Abstract
Background
For patients undergoing liver resection for colorectal metastases, specific clinico-pathological variables have been shown to be prognostic at baseline. This study analyses how the prognostic capability of these variables changes in a conditional survival model.
Methods
Retrospective review of a prospectively maintained database of patients who underwent an R0 resection of colorectal liver metastases from 1994 to 2004 at a single institution.
Results
In total, 807 patients were identified, with an 87-month median follow-up for survivors. Five- and 10-year disease-specific survivals (DSS) were 68% and 55%, respectively. The probability of further survival increased as the survival time increased. For 3-year survivors (n = 504), DSS were no longer significantly different between patients with a low (0–2) or high (3–5) clinical risk score (CRS, P = 0.19). On multivariate analysis, independent predictors of DSS for 3-year survivors were recurrence within the first 3 years after a liver resection, a pre-operative carcinoembryonic antigen (CEA) >200 ng/ml and disease-free interval <12 months prior to the diagnosis of liver metastasis. However, for those patients who were recurrence free at 1 year, no clinico-pathological variables retained prognostic significance.
Discussion
After 3 years of DSS and 1 year of recurrence-free survival, baseline clinico-pathological variables have a limited ability to predict future survival. Early post-operative recurrence appears to be the most useful single clinical feature in estimating conditional DSS.
Introduction
The liver is the most common site of distant metastasis from colorectal cancer, developing in approximately half of patients. A hepatic resection has become the standard therapy for resectable metastases, and remains the best chance for long-term survival and only chance of a cure.1 Previous studies have demonstrated that patients can be considered cured of their metastatic disease if they remain disease-free 10 years after a liver resection,2 which occurs in only about 20–25% of resected patients. On the other hand, 75% of all recurrences occur within the first 2 years post-operatively.3 Therefore, numerous studies have attempted to define clinical and histopathological variables associated with prognosis.4–15 These variables include nodal status and degree of differentiation of the primary colorectal tumour, synchronous compared with metachronous tumours, number and size of hepatic metastases and pre-operative concentration of carcinoembryonic antigen (CEA). From such variables, prognostic scoring systems have been developed, including the Clinical Risk Score (CRS)6 and the Memorial Sloan-Kettering Cancer Center (MSKCC) nomogram.16 While these systems have been validated at other institutions,17–19 their general applicability remains uncertain.15 Furthermore, substantial residual unexplained variability exists in these models, as their concordance indices (CI) are about 0.6.13,15,20
Traditional survival estimates are calculated from a single point in time, usually the time of diagnosis or operation. In contrast, conditional survival (CS) is defined as the probability of future survival based on survival time already accumulated, and includes only individuals who have survived to a pre-determined time of interest. CS has been studied across national populations21–24 and in specific cancers, including colorectal cancer25–28 and pancreatic adenocarcinoma.29,30 Only one study, by Nathan et al.20, has specifically analysed CS in patients who have undergone a liver resection for colorectal metastases. They demonstrated that CS improved over time but that the performance of various prognostic scoring systems declined as survival time increased. Several previous studies have demonstrated in other cancers28,31–33 that the prognostic value of clinicopathological variables diminishes as the survival time increases.
To date, analyses of conditional survival in cancer patients have examined all survivors, whether or not recurrence has occurred. No study has specifically examined patients who remain without evidence of recurrent disease after a curative resection, and how the prognostic ability of baseline risk factors changes as survival time increases. This is particularly relevant in patients who have undergone a curative-intent liver resection for colorectal metastases given the significant chance of recurrence,3 but also of long-term survival after recurrence owing to improved efficacy of modern chemotherapy and, in a minority of cases, resection of recurrent metastatic disease.
In this study of patients who have undergone a margin-negative liver resection for colorectal metastases, we sought to confirm that the prognostic value of risk-scoring systems decreased as the survival time increased. Further, we hypothesized that any clinico-pathological factors would have a limited prognostic ability to predict future overall and recurrence-free survival, specifically in the subset of patients who had survived without evidence of recurrent disease.
Materials and methods
Patients and data collection
With approval of the Institutional Review Board and in accordance with Health Insurance Portability and Accountability Act regulations, a prospectively maintained hepatobiliary database was used to identify all patients who underwent a resection of colorectal liver metastases at MSKCC from 1994–2004. Post-operative deaths (within 90 days of a hepatectomy) and those patients with margin-positive resections were excluded. Guidelines for resectability were medical fitness for a major laparotomy, no evidence of disseminated disease and a resection strategy encompassing all liver disease with an adequate remnant liver for recovery. A pre-operative extent-of-disease evaluation included chest/abdominal/pelvic computed tomography, and colonoscopy. 18F-fluorodeoxyglucose-positron emission tomography/CT (18FDG PET-CT) was used selectively. All patients were evaluated at a weekly multidisciplinary conference.
Variables studied included age, gender, site and node status of the primary, presence of lymphovascular invasion (LVI) or perineural invasion (PNI), synchronous compared with metachronous metastases, disease-free interval (DFI; defined as time elapsed from a primary resection to a hepatic recurrence), pre-hepatectomy CEA level, the presence or absence of bilobar disease, and the size and number of hepatic tumours. Post-operative variables evaluated were extent of resection and whether adjuvant hepatic-arterial infusional chemotherapy (HAIC) was administered. Accurate documentation of post-operative systemic chemotherapy in other patients was not available. The extent of the resection was recorded as major or minor, with a major resection defined as a resection of more than two segments. Post-operative follow-up included physical examination and cross-sectional imaging every 4 to 6 months.
