Abstract
Background
The significance of a positive margin in resected non-invasive pancreatic intraductal papillary mucinous neoplasms (IPMN) remains controversial. The aim of this study was to determine recurrence rates when dysplasia was present at the final surgical margin.
Methods
A prospectively maintained database identified 192 patients undergoing resection of non-invasive IPMN. Pathological, peri-operative and recurrence data were analysed.
Results
Ductal dysplasia was identified at the final surgical margin in 86 patients (45%) and defined as IPMN or Pancreatic Intraepithelial Neoplasia PanIN in 38 (20%) and 54 (28%) patients, respectively. At a median follow-up of 46 months, 40 (21%) patients recurred with 31 developing radiographical evidence of new cysts, 6 re-resected for IPMN and 3 diagnosed with pancreatic cancer within the remnant. Of those with margin dysplasia, 31% developed recurrent disease compared with 13% in those without dysplasia (P = 0.002). On multivariate analysis, margin dysplasia was associated with a three-fold increased risk of recurrence (P = 0.02). No relationship between dysplasia and development of pancreatic cancer was found.
Discussion
In this study, dysplasia at the margin after a pancreatectomy for non-invasive IPMN was associated with recurrence in the remnant gland, but not at the resection margin. While this finding may warrant closer follow-up, it does not identify a gland at higher risk for the subsequent development of invasive disease.
Introduction
Intraductal papillary mucinous neoplasms (IPMN) are defined as cystic lesions of the pancreas lined with mucin-producing epithelium with characteristic papillary proliferation.1–3 Typically classified as a branch duct or main duct type based on the distribution of pancreatic ductal involvement, IPMN are believed to represent a spectrum of pre-malignant to malignant lesions ranging from adenoma to carcinoma in situ and occasionally invasive adenocarcinoma.4,5 The treatment of IPMN remains controversial with most agreeing that small branch duct lesions can be followed with cross-sectional imaging whereas larger cysts or those with mural nodules or other solid components should be resected.6,7 Once the decision to operate has been made, approaches include parenchymal-preserving operations such as enucleation or a central pancreatectomy,8–10 pancreaticoduodenectomy, distal pancreatectomy, or in the case of diffuse disease, a total pancreatectomy.11,12
After a resection, pathological review of the surgical specimen focuses on identification of invasive carcinoma and assessment of the degree of dysplasia of the cyst lining and surgical margin. The latter has created considerable controversy regarding its oncological significance and the need for further resection in non-invasive IPMN.13,14 While some believe a positive margin represents residual pre-neoplastic tissue capable of malignant transformation, others suggest it is simply a marker of diffuse dysplasia throughout the gland with little oncological importance. Initial guidelines on the management of pancreatic cysts recommended frozen section evaluation of the margin during IPMN resections with re-excision for all but benign or adenomatous findings.7 The theoretical goal of a re-resection was to eliminate the potential risk of malignant transformation of dysplastic residual epithelium. A re-resection, however, is associated with increased morbidity and often results in continued margin positivity occasionally requiring a total pancreatectomy if a negative margin is to be achieved.14 Recent studies have questioned the significance of positive margins in non-invasive IPMN with regard to recurrence of both IPMN and pancreas cancer in the remnant gland.14–16 In a previous study from our institution of 78 patients who underwent resection of non-invasive IPMN, the risk of local recurrence after a median follow-up of 40 months was 8%.17 An association between margin status and recurrence was identified and recurrence was defined as radiographical appearance of pancreas cancer or an IPMN that required operative intervention.
In the present study, we evaluated clinical and pathological characteristics of 192 patients who underwent a resection for non-invasive IPMN. Recurrence included patients who developed radiographical evidence of new IPMN regardless of re-resection. Using these factors we identified predictors of both margin positivity and recurrent disease after a resection.
Methods
Patients
An institutional review board-approved prospectively maintained database of patients undergoing a pancreatic resection at Memorial Sloan-Kettering Cancer Center (MSKCC) was used to identify 192 patients who underwent a resection of non-invasive IPMN. Patients with invasive cancer and those undergoing a total pancreatectomy were excluded from analysis. All patients had disease confirmed by both radiographical and pathological analysis. Patient demographics, details on presentation as well as radiographical and follow-up data were collected and reviewed. Typically, a resection was recommended in the setting of main duct disease and branch duct cysts which were symptomatic, >2.5 cm in size, displaying evidence of growth, or when worrisome features such as mural nodules were present. After a resection, patients were followed with serial cross-sectional imaging assessing for evidence of new cystic or solid lesions.
