Abstract
We describe the vascular supply to the pharyngeal jaws and teeth in zebrafish, from larval stages to juveniles, using serial high quality semithin sections and 3D reconstructions. We have identified that the arterial blood supply to the last pair of branchial arches, which carries the teeth, issues from the hypobranchial artery. Surprisingly, the arteries supplying the pharyngeal jaws show an asymmetric branching pattern that is modified over ontogeny. Moreover, the blood vessel pattern that serves each jaw can best be described as a sinusoidal cavity encircling the bases of both the functional and replacement teeth. Capillaries branching from this sinusoidal cavity enter the pulp and constitute the intrinsic blood supply to the attached teeth. The role of these blood vessels during tooth development (whether instructive or nutritive) remains to be determined and requires further study. However, we have provided a firm morphological basis that will aid in the interpretation of experiments addressing this question.
Keywords: blood supply, hypobranchial artery, teeth, vascularization, zebrafish
Introduction
The zebrafish (Danio rerio) possesses numerous advantages (e.g. optical clarity of the embryo, ease of genetic manipulation, size, cost) that have rendered this small teleost an excellent model for the study of vertebrate development. Apart from its merits as a model organism for genetic, molecular, and developmental research (Lele & Krone, 1996; Roush, 1996), the zebrafish is also used as a model to study the processes of tooth development and continuous replacement (Huysseune et al. 1998; Van der Heyden et al. 2001, 2005; Verstraeten et al. 2010, 2013).
Unlike most tooth-bearing vertebrates, the zebrafish has no oral teeth but has its teeth implanted only on the ceratobranchials of the fifth branchial skeletal arch (Huysseune & Sire, 1998; Wautier et al. 2001; Stock, 2007), also called pharyngeal jaws. Zebrafish possess seven pharyngeal arches, with arch three to seven corresponding to branchial arches one to five. Pharyngeal arches three to six (also termed branchial arches one to four) are gill-bearing, whereas pharyngeal arch seven/branchial arch five is not gill-bearing but instead gives rise to the teeth.
The spatiotemporal pattern of tooth development and replacement in zebrafish has been well characterized. The zebrafish dentition consists of three rows of teeth on each body side, which extend rostro-caudally: a ventral row (V) of five teeth (1V–5V), a mediodorsal row (MD) of four teeth (1MD–4MD), and a dorsal row (D) of two teeth (1D–2D) (Van der Heyden & Huysseune, 2000). The first tooth bud already starts to develop at around 48 h post-fertilization (hpf) at position 4V and is quickly followed by the development of the tooth germs at positions 3V and 5V. The teeth in the two rostral positions, 2V and 1V, develop at 12 and 16 days post-fertization (dpf), respectively (Van der Heyden & Huysseune, 2000). Replacement of first-generation teeth starts already at 80 hpf (position 4V; Borday-Birraux et al. 2006). Contrary to first-generation teeth, replacement teeth do not arise directly from the pharyngeal epithelium, but rather from an epithelial invagination that develops from the crypt associated with the erupted predecessor tooth (Huysseune & Thesleff, 2004; Huysseune, 2006). This epithelial downgrowth is termed a successional lamina and is found in association with every functional tooth in the dentition. The distal end of this structure develops into a replacement tooth. The successional lamina itself is quickly taken up into the enamel organ of the developing tooth bud (Huysseune, 2006).
Blood vessels perfuse almost all tissues of the body and mediate critical exchange between tissues and blood. Moreover, endothelial cells have been shown to be involved in paracrine signalling with surrounding organ cells (Cleaver & Melton, 2003). Perivascular cells have been identified as a source of mesenchymal stem cells (reviewed in Crisan et al. 2012). Moreover, in tissue engineering, revascularization is important to achieve proper development of the tissue (Jain et al. 2005; Lechguer et al. 2008; Lovett et al. 2009). Hence, given the importance of the circulatory system in homeostasis, organ development, as well as in oxygen and nutrient supply, we can assume a role for blood vessels in tooth development and replacement. However, data regarding the vascular supply of teeth are scarce. Even in mammals, information is usually limited to the intrinsic blood supply of the tooth via the pulp cavity (Boyer & Neptune, 1962; Kindlova & Matena, 1962; Mattuella et al. 2007). Furthermore, there is a limited number of studies focusing on the role of blood vessels (whether instructive or permissive) in the process of tooth development and replacement (Miller, 1957). The role of blood vessels in tooth development and replacement in the zebrafish has never been addressed. Not even the vascular supply to the tooth-bearing pharyngeal jaws has been identified. Hence, to study the function of blood vessels during tooth development, we must first obtain a profound understanding of how blood is supplied to and drained from the dentigerous area.
Two features, one biological and one technological, might greatly advance our understanding of the vascular supply of zebrafish teeth. First, teeth are implanted on the last pair of skeletal branchial arches, which represent serial homologues to the more anterior branchial arches. Secondly, the choice of the zebrafish offers great advantages over other model organisms for studies of vascular biology. Indeed, the introduction of the zebrafish, Danio rerio, as a model organism has opened new avenues in vascular biology research that have traditionally been much more difficult to study, for example vascular development in vivo (Zon, 1995; Isogai et al. 2001; Munoz-Chapuli, 2011). Numerous genetic and developmental studies have focused on the zebrafish circulatory network (Hu et al. 2000; Weinstein, 2002; Kamei et al. 2004; Ellertsdottir et al. 2010; Ellett & Lieschke, 2010; Geudens & Gerhardt, 2011). As a result, a vascular atlas of the developing zebrafish embryo is now available. It demonstrates a strong similarity of the basic vascular pattern to that of other vertebrates, making the zebrafish an ideal model to study the genetic and molecular mechanisms regulating both angiogenesis and vasculogenesis (Isogai et al. 2001). In zebrafish, as in other teleosts, the cardiovascular system is a simple loop consisting of the heart, arteries, and veins, with a gill capillary network intercalated to oxygenate the blood. The heart consists of four parts: sinus venosus, atrium, ventricle, and bulbus arteriosus. Deoxygenated blood is pumped via the sinus venosus into the atrium and subsequently into the ventricle, then passes through the bulbus arteriosus into the ventral aorta (Hu et al. 2000, 2001). Next, the blood enters the aortic arches (AAs). In larvae, blood flows posteriorly from the bulbus arteriosus into the ventral aorta, passing from the root of AA1 to AA6 (Isogai et al. 2001; Anderson et al. 2008). However, in contrast to larvae, in juveniles and adults the blood flows anteriorly from AA6 to AA3 (Hu et al. 2001).