Statistical analysis
Statistical analyses were performed with the software package JMP (JMP, Cary, NC, USA) and R (http://www.r-project.org). Disease-specific survival (DSS) was calculated from the time of initial hepatectomy until cancer-related death. Survival curves were generated using the Kaplan–Meier method, with patients censored when lost to follow-up or upon death from non-cancer causes. Kaplan–Meier estimates of survival and Cox's proportional hazards models were then used to explore differences in survival among the strata established by the CRS,6 the MSKCC nomogram16 and the Nordlinger system.34 Conditional survival estimates represent the probability that a patient will survive an additional number of years, given that the patient has already survived a given amount of time. Conditional survival probabilities were calculated using Bayes’ rule and Kaplan–Meier estimates of unconditional survival. Univariate analysis for factors associated with DSS was conducted using the log-rank test. Variables significant at the 0.1 level were included in a multivariable Cox's proportional regression model. P-values <0.05 were considered statistically significant.
Results
Demographics and treatment
From January 1994 through to December 2004, a total of 951 patients underwent an initial hepatectomy for colorectal liver metastases. Of these, the 10 post-operative deaths (1%) and the 134 patients (14%) with positive margins were excluded. The remaining 807 patients make up the study population. The median follow-up for survivors, as measured from the time of hepatectomy, was 87 months. At last follow-up, 389 (48%) patients had died of the disease, 66 (8%) had died of other causes, 60 (7%) were alive with the disease and 292 (36%) were alive with no evidence of disease.
The demographic and tumor-related characteristics of the whole cohort of 807 patients are listed in Table 1. Most primary tumours were of advanced T stage [T3/T4 in 626 (78%) patients) and node positive (482 patients (60%)]. Ninety-seven patients (12%) presented with liver metastases at the time of diagnosis of their colorectal primary (synchronous disease). The majority (70%) of patients had a low CRS (score 0–2). Four per cent of the patients had extrahepatic metastases; these sites were resected at the time of the hepatectomy. A combined resection of the colorectal primary and liver metastases was performed in 44 of 97 patients (45%). Four hundred and eighty-four (60%) patients experienced recurrence, of which 198 (41%) occurred within the first year and 356 (74%) by 2 years after the liver resection.
Table 1.
Patient demographics
| Variable | Value |
|---|---|
| Gender | |
| Male | 466 (58%) |
| Female | 341 (42%) |
| Age | |
| Median age (years, range) | 63 (23–89) |
| >70 years old | 200 (25%) |
| Primary site | |
| Colon | 582 (72%) |
| Rectum | 225 (28%) |
| T stage of primary tumour | |
| 1 | 25 (3%) |
| 2 | 104 (13%) |
| 3 | 586 (73%) |
| 4 | 40 (5%) |
| Unknown | 52 (6%) |
| N stage of primary tumour | |
| N0 | 325 (40%) |
| N1,2 | 482 (60%) |
| Lymphovascular invasion of primary tumour | |
| Present | 356 (44%) |
| Absent | 199 (25%) |
| Unknown | 252 (31%) |
| Perineural invasion of primary tumour | |
| Present | 389 (48%) |
| Absent | 96 (12%) |
| Unknown | 322 (40%) |
| Tumour grade of primary tumour | |
| Low | 21 (3%) |
| Moderate | 635 (79%) |
| Poor | 67 (8%) |
| Unknown | 84 (10%) |
| Disease-free interval, months | |
| 0–12 | 444 (55%) |
| >12 | 363 (45%) |
| Size of largest metastasis, cm | |
| <5 | 554 (69%) |
| ≥5 | 253 (31%) |
| Number of liver metastases | |
| 1 | 397 (49%) |
| >1 | 410 (51%) |
| Pre-operative CEA level, ng/ml | |
| <200 | 642 (80%) |
| ≥200 | 76 (9%) |
| Unknown | 89 (11%) |
| Clinical Risk Score | |
| 0 | 55 (7%) |
| 1 | 239 (30%) |
| 2 | 267 (33%) |
| 3 | 184 (23%) |
| 4 | 51 (6%) |
| 5 | 11 (1%) |
| Clinical Risk Score: low versus high | |
| Low (0–2) | 561 (70%) |
| High (3–5) | 246 (30%) |
| Extent of liver resection | |
| Minor | 319 (40%) |
| Major | 488 (60%) |
| Extrahepatic disease resected | |
| Yes | 32 (4%) |
| No | 775 (96%) |
| Peri-operative chemotherapy | |
| Neoadjuvant | 179 (22%) |
| Adjuvant | |
| Systemic | 654 (81%) |
| HAI + systemic | 208 (26%) |
CEA, carcinoembryonic antigen; HAI, hepatic arterial infusion.
Conditional survival
Actuarial DSS was 68% at 5 years and 55% at 10 years. For those patients alive 1 year after the liver resection, the probability of surviving to 5 and 10 years after a liver resection increased to 70% and 56%, respectively (See Fig. 1a). For 3-year survivors, the probability of surviving to 5 and 10 years after a liver resection increased to 80% and 65%, respectively. Of the 345 patients (43% of the original cohort of 807 patients) who survived to 5 years, the probability of surviving to 10 years was 81%.
Figure 1.