Pathology
All surgical specimens were reviewed by gastrointestinal pathologists with extensive experience in pancreatic diseases. Cyst size was recorded based on the gross pathological measurement. Lesions were classified in accordance with World Health Organization (WHO) consensus guidelines as low-grade, moderate-grade or high-grade dysplasia. When possible, the epithelium was subtyped as gastric, intestinal, pancreatobiliary or oncocytic. Both imaging and pathological analyses were used to characterize the extent of involvement as a branch duct, main duct or mixed type. The uninvolved gland was assessed for the presence of fibrosis, pancreatitis and/or extra-cystic pancreatic intraepithelial neoplasia (PanIN). In addition to the primary lesion, a section of ductal epithelium at the transection margin was evaluated for the presence and grade of IPMN or PanIN. A positive margin was defined as IPMN or PanIN present at the final surgical margin regardless of degree of dysplasia. In selected patients, an intra-operative frozen section was performed and the results collected and compared with the final reported margin. When additional margins were taken, data regarding residual disease were recorded.
Statistical analysis
Nominal variables were evaluated using two-tailed χ2 or Fisher's exact tests when appropriate. Continuous variables were assessed with univariate logistic regression for parametric values and Wilcoxon's rank sum for non-parametric values. Multivariate logistic regression models were created using all factors with an alpha <0.10 on univariate analysis and variables previously identified as associated margin positivity or recurrence. Kaplan–Meier survival curves were created to determine differences in survival and P-values <0.05 were considered significant.
Results
Demographics
Between 1990 and 2010, 268 patients underwent a pancreatic resection for IPMN at MSKCC. Invasive cancer was identified on final pathology in 72 patients and these patients were excluded from further analysis. In 192 patients with non-invasive IPMN, the mean age at operation was 68 years with slightly more female patients (55%). Lesions were most often discovered incidentally during cross-sectional imaging or ultrasonography obtained for other purposes (54%). Symptoms were present in 88 patients (46%) at the time of presentation with the most commonly reported symptoms being pain (35%) and weight loss (11%). Every patient had pre-operative cross-sectional imaging with either a computed tomography scan or magnetic resonance imaging prior to intervention to assess size and extent of the disease as well as any worrisome features such as cyst wall thickening or mural nodules. Imaging revealed a dilated main pancreatic duct (>4 mm) in 55% of patients and nodularity of the cyst wall in 7%. Endoscopic ultrasound was performed in 38 patients (20%) with fluid aspiration and analysis revealing a mean cyst fluid carcinoembryonic antigen level of 1760 ng/ml (range 79–38530).
The most common indications for operative intervention were size at presentation (51%), enlargement of cysts on serial imaging (22%), biopsy suspicious for cancer or high grade dysplasia (16%) and radiographical evidence of a mass or nodule (7%). Operations included 125 pancreaticoduodenectomies, 53 distal pancreatectomies and 14 parenchymal-preserving procedures (enucleation or central pancreatectomy). After resection of the index lesion, 10 patients with multifocal IPMN were left with residual cysts which did not meet resection criteria.
Histopathology and margin status
On pathological evaluation, 23% of lesions were classified as low-grade, 46% moderate-grade and 31% high-grade lesions (Table 1). Gastric type epithelium was most common (62%), whereas intestinal, pancreatobiliary and oncocytic type was identified in 29%, 9% and 2%, respectively. Most lesions were classified as branch duct type (49%) and the rest main duct (25%) and mixed type (26%). The mean cyst size on gross examination was 2.7 cm. The unaffected gland was assessed for pathological abnormalities with evidence of pancreatitis discovered in 47% and fibrosis in 14%. PanIN was discovered in the extra-cystic pancreatic duct exclusive of the margin in 39% of specimens and ranged in degree from PanIN 1 (15%) to PanIN 2 (20%) and PanIN 3 (4%).
Table 1.
Pathological features of resected non-invasive intraductal papillary mucinous neoplasms (IPMN) in 192 patients
| Path | n = 192 |
|---|---|
| IPMN type | |
| Low grade (n = 44) | 23% |
| Moderate grade (n = 88) | 46% |
| High grade (n = 60) | 31% |
| Subtype | |
| Branch (n = 94) | 49% |
| Main (n = 47) | 25% |
| Mixed (n = 50) | 26% |
| Epithelium | |
| Gastric (n = 58) | 62% |
| Intestinal (n = 27) | 29% |
| Pancreato/biliary (n = 7) | 7% |
| Oncocytic (n = 2) | 2% |
| Size (mean) | 2.7 cm (±2.3) |
| Evidence of pancreatitis (n = 88) | 47% |
| Fibrosis (n = 26) | 14% |
| PanIN in the gland | |
| None (n = 113) | 61% |
| 1 (n = 28) | 15% |
| 2 (n = 37) | 20% |
| 3 (n = 8) | 4% |
The pancreatic ductal margin was assessed in each patient and the results are summarized in Table 2. IPMN or PanIN was discovered at the final surgical margin in 38 (20%) and 54 (28%) specimens, respectively. Six patients had both IPMN and PanIN identified. An intra-operative frozen section was performed in 44 cases (23%) and revealed residual disease in 17 patients (39% of frozen sections). This disease was most often low-grade IPMN (71% of positive frozen sections) with moderate- and high-grade IPMN found in 18% and 11% of patients, respectively. Of the 44 cases in which a frozen section was utilized, a re-resection was performed in 4 cases (9%) with 2 resulting in a negative final margin. When comparing frozen section results with those found on final pathology, there was concordance in 57% of cases resulting in a positive predictive value of 41.2% and a negative predictive value of 66.7%.