Zebrafish possess six pairs of AAs that connect the ventral aorta to the lateral dorsal aortae. AA1 develops at the onset of circulation and is a critical component of the initial circulation loop. In contrast, AA2 never fully develops and does not contribute to the final circulation. AA3–6 arise in branchial arch 1–4, respectively, and are called the branchial AAs, since they will supply blood to the gills (Isogai et al. 2001). More specifically, AA3 and AA4 connect to the bilateral dorsal aortae, whereas AA5 and AA6 merge before connecting to the beginning of the single midline dorsal aorta (Olson, 2000a,b; Isogai et al. 2001). In contrast, the fifth branchial arch does not bear gills in teleosts, and as a result is always neglected in descriptions of the vascular anatomy. Yet, in zebrafish, this is the tooth-bearing arch.
Here, we present a detailed morphological description of the spatial and temporal relationships between developing teeth, and their respective supplying arteries and draining veins in different life stages of the zebrafish. This description will serve as a reference for future research focusing on the functional link between the forming vasculature and the process of tooth replacement.
Material and methods
Animal husbandry
Wild-type zebrafish were bred and raised according to the methods described in Westerfield (1993). Briefly, fish were raised in a 14 h/10 h light/dark cycle at 28.5 °C. Embryos were obtained via natural mating and raised in egg water. Embryos, larvae and juvenile fish were sacrificed according to the Belgian law on the protection of laboratory animals (KB d.d. 13 September 2004) by an overdose of MS222 (3-aminobenzoic acid ethyl ester). Embryos/larvae 2–6 days post-fertilization (dpf), and juvenile zebrafish with standard length 4.2, 4.6, 6.0, 6.1, 6.4, 7.3, 7.4, 8.0, 8.3, 9.5, 11 mm and 25 mm (ranging from 12 to 75 dpf) were used. For early stages we use days post-fertilization because the fish were raised at the standard temperature recommended for developmental studies (Westerfield, 1993). However, in older fish, size variation becomes important and standard length gives a better indication of the developmental stage.
Tissue processing
Larvae and juvenile fish were processed according to Huysseune & Sire (1992). Briefly, they were fixed in a mixture of 1.5% glutaraldehyde and 1.5% paraformaldehyde buffered with 0.2 m cacodylate (pH 7.4) for 2 h at room temperature. Juvenile zebrafish were decalcified by adding 0.1 m EDTA to the fixative solution for one to several weeks at 4 °C. The decalcifying solution was refreshed every 2 days. After fixation, animals were rinsed in 0.2 m cacodylate buffer containing 10% sucrose, and postfixed for 2 h at room temperature with 1% OsO4 in 0.2 m cacodylate buffer containing 8% sucrose. After rinsing in the same buffer, specimens were dehydrated through a graded series of ethanol and embedded in Epon. Serial transverse and sagittal semithin (1 μm) sections were made using a Reichert-Jung ultra-cut ultramicrotome (Leica, Vienna, Austria). Sections were stained using toluidine blue, and mounted in DePeX (Gurr, BDH Laboratory, UK). All sections were examined using a Zeiss Axio Imager Microscope and photographed using an Axiocam MRC videocamera. Reconstructions were made using the software program Amira® (v5.3.3.). Each reconstruction is based on a series of around 250 semithin sections in which the individual blood vessels were traced and labelled. Based on these labels, the software program generates a 3D model. Finally, models were photographed from different perspectives using Rhinoceros® (v5.0).
Identification of blood vessels and nomenclature
To locate and identify blood vessels within the embryos and larvae we relied on the extensive work published by Isogai et al. (2001), which gives a detailed description of the vascular anatomy of the developing zebrafish.
Results
We will focus first on the arterial supply and venous drainage of the pharyngeal jaw region in zebrafish, and subsequently on the precise spatiotemporal relationship between blood vessels and the developing dentition.
Arterial blood supply to and venous drainage from the pharyngeal jaws
A 3D-reconstruction was done of the arteries supplying blood to the tooth-bearing region in a 6 dpf zebrafish, covering a distance of 220 μm along the anterior-posterior axis (Fig. 1A). We identified the hypobranchial artery (HA; Isogai et al. 2001), positioned just dorsal to the ventral aorta, as being responsible for providing blood to the teeth (Fig. 1A). Starting from the cranial-most end of the ventral aorta, the HA bifurcates into two arteries (Fig. 1B,C). Next, these paired vessels run posteriorly along either side of the ventral midline just dorsal to the ventral aorta (Fig. 1A). At the level of the bulbus arteriosus, they merge again into a single artery (Fig. 1E). Along their course, several connections exist between these paired arteries (Fig. 1A,D). In a 26 dpf fish (SL = 6.1 mm) we studied the HA in more detail at the level of the fifth ceratobranchials, where the most anteriorly positioned pharyngeal teeth are located. The HA is positioned just dorsal to the heart (Fig. 1F). However, just caudal to the bulbus arteriosus, this vessel splits up into a smaller left and a larger right branch (Fig. 1G). Detailed microscopical studies did not reveal any significant histological distinction between these two branches, such as differences in vessel wall architecture (data not shown). Immediately after this first split, the larger branch on the right side gives rise to a dorsal offshoot (Fig. 1H). Thus, whereas the smaller branch on the left body side is in itself responsible for the blood supply to the left fifth ceratobranchial, it is the dorsal offshoot of the larger right branch that reaches the teeth on the right side (Fig. 1I). The main branch on the right side eventually connects to the heart through the sinus venosus (Fig. 1A). All of the six individuals studied (100%), with a standard length 6.4, 7.3, 8.0, 9.5 and 11 mm and one specimen 6 dpf, displayed the same vascular pattern as described above.
Fig. 1.