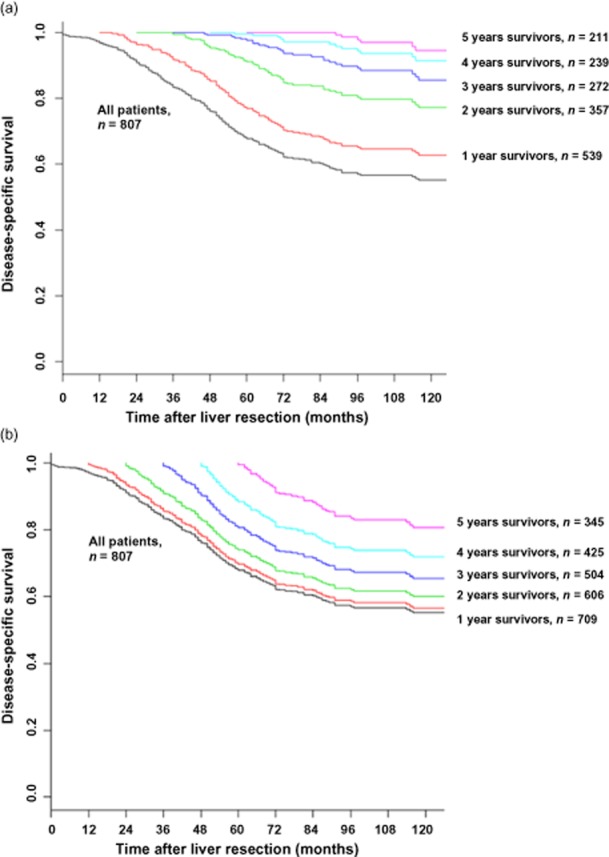
Conditional disease-specific survival for all survivors (a); and recurrence-free survivors (b)
The conditional survival for patients surviving without recurrence was higher than for all survivors. For those patients alive and recurrence free 1 year after a liver resection, the probabilities of surviving to 5 and 10 years after a liver resection were 78% and 64%, respectively (See Fig. 1b). For 3-year recurrence-free survivors, the probability of surviving to 5 and 10 years after a liver resection increased to 98% and 86%, respectively. Of the 211 patients (26% of the original cohort of 807 patients) who survived without recurrence to 5 years, the probability of surviving to 10 years was 95%.
Conditional survival and the clinical risk score for survivors
At baseline, patients with a high and low CRS had significantly different DSS (Fig. 2a, P < 0.0005). However, as survival time after liver resection increased, the difference in prognosis between the two groups decreased such that, among 3-year survivors, there was no significant difference in conditional survival between patients with a high and low CRS (Fig. 2b–d).
Figure 2.
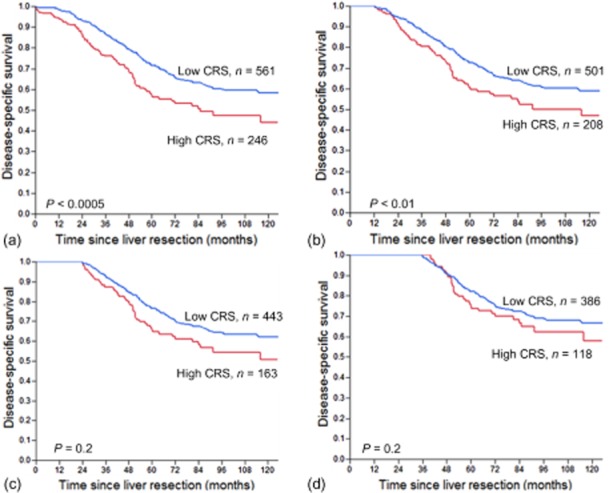
Disease-specific survival stratified by a low or high clinical risk score (CRS) at baseline (a), 1 year (b), 2 years (c) and 3 years (d) post-operatively
Analyses were performed to identify factors associated with further DSS for the 504 patients who had survived 3 years post-operatively (Table 2). Factors associated with further survival on univariate analysis included nodal status of the primary tumour, perineural invasion, the disease-free interval between diagnosis of the primary tumor and hepatic metastasis, CEA level and whether tumour recurrence had occurred within 3 years of the liver resection. On multivariate analysis, there were three independent factors associated with worse DSS: recurrence within 3 years of the liver resection [hazard ratio (HR) 8.6, 95% confidence interval (CI) 5.6–13.7], CEA > 200 ng/ml (HR 2.1, 95% CI 1.2–3.8) and disease-free interval < 12 months between the diagnosis of the primary tumour and hepatic metastasis (HR 1.4, 95% CI 1.0–2.1).
Table 2.