Table 2.
Pathology of the final surgical margin in 192 patients who underwent a resection for non-invasive intraductal papillary mucinous neoplasms (IPMN)
| Margin | |
|---|---|
| PanIN or IPMN at margin (n = 86) | 45% |
| IPMN at final margin | |
| None (n = 154) | 80% |
| Low grade (n = 17) | 9% |
| Moderate grade (n = 15) | 8% |
| High grade (n = 6) | 3% |
| PanIN at final margin | |
| None (n = 138) | 72% |
| 1 (n = 33) | 17% |
| 2 (n = 17) | 9% |
| 3 (4) | 2% |
| Frozen section (n = 44) | 23% |
| Path on frozen | |
| Negative (n = 27) | 61% |
| Low grade IPMN (n = 12) | 27% |
| Moderate grade IPMN (n = 3) | 7% |
| High grade IPMN (n = 1) | 2% |
| PanIN (n = 1) | 2% |
At a median follow-up of 46 months, 40 patients (21%) had recurrent disease (Table 3) defined as appearance of new cysts suspicious for IPMN on imaging (31 patients), pathological confirmation of IPMN on re-resection (6 patients) or the development of pancreatic cancer (3 patients). Patients with residual cysts after their index operation were not counted as recurrences unless they required a repeat operation (n = 1). Of the three patients who developed pancreas cancer, the original lesions displayed low- and high-grade dysplasia in 1 and 2 patients, respectively. Examination of the margins in the patients who developed cancer revealed high-grade dysplasia in one patient whereas the other 2 patients had negative margins. The discovery of cancer was at 10, 39 and 67 months after the resection and none developed cancer at the resected margin. Seven patients (4%) with presumed recurrent IPMN underwent a second operation during the follow-up period including 3 segmental resections and 3 completion pancreatectomies. One of the 6 patients had no evidence of recurrent IPMN on final pathological analysis.
Table 3.
Recurrence and follow-up data on 192 patients with a resected non-invasive intraductal papillary mucinous neoplasms (IPMN)
| Recurrence | |
|---|---|
| Median follow-up | 46 months |
| Recurrence (n = 40) | 21% |
| Location of recurrence | |
| Remnant gland (n = 32) | 80% |
| Margin (n = 3) | 8% |
| Remnant gland and margin (n = 3) | 8% |
| Unknown (n = 2) | 5% |
| Re-operation (n = 6) | 3% |
| Pancreas cancer (n = 3) | 2% |
When patients recurred, this was predominantly away from the surgical margin with only 8% having margin-only recurrence. The vast majority recurred elsewhere in the remnant gland (80%) or diffusely (8%) (Fig. 1). Of the six patients who recurred at the margin of resection, half had no evidence of PanIN or IPMN at the initial surgical margin.
Figure 1.
