during 2005–2012, by viral status
Table 4.
Univariate Cox regression analysis of factors associated with graft survival in patients transplanted for hepatocellular carcinoma
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Age | 1.01 (1.01–1.02) | <0.001 |
| MELD score | 1.01 (1.01–1.02) | <0.001 |
| BMI | 1.01 (1.00–1.02) | 0.138 |
| Gender | 0.406 | |
| Female | 1.00 | |
| Male | 1.05 (0.93–1.19) | |
| Race | 0.002 | |
| White | 1.00 | |
| African-American | 1.19 (1.01–1.40) | 0.036 |
| Asian | 0.73 (0.60–0.89) | 0.002 |
| Hispanic | 0.97 (0.85–1.12) | 0.688 |
| Other | 0.49 (0.50–1.24) | 0.305 |
| Cirrhosis | 0.742 | |
| No | 1.00 | |
| Yes | 0.98 (0.88–1.10) | |
| Diabetes | 0.017 | |
| No | 1.00 | |
| Yes | 1.14 (1.02–1.27) | |
| Lamivudine therapy | 0.089 | |
| No | 1.00 | |
| Yes | 0.30 (0.07–1.20) | |
| Hepatitis B immunoglobulin | 0.219 | |
| No | 1.00 | |
| Yes | 0.67 (0.36–1.27) | |
| Viral status | <0.001 | |
| HBV−/HCV− | 1.00 | |
| HBV+/HCV− | 0.67 (0.52–0.85) | 0.001 |
| HBV−/HCV+ | 1.13 (1.01–1.27) | 0.030 |
| HBV+/HCV+ | 0.97 (0.67–1.42) | 0.894 |
95% CI, 95% confidence interval; MELD, Model for End-stage Liver Disease; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus.
Table 5.
Univariate Cox regression analysis of factors associated with overall survival
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| Age | 1.02 (1.01–1.03) | <0.001 |
| MELD score | 1.02 (1.01–1.02) | <0.001 |
| BMI | 1.00 (0.99–1.01) | 0.478 |
| Gender | 0.909 | |
| Female | 1.00 | |
| Male | 1.01 (0.88–1.15) | |
| Race | <0.001 | |
| White | 1.00 | |
| African-American | 1.24 (1.04–1.48) | 0.018 |
| Asian | 0.72 (0.58–0.89) | 0.003 |
| Hispanic | 0.93 (0.80–1.09) | 0.368 |
| Other | 0.64 (0.37–1.10) | 0.105 |
| Cirrhosis | 0.844 | |
| No | 1.00 | |
| Yes | 0.99 (0.87–1.12) | |
| Diabetes | 0.010 | |
| No | 1.00 | |
| Yes | 1.17 (1.04–1.31) | |
| Lamivudine therapy | 0.803 | |
| No | 1.00 | |
| Yes | 0.95 (0.66–1.37) | |
| Hepatitis B immunoglobulin | 0.008 | |
| No | 1.00 | |
| Yes | 0.69 (0.53–0.91) | |
| Viral status | <0.001 | |
| HBV−/HCV− | 1.00 | |
| HBV+/HCV− | 0.68 (0.52–0.88) | 0.004 |
| HBV−/HCV+ | 1.14 (1.01–1.29) | 0.034 |
| HBV+/HCV+ | 1.05 (0.71–1.57) | 0.799 |
95% CI, 95% confidence interval; MELD, Model for End-stage Liver Disease; BMI, body mass index; HBV, hepatitis B virus; HCV, hepatitis C virus.
Multivariable (adjusted) analysis
Table 6 summarizes independent predictors of need for retransplantation and prognostic factors for GS and OS. Older patients were less likely to undergo retransplantation, and had poorer GS and OS. Patients with diabetes and those with higher MELD scores had poorer GS and OS, and African-Americans experienced poorer OS compared with patients of other races.
Table 6.