Factors associated with disease-specific survival after 3-year survival
| Factor (n) | 5-year conditional DSSa (%) | Univariate P | Multivariate P, HR (95% CI) |
|---|---|---|---|
| Gender | 0.73 | – | |
| Female (n = 214) | 65 | ||
| Male (n = 290) | 69 | ||
| Age, years | 0.54 | – | |
| ≤70 (n = 381) | 66 | ||
| >70 (n = 123) | 72 | ||
| Primary site | 0.46 | – | |
| Colon (n = 373) | 69 | ||
| Rectum (n = 166) | 65 | ||
| T stage of primary tumour | 0.79 | – | |
| 1, 2 (n = 89) | 70 | ||
| 3, 4 (n = 389) | 67 | ||
| N stage of primary tumour | 0.09 | 0.56 | |
| N0 (n = 232) | 72 | ||
| N1,2 (n = 272) | 64 | ||
| Lymphovascular invasion of primary tumour | 0.47 | – | |
| Present (n = 115) | 64 | ||
| Absent (n = 258) | 71 | ||
| Perineural invasion of primary tumour | 0.05 | 0.83 | |
| Present (n = 59) | 53 | ||
| Absent (n = 272) | 71 | ||
| Tumour grade of primary tumour | 0.54 | – | |
| Low (n = 15) | 65 | ||
| Moderate (n = 413) | 67 | ||
| Poor (n = 32) | 63 | ||
| DFI before liver metastasis, months | 0.07 | 0.04, 1.4 (1.0–2.1) | |
| 0–12 (n = 277) | 63 | ||
| >12 (n = 227) | 73 | ||
| Size of largest metastasis, cm | 0.16 | – | |
| <5 (n = 370) | 69 | ||
| ≥5 (n = 134) | 63 | ||
| Number of liver metastases | 0.67 | – | |
| 1 (n = 269) | 69 | ||
| >1 (n = 235) | 65 | ||
| Extent of liver resection | 0.91 | – | |
| Minor (n = 215) | 67 | ||
| Major (n = 289) | 68 | ||
| Extrahepatic disease resected | 0.45 | – | |
| Yes (n = 14) | 67 | ||
| No (n = 490) | 80 | ||
| Pre-operative CEA level, ng/ml | 0.005 | <0.01, 2.1 (1.2–3.8) | |
| <200 (n = 421) | 70 | ||
| ≥200 (n = 37) | 45 | ||
| Clinical Risk Score: | 0.19 | – | |
| Low (0–2) (n = 386) | 69 | ||
| High (3–5) (n = 118) | 62 | ||
| Pre-liver resection chemotherapy | 0.45 | ||
| Yes (n = 391) | 64 | ||
| No (n = 107) | 68 | – | |
| Adjuvant systemic chemotherapy | 0.69 | – | |
| Yes (n = 433) | 68 | ||
| No (n = 47) | 63 | ||
| Adjuvant hepatic arterial chemotherapy | 0.81 | – | |
| Yes (n = 160) | 65 | ||
| No (n = 336) | 69 | ||
| Recurrence within 3 years of a liver resection | <0.0001 | <0.0001, 8.6 (5.6–13.7) | |
| Yes (n = 232) | 38 | ||
| No (n = 272) | 90 | ||
Defined as the probability of surviving another 5 years, having already survived 3 years.
DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen; DFI, disease-free interval.
Conditional survival and the clinical risk score for recurrence-free survivors
A separate analysis was performed to examine conditional survival in the subset of survivors whose disease had not recurred. At 1 year recurrence-free survival after a liver resection and at all subsequent time points, the conditional survival of patients with a high and low CRS were not statistically significant (Fig. 3). For 1-year recurrence-free survivors, factors associated with further DSS on univariate analysis were perineural invasion, nodal status of the primary tumour, CEA level and the size of the largest hepatic metastasis (see Table 3). However, on multivariate analysis, no independent factors associated with DSS were found.
Figure 3.
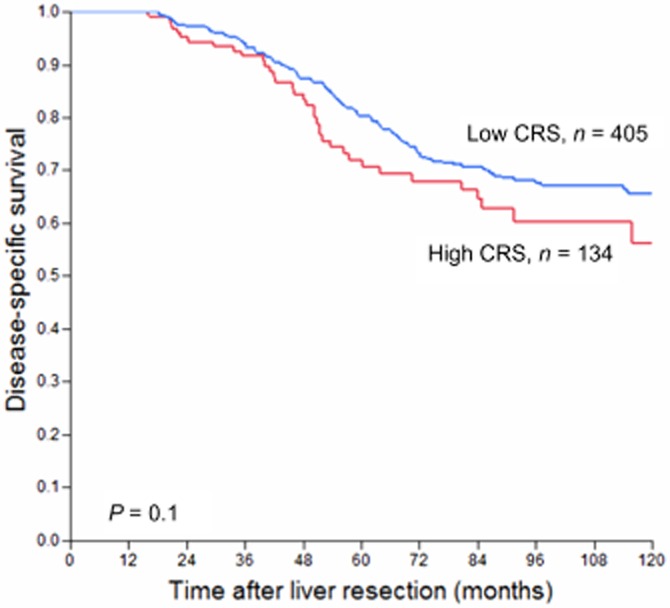
Disease-specific survival of patients who were recurrence free at 1 year post-operatively, stratified by low or high clinical risk score (CRS)
Table 3.