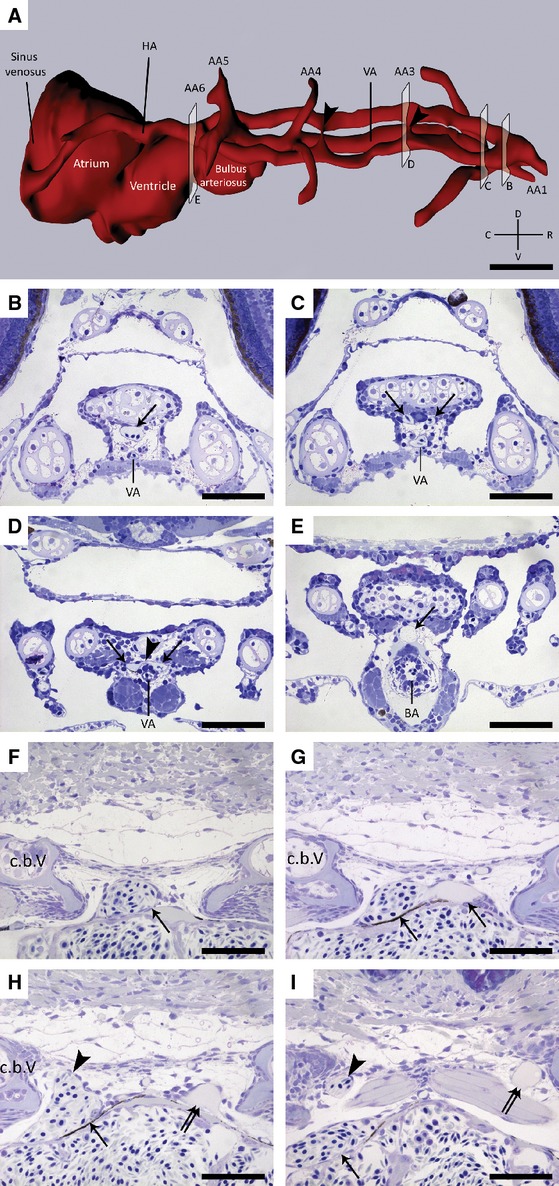
(A) 3D visualization of the vascular supply to the pharyngeal jaws in a 6 dpf zebrafish showing the position of the hypobranchial artery (HA) with regard to the ventral aorta (VA) and the different aortic arches (AA1–AA6). (B-E) Transverse, toluidine blue-stained sections of a wild-type zebrafish (6 dpf) in the regions indicated in (A). (B) Micrograph showing the position of the hypobranchial artery (arrow) dorsal to the ventral aorta (VA). (C,D) This artery bifurcates (arrows) and merges into a single unpaired median artery (arrow, E) at the level of the bulbus arteriosus (BA). Note the occasional connections (arrowheads in A,D) between the two vessels running along the ventral midline. (F-I) Transverse, toluidine blue-stained sections of a wild-type zebrafish (26 dpf, SL = 6.1 mm). (F) Micrograph illustrating the presence of the hypobranchial artery (arrow) in the vicinity of the tooth-bearing fifth ceratobranchials (c.b.V). (G) This artery splits in two branches (arrows) just caudal to the bulbus arteriosus. (H,I) Whereas the smaller branch on the left itself leads up to the teeth (double arrow), the larger branch on the right (arrow) gives rise to a dorsal offshoot (arrowhead), which serves the developing teeth of the right ceratobranchial. C, caudal; D, dorsal; R, rostral; V, ventral. Scale bars: 50 μm.
Apart from identifying the supplying arteries, we also determined the way in which blood is drained from this region (Fig. 2). The main branch on the right side itself connects to the sinus venosus and drains directly into the heart (Fig. 1A). The blood that supplies the teeth is drained via small capillaries positioned on the lateral posterior side of the fifth ceratobranchial (Fig. 2A). These capillaries branch off from the sinusoidal vessels (Fig. 2A') surrounding the teeth (see below), and merge into a single vessel (Fig. 2B). This vessel connects to the lateral dorsal aorta, just anterior to where the latter merges with its contralateral counterpart to form the single dorsal aorta that runs posteriorly along the body axis (Fig. 2C,D). Hence, the left and right pharyngeal jaw drain to bilateral vessels that each merge with their ipsilateral dorsal aorta, caudal to where AA5 and AA6 merge.
Fig. 2.
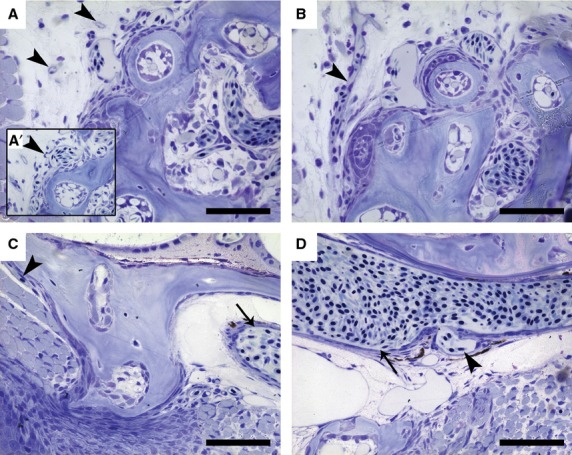
Sagittal, toluidine blue-stained sections of a wild-type zebrafish (SL = 9.5 mm). (A) Small capillaries (arrowheads) can be seen at the lateral posterior side of the dentigerous area in close proximity to the sinusoidal cavity. (A') These capillaries have been observed to branch off from the blood vessels surrounding the teeth (arrowhead). (B) These smaller capillaries merge into a single larger vessel (arrowhead). (C) Next, this vessel (arrowhead) runs mediodorsally towards the position of the dorsal aorta (arrow). (D) Finally, it connects (arrowhead) to the lateral dorsal aorta (arrow). In all sections anterior is to the right. Scale bars: 100 μm.
Spatiotemporal relationship between blood vessels and teeth
To identify the time at which blood vessels reach the dentigerous area during ontogeny, we studied wild-type zebrafish of increasing age, two individuals each at 3 and 6 dpf (2.4 and 2.8 mm, respectively), and three individuals each at 4.2, 4.6 and 6.1 mm SL (Fig. 3). Individuals of the same age displayed a similar vascular pattern. As stated above, blood is provided to the fifth tooth-bearing ceratobranchials via the HA. The first evidence of this blood vessel can be observed at 3 dpf. At this stage, the bifurcation into the two branches serving each of the paired pharyngeal jaws is already present (Fig. 3A). However, the dorsal offshoot on the right branch is still missing. The main branch itself on the right side already connects to the heart (Fig. 3B). At 6 dpf, the dorsal offshoot on the right side of the body can be observed (Fig. 3C). In addition, contrary to what we expect from the juvenile condition, we find the presence of a dorsal offshoot on the left side as well (Fig. 3D). Nonetheless, the main branch on the left side is less pronounced than the one on the right and does not connect to the sinus venosus. At this age, three functional teeth (3V1, 4V1, 5V1) and one replacement tooth (4V2) are present. Blood vessels are completely lacking in the vicinity of these teeth. At 4.2 mm SL (12 dpf), the branches on both the left and right sides of the body have dorsal offshoots that have reached the dentigerous area (Fig. 3E). This is the anterior end of the sinusoidal cavity, as we will describe for the juvenile condition. Furthermore, at this stage, the bases of the functional teeth at position 3V, 4V and 5V have become encircled by blood vessels. Nonetheless, the dental pulp of first-generation teeth (i.e. teeth developing directly from the pharyngeal epithelium; for example the tooth at position 3V) does not appear to contain vascular elements. At 4.6 mm SL (16 dpf), both of the two branches of the HA still give rise to a dorsal offshoot going towards the teeth (Fig. 3G). Teeth that have become functional at this stage (e.g. at position 2V) are now encircled by blood vessels at their base (Fig. 3H). Finally, at 6.1 mm SL (26 dpf), the situation resembles what we observe in juveniles. The dorsal offshoot on the left side is all that remains; the main branch is no longer present (Fig. 3I). Teeth that have become functional (e.g. at position 1V) are again encircled by blood vessels at their base (Fig. 3J).