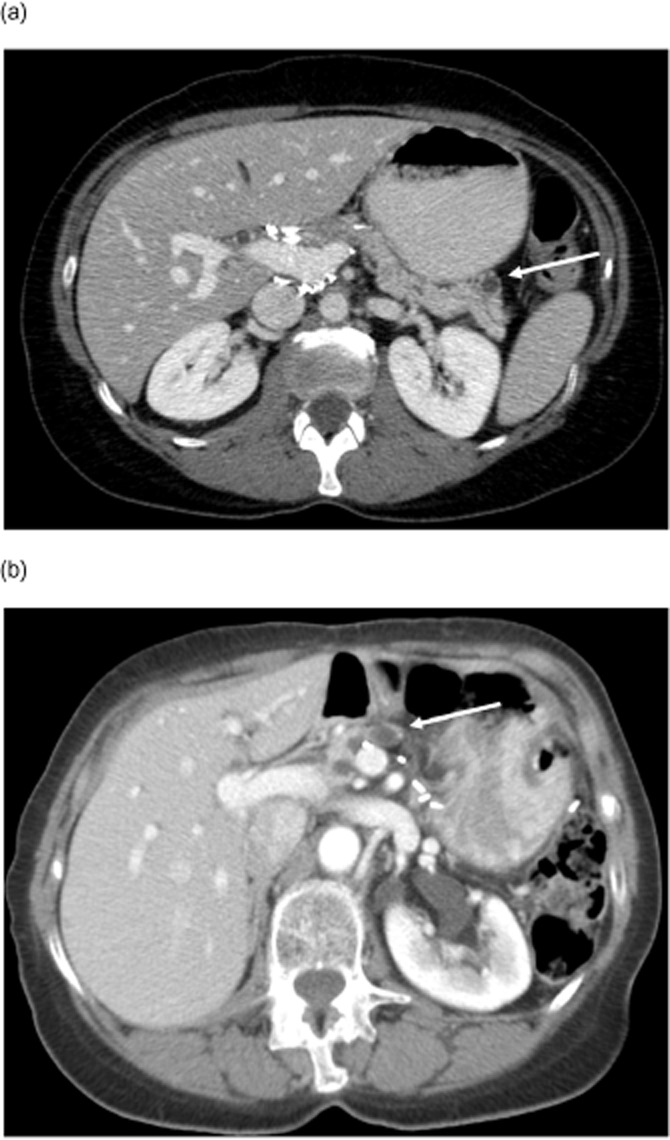
Location of recurrence of follow-up imaging. The white arrow marks a recurrent cyst. (a) Remnant recurrence. (b) Margin recurrence
Predictors of margin positivity
Univariate analysis of demographic and pathological data was performed to identify predictors of a positive final surgical margin after resection for non-invasive IPMN. The risk of a positive margin varied significantly with the degree of IPMN dysplasia. Patients with moderate- or high-grade dysplasia had a significantly increased risk of having a positive margin (50%) when compared with patients with low-grade dysplasia (22%) (P = 0.002) (Fig. 2). Other predictors of margin status included location of the cyst, epithelial type and the presence of PanIN in the extracystic pancreatic duct. There was a non-significant trend towards increased cyst size in patients with positive margins (2.5 versus 3 cm in negative and positive margins, respectively; P = 0.17). There was no difference in margin status between patients with main duct (47%) compared with branch duct IPMN (44%) (P = 0.86).
Figure 2.
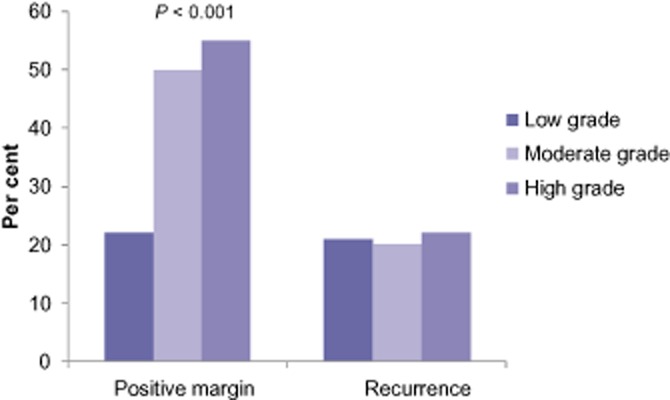
Margin status and recurrence by degree of dysplasia
Using multivariate regression (Table 4), the degree of dysplasia remained significant with high-grade IPMN associated with a 10-fold increase in positive margins (P = 0.03). The presence of extra-cystic PanIN also maintained significance with an odds ratio of 5.7 (P = 0.001).
Table 4.
Multivariate predictors of a positive margin
| Odds ratio | P-value | |
|---|---|---|
| Pain | 1.8 | 0.31 |
| Weight loss | 3.1 | 0.27 |
| Degree of dysplasia | 0.03 | |
| Adenoma | Referent | |
| Moderate | 3.2 | |
| High grade | 10.0 | |
| Location within the gland | 0.05 | |
| Head | Referent | |
| Body | 3.8 | |
| Tail | 4.1 | |
| Tissue subtype | 0.21 | |
| PanIN in the gland | 5.7 | 0.001 |
Predictors of disease recurrence
The above factors including margin status were used to identify predictors of recurrent disease after a pancreatectomy for IPMN (Table 5). On univariate analysis, the location of the cyst within the tail or body was associated with a significant increased rate of recurrence. When PanIN was present at the resection margin, the recurrence rate was nearly double (P = 0.02). If any dysplasia (IPMN or PanIN) was present the recurrence rate was 31% compared with 12% when a negative margin was found (P = 0.002). On multivariate analysis (Table 6), location within the body was associated with a 4.8-fold increased risk of recurrent disease (P = 0.01). When IPMN or PanIN was present at the final surgical margin, there was a three-fold increase (P = 0.02).
Table 5.