Factors associated with disease-specific survival after 1 year of recurrence-free survival
| Factor (n) | 5-year conditional DSSa (%) | Univariate P | Multivariate P, HR (95% CI) |
|---|---|---|---|
| Gender | 0.72 | – | |
| Female (n = 222) | 70 | ||
| Male (n = 317) | 73 | ||
| Age, years | 0.20 | – | |
| ≤70 (n = 404) | 70 | ||
| >70 (n = 135) | 76 | ||
| Primary site | 0.27 | – | |
| Colon (n = 373) | 73 | ||
| Rectum (n = 166) | 68 | ||
| T stage of primary tumour | 0.17 | – | |
| 1, 2 (n = 95) | 73 | ||
| 3, 4 (n = 415) | 70 | ||
| N stage of primary tumour | 0.04 | 0.10 | |
| N0 (n = 238) | 74 | ||
| N1,2 (n = 301) | 69 | ||
| Lymphovascular invasion of primary tumour | 0.58 | – | |
| Present (n = 117) | 71 | ||
| Absent (n = 267) | 74 | ||
| Perineural invasion of primary tumour | 0.02 | 0.12 | |
| Present (n = 56) | 62 | ||
| Absent (n = 285) | 74 | ||
| Tumour grade of primary tumour | 0.81 | – | |
| Low (n = 18) | 65 | ||
| Moderate (n = 431) | 71 | ||
| Poor (n = 36) | 76 | ||
| Disease-free interval, months | 0.11 | – | |
| 0–12 (n = 241) | 75 | ||
| >12 (n = 298) | 69 | ||
| Size of largest metastasis, cm | 0.07 | 0.19 | |
| <5 (n = 385) | 73 | ||
| ≥5 (n = 154) | 67 | ||
| Number of liver metastases | 0.47 | – | |
| 1 (n = 291) | 72 | ||
| >1 (n = 248) | 72 | ||
| Extent of liver resection | 0.73 | – | |
| Minor (n = 232) | 72 | ||
| Major (n = 307) | 72 | ||
| Extrahepatic disease resected | 0.67 | – | |
| Yes (n = 13) | 67 | ||
| No (n = 526) | 72 | ||
| Pre-operative CEA level, ng/ml | 0.06 | 0.27 | |
| <200 (n = 440) | 74 | ||
| ≥200 (n = 43) | 57 | ||
| Clinical Risk Score: | 0.1 | – | |
| Low (0–2) (n = 405) | 73 | ||
| High (3–5) (n = 134) | 68 | ||
| Pre-liver resection chemotherapy | 0.58 | – | |
| Yes (n = 98) | 72 | ||
| No (n = 434) | 71 | ||
| Adjuvant systemic chemotherapy | 0.98 | – | |
| Yes (n = 450) | 72 | ||
| No (n = 60) | 75 | ||
| Adjuvant hepatic arterial chemotherapy | 0.95 | – | |
| Yes (n = 156) | 71 | ||
| No (n = 372) | 73 | ||
Defined as the probability of surviving another 5 years, having already survived 1 year without recurrence.
DSS, disease-specific survival; HR, hazard ratio; CI, confidence interval; CEA, carcinoembryonic antigen.
In contrast to the lack of prognostic significance of traditional clinical variables, tumour recurrence within the first year after a liver resection was highly associated with worse DSS (Fig. 4). For those patients who were recurrence-free at 1 year post-operatively, the probability of surviving to 10 years post-operatively was 64%, compared with 24% for those patients who had recurred within the first year after a liver resection (P < 0.0001).
Figure 4.
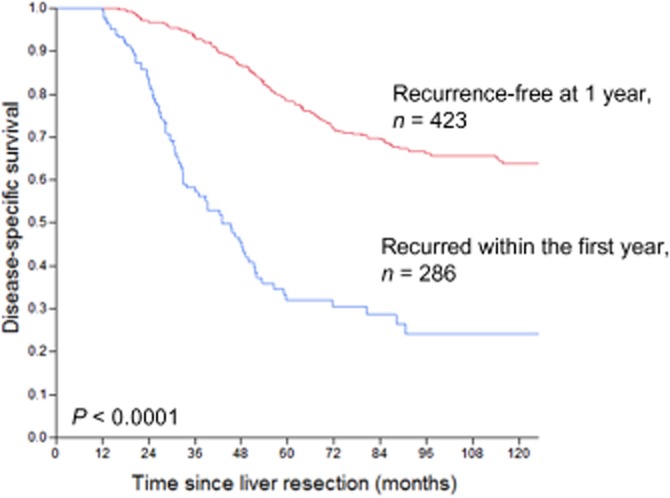
Disease-specific survival at 1 year post-operatively, stratified by recurrence within the first post-operative year
The MSKCC nomogram
A similar analysis was performed using the MSKCC nomogram.16 Patients were divided evenly into quartiles based on their predicted DSS, and, at baseline, these quartiles had significantly different survivals (Fig. 5a, P < 0.01). However, in spite of the baseline concordance index for the nomogram (0.587) being superior to that of the CRS (0.551), the nomogram could not provide prognostic discrimination between the quartiles for 3-year survivors (P = 0.12, data not shown). For recurrence-free survivors, at 1 year post-operatively, there was only a significantly different DSS for patients in the first versus the fourth quartile (P = 0.004), which was lost at 2 years of recurrence-free survival (Fig. 6, P = 0.42).
Figure 5.
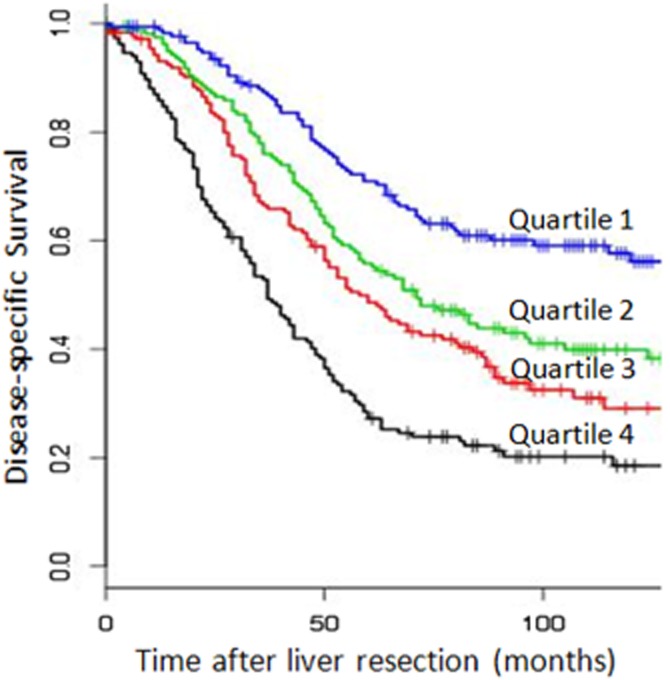
Disease-specific survival stratified by nomogram quartiles, at baseline
Figure 6.