Fig. 3.

Transverse, toluidine blue-stained sections of wild-type zebrafish 2.4–6.1 mm SL (3–26 dpf). (A,B) At 2.4 mm SL (3 dpf), both branches of the hypobranchial artery are present (arrows, A), the right branch already connecting to the heart (H) (arrow, B). (C,D) Next, at 2.8 mm SL (6 dpf), apart from the dorsal offshoot on the right branch (arrowhead, C), a dorsal offshoot can also be observed on the left side (arrowhead, D). Arrows indicate the two branches of the hypobranchial artery. (E-H) At 4.2 and 4.6 mm SL (12 and 16 dpf), blood is still supplied to the pharyngeal jaws via a dorsal offshoot of the branches on both sides of the body (arrowheads, E,G). Furthermore, starting from 4.2 mm SL onwards, blood vessels have reached the teeth and encircle the bases of the now functional, attached teeth (arrowheads, F,H). Both branches of the hypobranchial artery are still present (arrows, E-G). First-generation teeth, developing directly from the pharyngeal epithelium (asterisk, G), lack blood vessels in their immediate vicinity. (I,J) Finally, at 6.1 mm SL (26 dpf), the dorsal offshoot is all that remains on the left side and the main branch is no longer present (right arrowhead, I). However, the main branch on the right side is still clearly visible (arrow, I). The sinusoidal cavity has become more pronounced, encircling new teeth as they become functional (J, arrowheads). Scale bars: 50 μm.
To obtain a profound understanding of the spatiotemporal relationship between vascular elements and developing teeth, individual blood vessels were traced across serial semithin section of four different fish (SL = 6.4, 8.0, 11.0, and 25.0 mm). All specimens displayed the same pattern and a 3D reconstruction was done of the dentition and associated blood vessels of one of these fish (8.0 mm SL), covering a distance of 250 μm along the anterior-posterior axis. The resulting vascular pattern can be described as a sinusoidal cavity encircling the bases of both the functional and replacement teeth (Fig. 4A).
Fig. 4.
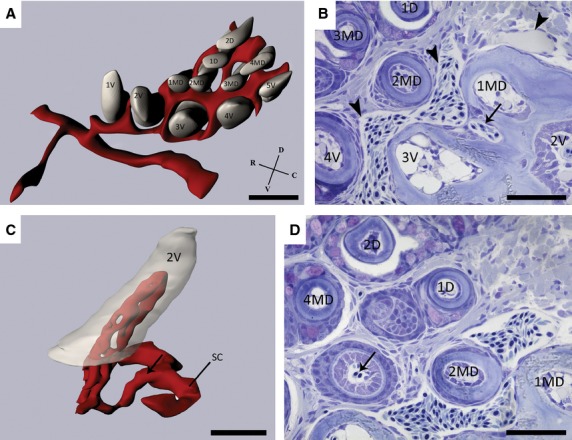
(A) 3D-reconstruction of a wild-type zebrafish dentition (SL = 8.0 mm) illustrating the elaborate anastomosing network of vessels (red) around the bases of the developing teeth (grey). Note the organization of the teeth in a ventral (1V–5V), mediodorsal (1MD–4MD) and dorsal (1D–2D) row. (B) Sagittal, toluidine blue-stained section of a wild-type zebrafish (SL = 9.5 mm) demonstrating the anastomosing network of blood vessels (arrowheads) surrounding the functional teeth (2V, 3V, 4V, 1MD, 2MD, 3MD and 1D). Moreover, note the branch (arrow) issuing from the sinusoidal cavity, which is responsible for the intrinsic blood supply to the functional tooth at position 2V. (C) Detailed reconstruction of the tooth at position 2V showing the blood vessels (red) branching off from the sinusoidal cavity (SC) and entering the pulpal cavity. The arrow indicates the same branch highlighted in (B). (D) Only replacement teeth in the late cytodifferentiation stage appear to contain evidence of blood vessels penetrating the pulpal cavity (arrow). C, caudal; D, dorsal; R, rostral; V, ventral. Scale bars: 50 μm.
The sinusoidal cavity on both sides of the body each connects to a branch of the hypobranchial artery, as described before. On each body side, a branch coming from this artery reaches the rostro-ventral side of the anterior-most tooth of the ventral row (1V) and is the beginning of a vessel, which bifurcates and anastomoses around the bases of the teeth, thus establishing a large, common sinusoidal cavity (Fig. 4A). With the exception of tooth 3V, 2MD and 3MD, the teeth are never fully enclosed by this network of blood vessels.
Apart from the vascular elements surrounding the dentition, blood vessels can also be seen entering the functional teeth (i.e. teeth ankylosed to the ceratobranchial bone) at the tooth base. These blood vessels come from branches (Fig. 4B) issuing from the sinusoidal cavity and are responsible for the intrinsic blood supply to the dental pulp. These branches always issue from the sinusoidal cavity at the postero-dorsal side of the tooth they will penetrate. This was the case for all the teeth studied (n = 22) in a juvenile fish (SL = 9.5 mm). Reconstruction of vascular elements inside the pulp cavity of a tooth at position 2V visualizes the way in which these branches issuing from the sinusoidal cavity are responsible for the intrinsic blood supply of the teeth (Fig. 4C). The dental organ does not appear to be vascularized. Indeed, blood vessels were never observed in the dental organ of any of the teeth studied (n = 22).
Developing replacement teeth only appear to become vascularized during the late cytodifferentiation/attachment stage (Fig. 4D). Of 22 teeth studied with their respective replacement teeth in different stages of development (i.e. initiation and morphogenesis, early and late cytodifferentiation, and attachment), only six appeared to contain blood vessels penetrating the pulp cavity (Fig. 4D). All of these replacement teeth were already in the late cytodifferentiation stage, close to attachment.