Multivariable analyses of prognostic factors for retransplantation, graft survival, and overall survival following liver transplantation for hepatocellular carcinoma
| Factors associated with retransplantation | ||
|---|---|---|
| Odds ratio (95% CI) | P-value | |
| Age | 0.98 (0.96–0.99) | 0.003 |
| Viral status | <0.001 | |
| HBV−/HCV− | 1.00 | |
| HBV+/HCV−a | 0.57 (0.30–1.08) | 0.087 |
| HBV−/HCV+a | 1.03 (0.77–1.36) | 0.859 |
| HBV+/HCV+ | 0.57 (0.18–1.83) | 0.342 |
| Factors associated with graft survival | ||
|---|---|---|
| Hazard ratio (95% CI) | P-value | |
| Age | 1.02 (1.01–1.02) | <0.001 |
| MELD score | 1.02 (1.01–1.02) | <0.001 |
| Diabetes | 0.019 | |
| No | 1.00 | |
| Yes | 1.14 (1.02–1.27) | |
| Viral status | <0.001 | |
| HBV−/HCV− | 1.00 | |
| HBV+/HCV−b | 0.76 (0.59–0.98) | 0.033 |
| HBV−/HCV+b | 1.25 (1.11–1.41) | <0.001 |
| HBV+/HCV+ | 1.03 (0.70–1.51) | 0.883 |
| Factors associated with overall survival | ||
|---|---|---|
| Hazard ratio (95% CI) | P-value | |
| Age | 1.02 (1.02–1.03) | <0.001 |
| MELD score | 1.02 (1.01–1.03) | <0.001 |
| Race | 0.022 | |
| White | 1.00 | |
| African-American | 1.22 (1.02–1.46) | 0.028 |
| Asian | 0.89 (0.70–1.13) | 0.343 |
| Hispanic | 0.90 (0.77–1.05) | 0.171 |
| Other | 0.65 (0.37–1.12) | 0.120 |
| Diabetes | 0.012 | |
| No | 1.00 | |
| Yes | 1.16 (1.03–1.31) | |
| Viral status | <0.001 | |
| HBV−/HCV− | 1.00 | |
| HBV+/HCV−c | 0.85 (0.63–1.13) | 0.263 |
| HBV−/HCV+c | 1.29 (1.13–1.47) | <0.001 |
| HBV+/HCV+ | 1.18 (0.79–1.76) | 0.424 |
95% CI, 95% confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-stage Liver Disease.
Significantly different odds ratios (P = 0.005) when directly compared.
Significantly different hazard ratios (P < 0.001) when directly compared.
Significantly different hazard ratios (P = 0.003) when directly compared.
After controlling for other factors, viral status was a significant independent predictor of outcome for all primary endpoints (Table 6). HBV−/HCV+ patients were retransplanted more often than HBV+/HCV− patients (P = 0.005). Graft longevity was lowest in HBV−/HCV+ patients and greatest in HBV+/HCV− patients. Both HBV−/HCV− and HBV+/HCV− patients lived longer after transplantation than HBV−/HCV+ patients. None of the antiviral therapies had a significant impact on outcome in final adjusted analyses.
Discussion
Using data based on modern selection criteria in HCC, this study finds that pre-transplant HBV and HCV status carries significant independent prognostic value. Mono-infected HCV+ patients achieve poorer GS and OS compared with both seronegative patients and mono-infected HBV+ patients.
Previous reports have attempted to recognize viral status as a useful prognostic tool in transplanted HCC patients, but results have been inconsistent.1,3 In a 2002 single-institution study, Moya et al.1 found that 5-year OS in transplanted HCC patients was worse in patients who were infected with HCV (59% versus 77%). Although it was anticipated that viral status would help to define the role of liver transplantation in HCC, there is no discussion of HBV or HCV infection in several recent reviews and consensus statements8–10 on the topic. Several series describing liver resection for HCC have also attempted to highlight the prognostic value of viral status. Whereas some series found that patients infected with HCV have increased rates of recurrence24 and poorer survival,25 others found that viral status did not impact outcomes.26 Pawlik et al.2 investigated the impacts of both HBV and HCV in resected HCC patients and found that infection with either virus was associated with bilobar disease. Patients mono-infected with HCV had a higher degree of liver fibrosis and less vascular invasion. Neither virus, however, had an impact on OS. In a recent meta-analysis of patients undergoing resection for HCC,27 both HBV and HCV infection were associated with poorer disease-free and overall survival.