Univariate predictors of recurrent disease
| Recurrence | P-value | |
|---|---|---|
| Gender | 0.29 | |
| Male | 17% | |
| Female | 24% | |
| Race | 0.56 | |
| White | 18.5% | |
| Non-white | 21% | |
| Pain at presentation | 0.43 | |
| Yes | 19% | |
| No | 24% | |
| Weight loss at presentation | 0.25 | |
| Yes | 10% | |
| No | 24% | |
| Dilated duct on pre-operative image | 0.37 | |
| Yes | 19% | |
| No | 26% | |
| Nodule or mass on pre-operative | 0.48 | |
| Yes | 31% | |
| No | 21% | |
| IPMN dysplasia | 0.97 | |
| Adenoma | 21% | |
| Moderate | 20% | |
| High grade | 22% | |
| Location of cyst | 0.004 | |
| Head | 15% | |
| Body | 41% | |
| Tail | 27% | |
| IPMN tissue type | 0.23 | |
| Gastric | 22% | |
| Intestinal | 22% | |
| Pancreatobiliary | 0% | |
| Oncocytic | 0% | |
| IPMN distribution | 0.83 | |
| Branch duct | 22% | |
| Main duct | 21% | |
| Mixed | 18% | |
| PanIN with the gland | 0.10 | |
| Yes | 27% | |
| No | 17% | |
| IPMN or PanIN at margin | 0.002 | |
| Yes | 31% | |
| No | 12% | |
Table 6.
Multivariate predictors of recurrent disease
| Odds ratio | P-value | |
|---|---|---|
| Location of cyst | 0.01 | |
| Head | Referent | |
| Body | 4.3 | |
| Tail | 1.8 | |
| Degree of dysplasia | 0.77 | |
| Adenoma | Referent | |
| Moderate | 1.3 | |
| High grade | 1.5 | |
| Distribution of dysplasia | 0.79 | |
| Branch duct | Referent | |
| Main duct | 0.8 | |
| Mixed type | 1.3 | |
| PanIN in gland | 1.3 | 0.50 |
| Any dysplasia at margin | 2.9 | 0.02 |
Kaplan–Meier curves were created to determine differences in recurrence-free and overall survival based on margin status (Fig. 3). Although patients with dysplasia at the margin had significantly worse disease-free survival (P = 0.001), this did not translate to a worse overall survival (P = 0.55).
Figure 3.
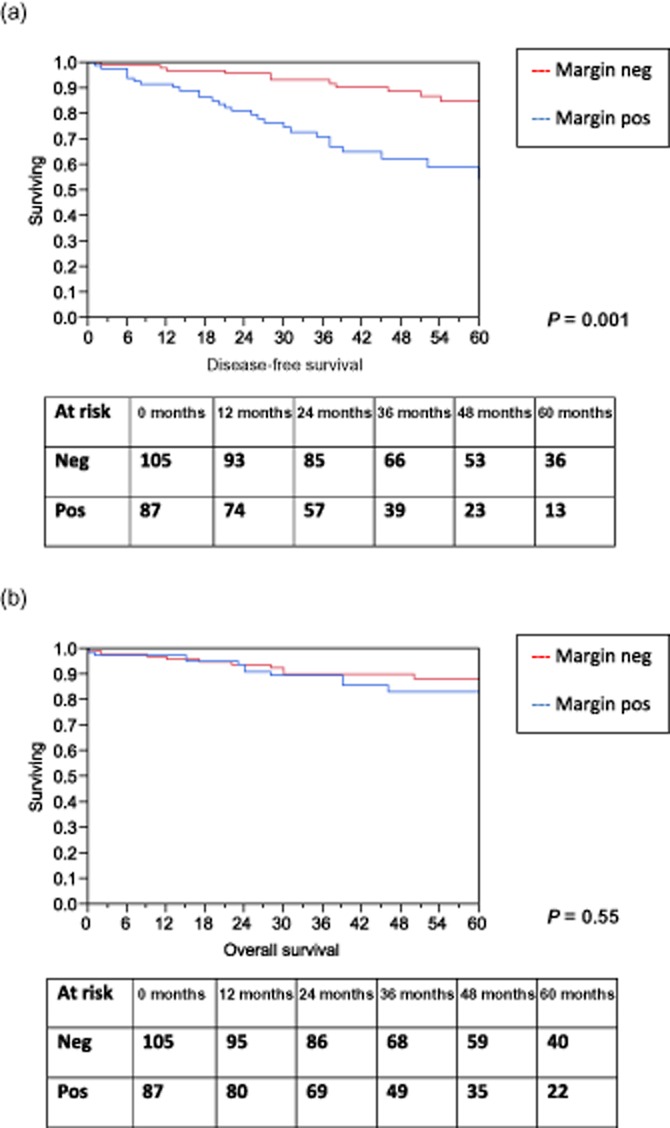
Overall and recurrence-free survival by margin status. (a) Recurrence-free survival by margin status. (b) Overall survival by margin status
Discussion
With increased utilization of cross-sectional imaging, the incidence of IPMN has risen dramatically over the past two decades.18,19 Lesions are often discovered when small and asymptomatic leading to considerable controversy in their management. At the crux of the debate is the potential for IPMN to progress to pancreatic adenocarcinoma which, when found in later stages, is almost universally fatal. In an attempt to standardize management, an International Consensus Guideline7 was published which recommended resection of suspected IPMN which were greater than 3 cm in size and/or had suspicious features such as mural nodules, a dilated pancreatic duct or positive cytology. An intra-operative frozen section was recommended at the time of operation with re-resection if the margin showed evidence of moderate- or high-grade dysplasia. Admittedly, this recommendation was made with little supporting data and the clinical significance of a positive margin remains controversial, particularly in non-invasive IPMN.