Discussion
This study aimed to improve our understanding of the way in which teeth are vascularized in a small teleost, the zebrafish. We determined the arterial blood supply to the pharyngeal jaws, as well as their venous drainage. We also elucidated the spatiotemporal relationship between the developing teeth and the surrounding blood vessels.
Arterial blood supply to the pharyngeal jaws
A median unpaired artery gives rise to branches that secure the blood supply to the pharyngeal teeth. Following the description of Isogai et al. (2001), together with our own microscopical observations, we have identified these branches as coming from the hypobranchial artery (HA; summarized in Fig. 5). In short, a pair of HA sprout from the first AA, carrying oxygenated blood in a rostro-medial direction. Oxygenated blood reaches AA1 through the dorsal aorta from the third and fourth AA, which are the main AAs supplying the cranial circulation (Isogai et al. 2001). Next, the pair of HA merge again at the extreme rostro-ventral midline, continuing as a single vessel over a short distance in a caudal direction along the ventral midline. In front of the rostral end of the ventral aorta (VA), this vessel splits again into a pair of HA. The two vessels run posteriorly, passing dorsal to AA1 and AA3, and ventral to AA4 through 6. In addition to what was already known about this vessel in zebrafish, we have established that prior to reaching the fifth ceratobranchials the pair of HA merges again into a single, median vessel. From then on, this vessel can be considered to be part of the coronary circulation, running over the surface of the heart and providing it with oxygen, and finally connecting to the sinus venosus. This seems evident since, in mice, coronary vessels have been shown to arise from angiogenic sprouts of the sinus venosus (Red-Horse et al. 2010). In addition, previous studies have shown that the coronary circulation in zebrafish derives cranially from the hypobranchial artery (Hu et al. 2001). However, we have determined that this vessel gives rise to branches supplying blood to the teeth, which has never before been observed.
Fig. 5.
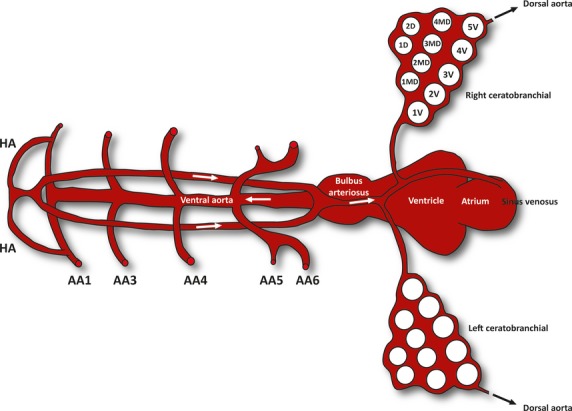
Schematic representation (dorsal view) of how blood is delivered through the hypobranchial artery (HA) to the tooth-bearing ceratobranchials in a juvenile zebrafish. AA1–AA6: aortic arches 1–6. Arrows indicate the direction of blood flow.
There are some remarkable differences in the vascular anatomy in zebrafish compared with another teleost, the rainbow trout (Oncorhynchus mykiss) whose vascular system has been well characterized (Isogai & Horiguchi, 1997), in particular with respect to the position of the hypobranchial artery. Whereas the HA in trout passes ventral to the ventral aorta, the HA in zebrafish is found on its dorsal side. Furthermore, the HA in trout splits in two coronary arteries as it passes under the bulbus arteriosus (Olson, 2000a). In zebrafish, too, the HA splits in two branches as it passes over the bulbus arteriosus. These two branches each give rise to dorsal offshoots running towards the teeth. However, at a size of 6.1 mm SL, the branch on the left is no longer present, leaving only the dorsal offshoot serving the teeth, and resulting in an asymmetric pattern of the arteries supplying the pharyngeal jaws. Thus, what appeared similar during development to the vascular plan in rainbow trout, is modified later in development, leaving only one coronary artery in zebrafish compared with two in rainbow trout. This can be understood by comparing the physiology of both species. Large, fast-swimming and predatory fish, such as trout, usually possess extensive coronary vessels, whereas zebrafish are neither large nor predator, and therefore have a lower oxygen demand on the heart (Farrell et al. 2012).
Furthermore, we have observed the presence of occasional connections between the two HA that pass just dorsal to the ventral aorta along the ventral midline (see Fig. 2A). However, this was only observed in younger specimens (e.g. 6 dpf in Fig. 1A), and never in juveniles. The vasculature in zebrafish is known to undergo extensive remodelling during development (Ellertsdottir et al. 2010; Herwig et al. 2011). Certain connections between blood vessels in zebrafish are known to become lost during development, being replaced by new connections. This is similar to the formation of the intersegmental veins, where angiogenic sprouts coming from the posterior cardinal vein will connect to existing segmental arteries, transforming them into segmental veins (Isogai et al. 2003). The connections between the two HA can thus be seen as transient structures that will disappear at a later developmental stage.
Venous drainage of the pharyngeal jaws
The coronary artery drains directly into the sinus venosus of the heart. The blood entering the sinusoidal cavity is collected through small capillaries that connect to the dorsal aorta just anterior to where the two lateral dorsal aortae merge into a single median vessel. This is analogous to the situation at the other branchial arches. Blood coming from the heart enters the gills through the afferent branchial arteries and connects to the dorsal aorta via the efferent branchial arteries (Olson, 2002; Skov & Bennett, 2005). However, in contrast to what occurs at the other branchial (gill-bearing) arches, the fifth (tooth-bearing) arch delivers deoxygenated blood to the dorsal aorta. We can only speculate about the level of deoxygenation as the blood passes through the pharyngeal jaws. In rainbow trout, only 20–28% of the oxygen transported to the tissues of the avascular retina is consumed (Waser & Heisler, 2004). Likewise, we assume that the oxygen levels in the blood are only partially depleted after leaving the dentigerous area in the zebrafish. Hence, the remaining oxygen would become redistributed via the dorsal aorta to the other organs and tissues.
Spatiotemporal relationship between blood vessels and teeth
A 3D reconstruction has provided a clear visual representation of the spatial relationship between vascular elements and teeth. Capillaries branching from the sinusoidal cavity enter the pulp and constitute the intrinsic blood supply of the functional (i.e. attached and erupted) teeth.