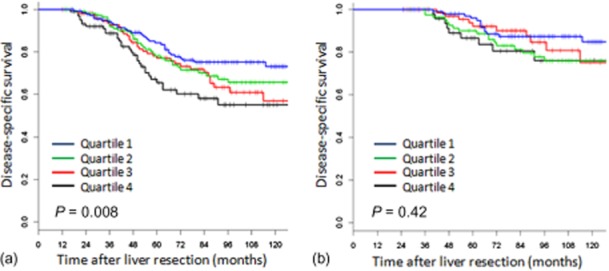
Disease-specific survival for recurrence-free survivors at 1 year (a) and 2 years (b) post-operatively, stratified by nomogram quartiles
In contrast to the lack of prognostic significance of traditional clinical variables, tumour recurrence within the first year after a liver resection was highly associated with worse DSS (Fig. 4). For those patients who were recurrence-free at 1 year post-operatively, the probability of surviving to 10 years post-operatively was 64%, compared with 24% for those patients who had recurred within the first year after a liver resection (P < 0.0001).
At baseline, the Nordlinger system did not provide prognostic stratification in our patient population (P = 0.61, data not shown).
Discussion
This study demonstrates that baseline clinico-pathological risk factors, whether combined into prognostic scoring systems or not, have little prognostic discriminatory value for even short-term survivors after a resection of colorectal liver metastases. This is most dramatically demonstrated for those patients who are alive without recurrence just 1 year post-operatively, at which time those patients with a baseline poor prognosis have the same DSS as those with a baseline good prognosis.
The main clinical utility of conditional survival analyses is in counselling patients who have already accumulated a survival time after treatment of their cancer. Similar to studies in other cancers,28,31,32,35–37 this study of patients who had undergone a margin-negative liver resection for colorectal metastasis found that, as survival time increased, so too did the probability of future survival. For a population of patients who, pre-operatively, have an approximately 25% chance of a 10-year survival, it is clinically meaningful, for example, to be able to advise 1-, 3- and 5-year survivors that their chance of reaching the 10-year survival time point has increased to 56%, 65% and 81%, respectively.
Although the CRS has been shown in most studies to stratify patients by outcome, its CI remains low (≤0.6),15 meaning that some patients with low CRS recur early, whereas those with a high CRS are not precluded from long-term survival or even a cure. This reflects the imperfect nature of clinico-pathological variables as surrogate markers of tumour biology. The analysis of the relationship between the CRS and conditional survival in the present study emphasizes this point. By 3 years of post-operative survival, prognostic stratification of the CRS is lost, and on multivariate analysis, while two pre-operative clinical variables (disease-free interval and CEA level) remain significant, it is the tumor's most recent biological history, namely evidence of recurrence after a liver resection, that is the most significant independent prognostic variable.
There is only one other study that examined conditional survival in patients who had undergone a resection for colorectal liver metastasis.20 This was a study of 949 patients from five hepatobiliary centres in the United States and Europe. Unlike the present study, 9% of patients had positive margins. Their study period of 1982–2008 bridged the introduction of modern chemotherapy in the mid-1990s. Details of patient treatment other than their resection, as well as tumour recurrence were not included. Their primary outcome measure was overall survival, whereas in this study, disease-specific survival was utilized. Therefore, as the present study comprises a more modern, margin-negative population with cancer-specific death as the primary endpoint, it may provide more accurate data for post-operative counselling. The study of Nathan et al.20 also compared three prognostic scoring systems: the CRS, the Nordlinger system and the MSKCC nomogram. Similar to our study, they found that the CRS and the MSKCC nomogram had poor-to-moderate discriminatory capacity at baseline (CI < 0.6 for both). This prognostic ability also decreased with increasing survival after a liver resection, although the time point at which all prognostic stratification was lost was not stated. The Nordlinger system in their study had a prognostic value at baseline that appeared to increase with survival time. However, in the present study, the Nordlinger system did not have a prognostic value even at baseline. Finally, in the Nathan et al.20 study, the prognostic value of early post-operative recurrence on future survival could not be examined.
The value of clinico-pathological variables in conditional survival analyses has been studied in other cancers. Studies in primary colon cancer,28 lymphoma32 and retroperitoneal sarcoma31,33 found that survival projections based on initial prognostic measures, akin to the CRS, converge with increased survival time. This convergence demonstrates that the baseline prognostic variables become less valuable over time. A recent study by Harshman et al.35 in patients with metastatic renal cell carcinoma treated with VEGF-targeted therapy demonstrated contrary findings. With a median follow-up of 20 months, the prognostic stratification of these patients into favourable, intermediate and poor risk groups remained significant (P < 0.0001) at 18 months. In contrast to the previous studies and the present one where conditional survival was analysed after potentially curative treatment (surgical resection for colon cancer and sarcoma, chemotherapy for lymphoma), patients in the study of Harshman et al.35 were treated with palliative chemotherapy. This difference in the goals (and efficacy) of treatment points to the impact of potentially curative therapy in altering the course of the disease.