There are two types of dysplastic epithelium found at the transected margin of the pancreas: IPMN and PanIN.2,20When IPMN is present, the degree of dysplasia is determined by irregularity of the papilla, nuclear crowding and mitosis and is described according to a WHO consensus statement as low- (adenomatous), moderate- or high-grade (carcinoma in situ).4 PanIN also represents dysplasia of the pancreatic ductal epithelium but differs from IPMN as it lacks papilla and does not result in radiographical or pathological appearance of cysts.20 PanIN is also described as having a spectrum of dysplasia ranging from minimal (PanIN 1) to moderate dysplasia (PanIN 2) and carcinoma in situ (PanIN 3).2 While most agree that low-grade IPMN and/or PanIN 1 at the surgical margin can be treated as benign, many contend that moderate- or high-grade dysplasia requires more radical resection including in some cases a total pancreatectomy.7,21–23 Most of these recommendations on the management of positive margins are based on anecdotes and small case series with no convincing evidence available.
In a single institution review of 19 patients undergoing segmental pancreatic resection for non-invasive IPMN, Raut et al.24 reported a 21% incidence of a positive final margin. At a median follow-up of 34.1 months, there were no instances of recurrence in this subset of patients leading the authors to conclude that a macroscopically negative resection was sufficient for non-invasive IPMN. Limitations of the study include the small sample size, a lack of details on post-operative imaging and determination of recurrence based solely on re-operation or biopsy. In a larger study by Fujii et al.,15 103 patients operated on for non-invasive IPMN were followed for evidence of recurrent disease. As in the previous studies, pathological confirmation of IPMN or cancer on re-operation or biopsy was needed to document recurrence. Twenty-seven per cent of patients had a positive surgical margin at initial resection ranging from adenoma to carcinoma in situ. At a median follow-up of 41 months, 10% of patients were found to have recurrent disease in the remnant gland. Of those who recurred, none did so at the surgical margin and recurrence was irrespective of the margin status at the time of initial operation. From this, the authors concluded that the status of the final surgical margin is not associated with recurrence after resection of non-invasive IPMN.
Because the number of patients and incidence of recurrence in studies of IPMN tends to be low, a meta-analysis was performed specifically addressing the issue of margin positivity.16 Twelve studies and 701 patients were included in the analysis with a cumulative recurrence rate of 4.9%. A statistically different recurrence rate existed between those patients with (9.6%) and without (3.7%) a positive surgical margin (P = 0.01). Based on these findings, the authors concluded that a resection to a microscopically negative margin was optimal in non-invasive IPMN. There was significant heterogeneity in the length of follow-up and definition of recurrence in the included studies and no mention of site of recurrence within the remnant gland.
A study from our institution in 200717 looked at the incidence of recurrent IPMN when PanIN or IPMN was present at the surgical margin. Of 72 patients with non-invasive IPMN, six patients recurred which was defined as development of metastatic pancreatic adenocarcinoma (3 patients) or re-operation for a new pancreatic cyst (3 patients). Of the six recurrences, four had positive surgical margins for IPMN and the incidence of recurrence in margin positive patients was 17%, compared with 2% in the margin negative population (P = 0.02). One of the difficulties identified in this paper was the ability to accurately identify IPMN on a frozen section of the pancreatic margin. In this study, a frozen section of the margin was performed in 27 patients, and the positive and negative predictive values of ‘IPMN at the margin’ on frozen section were 50% and 74%, respectively. These findings suggest that if we had extended the resection for a positive margin, then in half of the cases this would not have been necessary as the final pathology revealed no disease.
Missing from many of these prior series is inclusion of patients with recurrence visualized only on cross-sectional imaging as well as the significance of PanIN at the final surgical margin. In the present study, 20% of patients had surgical margins positive for IPMN with a majority exhibiting low- or moderate-grade dysplasia. PanIN was present at the margin in nearly 28% of patients with only 4 patients having high grade dysplasia (PanIN 3) present. In all, the overall margin positivity rate (IPMN and PanIN) was 45% which was similar to previous reports.17 Predictors of margin positivity included moderate- or high-grade dysplasia in the index lesion and PanIN present in the extra-cystic pancreatic duct. The latter suggests a diffuse duct process in which multiple or more widespread areas of epithelial irregularity exist, making dysplasia at the ductal margin more probable.