Comparatively little work has been conducted with respect to the vascular supply of teeth in common laboratory animals (rat, rabbit, hamster, opossum, monkey). Essentially, the blood supply of the teeth is considered to be coming from different sources, depending on the species (e.g. periosteal vessels, superior/inferior alveolar artery), and every tooth has its intrinsic supply which comprises a single or several smaller pulp vessels entering through the apical foramen (Perint, 1949; James, 1955; Cheng & Provenza, 1959; Adams, 1962a,b; Boyer & Neptune, 1962). Similar to what we see in zebrafish, Cutright & Bhaskar (1969) have demonstrated the presence of a vascular plexus encircling the primitive tooth germs in the area of the dental sac in rhesus monkeys. Branches coming from this plexus enter the dental pulp and secure the intrinsic blood supply of the tooth. It is possible that in zebrafish, due to the arrangement of the teeth in three rows (rather than one row, as in rhesus monkeys), the vascular plexus of each tooth has anastomosed to generate one large sinusoidal cavity, giving off branches that will constitute the intrinsic blood supply of the teeth.
Unlike in some other teleosts, we have not observed any evidence for capillaries penetrating the outer dental epithelium. In the Nile tilapia (Oreochromis niloticus), an advanced teleost, many capillaries can be seen closely associated with the dental epithelium (Prostak et al. 1993; Sasagawa, 1997). These capillaries are most likely involved in active transport of inorganic ions (Garant, 1970) and fluoride (Suga et al. 1983) during formation, mineralization and maturation of enameloid. A possible reason for the distinctive distribution of blood vessels in the enamel organ in the two species could be the small tooth size and especially the small size of the enameloid cap in zebrafish compared to the Nile tilapia. Transport of ions, e.g. through gap junctions (Huysseune & Sire, 1997), could be sufficient without involving the need for blood vessels.
The fact that the dentigerous area in zebrafish is so richly supplied with blood strongly suggests that the vascular system has an important role in the development and maintenance of the dentition. The extensive vascularization may even be connected with continuous replacement of teeth. However, the sinusoidal cavity develops relatively late and is not present when the first replacement tooth forms. In addition, blood vessels penetrating the pulp cavity have only been observed from second-generation teeth onwards, and only at the stage of cytodifferentiation, i.e. relatively late in tooth development. This suggests that the vasculature is not likely to be a trigger for tooth replacement as a process. Indeed, studies conducted in the past, albeit on mammals, have linked blood vessels to processes such as tooth eruption and exfoliation, rather than tooth initiation (Hunter, 1778; Tomes, 1882; Massler & Schour, 1941; Miller, 1957; Kaku et al. 2001). Interestingly, a study conducted in mice and rabbits regarding the role of vascularization in tooth eruption and resorption, has demonstrated a connection between the number of osteoclasts and the degree to which the dental and surrounding tissues are vascularized (Miller, 1957). This supports a role for blood vessels in tooth resorption rather than tooth initiation. The larval teeth in zebrafish do not appear to contain pulpal vessels, confirming the observations already made by Sire et al. (2002) for zebrafish and other species, nor has the sinusoidal cavity developed yet. Still, these teeth are resorbed. This contradicts a role for blood vessels in tooth resorption. However, the observation that first-generation teeth persist and coexist with their successors for several generations (Van der Heyden et al. 2000) might be linked to the lack of pulpal blood vessels in the larval teeth. The poor vascularization of first-generation teeth is probably connected to the small size of the dental pulp (approximately two to three cells wide), which is too small to accommodate vascular elements. Apart from a role in resorption, pulpal vessels can also serve a nutritive function. Later-generation teeth in zebrafish are larger in size than first-generation teeth, increasing the need for nutrient and oxygen supply via the circulatory system. It is safe to assume that they require ingrowth of capillaries to grow beyond a certain size. Likewise, tumours are not able to grow beyond a few millimetres without the ingrowth of capillaries from adjacent blood vessels (Folkman, 2001).
To determine the role of blood vessels in the process of tooth development and/or replacement, further research clearly needs to be done. For example, the study of vascular mutants or experiments involving the pharmaceutical blocking of vascular sprouts issuing from the sinusoidal cavity could help to elucidate the role of the vasculature in the dentition of zebrafish. Recent studies have shown the formation of supernumerary teeth in the zebrafish dentition, either when activating fgf signalling (Jackman et al. 2013) or when using retinoic acid (RA) treatments (Seritrakul et al. 2012). Exposure to exogenous RA has been shown to shift anteriorly the expression pattern of genes normally expressed in the tooth forming region (e.g. pitx2 and dlx2b), causing a dramatic anterior expansion of the pharyngeal teeth (Seritrakul et al. 2012). Those authors have linked this observation to a potential evolutionary role for RA in the dentition. In addition, the loss of fgf signalling in the oral epithelium has been hypothesized to be linked with cypriniform tooth loss (Stock et al. 2006). Both fgf and RA have already been shown to be involved in processes of blood vessel formation and are commonly targeted in cancer treatments to inhibit deregulated blood vessel formation (Cross & Claesson-Welsh, 2001; Hoffmann et al. 2007; Kim et al. 2012). Thus, loss of teeth or presence of supernumerary teeth resulting from inhibition, respectively overactivation, of these factors during tooth development, may well be mediated by the effect these factors have on the vasculature. It would be interesting to see whether these ectopic teeth are similarly vascularized (e.g. is there also a vascular plexus present?) and whether they can be replaced. However, the lack of any published detailed histology of these supernumerary teeth prevents us from making any conclusion regarding their vascularization. Such studies may also shed light on whether vascularization plays a role in evolutionary changes in number, position or cycling of teeth. For example, Sutton & Graze (1985) have hypothesized that the hydrodynamic pressure caused by blood flow in the dental pulp and the tissues surrounding the tooth, exerts a force towards the tooth and could cause it to move. Given the abundance of blood vessels in the dentigerous region as described here for zebrafish, hydrodynamic pressures as stated by the blood-vessel thrust theory (Sutton & Graze, 1985) could be involved in positioning of the teeth.
Concluding remarks
We have identified the blood supply to the pharyngeal jaws and revealed the presence of a remarkable vascular pattern serving the dentition in zebrafish. Having established this morphological baseline information, we can now engage in studies aiming to uncover the role of these blood vessels during tooth development and replacement. Based on the morphology, a role in initiation of replacement tooth formation seems unlikely, but this needs confirmation. The vasculature is probably more involved in tooth resorption and nutrition. However, further studies are required to reveal the role of the vasculature in a continuously replacing dentition, such as found in zebrafish.