As demonstrated in multiple retrospective series,38–44 recurrence after a resection for colorectal liver metastases occurs in 40–74% of patients, and is associated with a worse outcome. In the present study, the overall recurrence rate of 60%, with 40% of recurrences within the first year, is consistent with the published literature. A recent study from our institution3 examining the effect of recurrence on outcome found that a worse outcome was associated with a shorter time both between primary presentation and a hepatectomy and between a hepatectomy and recurrence, multiple sites of recurrence, non-lung recurrence, and inability to resect recurrent disease. In the present study, we found that in 3-year survivors, recurrence within 3 years was the most important independent prognostic variable associated with future DSS on multivariate analysis, with a HR 8.6. Two-thirds of patients who are recurrence free at 1 year and over 80% of patients recurrence free at 3 years will still be alive 10 years after a liver resection. This contrasts sharply with 10 year DSS of 24% and 38% for those who recur within 1 and 3 years, respectively. The novel and clinically useful finding from this study is that baseline (pre-operative) measures of poor prognosis are no longer discriminatory after just 1 year of recurrence-free survival. These data are useful and informative for physicians counselling patients in the years after a liver resection. From a research perspective, comparison of patients who recur early with those who do not may facilitate identification of new prognostic biomarkers.
The retrospective nature of this study produces several important limitations. First, there is missing data, especially regarding the pathological details of the primary tumour. The T stage and level of differentiation were unknown in 6% and 10%, respectively, with LVI and PNI unknown in 30–40%. While these variables are not part of the CRS, recent studies suggest that the histopathological features of the primary tumour may be more prognostic than characteristics of the liver metastases.13,45 Second, treatment regimens, especially use of neoadjuvant chemotherapy, cannot be standardized outside of the confines of a clinical trial. Thus, it is impossible to interpret the lack of a prognostic value of chemotherapy from this study.
In conclusion, conditional survival analyses provide useful prognostic information for counselling patients who have survived years after resection of colorectal liver metastasis. However, our ability to predict future survival after the survival time has been accrued remains poor, as demonstrated by the loss of the prognostic value of the CRS after 3 years of disease-specific survival and 1 year of recurrence-free survival. The presence or absence of recurrence appears to be the most useful single clinical feature in estimating conditional DSS.
Conflicts of interest
None declared.
References
- 1.House MG, Ito H, Gonen M, Fong Y, Allen PJ, DeMatteo RP, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi: 10.1016/j.jamcollsurg.2009.12.040. 52–5. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 3.D'Angelica M, Kornprat P, Gonen M, DeMatteo RP, Fong Y, Blumgart LH, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18:1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 4.Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–639. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 5.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. discussion 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, et al. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001;96:3178–3184. doi: 10.1111/j.1572-0241.2001.05278.x. [DOI] [PubMed] [Google Scholar]
- 8.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minagawa M, Yamamoto J, Kosuge T, Matsuyama Y, Miyagawa S, Makuuchi M. Simplified staging system for predicting the prognosis of patients with resectable liver metastasis: development and validation. Arch Surg. 2007;142:269–276. doi: 10.1001/archsurg.142.3.269. discussion 77. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Analysis of preoperative prognostic factors for long-term survival after hepatic resection of liver metastasis of colorectal carcinoma. J Gastrointest Surg. 2005;9:374–380. doi: 10.1016/j.gassur.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 11.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 12.Schindl M, Wigmore SJ, Currie EJ, Laengle F, Garden OJ. Prognostic scoring in colorectal cancer liver metastases: development and validation. Arch Surg. 2005;140:183–189. doi: 10.1001/archsurg.140.2.183. [DOI] [PubMed] [Google Scholar]
- 13.Tan MC, Castaldo ET, Gao F, Chari RS, Linehan DC, Wright JK, et al. A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by positron emission tomography with [18F]fluoro-2-deoxy-D-glucose: role of primary tumor variables. J Am Coll Surg. 2008;206:857–868. doi: 10.1016/j.jamcollsurg.2007.12.023. discussion 68–9. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Shimada H, Ueda M, Matsuo K, Endo I, Togo S. Long-term characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol. 2007;14:1336–1346. doi: 10.1245/s10434-006-9071-3. [DOI] [PubMed] [Google Scholar]
- 15.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kattan MW, Gonen M, Jarnagin WR, DeMatteo R, D'Angelica M, Weiser M, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282–287. doi: 10.1097/SLA.0b013e31815ed67b. [DOI] [PubMed] [Google Scholar]
- 17.Mala T, Bohler G, Mathisen O, Bergan A, Soreide O. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome? World J Surg. 2002;26:1348–1353. doi: 10.1007/s00268-002-6231-x. [DOI] [PubMed] [Google Scholar]