Co-infection with both HBV and HCV (HBV+/HCV+) has a perplexing link with risk for and prognosis in HCC. Epidemiologically, several series have noted an additive or synergistic risk for HCC in co-infected patients.14,16,17 Patients with HCV who show serologic evidence of prior HBV infection tend to have higher rates of non-cirrhotic HCC, poorly differentiated HCC, and poorer survival rates after resection.14,28 The X gene of HBV has been implicated in the synergistic risk for HCC in co-infected patients; however, these mechanisms are not firmly established.14 Given these associations between co-infection and risk for HCC, it is somewhat surprising that several series have described improved outcomes in HBV+/HCV+ patients after liver transplantation. In small studies, co-infected patients have exhibited GS21 and OS22,23 superior to those in their HBV−/HCV+ counterparts. Although some viruses are known to inhibit the replication of other secondary viruses at a molecular level, HBV and HCV have not been shown to interfere with the replication of one another in vitro.18 If viral interference truly exists in HBV+/HCV+ patients after transplantation, it most likely occurs through host adaptive or innate immune properties. In the present study, the co-infected population was relatively small (n = 138). Although HBV+/HCV+ patients showed trends toward fewer retransplantations, prolonged GS and prolonged OS compared with HBV−/HCV+ patients, these differences were not statistically significant. Few patients were treated with antiviral therapy and, despite its theoretical benefit, improved outcomes were not demonstrated.
In addition to viral status, several other variables (age, MELD score, presence of diabetes and race) were found to influence the primary endpoints in the present study. The presence of diabetes has been shown to increase risk for HCC in patients with concurrent HBV or HCV infection or alcoholic cirrhosis,29 but has not previously been associated with outcome following liver transplantation for HCC. This oncogenic association may be linked to obesity and metabolic syndrome, and the risk for HCC associated with non-alcoholic steatohepatitis.14 Indeed, one study linked higher BMI to early death in seronegative patients who underwent transplant,30 but another study found higher BMI to have no influence on short- or longterm outcomes.31 In the present study, BMI did not show prognostic significance in adjusted models. Race has also been associated with outcome after liver transplantation for HCC. African-American patients have higher rates of chronic graft failure, poorer GS and poorer OS.32–34 Although the present study did not find race to be independently predictive of retransplantation rates or GS, it did establish a poorer OS in African-Americans. Increased age also predicted poorer GS and OS in this study, a finding that aligns with those cited in previous reports.32
Initially, the present study was designed to also examine the influence of donor HBV and HCV status on outcomes; however, no donors in this study sample were coded as HBV+ or HCV+. Registry datasets such as that of UNOS are known to suffer from miscoding; nonetheless, no conclusions can be drawn about the impact of donor viral status on the primary endpoints of the current study. This registry also lacks potentially informative data on viral loads; accordingly, response to antiviral medications cannot be assessed. Although the study population was intentionally limited, complete records of viral status were available for 7060 (91%) of the 7742 patients who were transplanted for HCC from 2005. The generalizability of these results assumes that all transplanted patients met modern selection criteria, but this dataset does not allow for specific adjustment for several oncologic staging parameters, such as tumour size, multifocality and vascular invasion. Because the timing of recurrence was not recorded, recurrence-free and disease-free survival analyses are not possible.
In conclusion, HBV and HCV viral status influences prognosis in patients undergoing liver transplantation for HCC. After adjusting for other covariates, HBV−/HCV+ patients achieve poorer GS and OS compared with both HBV−/HCV− and HBV+/HCV− patients. Both patients and physicians should understand the ramifications of viral status on post-transplant outcomes in HCC patients, especially in an era in which the allocation of grafts is carefully scrutinized.