Acknowledgments
We thank Mieke Soenens and Dennis Vlaeminck for expert technical assistance, and the lab of Evolutionary Developmental Biology for support and discussions. J.C. acknowledges a grant from the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
References
- Adams D. The blood supply to developing dentine. Arch Oral Biol. 1962a;7:773–774. doi: 10.1016/0003-9969(62)90127-9. [DOI] [PubMed] [Google Scholar]
- Adams D. The blood supply to the enamel organ of the rodent incisor. Arch Oral Biol. 1962b;7:279–286. doi: 10.1016/0003-9969(62)90018-3. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Pham VN, Vogel AM, et al. Loss of unc45a precipitates arteriovenous shunting in the aortic arches. Dev Biol. 2008;318:258–267. doi: 10.1016/j.ydbio.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borday-Birraux V, Van der Heyden C, Debiais-Thibaud M, et al. Expression of Dlx genes during the development of the zebrafish pharyngeal dentition: evolutionary implications. Evol Dev. 2006;8:130–141. doi: 10.1111/j.1525-142X.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Boyer CC, Neptune CM. Patterns of blood supply to teeth and adjacent tissues. J Dent Res. 1962;41:158–171. [Google Scholar]
- Cheng TC, Provenza DV. Histologic observations on the morphology of the blood vessels of canine and human tooth pulp. J Dent Res. 1959;38:552–557. doi: 10.1177/00220345590380031801. [DOI] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Crisan M, Corselli M, Chen WC, et al. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16:2851–2860. doi: 10.1111/j.1582-4934.2012.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- Cutright DE, Bhaskar SN. Pulpal vasculature as demonstrated by a new method. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1969;27:678–683. doi: 10.1016/0030-4220(69)90104-2. [DOI] [PubMed] [Google Scholar]
- Ellertsdottir E, Lenard A, Blum Y, et al. Vascular morphogenesis in the zebrafish embryo. Dev Biol. 2010;341:56–65. doi: 10.1016/j.ydbio.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Ellett F, Lieschke GJ. Zebrafish as a model for vertebrate hematopoiesis. Curr Opin Pharmacol. 2010;10:563–570. doi: 10.1016/j.coph.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Farrell AP, Farrell ND, Jourdan H. A perspective on the evolution of the coronary circulation in fishes and the transition to terrestrial life. In: Sedmera D, Wang T, et al., editors. Ontogeny and Phylogeny of the Vertebrate Heart. New York: Springer; 2012. pp. 75–102. [Google Scholar]
- Folkman J. Angiogenesis-dependent diseases. Semin Oncol. 2001;28:536–542. doi: 10.1016/s0093-7754(01)90021-1. [DOI] [PubMed] [Google Scholar]
- Garant PR. Observations on the ultrastructure of the ectodermal component during odontogenesis in Helostoma temmincki. Anat Rec. 1970;166:167–187. doi: 10.1002/ar.1091660206. [DOI] [PubMed] [Google Scholar]
- Geudens I, Gerhardt H. Coordinating cell behaviour during blood vessel formation. Development. 2011;138:4569–4583. doi: 10.1242/dev.062323. [DOI] [PubMed] [Google Scholar]
- Herwig L, Blum Y, Krudewig A, et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21:1942–1948. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Hoffmann S, Rockenstein A, Ramaswamy A, et al. Retinoic acid inhibits angiogenesis and tumor growth of thyroid cancer cells. Mol Cell Endocrinol. 2007;264:74–81. doi: 10.1016/j.mce.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Hu N, Sedmera D, Yost HJ, et al. Structure and function of the developing zebrafish heart. Anat Rec. 2000;260:148–157. doi: 10.1002/1097-0185(20001001)260:2<148::AID-AR50>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hu N, Yost HJ, Clark EB. Cardiac morphology and blood pressure in the adult zebrafish. Anat Rec. 2001;264:1–12. doi: 10.1002/ar.1111. [DOI] [PubMed] [Google Scholar]
- Hunter J. The Natural History of the Human Teeth. London: J. Johnson; 1778. [Google Scholar]
- Huysseune A. Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int J Dev Biol. 2006;50:637–643. doi: 10.1387/ijdb.052098ah. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J-Y. Development of cartilage and bone tissues of the anterior part of the mandible in cichlid fish: a light and TEM study. Anat Rec. 1992;233:357–375. doi: 10.1002/ar.1092330304. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire JY. Structure and development of first-generation teeth in the cichlid Hemichromis bimaculatus (Teleostei, Cichlidae) Tissue Cell. 1997;29:679–697. doi: 10.1016/s0040-8166(97)80044-4. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in nonmammalian vertebrates. Eur J Oral Sci. 1998;106:437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Thesleff I. Continuous tooth replacement: the possible involvement of epithelial stem cells. BioEssays. 2004;26:665–671. doi: 10.1002/bies.20039. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Van der Heyden C, Sire JY. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae) Anat Embryol. 1998;198:289–305. doi: 10.1007/s004290050185. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M. The earliest stages in the development of the circulatory system of the rainbow trout Oncorhynchus mykiss. J Morphol. 1997;233:215–236. doi: 10.1002/(SICI)1097-4687(199709)233:3<215::AID-JMOR2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, et al. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Jackman WR, Davies SH, Lyons DB, et al. Manipulation of Fgf and Bmp signaling in teleost fishes suggests potential pathways for the evolutionary origin of multicuspid teeth. Evol Dev. 2013;15:107–118. doi: 10.1111/ede.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Au P, Tam J, et al. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- James WW. The blood capillary system of the odontoblast layer of the dental pulp. J Anat. 1955;89:547–549. [PMC free article] [PubMed] [Google Scholar]
- Kaku M, Kohno S, Kawata T, et al. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J Dent Res. 2001;80:1880–1883. doi: 10.1177/00220345010800100401. [DOI] [PubMed] [Google Scholar]
- Kamei M, Isogai S, Weinstein BM. Imaging blood vessels in the zebrafish. Methods in Cell Biology. 2004;76:51–74. doi: 10.1016/s0091-679x(04)76004-5. [DOI] [PubMed] [Google Scholar]
- Kim JM, Shin HI, Cha SS, et al. DJ-1 promotes angiogenesis and osteogenesis by activating FGF receptor-1 signaling. Nat Commun. 2012;3:1296. doi: 10.1038/ncomms2313. [DOI] [PubMed] [Google Scholar]