This concept of a pancreatic duct ‘field defect’ is supported by previous studies of pathological analysis of resected specimens where multicentric ductal dysplasia was frequently encountered.25 Further evidence comes from clinical observations that pancreatic adenocarcinoma can develop at sites distant from the index cyst in patients after pancreatic resection for IPMN as well as those followed radiographically for small cysts.17,26 In the present series, location of the resected cyst was also associated with margin status with body and tail lesions having a near four-fold increase in margin positivity. One possible explanation for this was the 67% incidence in positive margins in those undergoing a central pancreatectomy for body lesions.
A frozen section was used sparingly in this study with 23% of margins assessed intra-operatively. Of those, 39% were positive with a vast majority showing evidence of low-grade dysplasia and only four patients exhibiting moderate- or high-grade dysplasia. Re-resection of the margin was performed in four patients with pathological clearance achieved in only two of those. We again have demonstrated the relatively poor accuracy of a frozen section in identifying dysplasia at the margin after resection of IPMN.
At a median follow-up of 46 months, 21% of patients had recurrent disease with a majority of these showing evidence of new cysts presumed to be IPMN on imaging. Six patients underwent re-operation which confirmed recurrent non-invasive IPMN in five and three developed pancreas adenocarcinoma. Predictors of recurrent disease included location of the index lesion in the body or tail or dysplasia at the final surgical margin. The latter was associated with a three-fold increased risk and seemed to be independent of the degree of dysplasia at the margin. Of note, while the degree of dysplasia of the resected IPMN was associated with the rate of margin positivity, it did not seem to be associated with recurrence rates (Fig. 2). To further clarify the association of margin status on recurrence, all cross-sectional imaging and operative reports were re-reviewed to determine whether disease developed at the transection margin or elsewhere in the gland. Of the 40 patients who recurred, 6 had new lesions develop adjacent to the prior resection with only 3 having disease restricted to the transection margin alone. The remainder recurred elsewhere in the remnant gland. Of the 6 patients that had a margin recurrence, half had a negative surgical margin at in their initial resection. These findings suggest that although a positive margin is associated with recurrent disease, it is likely a marker of diffuse ductal instability and not a local oncologic failure. The low incidence of disease recurrence at the actual site of resection suggests no need to achieve a microscopically negative margin in non-invasive IPMN making an intra-operative frozen section and re-resection unnecessary. This is further supported by the observation that, although margin positive patients had a worse recurrence-free survival, there was no difference in overall-survival highlighting the relative benign nature of non-invasive IPMN (Fig. 3).
Current guidelines recommend stratification of surveillance of the remnant pancreas based on findings from the initial operation.6 High-risk patients are assessed every 3–9 months with cross-sectional imaging whereas low-risk patients may be screened annually. Because a margin negative resection is associated with a low rate of recurrence, perhaps the interval between screening may be lengthened in this population, although long-term follow-up supporting this conclusion is lacking. In conclusion, these data suggest that when dysplasia is present at multiple locations within the pancreas such as the surgical margin and/or extra-cystic duct, patients are at increased risk of developing recurrent IPMN supporting the concept of a ‘field defect’. Although these patients may require closer follow-up with cross-sectional imaging to identify recurrent lesions, the overall risk of progression to invasive disease is low, and therefore an extended resection or reoperation does not seem justified at this time.
Conflicts of interest
None declared.