Acknowledgments
This work was supported in part by the Health Resources and Services Administration, US Department of Health and Human Services (contract 234-2005-370011C). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services. The mention of trade names, commercial products or organizations does not imply their endorsement by the US Government.
Conflicts of interest
None declared.
References
- 1.Moya A, Berenguer M, Aguilera V, Juan FS, Nicolas D, Pastor M, et al. Hepatocellular carcinoma: can it be considered a controversial indication for liver transplantation in centres with high rates of hepatitis C? Liver Transpl. 2002;8:1020–1027. doi: 10.1053/jlts.2002.35664. [DOI] [PubMed] [Google Scholar]
- 2.Pawlik TM, Poon RT, Abdalla EK, Sarmiento JM, Ikai I, Curley SA, et al. Hepatitis serology predicts tumour and liver-disease characteristics but not prognosis after resection of hepatocellular carcinoma. J Gastrointest Surg. 2004;8:794–804. doi: 10.1016/j.gassur.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Luna H, Balan V, Sharma P, Byrne T, Mulligan D, Rakela J, et al. Hepatitis C virus infection with hepatocellular carcinoma: not a controversial indication for liver transplantation. Transplantation. 2004;78:580–583. doi: 10.1097/01.tp.0000129797.30999.69. [DOI] [PubMed] [Google Scholar]
- 4.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl. 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225–235. doi: 10.1007/s00535-005-1566-3. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 8.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cauchy F, Fuks D, Belghiti J. HCC: current surgical treatment concepts. Langenbecks Arch Surg. 2012;397:681–695. doi: 10.1007/s00423-012-0911-2. [DOI] [PubMed] [Google Scholar]
- 10.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl):75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 11.Koniaris LG, Levi DM, Pedroso FE, Franceschi D, Tzakis AG, Santamaria-Barria JA, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254:527–537. doi: 10.1097/SLA.0b013e31822ca66f. discussion 537–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung JY, Zhu AX, Gordon FD, Pratt DS, Mithoefer A, Garrigan K, et al. Liver transplantation outcomes for early-stage hepatocellular carcinoma: results of a multicentre study. Liver Transpl. 2004;10:1343–1354. doi: 10.1002/lt.20311. [DOI] [PubMed] [Google Scholar]
- 13.Akahoshi H, Taura N, Ichikawa T, Miyaaki H, Akiyama M, Miuma S, et al. Differences in prognostic factors according to viral status in patients with hepatocellular carcinoma. Oncol Rep. 2010;23:1317–1323. doi: 10.3892/or_00000766. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan DE, Reddy KR. Rising incidence of hepatocellular carcinoma: the role of hepatitis B and C; the impact on transplantation and outcomes. Clin Liver Dis. 2003;7:683–714. doi: 10.1016/s1089-3261(03)00060-6. [DOI] [PubMed] [Google Scholar]
- 15.Huo TI, Huang YH, Hsia CY, Su CW, Lin HC, Hsu CY, et al. Characteristics and outcome of patients with dual hepatitis B and C-associated hepatocellular carcinoma: are they different from patients with single virus infection? Liver Int. 2009;29:767–773. doi: 10.1111/j.1478-3231.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- 16.Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, et al. Occurrence of hepatocellular carcinoma and decompensation in Western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77–82. doi: 10.1002/hep.1840210114. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JY, Dai M, Wang X, Lu WQ, Li DS, Zhang MX, et al. A case–control study of hepatitis B and C virus infection as risk factors for hepatocellular carcinoma in Henan, China. Int J Epidemiol. 1998;27:574–578. doi: 10.1093/ije/27.4.574. [DOI] [PubMed] [Google Scholar]