- Kindlova M, Matena V. Blood vessels of the rat molar. J Dent Res. 1962;41:650–660. doi: 10.1177/00220345620410031801. [DOI] [PubMed] [Google Scholar]
- Lechguer AN, Kuchler-Bopp S, Hu B, et al. Vascularization of engineered teeth. J Dent Res. 2008;87:1138–1143. doi: 10.1177/154405910808701216. [DOI] [PubMed] [Google Scholar]
- Lele Z, Krone PH. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol Adv. 1996;14:57–72. doi: 10.1016/0734-9750(96)00004-3. [DOI] [PubMed] [Google Scholar]
- Lovett M, Lee K, Edwards A, et al. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15:353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massler M, Schour I. Studies in tooth development: theories of eruption. Am J Orthod Oral Surg. 1941;27:552–576. [Google Scholar]
- Mattuella LG, Bento LW, de Figueiredo JAP, et al. Vascular endothelial growth factor and its relationship with the dental pulp. J Endod. 2007;33:524–530. doi: 10.1016/j.joen.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Miller BG. Investigations of the influence of vascularity and innervation on tooth resorption and eruption. J Dent Res. 1957;36:669–676. doi: 10.1177/00220345570360050501. [DOI] [PubMed] [Google Scholar]
- Munoz-Chapuli R. Evolution of angiogenesis. Int J Dev Biol. 2011;55:345–351. doi: 10.1387/ijdb.103212rm. [DOI] [PubMed] [Google Scholar]
- Olson KR. Chapter 10 – Circulatory system. In: Gary KO, editor. The Laboratory Fish. London: Academic Press; 2000a. pp. 161–171. [Google Scholar]
- Olson KR. Chapter 22 – Circulatory system. In: Gary KO, editor. The Laboratory Fish. London: Academic Press; 2000b. pp. 369–378. [Google Scholar]
- Olson KR. Vascular anatomy of the fish gill. J Exp Zool. 2002;293:214–231. doi: 10.1002/jez.10131. [DOI] [PubMed] [Google Scholar]
- Perint J. Detailed roentgenologic examination of the blood supply in the jaws and teeth by applying radiopaque solutions. Oral Surg Oral Med Oral Pathol. 1949;2:2–20. doi: 10.1016/0030-4220(49)90121-8. [DOI] [PubMed] [Google Scholar]
- Prostak KS, Seifert P, Skobe Z. Enameloid formation in 2 tetraodontiform fish species with high and low fluoride contents in enameloid. Arch Oral Biol. 1993;38:1031–1044. doi: 10.1016/0003-9969(93)90164-h. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, et al. Coronary arteries form by developmental reprogramming of venous cells. Nature. 2010;464:549–554. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roush W. Zebrafish embryology builds better model vertebrate. Science. 1996;272:1103. doi: 10.1126/science.272.5265.1103. [DOI] [PubMed] [Google Scholar]
- Sasagawa I. Fine structure of the cap enameloid and of the dental epithelial cells during enameloid mineralisation and early maturation stages in the tilapia, a teleost. J Anat. 1997;190:589–600. doi: 10.1046/j.1469-7580.1997.19040589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seritrakul P, Samarut E, Lama TTS, et al. Retinoic acid expands the evolutionarily reduced dentition of zebrafish. FASEB J. 2012;26:5014–5024. doi: 10.1096/fj.12-209304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sire JY, Davit-Beal T, Delgado S, et al. First-generation teeth in nonmammalian lineages: evidence for a conserved ancestral character? Microsc Res Tech. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- Skov PV, Bennett MB. Branchial vascular pathways in two species of Tetraodontiformes and the concept of secondary vessels and nutrient arteries. Zoomorphology. 2005;124:79–88. [Google Scholar]
- Stock DW. Zebrafish dentition in comparative context. J Exp Zool B Mol Dev Evol. 2007;308B:523–549. doi: 10.1002/jez.b.21187. [DOI] [PubMed] [Google Scholar]
- Stock DW, Jackman WR, Trapani J. Developmental genetic mechanisms of evolutionary tooth loss in cypriniform fishes. Development. 2006;133:3127–3137. doi: 10.1242/dev.02459. [DOI] [PubMed] [Google Scholar]
- Suga S, Taki Y, Wada K. Fluoride concentration in the teeth of perciform fishes and its phylogenetic significance. Jpn J Ichthyol. 1983;30:81–93. [Google Scholar]
- Sutton PRN, Graze HR. The blood-vessel thrust theory of tooth eruption and migration. Med Hypotheses. 1985;18:289–295. doi: 10.1016/0306-9877(85)90030-1. [DOI] [PubMed] [Google Scholar]
- Tomes CS., Sir . A Manual of Dental Anatomy, Human and Comparative. London: J. & A. Churchill; 1882. [Google Scholar]
- Van der Heyden C, Huysseune A. Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae) Dev Dyn. 2000;219:486–496. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1069>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Van der Heyden C, Huysseune A, Sire JY. Development and fine structure of pharyngeal replacement teeth in juvenile zebrafish (Danio rerio) (Teleostei, Cyprinidae) Cell Tissue Res. 2000;302:205–219. doi: 10.1007/s004410000180. [DOI] [PubMed] [Google Scholar]
- Van der Heyden C, Wautier K, Huysseune A. Tooth succession in the zebrafish (Danio rerio. Arch Oral Biol. 2001;46:1051–1058. doi: 10.1016/s0003-9969(01)00064-4. [DOI] [PubMed] [Google Scholar]
- Van der Heyden C, Allizard F, Sire JY, et al. Tooth development in vitro in two teleost fish, the cichlid Hemichromis bimaculatus and the cyprinid Danio rerio. Cell Tissue Res. 2005;321:375–389. doi: 10.1007/s00441-004-1036-x. [DOI] [PubMed] [Google Scholar]
- Verstraeten B, Sanders E, van Hengel J, et al. Zebrafish teeth as a model for repetitive epithelial morphogenesis: dynamics of E-cadherin expression. BMC Dev Biol. 2010;10:58–68. doi: 10.1186/1471-213X-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten B, van Hengel J, Sanders E, et al. N-cadherin is required for cytodifferentiation during zebrafish odontogenesis. J Dent Res. 2013;92:365–370. doi: 10.1177/0022034513477424. [DOI] [PubMed] [Google Scholar]
- Waser WP, Heisler N. Oxygen delivery to the fish eye: blood flow in the pseudobranchial artery of rainbow trout (Oncorhynchus mykiss. Fish Physiol Biochem. 2004;30:77–85. [Google Scholar]
- Wautier K, Van der Heyden C, Huysseune A. A quantitative analysis of pharyngeal tooth shape in the zebrafish (Danio rerio, Teleostei, Cyprinidae) Arch Oral Biol. 2001;46:67–75. doi: 10.1016/s0003-9969(00)00091-1. [DOI] [PubMed] [Google Scholar]
- Weinstein BM. Plumbing the mysteries of vascular development using the zebrafish. Semin Cell Dev Biol. 2002;13:515–522. doi: 10.1016/s1084952102001052. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene: University of Oregon Press; 1993. p. 385. [Google Scholar]
- Zon LI. Developmental biology of hematopoiesis. Blood. 1995;86:2876–2891. [PubMed] [Google Scholar]