References
- 1.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hara H, Suda K. Review of the cytologic features of noninvasive ductal carcinomas of the pancreas: differences from invasive ductal carcinoma. Am J Clin Pathol. 2008;129:115–129. doi: 10.1309/PHV2244LC8B0P7TR. [DOI] [PubMed] [Google Scholar]
- 3.Katabi N, Klimstra DS. Intraductal papillary mucinous neoplasms of the pancreas: clinical and pathological features and diagnostic approach. J Clin Pathol. 2008;61:1303–1313. doi: 10.1136/jcp.2007.049361. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa T, Kloppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794–799. doi: 10.1007/s00428-005-0039-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhai J, Sarkar R, Ylagan L. Pancreatic mucinous lesions: a retrospective analysis with cytohistological correlation. Diagn Cytopathol. 2006;34:724–730. doi: 10.1002/dc.20561. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 8.Hwang HK, Park JS, Kim JK, Park CM, Cho SI, Yoon DS. Comparison of efficacy of enucleation and pancreaticoduodenectomy for small (<3 cm) branch duct type intraductal papillary mucinous neoplasm located at the head of pancreas and the uncinate process. Yonsei Med J. 2012;53:106–110. doi: 10.3349/ymj.2012.53.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652. [DOI] [PubMed] [Google Scholar]
- 10.Crippa S, Bassi C, Warshaw AL, Falconi M, Partelli S, Thayer SP, et al. Middle pancreatectomy: indications, short- and long-term operative outcomes. Ann Surg. 2007;246:69–76. doi: 10.1097/01.sla.0000262790.51512.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnelldorfer T, Sarr MG, Nagorney DM, Zhang L, Smyrk TC, Qin R, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. [DOI] [PubMed] [Google Scholar]
- 12.Turrini O, Schmidt CM, Pitt HA, Guiramand J, Aguilar-Saavedra JR, Aboudi S, et al. Side-branch intraductal papillary mucinous neoplasms of the pancreatic head/uncinate: resection or enucleation? HPB. 2011;13:126–131. doi: 10.1111/j.1477-2574.2010.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada K, Imaizumi T, Hirabayashi K, Matsuyama M, Yazawa N, Dowaki S, et al. The distance of tumor spread in the main pancreatic duct of an intraductal papillary-mucinous neoplasm: where to resect and how to predict it. J Hepatobiliary Pancreat Sci. 2010;17:516–522. doi: 10.1007/s00534-009-0257-5. [DOI] [PubMed] [Google Scholar]
- 14.Salvia R, Bassi C, Falconi M, Serini P, Crippa S, Capelli P, et al. Intraductal papillary mucinous tumors of the pancreas. Surgical treatment: at what point should we stop? JOP. 2005;6(1 Suppl):112–117. [PubMed] [Google Scholar]
- 15.Fujii T, Kato K, Kodera Y, Kanda M, Nagai S, Yamada S, et al. Prognostic impact of pancreatic margin status in the intraductal papillary mucinous neoplasms of the pancreas. Surgery. 2010;148:285–290. doi: 10.1016/j.surg.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Leng KM, Wang ZD, Zhao JB, Cui YF, Zhong XY. Impact of pancreatic margin status and lymph node metastases on recurrence after resection for invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Dig Surg. 2012;29:213–225. doi: 10.1159/000339334. [DOI] [PubMed] [Google Scholar]
- 17.White R, D'Angelica M, Katabi N, Tang L, Klimstra D, Fong Y, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204:987–993. doi: 10.1016/j.jamcollsurg.2006.12.040. discussion 93–95. [DOI] [PubMed] [Google Scholar]
- 18.Klibansky DA, Reid-Lombardo KM, Gordon SR, Gardner TB. The clinical relevance of the increasing incidence of intraductal papillary mucinous neoplasm. Clin Gastroenterol Hepatol. 2012;10:555–558. doi: 10.1016/j.cgh.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S, Sclabas G, Reid-Lombardo KM. Population-based epidemiology, risk factors and screening of intraductal papillary mucinous neoplasm patients. World J gastrointest Surg. 2010;2:314–318. doi: 10.4240/wjgs.v2.i10.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longnecker DS, Adsay NV, Fernandez-del Castillo C, Hruban RH, Kasugai T, Klimstra DS, et al. Histopathological diagnosis of pancreatic intraepithelial neoplasia and intraductal papillary-mucinous neoplasms: interobserver agreement. Pancreas. 2005;31:344–349. doi: 10.1097/01.mpa.0000186245.35716.18. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C, Sarr MG, Lillemoe KD, Reber HA. Natural history of intraductal papillary mucinous neoplasms (IPMN): current evidence and implications for management. J Gastrointest Surg. 2008;12:645–650. doi: 10.1007/s11605-007-0447-x. [DOI] [PubMed] [Google Scholar]
- 22.Rivera JA, Fernandez-del Castillo C, Pins M, Compton CC, Lewandrowski KB, Rattner DW, et al. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms. A single malignant clinicopathologic entity. Ann Surg. 1997;225:637–644. doi: 10.1097/00000658-199706000-00001. discussion 44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loftus EV, Jr, Olivares-Pakzad BA, Batts KP, Adkins MC, Stephens DH, Sarr MG, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996;110:1909–1918. doi: 10.1053/gast.1996.v110.pm8964418. [DOI] [PubMed] [Google Scholar]
- 24.Raut CP, Cleary KR, Staerkel GA, Abbruzzese JL, Wolff RA, Lee JH, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Izawa T, Obara T, Tanno S, Mizukami Y, Yanagawa N, Kohgo Y. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001;92:1807–1817. doi: 10.1002/1097-0142(20011001)92:7<1807::aid-cncr1697>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Lafemina J, Katabi N, Klimstra D, Correa-Gallego C, Gaujoux S, Kingham TP, et al. Malignant progression in IPMN: a cohort analysis of patients initially selected for resection or observation. Ann Surg Oncol. 2013;20:440–447. doi: 10.1245/s10434-012-2702-y. [DOI] [PubMed] [Google Scholar]