- 18.Bellecave P, Gouttenoire J, Gajer M, Brass V, Koutsoudakis G, Blum HE, et al. Hepatitis B and C virus co-infection: a novel model system reveals the absence of direct viral interference. Hepatology. 2009;50:46–55. doi: 10.1002/hep.22951. [DOI] [PubMed] [Google Scholar]
- 19.Sagnelli E, Coppola N, Messina V, Di Caprio D, Marrocco C, Marotta A, et al. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology. 2002;36:1285–1291. doi: 10.1053/jhep.2002.36509. [DOI] [PubMed] [Google Scholar]
- 20.Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024–1029. doi: 10.1053/j.gastro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Waki K, Sugawara Y, Tamura S, Mieno MN, Yamashiki N, Kadowaki T, et al. Outcome of liver transplantation for recipients with hepatitis B and hepatitis C virus co-infection: analysis of the UNOS data. Transplantation. 2011;92:809–814. doi: 10.1097/TP.0b013e31822d4dc3. [DOI] [PubMed] [Google Scholar]
- 22.Huang EJ, Wright TL, Lake JR, Combs C, Ferrell LD. Hepatitis B and C co-infections and persistent hepatitis B infections: clinical outcome and liver pathology after transplantation. Hepatology. 1996;23:396–404. doi: 10.1002/hep.510230302. [DOI] [PubMed] [Google Scholar]
- 23.Rifai K, Wedemeyer H, Rosenau J, Klempnauer J, Strassburg CP, Manns MP, et al. Longer survival of liver transplant recipients with hepatitis virus co-infections. Clin Transplant. 2007;21:258–264. doi: 10.1111/j.1399-0012.2006.00636.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Li H, Qin Y, Wang PP, Hao X. Comparison of surgical outcomes for small hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a Chinese experience. J Gastroenterol Hepatol. 2007;22:1936–1941. doi: 10.1111/j.1440-1746.2006.04619.x. [DOI] [PubMed] [Google Scholar]
- 25.Kondo K, Chijiiwa K, Funagayama M, Kai M, Otani K, Ohuchida J. Differences in longterm outcome and prognostic factors according to viral status in patients with hepatocellular carcinoma treated by surgery. J Gastrointest Surg. 2008;12:468–476. doi: 10.1007/s11605-007-0402-x. [DOI] [PubMed] [Google Scholar]
- 26.Kao WY, Su CW, Chau GY, Lui WY, Wu CW, Wu JC. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg. 2011;35:858–867. doi: 10.1007/s00268-010-0928-z. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Si X, Wu L, Su X, Li B, Zhang Z. Influence of viral hepatitis status on prognosis in patients undergoing hepatic resection for hepatocellular carcinoma: a meta-analysis of observational studies. World J Surg Oncol. 2011;9:108. doi: 10.1186/1477-7819-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubo S, Nishiguchi S, Hirohashi K, Tanaka H, Tsukamoto T, Hamba H, et al. Clinical significance of prior hepatitis B virus infection in patients with hepatitis C virus-related hepatocellular carcinoma. Cancer. 1999;86:793–798. doi: 10.1002/(sici)1097-0142(19990901)86:5<793::aid-cncr14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case–control study among United States veterans. Am J Gastroenterol. 2001;96:2462–2467. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 30.Wigg AJ, Gunson BK, Mutimer DJ. Outcomes following liver transplantation for seronegative acute liver failure: experience during a 12-year period with more than 100 patients. Liver Transpl. 2005;11:27–34. doi: 10.1002/lt.20289. [DOI] [PubMed] [Google Scholar]
- 31.Yoo HY, Molmenti E, Thuluvath PJ. The effect of donor body mass index on primary graft non-function, retransplantation rate, and early graft and patient survival after liver transplantation. Liver Transpl. 2003;9:72–78. doi: 10.1053/jlts.2003.50006. [DOI] [PubMed] [Google Scholar]
- 32.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 33.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002;359:287–293. doi: 10.1016/S0140-6736(02)07494-9. [DOI] [PubMed] [Google Scholar]
- 34.Nair S, Thuluvath PJ. Does race-matched liver transplantation offer any graft survival benefit? Transplant Proc. 2001;33:1523–1524. doi: 10.1016/s0041-1345(00)02581-1. [DOI] [PubMed] [Google Scholar]