- 18.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–1172. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 19.Reddy SK, Kattan MW, Yu C, Ceppa EP, de la Fuente SG, Fong Y, et al. Evaluation of peri-operative chemotherapy using a prognostic nomogram for survival after resection of colorectal liver metastases. HPB. 2009;11:592–599. doi: 10.1111/j.1477-2574.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–764. doi: 10.1016/j.jamcollsurg.2009.12.041. 64-6. [DOI] [PubMed] [Google Scholar]
- 21.Ellison LF, Bryant H, Lockwood G, Shack L. Conditional survival analyses across cancer sites. Health Rep. 2011;22:21–25. [PubMed] [Google Scholar]
- 22.Janssen-Heijnen ML, Gondos A, Bray F, Hakulinen T, Brewster DH, Brenner H, et al. Clinical relevance of conditional survival of cancer patients in europe: age-specific analyses of 13 cancers. J Clin Oncol. 2010;28:2520–2528. doi: 10.1200/JCO.2009.25.9697. [DOI] [PubMed] [Google Scholar]
- 23.Merrill RM, Hunter BD. Conditional survival among cancer patients in the United States. Oncologist. 2010;15:873–882. doi: 10.1634/theoncologist.2009-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu XQ, Baade PD, O'Connell DL. Conditional survival of cancer patients: an Australian perspective. BMC Cancer. 2012;12:460. doi: 10.1186/1471-2407-12-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41:1097–1106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 26.Wang SJ, Fuller CD, Emery R, Thomas CR. Conditional survival in rectal cancer: a SEER database analysis. Gastrointest Cancer Res. 2007;1:84–89. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang SJ, Wissel AR, Luh JY, Fuller CD, Kalpathy-Cramer J, Thomas CR., Jr An interactive tool for individualized estimation of conditional survival in rectal cancer. Ann Surg Oncol. 2011;18:1547–1552. doi: 10.1245/s10434-010-1512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamboni BA, Yothers G, Choi M, Fuller CD, Dignam JJ, Raich PC, et al. Conditional survival and the choice of conditioning set for patients with colon cancer: an analysis of NSABP trials C-03 through C-07. J Clin Oncol. 2010;28:2544–2548. doi: 10.1200/JCO.2009.23.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent TS, Sachs TE, Sanchez N, Vollmer CM, Jr, Callery MP. Conditional survival in pancreatic cancer: better than expected. HPB. 2011;13:876–880. doi: 10.1111/j.1477-2574.2011.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo SC, Nathan H, Cameron JL, Olino K, Edil BH, Herman JM, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer. 2012;118:2674–2681. doi: 10.1002/cncr.26553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott AM, Habermann EB, Parsons HM, Tuttle T, Al-Refaie W. Prognosis for primary retroperitoneal sarcoma survivors: a conditional survival analysis. Cancer. 2012;118:3321–3329. doi: 10.1002/cncr.26665. [DOI] [PubMed] [Google Scholar]
- 32.Moller MB, Pedersen NT, Christensen BE. Conditional survival of patients with diffuse large B-cell lymphoma. Cancer. 2006;106:2165–2170. doi: 10.1002/cncr.21877. [DOI] [PubMed] [Google Scholar]
- 33.Parsons HM, Habermann EB, Tuttle TM, Al-Refaie WB. Conditional survival of extremity soft-tissue sarcoma: results beyond the staging system. Cancer. 2011;117:1055–1060. doi: 10.1002/cncr.25564. [DOI] [PubMed] [Google Scholar]
- 34.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 35.Harshman LC, Xie W, Bjarnason GA, Knox JJ, MacKenzie M, Wood L, et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol. 2012;13:927–935. doi: 10.1016/S1470-2045(12)70285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karakiewicz PI, Suardi N, Capitanio U, Isbarn H, Jeldres C, Perrotte P, et al. Conditional survival predictions after nephrectomy for renal cell carcinoma. J Urol. 2009;182:2607–2612. doi: 10.1016/j.juro.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 37.Thompson RH, Leibovich BC, Lohse CM, Cheville JC, Zincke H, Blute ML, et al. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: the D-SSIGN score. J Urol. 2007;177:477–480. doi: 10.1016/j.juro.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 38.Bozzetti F, Doci R, Bignami P, Morabito A, Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg. 1987;205:264–270. doi: 10.1097/00000658-198703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 40.Metcalfe M, Mann C, Mullin E, Maddern G. Detecting curable disease following hepatectomy for colorectal metastases. Aust N Z J Surg. 2005;75:524–527. doi: 10.1111/j.1445-2197.2005.03421.x. [DOI] [PubMed] [Google Scholar]
- 41.Mutsaerts EL, van Ruth S, Zoetmulder FA, Rutgers EJ, Hart AA, van Coevorden F. Prognostic factors and evaluation of surgical management of hepatic metastases from colorectal origin: a 10-year single-institute experience. J Gastrointest Surg. 2005;9:178–186. doi: 10.1016/j.gassur.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi S, Inoue K, Konishi M, Nakagouri T, Kinoshita T. Prognostic factors for poor survival after repeat hepatectomy in patients with colorectal liver metastases. Surgery. 2003;133:627–634. doi: 10.1067/msy.2003.151. [DOI] [PubMed] [Google Scholar]
- 43.Topal B, Kaufman L, Aerts R, Penninckx F. Patterns of failure following curative resection of colorectal liver metastases. Eur J Surg Oncol. 2003;29:248–253. doi: 10.1053/ejso.2002.1421. [DOI] [PubMed] [Google Scholar]
- 44.Ueno H, Mochizuki H, Hashiguchi Y, Hatsuse K, Fujimoto H, Hase K. Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br J Surg. 2004;91:327–333. doi: 10.1002/bjs.4429. [DOI] [PubMed] [Google Scholar]
- 45.Cardona K, Mastrodomenico P, D'Amico F, Shia J, Gonen M, Weiser MR, et al. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol. 2013;20:148–154. doi: 10.1245/s10434-012-2540-y. [DOI] [PubMed] [Google Scholar]


